Abstract
The nuclear pore complex (NPC) is a huge protein complex embedded in the nuclear envelope. It has central functions in nucleocytoplasmic transport, nuclear framework, and gene regulation. Nucleoporin 107 kDa (NUP107) is a component of the NPC central scaffold and is an essential protein in all eukaryotic cells. Here, we report on biallelic NUP107 mutations in nine affected individuals who are from five unrelated families and show early-onset steroid-resistant nephrotic syndrome (SRNS). These individuals have pathologically focal segmental glomerulosclerosis, a condition that leads to end-stage renal disease with high frequency. NUP107 is ubiquitously expressed, including in glomerular podocytes. Three of four NUP107 mutations detected in the affected individuals hamper NUP107 binding to NUP133 (nucleoporin 133 kDa) and NUP107 incorporation into NPCs in vitro. Zebrafish with nup107 knockdown generated by morpholino oligonucleotides displayed hypoplastic glomerulus structures and abnormal podocyte foot processes, thereby mimicking the pathological changes seen in the kidneys of the SRNS individuals with NUP107 mutations. Considering the unique properties of the podocyte (highly differentiated foot-process architecture and slit membrane and the inability to regenerate), we propose a “podocyte-injury model” as the pathomechanism for SRNS due to biallelic NUP107 mutations.
Introduction
Nephrotic syndrome (NS) is a renal disease caused by disruption of the glomerular filtration barrier, which results in massive proteinuria, hypoalbuminemia, and dyslipidemia. Idiopathic NS occurs in 16/100,000 children.1 Most children with idiopathic NS respond well to steroids, but 10%–20% of affected children are categorized as having steroid-resistant NS (SRNS).2–6 SRNS is a clinically and genetically heterogeneous renal disorder that might have an immunological, structural, or functional etiology.2,5,7–9 Higher rates of genetic delineation are expected in early-onset SRNS.7 Clinical differences in SRNS have been suggested to depend on its age of onset.7 Current medical management and prognosis in NS are based largely on the histological diagnosis. Effective SRNS treatments are not well established, and renal transplantation is eventually required. Importantly, 63%–73% of those with childhood-onset SRNS show pathologically focal segmental glomerulosclerosis (FSGS),which carries a great risk of progression to end-stage renal disease (ESRD).1,6,8,10 To date, at least 27 genes are associated with SRNS, thereby expanding our knowledge of the pathomechanisms involved in SRNS and podocyte development and function.11 Although SRNS is the leading cause of ESRD in children worldwide, approximately 70% of those with childhood-onset SRNS are genetically uncharacterized.7,11 We describe here an additional genetic cause of early-onset SRNS and propose its possible pathomechanism.
Material and Methods
Human Subjects
A total of 18 families (10 with affected siblings and 8 with a single affected individual) who lack any known genetic causes of SRNS (in 27 known genes) were recruited to this study. They presented with non-syndromic early-onset SRNS with onset ages between 1 and 11 years. The clinical aspects of 7 of the 18 families have been described previously.12 Affected individuals were resistant to standard steroid therapy but were partially responsive to immunosuppressive drugs. At least ten affected individuals in eight families underwent renal transplants and have had no recurrence of SRNS to date. All samples were collected after written informed consent was obtained. The study protocol was approved by the institutional review boards of Yokohama City University School of Medicine, Kansai Medical University, RIKEN, Tokyo Women’s Hospital, and Kobe University.
DNA Extraction
Peripheral-blood leukocytes or saliva from affected individuals and their families was collected. Genomic DNA was extracted with a QIAamp DNA Blood Max Kit (QIAGEN) or Oragene DNA (DNA Genoteck) according to the instructions of each manufacturer.
Whole-Exome Sequencing and Informatics Analyses
Whole-exome sequencing (WES) was performed on affected individuals (one individual from each family) and their parents when the samples were available, as reported previously.13 In brief, 3-μg samples of genomic DNA were sheared with the Covaris S2 system (Covaris); genome partitioning was performed with SureSelect Human All Exon V5 (Agilent Technology) according to the manufacturer’s instructions. Prepared samples were run on a HiSeq 2000 instrument (Illumina) with 101-bp paired-end reads and 7-bp index reads. The sequence reads were mapped to the human reference sequence (GRCh37) by Novoalign 3.00. Next, PCR duplication and variant calls were processed by Picard and the Genome Analysis Toolkit. Ten of the 18 families have multiple affected children, suggesting the autosomal-recessive model, in which homozygous or compound-heterozygous variants are focused in each affected individual. Genetic variants in exons and canonical splice sites (±2 bp) with a minor allele frequency (MAF) of >0.005 in the NHLBI Exome Sequencing Project Exome Variant Server (EVS), Exome Aggregation Consortium (ExAC) Browser, Human Genetic Variation Database (HGVD, which is a public exome database for the Japanese population), or in-house Japanese exome data (n = 575) were removed from the candidates. Genes that harbor recessive variants detected commonly in two or more probands were selected. Candidate recessive variants were checked in each family by Sanger sequencing for confirmation that such variants co-segregated with the disease.
Haplotype Analysis
To determine the haplotype associated with c.2492A>C (p.Asp831Ala), which was found commonly in the five families, we amplified samples of genomic DNA or whole-genome-amplified DNA with 13 microsatellite markers (D12S364, S12S310, D12S1617, D12S345, D12S85, D12S368, D12S83, D12S326, D12S351, D12S346, D12S78, D12S79, and D12S86) from the ABI PRISM Linkage Mapping Set (Life Technologies).The PCR products were run on a 3500xl Genetic Analyzer (Life Technologies) and analyzed with GeneMapper 5 software (Life Technologies). Additionally, informative SNPs were chosen from the WES data for each affected individual and used thereafter for constructing haplotype blocks.
Expression of Human NUP107
NUP107 (nucleoporin 107 kDa; GenBank: NM_020401.2; MIM: 607617) expression in human embryos and adults was checked by a TaqMan Gene Expression Assay with two probe sets (Hs00914854_g1 and Hs00220703_m1 from Life Technologies) internally standardized by beta actin (Life Technologies). cDNA from human fetal and adult tissues was purchased from Clontech. qPCR was performed by a Rotor-Gene Q instrument (QIAGEN), the data from which was analyzed by the ΔΔCt method with Rotor-Gene 6000 Series software (QIAGEN). The experiments were done in duplicate. The expression level of each tissue represents the mean value of the duplicates.
Histopathology and Transmission Electron Microscopy on Samples from Individuals with Early-Onset SRNS
We stained 3-μm-thick sections cut from paraffin-embedded biopsied kidney tissues with H&E, periodic acid-Schiff stain, and periodic acid methenamine silver stain according to standard methods. For transmission electron microscopy, 1-mm renal-biopsy specimen cubes were fixed in 2% phosphate-buffered glutaraldehyde (pH 7.3) at room temperature, dehydrated in an alcohol gradient, and embedded in Epon-Araldite resin. Sections of 1-μm thickness were cut with an ultra-microtome (Ultracut UCT, Leica), stained with toluidine blue, and examined with a light microscope. Ultrathin sections (60–90 nm) stained by lead citrate were examined with a JEM1011 transmission electron microscope (JEOL). The TUNEL method was used to detect apoptotic cells on tissue sections with an in situ apoptosis detection kit (Takara) according to the manufacturer’s instruction.
Immunofluorescence Microscopy
We deparaffinized and rehydrated 3-μm-thick paraffin sections of a necropsy specimen and then autoclaved them in target retrieval solution (S1700, Dako) for 15 min at 105°C. The sections were subjected to immunofluorescence labeling with primary antibodies including rabbit anti-NUP107 mAb (1.5 μg/ml, EPR12241, ab182559, Abcam), mouse anti-WT1 mAb (1:100, WT49, NCL-L-WT1-562, Leica), and mouse anti-Ezrin mAb (1:500, 3C12, E8897, lot 102K4824, Sigma-Aldrich). Normal rabbit and mouse immunoglobulins (IgGs) (sc-2027 [lot L1212] and sc-2025 [lot H1512], respectively, Santa Cruz) were used for negative controls. The CSAII kit (K1497, DAKO) was used for signal amplification of WT1, and other primary antibodies were visualized with Alexa555-conjugated anti-rabbit (1 μg/ml) or Alexa647-conjugated anti-mouse IgG (2 μg/ml) secondary antibodies (A21429 or A21236, respectively, Life Technologies), and then samples were mounted with ProLong Gold antifade reagent (P36930, Life Technologies). Single optical sections were acquired at 16-bit data depth with a confocal microscope system (AxioImager.Z1 microscope with LSM 700 laser scanner, Carl Zeiss) equipped with a C-Apochromat water immersion objective (40×, 1.2 numerical aperture [NA], Carl Zeiss); images were arranged with Photoshop CS5 (Adobe Systems).
Expression Vectors
Mammalian expression vectors were prepared with the Gateway system (Life Technologies). The NUP107 open reading frame was amplified by PCR with human cDNA derived from a human lymphoblastoid cell line. The PCR product was introduced into the Gateway pDONR221 vector (Life Technologies), and its sequence was confirmed by Sanger sequencing. For mutagenesis, a QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) was used. After confirming appropriate mutagenesis, we performed LR recombination to create a mammalian expression vector (pcDNA-DEST53, Life Technologies) to produce N-terminally GFP-fused NUP107 proteins. Among four NUP107 mutations observed in this cohort, c.969+1G>A was mimicked by c.969_970insTAG, which created the nonsense codon just after the mutation (p.Asp324∗). Whereas two truncating mutations (c.969+1G>A and c.1079_1083delAAGAG [p.Glu360Glyfs∗6]) are thought unlikely to be present in vivo because of nonsense-mediated decay, these constructs were used as controls for the binding loss, given that C-terminally truncated proteins reportedly lose the NUP107-NUP133 interaction.14
Cell-free Protein Synthesis and In Vitro Pull-Down Assays
In vitro transcription and cell-free protein synthesis were performed as described previously.15,16 In vitro transcription templates for wild-type or mutant NUP107 were amplified by slit-primer PCR. For generation of transcription templates, the first PCR was performed with 50 ng/μl of each plasmid, 100 nM of the S1 common primer (5′-CCACCCACCACCACCAACAAAAAAGCAGGCTATG-3′), and 100 nM of the vector-specific reverse primer (5′-ATCTTTTCTACGGGGTCTGA-3′). The second PCR was performed with the first PCR product as a template with 100 μM of the SPu primer (5′-GCGTAGCATTTAGGTGACACT-3′), 100 μM of the vector-specific reverse primer (5′-ACGTTAAGGGATTTTGGTCA-3′), and 1 μM of either the deSP6-E02-FLAG-tagged primer or the biotin-ligation site (bls) primer for the addition of the nucleotide sequences of the FLAG tag or the bls tag, respectively (FLAG tagged: 5′-GGTGACACTATAGAACTCACCTATCTCTCTACACAAAACATTTCCCTACATACAACTTTCAACTTCCTATTATGGACTACAAGGATGACGATGACAAGCTCCACCCACCACCACCAATG-3′; bls tagged: 5′-GGTGACACTATAGAACTCACCTATCTCTCTACACAAAACATTTCCCTACATACAACTTTCAACTTCCTATTATGGGCCTGAACGACATCTTCGAGGCCCAGAAGATCGAGTGGCACGAACTCCACCCACCACCACCAATG-3′).
An ENDEXT Wheat Germ Expression Kit (CellFree Sciences) was used for cell-free protein synthesis according to the manufacturer’s instructions for the bilayer translation method. Biotinylated proteins were produced as described previously.17
Biotinylated wild-type or altered NUP107 was mixed with FLAG-NUP133 (nucleoporin 133 kDa; GenBank: NM_018230.2; MIM: 607613) in lysis buffer containing 25 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 2% Triton X-100, 1 mM DTT, and 10 mg/ml BSA. After incubation for 1 hr at 26°C, streptavidin MagneSphere beads (Promega) were added, and the mixture was incubated for 30 min at room temperature. After three washes with lysis buffer, bound proteins were eluted from the beads with 20 μl of 2× SDS sample buffer. Bound proteins were separated by SDS-PAGE followed by immunoblotting with an anti-FLAG antibody (Sigma-Aldrich) or a Streptavidin-HRP conjugate (GE Healthcare). Proteins on the blot were detected with Immobilon Western Chemiluminescent HRP Substrate (Millipore) and FluorChem FC2 (Alpha Innotech) in accordance with the protocol from each manufacturer.
Immunoprecipitation
The cell lysate used for immunoprecipitation was prepared according to a method reported previously18,19 with a slight modification. In brief, HeLa cells were transfected with the wild-type or altered N-terminally GFP-fused NUP107 construct by Viafect (Promega) according to the manufacturer’s instructions. The cells were lysed with lysis buffer containing 10 mM Tris-HCl (pH 7.4), 400 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM DTT supplemented with complete proteinase inhibitor cocktail (Roche Diagnostics GmbH), and PhosSTOP (Roche Diagnostics); sonicated; and then incubated for 30 min at 4°C. For debris removal, the crude lysate was centrifuged at 20,630 × g for 20 min at 4°C. After collection, the supernatant was diluted 3.75× in dilution buffer (10 mM Tris-HCl [pH 7.4], 2 mM EDTA, 1 mM DTT, complete proteinase inhibitor cocktail, and PhosSTOP). For immunoprecipitation of the GFP-fused NUP107, mouse anti-GFP antibody (11-814-460-001, Roche Diagnostics) and Protein G Sepharose beads (17-0618-01, GE Healthcare) were added. After incubation for 2 hr at 4°C, the beads were washed with wash buffer (lysis buffer diluted 3.75× in dilution buffer). After the protein-bound beads were boiled, they were run on an SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane (Millipore). Membranes prepared in this manner were incubated in 0.2% Casein in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for blocking. The membrane was probed with rabbit anti-GFP primary antibody (598, MBL) diluted at 1:1,000 and mouse anti-NUP133 (M00055746-M01, Abnova) diluted at 1:500 followed by secondary antibodies HRP-rabbit anti-rat IgG (A5795, Sigma-Aldrich) and HRP-goat anti-mouse IgG (170-6516, Bio-Rad) both diluted at 1:3,000 with 0.2% Casein in TBS-T. For obtaining protein signals, Immobilon Western Chemiluminescent HRP Substrate (Millipore) was used as a chemiluminescence substrate.
Subcellular Localization of NUP107
HeLa cells cultured in DMEM (Life Technologies) containing 10% fetal bovine serum (Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2 on poly-L-lysine-coated coverslips (Wako) were transfected with the wild-type or altered N-terminally GFP-fused NUP107 vector with the use of Viafect (Promega). After incubation for 48 hr, the cells were washed with pre-warmed PBS at 37°C and then fixed with pre-warmed 2% paraformaldehyde (Wako) in PBS at 37°C for 10 min. The cells were treated with 0.5% Triton X-100 in PBS for 2.5 min and then incubated with 5% normal goat serum (NGS, Merck Millipore) in PBS for 1 hr. After blocking, the cells were reacted with the primary antibody (MAb414 [mouse anti-nuclear pore complex (NPC) proteins], MMS-120P, Covance) diluted at 1:3,000 in 1% NGS in PBS for 2 hr, washed with PBS, and then reacted with the secondary antibody (Alexa Fluor 594 goat anti-mouse IgG, A11032, Life Technologies) in 1% NGS in PBS for 2 hr. After staining, the cells were mounted in paraphenylenediamine solution (80% glycerol in PBS and 1 mg/ml paraphenylenediamine, 11873580001, Roche Diagnostics). Images were captured with a DeltaVision microscope (Applied Precision) equipped with a Plan Apo objective lens (100×, 1.35 NA, Olympus) and a Cool Snap HQ2 CCD camera (Photometrics).
Zebrafish Knockdown by Microinjection of Morpholino Oligonucleotides
The antisense morpholino oligonucleotides (MOs) for nup107 translation blocking (TB) (5′-AAGTCTGACTCCATTCCATATTGTC-3′)20 and for nup107 splice blocking (SB) (5′-ATACATTTAAGCTCACCTCTCTGAC-3′) and a standard MO control (5′-CCTCTTACCTCAGTTACAATTTATA-3′) obtained from Gene Tools were injected into 1- to 2-cell-stage embryos, each at a final concentration of 0.25 mM. The experiment was authorized by the Institutional Committee for Fish Experiments at the National Research Institute of Fisheries Science.
RNA Isolation and RT-PCR Analysis
Total RNA was extracted from embryos at 24 hr post-fertilization (hpf) with TRIzol reagent according to the manufacturer’s (Life Technologies) protocol. Double-stranded cDNA was synthesized with M-MLV reverse transcriptase (Promega) and then amplified by PCR with ExTaq (Takara). For detecting the splicing mutation (caused by the MO injections) in nup107 exon 24, the following primers were used: 5′-TGAACTGTCCTCCGGTGAAG-3′ (forward) and 5′-TGCGATGATGTCAGCAAGAC-3′ (reverse). For the PCR amplifications, the initial denaturing step at 94°C for 5 min was followed by 29 cycles of 30 s at 94°C, 30 s at 61°C, 30 s at 72°C, and a final extension of 7 min at 72°C. PCR products were separated on 3% agarose gels.
Histopathology and Transmission Electron Microscopy of Zebrafish
Larvae injected with control MO, nup107-TB MO, and nup107-SB MO at 5.5 days after fertilization were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight. After fixation, the samples were washed three times with 0.1 M cacodylate buffer for 30 min each and then postfixed with 2% osmium tetroxide in 0.1 M cacodylate buffer at 4°C for 3 hr. The samples were dehydrated in graded ethanol solution (50%, 70%, 90%, and 100%), infiltrated with propylene oxide (PO) two times for 30 min each, immersed in a 70:30 mixture of PO and resin (Quetol-812, Nisshin EM) for 1 hr, and then kept in an open-capped tube so that volatile PO would evaporate overnight. The samples were transferred to fresh 100% resin and polymerized at 60°C for 48 hr. The polymerized resins were cut into semi-thin (1.5-μm) sections with an Ultracut UCT (Leica) and then stained with 0.5% toluidine blue. Ultra-thin (70-nm) sections were cut on an Ultracut UCT (Leica) ultramicrotome and mounted on copper grids. The sections were stained with 2% uranyl acetate at room temperature for 15 min, washed with distilled water, and stained with lead stain solution (Sigma-Aldrich) at room temperature for 3 min. The grids were observed with a transmission electron microscope (JEM-1400Plus, JEOL) at 80 kV.
Molecular-Dynamics Simulation of the p.Asp831Ala Substitution in NUP107
Molecular-dynamics (MD) simulations of the wild-type and p.Asp831Ala Nup107 were carried out with the program package GROMACS (Groningen Machine for Chemical Simulation) version 5.0 with the Optimized Potentials for Liquid Simulations all-atom force field based on the local Møller-Plesset perturbation theory (OPLS-AA/L).21 The starting structure of NUP107 was extracted from the crystal structure of the NUP107-NUP133 complex (PDB: 3CQC). The missing regions in NUP107 were modeled with the Phyre2 modeling server,22 and the p.Asp831Ala substitution was introduced with FoldX software.23 The wild-type and altered NUP107 molecules were solvated with simple-point-charge water molecules in a cubic box extending at least 1.0 nm from the protein surface. Sodium ions were added to neutralize the systems, which were then subjected to energy minimization for 50,000 steps by steepest descent. The minimized systems were then equilibrated by position-restrained MD simulation for soaking the water molecules in the macromolecules in two steps as follows: an NVT ensemble (constant number of particles, volume, and temperature) for 100 ps and an NPT ensemble (constant number of particles, pressure, and temperature) for 4,000 ps each at 310 K. The well-equilibrated systems were then subjected to MD simulations for 30 ns each at 310 K without any restrictions. In all simulations, for maintaining a constant temperature of 310 K, temperature coupling using velocity rescaling with a stochastic term24 was employed with a coupling constant τ of 0.1 ps. Van der Waals interactions were modeled with 6–12 Lennard-Jones potentials with a 1.4-nm cutoff. Long-range electrostatic interactions were calculated with the particle-mesh Ewald method25 with a 1.4-nm cutoff for the real-space term. Covalent bonds were constrained with the LINCS algorithm.26
Results
Pathogenic Mutations Detected by WES
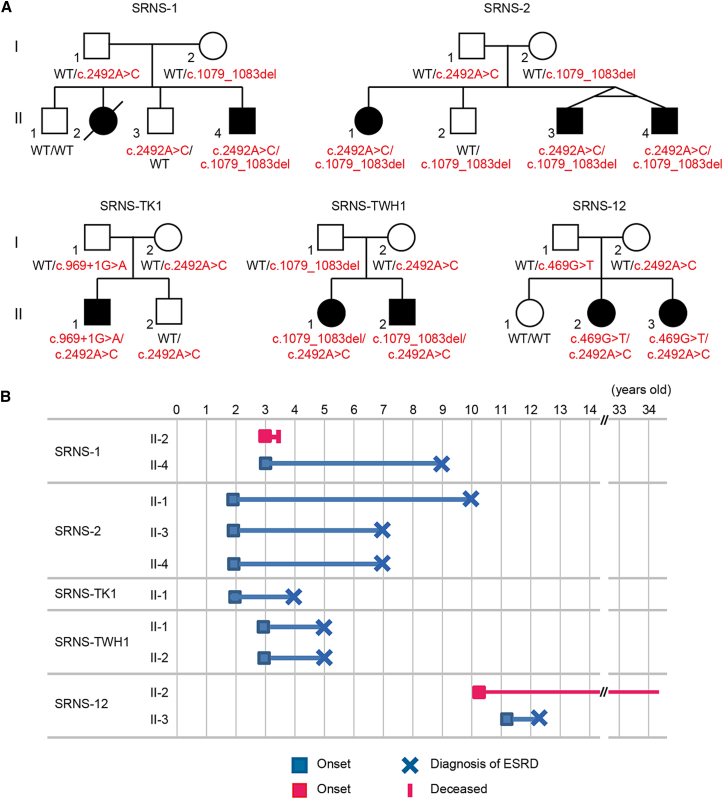
To identify the genetic cause of early-onset SRNS, we performed WES on 18 probands. Because we found multiple affected siblings in ten families, we speculated on an autosomal-recessive inheritance pattern for SRNS and focused on the recessive variants shared by two or more families with well-performed WES data (Tables S1–S3, S4, and S5). Biallelic mutations in NUP107, which encodes NUP107, were common in five families, and the mutation co-segregated perfectly with the affected state in all five families (Figure 1A, Table 1, and Figure S1). None of the other families in our cohort had any pathological variants in NUP107 or any other known genes associated with SRNS, as listed in Table S6.
Figure 1.
Genetic Analysis and Clinical Course of Early-Onset SRNS in Affected Individuals with NUP107 Mutations
(A) Familial pedigrees and NUP107 mutations. Mutant alleles are colored in red. WT indicates the wild-type allele. Filled and unfilled symbols represent affected and unaffected members, respectively.
(B) Clinical course of the affected individuals. The onset of renal symptoms and diagnosis of ESRD are represented by squares and crosses, respectively. Blue and red horizontal bars indicate the period leading to ESRD and the period before completed ESRD, respectively. SRNS-1 II-2 died from a viral infection before the advent of ESRD.
Table 1.
Clinical and Genetic Summary of SRNS-Affected Families Harboring NUP107 Mutations
| Family | Individual | Mutation | Age at Onset (Years) | Age at Diagnosis of ESRD (Years) | Treatment | Histology (Subtype, Age in Years) |
|---|---|---|---|---|---|---|
| SRNS-1a | II-2b | ND | 3 | NA | Pred | FSGS (NOS, 3) |
| II-4 | c.[1079_1083del];[2492A>C] | 3 | 9 | Pred, CyA, CPA | FSGS (NOS, 3) | |
| SRNS-2a | II-1 | c.[1079_1083del];[2492A>C] | 2 | 10 | Pred, CPA | MCNS (NOS, 2), FSGS (NOS, 4) |
| II-3 | c.[1079_1083del];[2492A>C] | 2 | 7 | Pred | MCNS (2) | |
| II-4 | c.[1079_1083del];[2492A>C] | 2 | 7 | Pred | FSGS (NOS, 2) | |
| SRNS-TK1 | II-1 | c.[969+1G>A];[2492A>C] | 2 | 4 | Pred, CyA, CPA | FSGS (NOS, 2) |
| SRNS-TWH1 | II-1 | c.[1079_1083del];[2492A>C] | 3 | 5 | Pred, ARB, PP | FSGS (collapsing, 3) |
| II-2 | c.[1079_1083del];[2492A>C] | 3 | 5 | Pred, CyA, ARB | FSGS (collapsing, 3) | |
| SRNS-12a | II-2 | c.[469G>T];[2492A>C] | 10 | NA | ARB | ND |
| II-3 | c.[469G>T];[2492A>C] | 11 | 12 | Pred, ARB | FSGS (NOS, 11) |
Abbreviations are as follows: ARB, AT II receptor blocker; collapsing, collapsing variants; CPA, cyclophosphamide; CyA, cyclosporine A; ESRD, end-stage renal disease; FSGS, focal segmental glomerulosclerosis; MCNS, minimal-change nephrotic syndrome; NA, not applicable; ND, not determined; NOS, non-specific type; PP, plasmapheresis; Pred, prednisone.
These families appear in a previous report by Kitamura et al.12
This individual died from a viral infection at the age of 3 years.
We identified a total of four NUP107 mutations, including two missense mutations (c.469G>T [p.Asp157Tyr] and c.2492A>C [p.Asp831Ala]), one 5-bp deletion (c.1079_1083delAAGAG [p.Glu360Glyfs∗6]), and one splice-donor-site mutation (c.969+1G>A) (Table 2). Heterozygous c.2492A>C was common in all five families. The two missense mutations altered evolutionally conserved amino acids (Figure S2) and were predicted to be pathogenic by web-based programs PolyPhen-2 and MutationTaster (Table 2). Furthermore, p.Asp831Ala resides within the Nup84-Nup100 domain (Figure S3). The 5-bp deletion was subjected to nonsense-mediated mRNA decay and probably led to a lack of protein synthesis (Figure S4). The splicing mutation (c.969+1G>A) causes a loss of the intrinsic splicing donor site (Figure S5). All four variants were examined in the EVS, ExAC Browser, HGVD, and in-house Japanese exome database (n = 575). The c.1079_1083delAAGAG variant was observed at frequencies of 0.0000083 in the ExAC Browser and 0.0008696 in the in-house Japanese exome data. Another variant, c.2492A>C, was observed at a frequency of 0.0013587 only in HGVD, but not in the EVS, ExAC Browser, or in-house Japanese exome data (Table 2). The other mutations (c.469G>T and c.969+1G>A) were never observed in any of four variant databases. Among 881 NUP107 variants registered in the ExAC Browser, a total of 31 variants with a MAF ≥ 0.005 were in non-coding regions (intronic but not in canonical acceptor or donor sites or UTRs) or were synonymous variants (Table S7). Furthermore, 36 loss-of-function variants in NUP107 are not homozygous (all heterozygous; Table S8). Therefore, this genetic evidence strongly suggests that biallelic NUP107 mutations could lead to autosomal-recessive SRNS.
Table 2.
NUP107 Mutations in Affected Individuals with Early-Onset SRNS
| Mutation | Amino Acid Change | PolyPhen-2 | PyloP | MutationTaster | Grantham | EVS | ExAC | HGVD | In-House Exomesa(n = 575) |
|---|---|---|---|---|---|---|---|---|---|
| c.469G>T | p.Asp157Tyr | 0.712 | 2.84 | 0.998403 | 160 | 0 | 0 | 0 | 0 |
| c.969+1G>A | splice site | NA | NA | NA | NA | 0 | 0 | 0 | 0 |
| c.1079_1083delAAGAG | p.Glu360Glyfs∗6 | NA | NA | NA | NA | 0 | 0.0000083 | 0 | 0.0008696 |
| c.2492A>C | p.Asp831Ala | 1.000 | 1.952 | 0.99995 | 126 | 0 | 0 | 0.0013587 | 0 |
Mutations were annotated according to NUP107 cDNA (GenBank: NM_020401.2). Abbreviations are as follows: EVS, NHLBI Exome Sequencing Project Exome Variant Server; HGVD, Human Genetics Variation Database (the public exome database of the Japanese population).
In-house exome database of Japanese control individuals.
A Common Haplotype Harboring c.2492A>C
Interestingly, all affected individuals carry c.2492A>C heterozygously. To determine whether c.2492A>C was derived from an ancestral chromosome, we constructed the haplotype in all families by using informative microsatellite markers and SNPs. We confirmed that a 412-kb haplotype was shared by all five families (Figure S6). Considering the extreme rarity of c.2492A>C in different whole-exome databases, c.2492A>C is likely to be specific to East Asians.
Clinical Characterization of NUP107-Related SRNS
Noticeably, the clinical course of affected individuals with NUP107 mutations was similar (Figure 1B and the supplemental note). In brief, the four families consistently showed early-onset SRNS whereby NS first manifested itself at age 2–3 years and ESRD became evident before age 10 years. One family (SRNS-12) showed an exceptionally late onset of NS, which appeared after 10 years of age, and renal function has been relatively preserved at the current 34 years of age. Renal biopsies revealed histopathological FSGS in all affected individuals (Figure 2, Table 1, and Figure S7). Depletion of NUP107 was shown to lead to apoptosis in eukaryotes,20,27 and we observed apoptotic changes in the renal biopsy samples from SRNS individuals (SRNS-TWH1 II-1 and II-2) with NUP107 mutations. Cells with the characteristic morphological features, such as nuclear shrinkage and fragmentation, were occasionally found in the glomeruli and renal tubules (Figure S8). Some of these cells could be TUNEL positive (apoptotic), although we failed to recognize TUNEL-positive cells in the glomeruli of the few biopsied specimens, given that only ten glomeruli were observed (data not shown). Among them, five individuals underwent renal transplants and have experienced no recurrence of SRNS to date. Additionally, none of them showed neurological phenotypes.
Figure 2.
Kidney Histopathology of Affected Individuals with Biallelic NUP107 Mutations
(A–C) Light micrographs of kidney biopsy specimens from SRNS-TWH II-1. (A) A low-power view (periodic acid-Schiff stain, 100× magnification) of two representative abnormal glomeruli (arrows). Half of the glomerulus is sclerosed (arrowheads). (B and C) Enlarged images (periodic acid methenamine silver stain, 400× magnification) show the collapse of glomerular tufts with hypertrophy and hyperplasia of the glomerular epithelial cells that fill the urinary space. Tubular injury accompanying atrophy of epithelia and interstitial fibrosis is noted.
(D–F) Electron micrographs of biopsy specimens from SRNS-2 II-1 (D), SRNS-2 II-3 (E), and SRNS-2 II-4 (F). Effacement of podocyte foot processes and some mesangial expansion with sub-endothelial electron-dense deposits are apparent. The thickness of the glomerular basement membrane appears normal and shows no evidence of splitting, lamellation, or fragmentation, thereby excluding the possibility of a primary basement-membrane defect. Accumulation of storage materials and dysmorphic mitochondria were not found in the podocyte cytoplasm. Abbreviations are as follows: E, endothelial cell; M, mesangial cell; P, podocyte; Pa, papillary epithelia. Arrowheads indicate effacement of podocyte foot processes, yellow arrows represent electron dense deposits, black arrows show flattened podocyte foot processes, and yellow asterisks show paramesangial deposits.
Scale bars represent100 μm (A), 40 μm (B and C), 2 μm (D and E), and 5 μm (F).
NUP107 Function and NUP107 Expression in Humans
NUP107 is an essential component of the NPC, which is one of the largest protein complexes (∼125 MDa in vertebrates) in eukaryotes and comprises ∼30 nucleoporins embedded in the nuclear envelope.28,29 It facilitates the efficient transfer of macromolecules between the nucleus and cytoplasm in a highly selective manner and plays pivotal roles in the nuclear framework and gene expression.28,30–33 Although some nucleoporins have tissue specificity,34 NUP107 and NUP107 are ubiquitously expressed as the core gene and the essential scaffold protein, respectively, of the NPC.29,35–37 As the results of the TaqMan expression assay show, NUP107 is expressed ubiquitously in most human fetal and adult tissues, including the kidney (Figure S9). To evaluate the physiological relevance of NUP107 in human podocytes, we examined the intracellular localization of NUP107, along with WT1 (a podocyte-specific transcription factor38) and Ezrin (a marker protein for apical domains of epithelial cells39), in human podocytes. Confocal microscopy demonstrated that NUP107 co-localized with WT1 and was distributed in a speckle-like pattern in the nuclei of human podocytes surrounding the glomerular capillary tufts (Figure S10). In addition to podocytes, most other cell types showed a similar staining pattern for NUP107. These data suggest that NUP107 has an important function for renal filtration in human podocytes. A direct link between NUP107 and renal disease has never been shown, but NUP107 knockdown in HeLa cells altered the localization of ELYS, and this affected the proper localization of lamin A/C,19 an alteration in which caused FSGS.40
Effect of the Common NUP107 p.Asp831Ala Substitution on the Structure of the Protein and Its Binding to NUP133
To evaluate the effect of p.Asp157Tyr and p.Asp831Ala substitutions from a structural viewpoint, we mapped the variant positions on the crystal structure of the yeast Sec13-Nup145C-Nup84 complex (PDB: 3IKO),41 which is analogous to the human SEC13-NUP96-NUP107 complex (NUP96 is the C-terminal half product of NUP98 [GenBank: NM_016320.4; MIM: 601021], processed after translation42,43) and the human NUP107-NUP133 complex (PDB: 3CQC).14 Asp157 is predicted to reside on the surface of the protein, suggesting that the p.Asp157Tyr substitution does not affect the folded structure of NUP107 (Figure S11). However, because this protein interacts with many other proteins,44 the possibility that the p.Asp157Tyr substitution might impair these interactions cannot be excluded, although no such changed interaction for this particular variant site has been reported. Because the Asp831 side chain forms hydrogen bonds with the Arg842 side chain, the p.Asp831Ala substitution is considered to disrupt these hydrogen bonds. To evaluate the effects of this variant on the structure of NUP107, we performed MD simulations for wild-type and altered NUP107 in solution. In this substitution, a region around the variant site and a region involved in interactions with NUP133 (amino acid residues 881–890) both showed more fluctuations than did those same regions in the wild-type protein (Figure S12). This NUP133-interacting region is considered to be structurally correlated with the variant site through van der Waals contacts (Figure S12B). The results from the MD simulations suggest that the p.Asp831Ala substitution impairs the molecular interaction between NUP107 and NUP133.
Impaired Function of the Altered NUP107
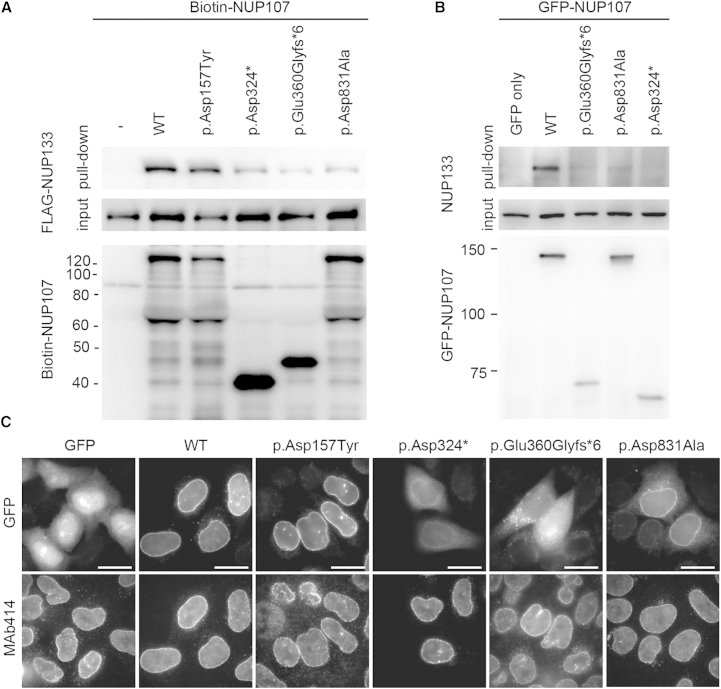
Because NUP107 interacts with NUP133 via its C-terminal tail,14 we investigated the mutational effects on the protein-protein interaction between NUP107 and NUP133 in vitro. We used an in vitro pull-down assay with recombinant proteins produced in a wheat germ cell-free system to determine the contribution of the C-terminal region of NUP107. Consistent with a previous report,14 the altered NUP107 that lacked a third of the C-terminal region (amino acids 645–925) did not bind to NUP133 as tightly as wild-type NUP107 under equilibrium conditions (Figure S13). Likewise, two truncated NUP107 proteins with extensively shorter C termini (p.Asp324∗ and p.Glu360Glyfs∗6) also showed weaker binding to NUP133. Notably, a p.Asp831Ala protein with an altered C terminus exhibited significantly reduced binding to NUP133, whereas a p.Asp157Tyr protein with an altered N terminus retained full binding activity (Figure 3A). Wild-type GFP-fused NUP107, which was transiently produced by a mammalian expression vector, was bound to endogenous NUP133 in HeLa cells, and the p.Asp831Ala protein was also bound to NUP133 but weakly in comparison to the wild-type (Figure 3B). Observation of the intracellular localization of altered GFP-NUP107 indicated that the two truncated proteins were distributed mainly in the cytoplasm, whereas the wild-type protein was clearly localized in the nuclear envelope (Figure 3C). The p.Asp831Ala altered protein was localized in the nuclear envelope and cytoplasm (Figure 3C). These results are consistent with the impaired interaction observed between the altered NUP107 and NUP133.
Figure 3.
Decreased Intermolecular Interactions between NUP107 and NUP133
(A) In vitro protein-protein binding assay of altered NUP107 with NUP133. The FLAG-tagged NUP133 mixed with biotinylated altered NUP107 proteins was subjected to a pull-down assay with streptavidin magnetic beads. The bound proteins were separated by SDS-PAGE and then detected with an anti-FLAG antibody or with streptavidin-horseradish peroxidase. The corresponding protein inputs are shown in the middle and bottom panels.
(B) Evaluation of the interaction between NUP107 and NUP133 with the use of wild-type NUP107 and its alterations. Wild-type GFP-NUP107 or its alterations were transiently produced in HeLa cells and precipitated with an anti-GFP antibody. The NUP107-NUP133 interaction was analyzed via immunoblotting using the antibodies indicated.
(C) Subcellular localization of NUP107 or its alterations. For visualizing localization of altered or wild-type GFP-NUP107 in HeLa cells, the cells were fixed and stained with a MAb414 antibody recognizing the NPC on the nuclear envelopes. Scale bars represent 20 μm.
The following abbreviation is used: WT, wild-type.
Zebrafish with nup107 Knockdown Have Glomerular Abnormalities Mimicking SRNS
Reportedly, zebrafish with homozygous nup107 mutations and morphants with nup107 knockdown produced with anti-sense MOs each similarly showed a thin pharyngeal skeleton, unfolded intestine, and loss of swim bladder and died on days 5 and 6.20 However, the specific renal phenotype was not investigated. Therefore, we injected the nup107-TB MO or nup107-SB MO to create an in-frame (15-bp) deletion at exon 24 to mimic the commonly shared missense mutation (c.2492A>C [p.Asp831Ala]) and then carefully observed the renal phenotype in vivo (Figures S14 and S15). As reported previously,20 neither of the zebrafish morphants developed edema until they died at around days 5 and 6 (Figure S14A). Furthermore, we sought to identify the glomerular filtration impairment in knockdown zebrafish (nup107-TB MO) but did not observe any traces of recognizable protein leakage in glomeruli at 96 hpf (data not shown). Although zebrafish might not be the best animal model for generating renal phenotypes, in a microscopic section of the nup107-SB morphant, we were able to find supportive findings in that the glomeruli were generally underdeveloped and showed hypoplastic or poorly organized capillary vessels and mesangial regions (Figures S14C–S14E). Electron microscopy revealed abnormally shaped foot processes and collapse of the capillary lumen in both morphants (Figures S14F–S14K and S16). Because these observations are similar to those from humans with FSGS, the zebrafish morphants might reflect the renal changes caused by the NUP107 mutation.
Unchanged NPC Localization in Lymphoblastoid Cells from Affected Individuals with NUP107 Mutations
Reportedly, NUP107 depletion results in decreased or absent NPCs.29,36 However, a lymphoblastoid cell line derived from affected individuals showed no apparent NPC loss or abnormality by immunohistochemistry analysis (data not shown), which indicates that some residual functions of altered NUP107 might persist in the cells of affected individuals, at least under non-stressful conditions. NUP107 is an essential scaffold protein in the NPC, a structure that is evolutionary conserved from yeast to vertebrates.29,36 Therefore, in the null state, NUP107 mutants might be lethal in humans.
Discussion
In this study, we have shown that biallelic NUP107 mutations cause early-onset SRNS in humans. Affected individuals with NUP107 mutations usually developed SRNS at 2–3 years of age and progressed to ESRD before 10 years of age but experienced no recurrence of the disease after renal transplantation. How do NUP107 mutations cause a glomerular phenotype in humans? This might be partly explained by the specific properties of podocytes, which are highly differentiated with a unique architecture (foot processes and slit membranes).45,46 In affected individuals with NUP107 mutations, insufficient NUP107 function could cause immature and/or hypoplastic podocytes, or at least functionally impaired podocytes that are progressively destroyed by increased filtration pressure after birth. Interestingly, nuclear-envelope proteins, including NPCs, are closely associated with mechanotransduction signaling,47,48 and mechanical stretching decreases podocyte proliferation and cell-body size by reorganizing the actin cytoskeleton in vitro.49,50 Thus, increased post-natal capillary pressure leading to mechanical stretching of vulnerable podocytes might accelerate glomerulus damage. Furthermore, mature podocytes do not regenerate.51,52 Thus, the core pathological condition of SRNS caused by NUP107 mutations is a structural abnormality, which correlates well with the early SRNS onset in childhood, its steroid resistance, and its lack of post-transplant relapse (Figure S17).
Recently, a homozygous missense mutation (c.303G>A [p.Met101Ile]) was reported in an affected individual who is from a consanguineous family and presents with global developmental delay and early-onset FSGS.53 However, none of our affected individuals with NUP107 recessive mutations show neurological impairment. Additional genetic factors might be involved in the neurological symptoms of the consanguineous family. Alternatively, different mutations could cause an additional neurological phenotype. This mutation has been suggested to lead to abnormal splicing (and possibly a nearly null function), although no direct evidence has been shown.53 As for p.Asp157Tyr, we could not find direct evidence of its functional impairment experimentally. However, it could be a hypomorphic variant; if so, this might explain the milder phenotype in the SRNS-12 family, who carries both missense mutations (c.469G>T [p.Asp157Tyr] and c.2492A>C [p.Asp831Ala]). Thus, it is possible that the residual NUP107 function left by missense mutations (including c.469G>T [p.Asp157Tyr]) is related to the late onset age and/or milder severity of the disease. It is intriguing that mutations in NUP107, which encodes an essential nucleoporin of the NPC, lead to a kidney-specific disease in humans.
In summary, biallelic NUP107 mutations cause early-onset SRNS for which renal transplantation is the only effective treatment. Access to genetic information is useful for proper clinical management of NS. Therefore, screening NUP107 mutations in SRNS individuals with broad ranges of clinical severity is strongly encouraged. Furthermore, we did not identify the genetic cause in six pairs of affected siblings and seven single affected individuals in our cohort, which implies a heterogenetic etiology for early-onset SRNS. Further research is necessary to uncover the whole picture of this type of SRNS.
Acknowledgments
We are grateful to all the affected individuals and their families who participated in this study. We also thank our clinical colleagues who supported the participating families: Dr. Makoto Endo (Laboratory of Fish Health Management, Tokyo University of Marine Science and Technology) for morphological evaluation of zebrafish and Ms. Sugimoto, Ms. Takabe, and Mr. Mitsui for technical assistance. This work was supported in part by a grant for Research on Measures for Intractable Diseases, a grant for Comprehensive Research on Disability Health and Welfare, and the Strategic Research Program for Brain Science from the Japan Agency for Medical Research and Development; a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) (24118007) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Grants-in-Aid for Scientific Research (A, B, and C) and for Challenging Exploratory Research from the Japan Society for the Promotion of Science; the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program of the Project for Developing Innovation Systems from the Japan Science and Technology Agency; the Takeda Science Foundation; the Osaka Kidney Foundation; and grant HI12C0014 from the Korean Health Technology R&D Project, Ministry of Health & Welfare. K.I. also received grants from Pfizer Japan, Daiichi Sankyo, the Japan Blood Product Organization, Miyarisan Pharmaceutical, AbbVie, CSL Behring, JCR Pharmaceuticals, and Teijin Pharma; and consulting fees from Chugai Pharmaceutical and Astellas Pharma. N.Y. received grants from Novartis Pharma KK and Asahi Kasei Pharma.
Published: September 24, 2015
Footnotes
Supplemental Data include a supplemental note, 17 figures, and 8 tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.08.013.
Contributor Information
Hiroyasu Tsukaguchi, Email: tsukaguh@hirakata.kmu.ac.jp.
Naomichi Matsumoto, Email: naomat@yokohama-cu.ac.jp.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes FTP site, ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/technical/reference/README.human_g1k_v37.fasta.txt
ExAC Browser, http://exac.broadinstitute.org/
Genome Analysis Toolkit, http://www.broadinstitute.org/gatk
NHLBI Exome Sequencing Project Exome Variant Server, http://evs.gs.washington.edu/EVS/
Novoalign, http://www.novocraft.com
OMIM, http://www.omim.org
Picard, http://picard.sourceforge.net
UCSC Genome Browser, https://genome.ucsc.edu/
Supplemental Data
References
- 1.Gipson D.S., Massengill S.F., Yao L., Nagaraj S., Smoyer W.E., Mahan J.D., Wigfall D., Miles P., Powell L., Lin J.J. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 2.Bullich G., Trujillano D., Santín S., Ossowski S., Mendizábal S., Fraga G., Madrid Á., Ariceta G., Ballarín J., Torra R. Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: mutations in multiple glomerular genes may influence disease severity. Eur. J. Hum. Genet. 2015;23:1192–1199. doi: 10.1038/ejhg.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinney P.A., Feltbower R.G., Brocklebank J.T., Fitzpatrick M.M. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr. Nephrol. 2001;16:1040–1044. doi: 10.1007/s004670100021. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.S., Bellew C.A., Silverstein D.M., Aviles D.H., Boineau F.G., Vehaskari V.M. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int. 2005;68:1275–1281. doi: 10.1111/j.1523-1755.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 5.Saleem M.A. New developments in steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 2013;28:699–709. doi: 10.1007/s00467-012-2239-0. [DOI] [PubMed] [Google Scholar]
- 6.Zagury A., Oliveira A.L., Montalvão J.A., Novaes R.H., Sá V.M., Moraes C.A., Tavares Mde.S. Steroid-resistant idiopathic nephrotic syndrome in children: long-term follow-up and risk factors for end-stage renal disease. J. Bras. Nefrol. 2013;35:191–199. doi: 10.5935/0101-2800.20130031. [DOI] [PubMed] [Google Scholar]
- 7.Trautmann A., Bodria M., Ozaltin F., Gheisari A., Melk A., Azocar M., Anarat A., Caliskan S., Emma F., Gellermann J., PodoNet Consortium Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin. J. Am. Soc. Nephrol. 2015;10:592–600. doi: 10.2215/CJN.06260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovric S., Fang H., Vega-Warner V., Sadowski C.E., Gee H.Y., Halbritter J., Ashraf S., Saisawat P., Soliman N.A., Kari J.A., Nephrotic Syndrome Study Group Rapid detection of monogenic causes of childhood-onset steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 2014;9:1109–1116. doi: 10.2215/CJN.09010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machuca E., Benoit G., Antignac C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum. Mol. Genet. 2009;18(R2):R185–R194. doi: 10.1093/hmg/ddp328. [DOI] [PubMed] [Google Scholar]
- 10.Mekahli D., Liutkus A., Ranchin B., Yu A., Bessenay L., Girardin E., Van Damme-Lombaerts R., Palcoux J.B., Cachat F., Lavocat M.P. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr. Nephrol. 2009;24:1525–1532. doi: 10.1007/s00467-009-1138-5. [DOI] [PubMed] [Google Scholar]
- 11.Sadowski C.E., Lovric S., Ashraf S., Pabst W.L., Gee H.Y., Kohl S., Engelmann S., Vega-Warner V., Fang H., Halbritter J., SRNS Study Group A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamura A., Tsukaguchi H., Iijima K., Araki J., Hattori M., Ikeda M., Honda M., Nozu K., Nakazato H., Yoshikawa N. Genetics and clinical features of 15 Asian families with steroid-resistant nephrotic syndrome. Nephrol. Dial. Transplant. 2006;21:3133–3138. doi: 10.1093/ndt/gfl347. [DOI] [PubMed] [Google Scholar]
- 13.Tsurusaki Y., Koshimizu E., Ohashi H., Phadke S., Kou I., Shiina M., Suzuki T., Okamoto N., Imamura S., Yamashita M. De novo SOX11 mutations cause Coffin-Siris syndrome. Nat. Commun. 2014;5:4011. doi: 10.1038/ncomms5011. [DOI] [PubMed] [Google Scholar]
- 14.Boehmer T., Jeudy S., Berke I.C., Schwartz T.U. Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex. Mol. Cell. 2008;30:721–731. doi: 10.1016/j.molcel.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takai K., Sawasaki T., Endo Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat. Protoc. 2010;5:227–238. doi: 10.1038/nprot.2009.207. [DOI] [PubMed] [Google Scholar]
- 16.Sawasaki T., Morishita R., Gouda M.D., Endo Y. Methods for high-throughput materialization of genetic information based on wheat germ cell-free expression system. Methods Mol. Biol. 2007;375:95–106. doi: 10.1007/978-1-59745-388-2_5. [DOI] [PubMed] [Google Scholar]
- 17.Sawasaki T., Kamura N., Matsunaga S., Saeki M., Tsuchimochi M., Morishita R., Endo Y. Arabidopsis HY5 protein functions as a DNA-binding tag for purification and functional immobilization of proteins on agarose/DNA microplate. FEBS Lett. 2008;582:221–228. doi: 10.1016/j.febslet.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawryluk-Gara L.A., Shibuya E.K., Wozniak R.W. Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol. Biol. Cell. 2005;16:2382–2394. doi: 10.1091/mbc.E04-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clever M., Funakoshi T., Mimura Y., Takagi M., Imamoto N. The nucleoporin ELYS/Mel28 regulates nuclear envelope subdomain formation in HeLa cells. Nucleus. 2012;3:187–199. doi: 10.4161/nucl.19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X., Yang S., Han Y., Zhao X., Zhao L., Tian T., Tong J., Xu P., Xiong C., Meng A. Loss of zygotic NUP107 protein causes missing of pharyngeal skeleton and other tissue defects with impaired nuclear pore function in zebrafish embryos. J. Biol. Chem. 2012;287:38254–38264. doi: 10.1074/jbc.M112.408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A.E., Berendsen H.J. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 22.Kelley L.A., Sternberg M.J. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 23.Guerois R., Nielsen J.E., Serrano L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J. Mol. Biol. 2002;320:369–387. doi: 10.1016/S0022-2836(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 24.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 25.Darden T., York D., Pedersen L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 26.Hess B., Bekker H., Berendsen H.J.C., Fraaije J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1998;18:1463–1472. 10.1002/(SICI)1096-987X(199709)18:12<1463:AID-JCC4>3.0.CO;2-H. [Google Scholar]
- 27.Banerjee H.N., Gibbs J., Jordan T., Blackshear M. Depletion of a single nucleoporin, Nup107, induces apoptosis in eukaryotic cells. Mol. Cell. Biochem. 2010;343:21–25. doi: 10.1007/s11010-010-0494-6. [DOI] [PubMed] [Google Scholar]
- 28.Antonin W., Ellenberg J., Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. doi: 10.1016/j.febslet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 29.Boehmer T., Enninga J., Dales S., Blobel G., Zhong H. Depletion of a single nucleoporin, Nup107, prevents the assembly of a subset of nucleoporins into the nuclear pore complex. Proc. Natl. Acad. Sci. USA. 2003;100:981–985. doi: 10.1073/pnas.252749899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoelz A., Debler E.W., Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 31.Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 32.Fried H., Kutay U. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 2003;60:1659–1688. doi: 10.1007/s00018-003-3070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strambio-De-Castillia C., Niepel M., Rout M.P. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 34.Ori A., Banterle N., Iskar M., Andrés-Pons A., Escher C., Khanh Bui H., Sparks L., Solis-Mezarino V., Rinner O., Bork P. Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Mol. Syst. Biol. 2013;9:648. doi: 10.1038/msb.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bui K.H., von Appen A., DiGuilio A.L., Ori A., Sparks L., Mackmull M.T., Bock T., Hagen W., Andrés-Pons A., Glavy J.S., Beck M. Integrated structural analysis of the human nuclear pore complex scaffold. Cell. 2013;155:1233–1243. doi: 10.1016/j.cell.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 36.Walther T.C., Alves A., Pickersgill H., Loïodice I., Hetzer M., Galy V., Hülsmann B.B., Köcher T., Wilm M., Allen T. The conserved Nup107-160 complex is critical for nuclear pore complex assembly. Cell. 2003;113:195–206. doi: 10.1016/s0092-8674(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 37.González-Aguilera C., Askjaer P. Dissecting the NUP107 complex: multiple components and even more functions. Nucleus. 2012;3:340–348. doi: 10.4161/nucl.21135. [DOI] [PubMed] [Google Scholar]
- 38.Mundlos S., Pelletier J., Darveau A., Bachmann M., Winterpacht A., Zabel B. Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- 39.Saotome I., Curto M., McClatchey A.I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Thong K.M., Xu Y., Cook J., Takou A., Wagner B., Kawar B., Ong A.C. Cosegregation of focal segmental glomerulosclerosis in a family with familial partial lipodystrophy due to a mutation in LMNA. Nephron Clin. Pract. 2013;124:31–37. doi: 10.1159/000354716. [DOI] [PubMed] [Google Scholar]
- 41.Nagy V., Hsia K.C., Debler E.W., Kampmann M., Davenport A.M., Blobel G., Hoelz A. Structure of a trimeric nucleoporin complex reveals alternate oligomerization states. Proc. Natl. Acad. Sci. USA. 2009;106:17693–17698. doi: 10.1073/pnas.0909373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontoura B.M., Blobel G., Matunis M.J. A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol. 1999;144:1097–1112. doi: 10.1083/jcb.144.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loïodice I., Alves A., Rabut G., Van Overbeek M., Ellenberg J., Sibarita J.B., Doye V. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol. Biol. Cell. 2004;15:3333–3344. doi: 10.1091/mbc.E03-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 45.Pavenstädt H., Kriz W., Kretzler M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 46.Quaggin S.E., Kreidberg J.A. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 47.Swift J., Discher D.E. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 2014;127:3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedorchak G.R., Kaminski A., Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014;115:76–92. doi: 10.1016/j.pbiomolbio.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Endlich N., Kress K.R., Reiser J., Uttenweiler D., Kriz W., Mundel P., Endlich K. Podocytes respond to mechanical stress in vitro. J. Am. Soc. Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 50.Petermann A.T., Hiromura K., Blonski M., Pippin J., Monkawa T., Durvasula R., Couser W.G., Shankland S.J. Mechanical stress reduces podocyte proliferation in vitro. Kidney Int. 2002;61:40–50. doi: 10.1046/j.1523-1755.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 51.Kriz W. Progressive renal failure--inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol. Dial. Transplant. 1996;11:1738–1742. [PubMed] [Google Scholar]
- 52.Nagata M., Nakayama K., Terada Y., Hoshi S., Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am. J. Pathol. 1998;153:1511–1520. doi: 10.1016/s0002-9440(10)65739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alazami A.M., Patel N., Shamseldin H.E., Anazi S., Al-Dosari M.S., Alzahrani F., Hijazi H., Alshammari M., Aldahmesh M.A., Salih M.A. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep. 2015;10:148–161. doi: 10.1016/j.celrep.2014.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.