Abstract
A 57-year-old man presented with unproductive cough and dyspnea for 6 months in Fujian Province, China. His misuse of a large amount of steroids (accumulated dose equivalent to 3530 mg prednisolone) resulted in Talaromyces marneffei infection. Chest computed tomographic scan revealed diffuse interstitial and multiple cavitary lung lesions. Treatment with amphotericin B combined with itraconazole resulted in total recovery, with marked regression of lung lesions.
Keywords: Diffuse interstitial lung lesions, multiple cavitary lung lesions, non-HIV patient, pulmonary fungi infection, Talaromyces marneffei
A 57-year-old man presented with unproductive cough and dyspnea for 6 months in Fujian Province, China, on 2 March 2013. He was first misdiagnosed as having idiopathic pulmonary fibrosis because of the diffuse lung lesions observed on chest computed tomographic (CT) scan (Fig. 1a) and was treated with a large amount of steroids (accumulated dose equivalent to 3530 mg prednisolone in 54 days). However, his dyspnea became severe, and he had an unresolved fever during the post–rainy season conditions present 10 days before he was admitted to our hospital.
Fig. 1.
Diffuse interstitial and multiple cavitary lung lesions due to Talaromyces marneffei infection on computed tomography (CT). (a) Chest CT revealing interstitial lung lesions at lower lobes of both lungs on 2 February 2013. (b) Chest CT showing progressive diffuse interstitial lung lesions and novel multiple cavitary lesions (arrow) at all lobes of both lungs on 1 April 2013. (c) Chest CT revealing marked regression of interstitial and cavitary lung lesions after treatment on 6 May 2013. (d) Chest CT showing near-normal findings except for residual fibrosis in both lungs after 5 months' follow-up on 9 September 2013.
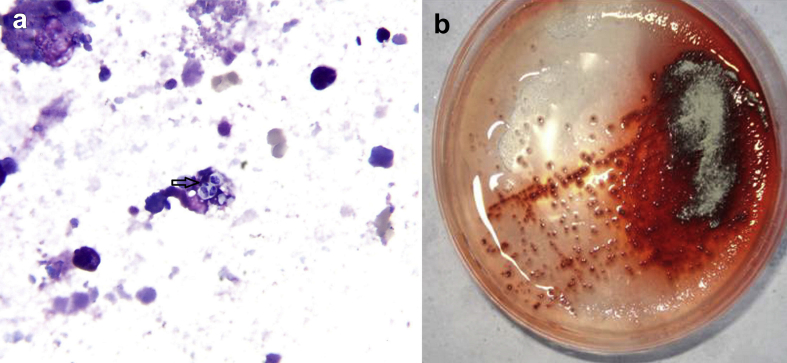
Physical examination at this time revealed tachypnea, slight cyanosis, respiratory rate of 28 breaths per minute, and a few fine crepitation rales heard over the lower lobe of each lung. No other abnormalities were noted, and no skin lesions or lymphadenopathies were observed. He did not have any heart disease. Laboratory test results were as follows: serum brain natriuretic peptide was normal; white blood cell counts were 12.1 × 109/L with 80% neutrophils. Chest CT scan revealed diffuse interstitial and multiple cavitary lung lesions (Fig. 1b). Arterial blood gas analysis revealed a low Pao2/Fio2 ratio of 160 mm Hg (pH 7.435, Paco2 31.4 mm Hg, Pao2 65.6 mm Hg, Hco3− 21.3 mmol/L under the condition of Fio2 41% oxygen), suggesting severe adult respiratory distress syndrome. Plasma HIV antibody and autoimmune antibody tests were negative; cellular and humoral immunity tests were mostly close to normal. Although the serum aspergillus galactomannan antigen assay was normal (enzyme immunoassay), the bronchoalveolar lavage (BAL) smear showed some intracellular yeastlike cells in macrophages (Fig. 2a), and fungal cultures of the BAL and blood samples yielded Talaromyces marneffei (Fig. 2b).
Fig. 2.
Bronchoalveolar lavage (BAL) smear, BAL and blood culture showing Talaromyces marneffei. (a) Bronchoalveolar lavage smear showing some intracellular yeastlike cells in macrophages (arrow), with characteristic septate forms (Wright stain; original magnification, ×1000). A high-resolution version of this slide for use with the Virtual Microscope is available as eSlide VM00734. (b) BAL and blood culture at 25°C on Sabouraud dextrose agar plate yielded fungal colonies with distinctive red diffusible pigment.
The pathogen's gene fragment was obtained by PCR amplification of 16S RNA of T. marneffei both from sputum and BAL (PM-F: CTA CAT ATG ATG TCG TAC CGG GCT CCC TT, PM-R: CAT CTC GAG CAG ACC AGC TTC TTC GCC), and its DNA sequence by direct sequencing shared 100% homology with T. marneffei ATCC 18224 (GenBank XM_002148843) and 85% homology with Talaromyces stipitatus ATCC 10500 (GenBank XM_002485272) from GenBank [1]. Therefore, further examination of the organism's DNA sequence was confirmed as T. marneffei. The patient was provided liposomal amphotericin B (1 mg/kg daily) combined with itraconazole (200 mg/d) for 18 days with a gradual reduction of the glucocorticoid dose, followed by itraconazole at a daily oral dose of 200 mg twice a day as secondary prophylaxis. He experienced dyspnea relief and recovered gradually. When his oxygenation index exceeded 300 mm Hg (Fig. 1c), he was discharged from the hospital. He has been followed up for 17 months without recurrence, his Spo2 was 99% under air and his chest CT scan was nearly normal except for residual fibrosis in both lungs (Fig. 1d).
Talaromyces marneffei is an emerging dimorphic human pathogenic fungus endemic to Southeast Asian areas such as India, Southeast China and Taiwan [2,3]. Most disseminated T. marneffei infections have been diagnosed in HIV-related patients [4], and some have also been found in immunosuppressed patients [5]. The case described here occurred in a non-HIV patient with diffuse interstitial lung disease, who was treated inappropriately with large accumulated doses of steroids. We presumed that his T. marneffei infection was related to his immunocompromised condition, the result of his use of these immunosuppressive agents and the moist environment of the concurrent rainy season. However, the number of doses of prednisone equivalents that were required for the infection to appear and why the patient's immunity tests were not abnormal under his immunocompromised condition need further investigation.
Unlike cases occurring in HIV patients, our patient with T. marneffei infection had lungs that were seriously impaired and presented with a significant adult respiratory distress syndrome. However, he had no skin lesions, lymphadenopathies or any opportunistic infections. Instead, the patient's pulmonary images indicated rare diffuse interstitial and multiple cavitary lung lesions, comparable to those in a previous report by Hung et al. of a cavitary T. marneffei (Penicillium marneffei) pneumonia in an HIV patient [6]. Our patient received a combination of liposomal amphotericin B and itraconazole for a longer period than suggested in reference guidelines as an initial treatment because of his severe condition, and he responded well in spite of some adverse gastrointestinal effects. Because the patient should have recovered from his immunocompromised condition after the glucocorticoid withdrawal, the necessity for the course of secondary prophylaxis is controversial.
Conflict of Interest
None declared.
Acknowledgement
Supported in part by National Grant of China (grant 2014ZX10004005) also supported by Key Grant of Fujian Province (grant 2012Y0016).
Contributor Information
Y.S. Chen, Email: slyyywb@126.com.
S.X. Cai, Email: hxkcai@126.com.
References
- 1.Nierman W.C., Fedorova-Abrams N.D., Andrianopoulos A. Genome sequence of the AIDS-associated pathogen Penicillium marneffei (ATCC18224) and its near taxonomic relative Talaromyces stipitatus (ATCC10500) Genome Announc. 2015;3(1) doi: 10.1128/genomeA.01559-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrison R.G., Boyd K.S. Dimorphism of Penicillium marneffei as observed by electron microscopy. Can J Microbiol. 1973;19:1305–1309. doi: 10.1139/m73-209. [DOI] [PubMed] [Google Scholar]
- 3.DiSalvo A.F., Fickling A.M., Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. 1973;60:259–263. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 4.Bulterys P.L., Le T., Quang V.M., Nelson K.E., Lloyd-Smith J.O. Environmental predictors and incubation period of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2013;56:1273–1279. doi: 10.1093/cid/cit058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J.N., Lin H.H., Lai C.H., Wang J.L., Yu T.J. Renal transplant recipient infected with Penicillium marneffei. Lancet Infect Dis. 2010;10:138. doi: 10.1016/S1473-3099(10)70005-0. [DOI] [PubMed] [Google Scholar]
- 6.Hung C.C., Chang S.Y., Sun H.Y., Hsueh P.R. Cavitary pneumonia due to Penicillium marneffei in an HIV-infected patient. Am J Respir Crit Care Med. 2013;187:e3–e4. doi: 10.1164/rccm.201202-0321IM. [DOI] [PubMed] [Google Scholar]