Abstract
Neurological soft signs have been considered one of the promising neurological endophenotypes for schizophrenia. However, most previous studies have employed clinical rating data only. The present study aimed to examine the neurobiological basis of one of the typical motor coordination signs, the Fist–Edge–Palm (FEP) task, in patients with first-episode schizophrenia and their non-psychotic first degree relatives. Thirteen patients with first-episode schizophrenia, 14 non-psychotic first-degree relatives and 14 healthy controls were recruited. All of them were instructed to perform the FEP task in a 3 T GE Machine. Psychophysiological interaction (PPI) analysis was used to evaluate the functional connectivity between the sensorimotor cortex and frontal regions when participants performed the FEP task compared to simple motor tasks. In the contrast of palm-tapping (PT) vs. rest, activation of the left frontal–parietal region was lowest in the schizophrenia group, intermediate in the relative group and highest in the healthy control group. In the contrast of FEP vs. PT, patients with schizophrenia did not show areas of significant activation, while relatives and healthy controls showed significant activation of the left middle frontal gyrus. Moreover, with the increase in task complexity, significant functional connectivity was observed between the sensorimotor cortex and the right frontal gyrus in healthy controls but not in patients with first episode schizophrenia. These findings suggest that activity of the left frontal–parietal and frontal regions may be neurofunctional correlates of neurological soft signs, which in turn may be a potential endophenotype of schizophrenia. Moreover, the right frontal gyrus may play a specific role in the execution of the FEP task in schizophrenia spectrum disorders.
Keywords: Neurological soft sign, First episode schizophrenia, First degree relatives, Endophenotype
Highlights
-
•
Examine the neurobiological basis of the typical Fist–Edge–Palm (FEP) signs
-
•
Patients with first-episode schizophrenia showed functional connectivity of the FEP signs.
-
•
Right frontal gyrus plays a specific role in the FEP in patients and non-psychotic first-degree relatives.
1. Introduction
Neurological soft signs (NSS) have long been considered one of the functional features (Tsuang and Faraone, 1999; Tsuang et al., 1991) and endophenotypes (Chan and Gottesman, 2008; Chan et al., 2010a) of schizophrenia spectrum disorders. However, most of the studies of NSS have been limited to the use of clinical ratings. Instead, recent findings from both structural and functional imaging studies have shown that NSS may be associated with specific brain alterations. Specifically, in individuals with psychosis, more NSS have been associated with smaller volumes of the inferior frontal lobes (Thomann et al., 2009a), the pre-central gyrus (Heuser et al., 2011; Mouchet-Mages et al., 2011), the global cortical sulci (Dazzan et al., 2004; Gay et al., 2013) as well as the cerebellum (Ho et al., 2004; Thomann et al., 2009a,b). In addition, NSS may be responsible for some of the observed clinical manifestations in schizophrenia (Keshavan et al., 2003; Mouchet-Mages et al., 2011; Schroder et al., 1999). A recent meta-analysis of structural and functional imaging findings suggests that NSS are associated with volume reductions of the pre-central gyrus, the cerebellum, the inferior frontal gyrus and the thalamus. Furthermore, the same meta-analysis also suggests that functional imaging studies support an association between NSS and altered neural activation in the inferior frontal gyrus, the bilateral putamen, the cerebellum and the superior temporal gyrus in patients with schizophrenia (Zhao et al., 2014). Taken together, these findings support the presence of a dysfunction of neural circuitry that underlies the presence of these minor neurological abnormalities, which are already present at illness onset.
On the other hand, empirical findings have also shown that non-psychotic first-degree relatives of schizophrenia patients exhibit a higher prevalence of NSS compared to healthy controls (Egan et al., 2001; Gourion et al., 2004; Lawrie et al., 2001). A meta-analysis comparing the prevalence of NSS between patients with schizophrenia, non-psychotic first-degree relatives and healthy controls indicated a mean effect size of 0.8 and 0.97 for patients and their non-psychotic first-degree relatives and for non-psychotic first-degree relatives and controls respectively (Chan et al., 2010b). These results are consistent with the argument that NSS are familial in nature and segregate with the illness. However, no studies had examined the brain correlates of NSS in healthy relatives of patients with schizophrenia.
Furthermore, all the aforementioned neuroimaging studies in schizophrenia patients used conventional subtraction analysis and did not examine the underlying connectivity between the brain regions identified. Given that some NSS, such as the Fist–Edge–Palm (FEP) task (first described by Luria, see Heuser et al., 2011), involve the functional integration or regulation of areas involved in motor sequencing rather than being directly activated, a connectivity approach is more appropriate to identify the specific network involved. Only one study (Rao et al., 2008) adopted a psychophysiological interaction (PPI) (Friston et al., 1997) method to re-analyze their previous findings on the FEP task in healthy volunteers (Chan et al., 2006). This new analysis showed enhanced functional connectivities between the left- and right sensorimotor cortices (SMC) and the right inferior and middle prefrontal cortices during FEP task performance compared to a simple palming task. These findings suggest a regulatory role of the prefrontal cortex on FEP task execution.
In this study, we examined whether prefrontal regions are involved in the integration or regulation of neural activity underlying motor sequencing in patients with first-episode schizophrenia and their non-psychotic first degree relatives (FDR). More specifically, we aimed to identify any frontal regions where coupling in these areas and the sensorimotor cortex (SMC) significantly differed between the complex FEP task and a simple control motor task using PPI analysis. We hypothesized that patients with first-episode schizophrenia and their non-psychotic FDRs would show a reduced dysfunction of the prefrontal cortex and SMC while performing the FEP task.
2. Method
2.1. Participants
Thirteen right-handed first-episode schizophrenia patients were recruited from the Mental Health Center, Peking University, Beijing, for the study. Participants were recruited to the study if they met the following inclusion criteria: a) diagnosis of schizophrenia ascertained by experienced psychiatrists according to the DSM-IV (APA, 1994); b) aged 18–40 years; and c) illness duration within 2 years. The exclusion criteria were: a) a history of neurological disorders; b) a lifetime history of substance abuse and c) an estimated IQ lower than 70. Current symptom severity was assessed with the Positive and Negative Syndrome Scale (Kay et al., 1987).
Fourteen non-psychotic FDRs of the patients taking part in the study were also invited to participate. Potential participants were excluded if they a) met the DSM-IV criteria for substance abuse; b) were suffering from any clinically unstable medical disorder; c) had a history of head injury (past or present); and d) had an estimated IQ lower than 70.
Fourteen healthy controls matched with the patients in age, gender and handedness were recruited from local universities through advertisements. The exclusion criteria were the same as those for the FDRs. Written informed consent was obtained from all participants after explanation of the study procedures. The Institutional Review Board of the Institute of Psychology, the Chinese Academy of Sciences, approved the study protocol. Their demographic information is shown in Table 1.
Table 1.
Demographic and clinical information.
| SCZ (n = 13) |
Relatives (n = 14) | Controls (n = 14) | |
|---|---|---|---|
| Age | 20.08 ± 3.38 | 44.43 ± 7.68 | 21.71 ± 3.81 |
| Gender (M:F) | 5:8 | 6:8 | 8:6 |
| Education | 12.23 ± 2.20 | 9.54 ± 4.41 | 13.43 ± 1.50 |
| IQ estimates | 107.54 ± 14.04 | 91.86 ± 22.81 | 118.07 ± 15.38 |
| Handedness (%right) | 100% | 100% | 100% |
| Duration of illness (years) | 1.67 ± 0.81 | ||
| CPZ (mg/day) | 170.00 ± 97.75 | ||
| PANSS | |||
| Positive symptoms | 16.10 ± 5.13 | ||
| Negative symptoms | 20.70 ± 4.64 | ||
| General psychopathology | 31.20 ± 6.16 |
2.2. NSS measures
Behavioral neurological soft signs were examined with the abridged version of the Cambridge Neurological Inventory (CNI) (Chan et al., 2009). This abridged version offers instructions for eliciting and rating a comprehensive range of NSS in motor coordination, sensory integration and disinhibition. Scoring was done according to standardized anchor points to indicate “normal” response (0) and “abnormal” response (1).
2.3. NSS task and procedure
The FEP task has been described elsewhere (Chan et al., 2006; Rao et al., 2008) (for the earliest description, please see Luria, 1966; in Heuser et al., 2011). In brief, the task consisted of three right-hand motor tasks which varied in complexity. In the simple palm tapping (PT) task, participants were required to repeat only one right palm tapping in the prone position. In the intermediate complex pronation/supination (PS) task, participants were required to perform right palm tapping in the prone and supine positions alternatively. In the complex FEP task, participants were required to successivley place their right hand in a fist resting position vertically (fist), a palm resting position vertically (edge), and a palm resting position horizontally (palm). A resting condition (A) without any hand movement when participants were asked to focus on the screen was used as the control baseline of the PT task, and the PT task was in turn used as the control baseline of the PS and FEP tasks. Participants were asked to practice the motor actions correctly at a constant rate before entering the scanner. During scanning, their performance was monitored by the experimenter through the window of the scanner room. In the formal imaging task, participants were instructed to perform the three tasks at a similar pace throughout the entire experiment. PT and PS tasks were executed in 1 s and the FEP task was executed in 1.5 s. The experimenter monitored the task performance outside the scanner room to ensure that the participants performed the correct hand movements.
This study utilized a block design. First, a resting condition lasted for 20 s and then 6 s of counting backward reminded the participants to get ready for their hand movement. After backward counting, the PT or PS or FEP task lasted for 40 s. One of these three hand movements would be presented on the screen. Participants were required to conduct the hand movements according to the demonstration on the screen. Each hand movement task would be conducted twice in each run. There were a total of three runs. The sequence of the three hand movement tasks was optimized and counterbalanced within the three runs.
2.4. Imaging data acquisition
The functional imaging data was originally acquired in a GE 3 T Sigma scanner (General Electric, Waukesha, WI, USA) with a standard GE birdcage-type RF coil using a standard T2*-weighted EPI sequence. The EPI parameters were: TR = 2 s TE = 60 ms, FOV = 24 × 24 cm, Matrix = 64 × 64, flip angle = 60°, 22 axial slices (5 mm thick/1.2 mm sp, from superior to inferior). The spatial resolution for the functional images was 3.75 × 3.75 × 6.2 mm. High-resolution anatomical images were also obtained using the standard T1-weighted sequence (66 axial slices, 2.0 mm thick/interleaved, FOV = 24 × 24 cm, Matrix = 256 × 256).
2.5. Conventional analysis of fMRI data
Images were analyzed with statistical parametric mapping software (SPM8, Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab 2009b (Mathworks Inc., Sherborn, MA, USA). Three dummy scans in the beginning of the experiment were removed automatically from the dataset. Pre-processing included motion correction (re-alignment). Images were registered to the first ICBM 152, which was based on 152 brains and was created by the Montreal Neurological Institute (MNI) with a 2 × 2 × 2 mm resolution. In the final step of pre-processing, the images were spatially smoothed by an isotropic Gaussian Kernel with FWHM of 8 mm. Conventional analyses were first conducted at the individual-level using voxel-wise general linear modeling (GLM) and four T-contrasts were defined between the tasks and the corresponding baselines, i.e., PT vs. rest, PS vs. rest, FEP vs. rest and FEP vs. PT. Group-level random effect analyses were then conducted using one-way ANOVA. Data from the contrasts of the first level model in the healthy control group, the schizophrenia group and the FDR group were entered into this model. A threshold of AlphaSim corrected p < 0.01 and cluster size larger than 15 voxels were used to identify the activations associated with each contrast. Furthermore, we used a regression model to find the regions with group difference. We set up an F contrast to find the regions with main effect of group difference. Signal change percentage was retrieved from the ROIs with group difference for post-hoc analysis.
2.6. PPI analysis
PPI analysis was used to estimate functional integration during task execution under different motor complexity conditions. The left SMC was determined a-priori as the reference region for the PPI analysis because the motor tasks in the present study were only completed with the right hand. This region was defined by using a sphere with a radius of 8 mm and a center at the peak activation in the left SMC activation (MNI coordinates = [−36 −28 52], from the conventional analysis of the contrast of PT vs. rest). We performed voxel-wise PPI analysis at individual-level for the left SMC to see if any other brain areas connected to the SMC showed a significant increase in functional coupling (the slope of regression) during the FEP task compared with a control task (e.g., the PT or PS task). For each participant, the activation time course signal in the reference region (i.e., the first eigenvariate time series, adjusted by effect of interest) was extracted from the conventional GLM and entered into the PPI analysis as the first regressor representing the physiological variable. A second regressor representing the motor tasks with different complexity was entered into the PPI analysis as the psychological variable. The psychophysiological interaction between task complexity and activation signal in the reference region was designated as the regressor of interest in the PPI analysis. Thereafter we performed group-level random effect analysis on the individual results using one sample t-tests for the contrast FEP vs. PT. Group-level paired t-tests were conducted for the contrast FEP vs. PS. Areas of significant activation were identified at a threshold of uncorrected p < 0.001 and cluster size larger than 15 voxels.
3. Results
3.1. Behavioral performance
The CNI total scores and the three subscale scores are summarized in Table 2. There was no significant difference between patients with schizophrenia, their FDRs and healthy controls in the three subscales and total score of CNI.
Table 2.
Descriptive CNI performances of the patients with schizophrenia, non-psychotic first-degree relatives and healthy controls.
| CNI scores | SCZ (n = 13) Mean (SD) |
Relatives (n = 14) Mean (SD) |
Controls (n = 14) Mean (SD) |
F value |
|---|---|---|---|---|
| Motor coordination | 1. 96 (2.02) | 1.29 (1.68) | 1.14 (1.17) | 0.399, n.s. |
| Sensory integration | 1.08 (1.44) | 1.71 (1.20) | 1.57 (1.40) | 0.820, n.s. |
| Disinhibition | 1.62 (1.76) | 1.07 (1.44) | 0.64 (0.84) | 1.658, n.s. |
| Total score | 4.38 (3.91) | 4.07 (3.50) | 3.36 (2.41) | 0.344, n.s. |
3.2. Conventional analysis
Conventional activation of the PT, PS and FEP tasks in the three groups, after co-varying for age, is shown in Table 3. The left frontal parietal region was significantly activated when participants performed the PT, PS and FEP tasks with their right hand. Moreover, in the PT–rest contrast, activation in the left frontal parietal region, together with the left medial frontal region, were lowest in patients with schizophrenia, intermediate in the FDRs and highest in healthy controls (SCZ < REL < HC). We further used a regression model and conducted a post-hoc analysis to identify the regions that were different across groups both linearly and non-linearly. In the PT–rest contrast, we confirmed that the left frontal precentral gyrus (−30 −34 64, k = 100, F(2,37) = 20.23) was linearly activated in all three groups (SCZ < REL < HC). The left medial frontal region (HC = Rel > SCZ, −8 −18 64, k = 136, F(2,37) = 15.59) and the left middle temporal gyrus (HC > SCZ = Rel, −48 −68 10, k = 124, F(2,37) = 11.7) were non-linearly activated in all three groups. Fig. 1 illustrates the signal change percentage in these three ROIs in the three groups.
Table 3.
Activation results in conventional analysis (AlphaSim corrected: p < 0.01, controlling for age).
| Condition | Group | k | T | MNI (x, y, z) | Brain region |
|---|---|---|---|---|---|
| PT–rest | HC | 1387 | 10.45 | −32 −32 64 | Left frontal precentral gyrus |
| 207 | 7.08 | 52 −66 −2 | Right inferior temporal gyrus | ||
| 1095 | 6.44 | −48 −26 18 | Left insula | ||
| 790 | 6.18 | −8 −14 64 | Left medial frontal gyrus | ||
| 444 | 4.97 | −48 −68 8 | Left middle temporal gyrus | ||
| 104 | 4.56 | 58 −32 20 | Right postcentral gyrus | ||
| SCZ | 228 | 4.45 | −36 −28 50 | Left frontal precentral gyrus | |
| Rel | 818 | 6.83 | −32 −24 56 | Left frontal precentral gyrus | |
| 170 | 4.43 | 4 −64 −20 | Right declive | ||
| HC > Rel > SCZ | 156 | 5.74 | −30 −34 64 | Left frontal precentral gyrus | |
| 232 | 5.13 | −6 −16 64 | Left medial frontal gyrus | ||
| 525 | 4.49 | −60 −26 12 | Left superior temporal gyrus | ||
| 514 | 4.47 | −48 −68 8 | Left middle temporal gyrus | ||
| 261 | 4.14 | −16 58 22 | Left superior frontal gyrus | ||
| PS–rest | HC | 3641 | 11.25 | −32 −32 62 | Left frontal precentral gyrus |
| 355 | 4.79 | 52 −64 −4 | Right middle temporal gyrus | ||
| 121 | 4.63 | 58 −32 20 | Right postcentral gyrus | ||
| 259 | 4.19 | −50 −26 16 | Left postcentral gyrus | ||
| SCZ | 1276 | 6.67 | −34 −32 62 | Left frontal precentral gyrus | |
| 114 | 4.23 | −10 −12 64 | Left medial frontal gyrus | ||
| Rel | 380 | 5.77 | −34 −26 58 | Left frontal precentral gyrus | |
| HC > Rel > SCZ | None | ||||
| FEP–rest | HC | 2230 | 9.45 | −34 −32 64 | Left frontal precentral gyrus |
| 656 | 6.29 | −6 −8 56 | Left medial frontal gyrus | ||
| 497 | 5.96 | 60 –32 24 | Right inferior parietal gyrus | ||
| 542 | 5.74 | 52 –64 −4 | Right middle temporal gyrus | ||
| 122 | 4.51 | 36 –60 56 | Right superior parietal gyrus | ||
| 130 | 3.98 | −48 −74 −2 | Left inferior occipital gyrus | ||
| 163 | 3.94 | −48 −28 18 | Left superior temporal gyrus | ||
| SCZ | 857 | 5.23 | −38 −30 58 | Left postcentral gyrus | |
| Rel | 733 | 5.94 | −34 −24 56 | Left frontal precentral gyrus | |
| 479 | 5.3 | 0 –2 58 | Left medial frontal gyrus | ||
| 150 | 4.29 | 4 –66 −18 | Right declive | ||
| 116 | 3.87 | −56 −20 8 | Left superior temporal gyrus | ||
| HC > Rel > SCZ | 216 | 4.32 | −34 −84 0 | Left middle occipital gyrus | |
| FEP–PT | HC | 302 | 5.22 | −52 −36 52 | Left postcentral gyrus |
| 1422 | 4.97 | 32 –60 58 | Right superior parietal gyrus | ||
| 343 | 4.56 | −28 −62 52 | Left precuneus gyrus | ||
| 160 | 4.5 | 24 –14 58 | Right middle frontal gyrus | ||
| 144 | 4.47 | −26 −14 58 | Left middle frontal gyrus | ||
| 255 | 4.16 | 46 –54 −10 | Right temporal gyrus | ||
| SCZ | 952 | 5.81 | −28 −62 54 | Left precuneus gyrus | |
| 149 | 4.4 | 30 –58 56 | Right superior parietal gyrus | ||
| Rel | 364 | 5.66 | −30 −2 54 | Left middle frontal gyrus | |
| HC > Rel > SCZ | None | ||||
| FEP-PS | HC | None | |||
| Rel | 145 | 4.52 | −30 −84 −14 | Left middle occipital gyrus | |
| 222 | 4.33 | −30 −2 58 | Left middle frontal gyrus | ||
| 147 | 4.03 | 0 –2 60 | Left medial frontal gyrus | ||
| SCZ | None | ||||
| HC > Rel > SCZ | None |
Fig. 1.
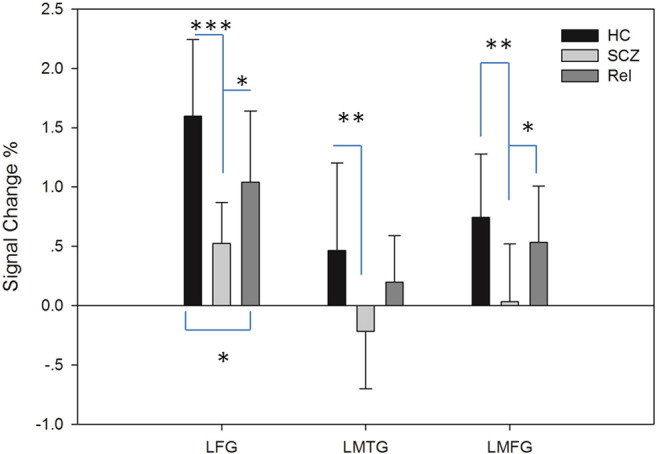
Signal change percentage of three ROIs in the three groups. Note: HC: healthy control, SCZ: schizophrenia, Rel: relative; LFG: left frontal precentral gyrus, LMTG: left middle temporal gyrus and LMFG: left medial frontal gyrus; ***p < 0.001, **p < 0.01 and *p < 0.05; error bar: standard deviation.
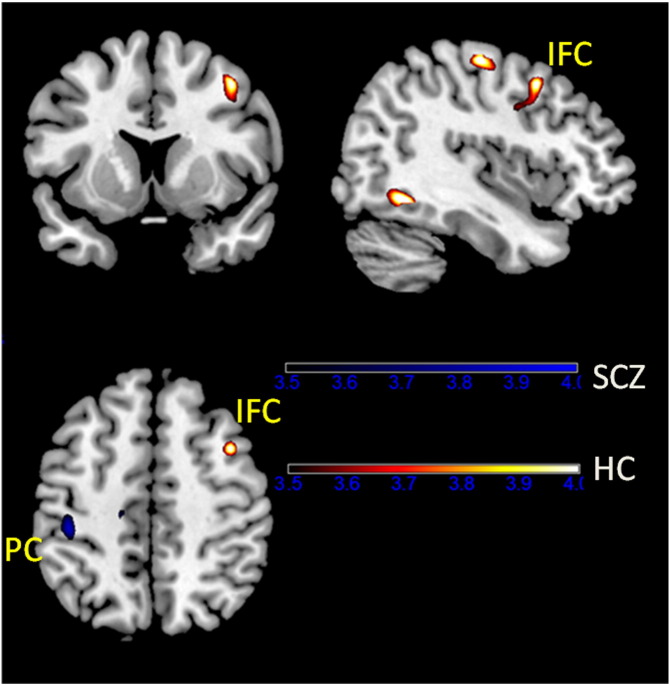
The activations associated with task complexity were examined in the FEP–PS and the FEP–PT contrasts. In the FEP–PT contrast, the bilateral middle frontal regions were activated in the healthy control group. The left middle frontal region was activated in the FDR group, but there was no frontal region activation in first episode schizophrenia patients. Fig. 2 illustrates the frontal activation in both healthy controls and FDRs. In the FEP–PS contrast, the left middle frontal region was also activated in the FDR group, but not in the healthy control group or the schizophrenia group.
Fig. 2.
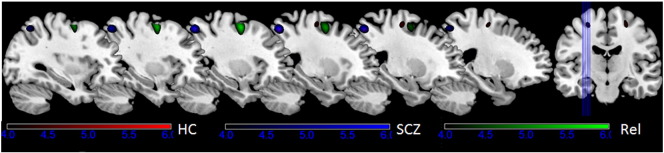
Brain activation of the three groups in the FEP vs. PT contrast. Both healthy controls and first-degree relatives of patients with schizophrenia activated the left middle frontal gyrus but patients with schizophrenia did not. Note: HC: healthy control, SCZ: patients with first episode schizophrenia and Rel: first-degree relatives.
3.3. PPI analysis
The PPI analysis identified right frontal regions in which the activity showed significant activation coupling to activity in the left SMC during performance of the more complex FEP task relative to the simple PT or PS tasks in the healthy control group, but not in the schizophrenia group. The results are shown in Table 4 and Fig. 3.
Table 4.
Regions that showed greater functional connectivity to the left sensorimotor cortex (SMC) during more complex motor tasks comparing with simpler motor tasks (FEP vs. PS; FEP vs. PT; and PS vs. PT).
| Group | Region | Peak MNI coordinates X, Y, Z |
Peak T score | Cluster size |
|---|---|---|---|---|
| FEP vs. PS | ||||
| HC | Right inferior frontal gyrus | 42 4 26 | 3.59 | 66 |
| HC > SCZ | Left occipital lobe | −48 −78 8 | 4.02 | 72 |
| FEP vs. PT | ||||
| HC | Right frontal lobe | 32 −14 58 | 3.98 | 438 |
| SCZ | Left parietal lobe | −42 −28 52 | 3.27 | 18 |
Fig. 3.
Functional connectivity results in the FEP vs. PT contrast. In healthy controls rather than patients with schizophrenia, the right inferior frontal gyrus was found to modulate the activation of the left SMC with increase in task complexity. Note: HC: healthy control, SCZ: patients with first episode schizophrenia, IFC: inferior frontal cortex and PC: parietal cortex.
4. Discussion
In this study, we report for the first time the neural activation and connectivity elicited by execution of the FEP task in patients with first episode schizophrenia and their healthy first degree relatives. Our main finding is that patients with first episode schizophrenia do not show, in comparison with healthy controls and their FDRs, activation of the left middle frontal gyrus in the execution of the FEP versus a simpler motor task like the PT. This provides evidence of a frontal dysfunction in these patients when performing a complex motor task. The right inferior frontal gyrus was found to modulate the activation of the sensorimotor cortex with the increase in motor complexity in healthy controls. A frontal dysfunction was also demonstrated by our second main finding, suggesting that with increase in task difficulty, patients with first episode schizophrenia do not show functional connectivity between the sensorimotor cortex and the right frontal gyrus, in contrast to healthy controls.
The presence of a prefrontal dysfunction in schizophrenia is supported by extensive evidence from both structural and functional neuroimaging studies. Prefrontal areas have been consistently reported as reduced in volume in patients with schizophrenia (Chan et al., 2011; Dazzan et al., 2004). Furthermore, fMRI studies have shown altered activation of frontal areas during executive and working memory tasks, particularly with increasing capacity demand (Barch et al., 2012; Callicott et al., 2000; Perry et al., 2001). At a functional level, patients with schizophrenia also show an excess of signs reflecting frontal release, such as abnormalities in eye movements and short-term memory deficits, which again point to a frontal cortical alteration (Hyde et al., 2007). In this study, we tested the activation of frontal areas during a complex motor task, the FEP task, which has long been considered in neurological and neurocognitive studies as indicative of frontal lobe lesions (Luria, 1966). Two previous fMRI studies have reported that in healthy individuals, the execution of this task did not induce activation of prefrontal areas, but induced activation of other parts of the cortex, including the sensorimotor areas (Chan et al., 2006; Umetsu et al., 2002). A subsequent study, using the same PPI approach that we used in this study, showed that this finding could reflect an indirect involvement of the prefrontal cortex, which could exert an integrative and regulatory function on neural circuitry involved in complex motor sequences. We found here that in healthy individuals, the execution of the FEP task, compared to a simpler motor act, was indeed associated with both direct activation of the prefrontal cortex, and greater coupling of prefrontal areas and sensorimotor cortex. However, this was not the case in patients with schizophrenia. Interestingly, a recent meta-analysis of the brain connectome suggests that the frontal lobe represents a hub (a region highly interconnected with other brain regions and valuable for integrative information processing and adaptive behavior) particularly affected in patients with schizophrenia (Crossley et al., 2014). There is a paucity of studies on brain activation during complex motor tasks in these patients. However, neuroimaging studies that examined gross motor coordination signs such as finger-to-thumb operation tests in these patients have reported reduced activation in the SMC and the supplementary motor area (SMA) in comparison to healthy controls (Schröder et al., 1995). A later similar study used pronation/supination tests and found significant reduced activation in the SMC in patients with schizophrenia compared to healthy controls (Schroder et al., 1999). The lack of activation we found with a task like the FEP, which requires fine motor coordination and requires executive processes like inhibition, planning and updating, could be interpreted as part of the hypofrontality hypothesis of schizophrenia reflecting a reduced function of the prefrontal cortex (Callicott et al., 2000).
It is interesting that we found a dose–response relationship of the left frontal parietal activation across patients, their FDRs and healthy controls. Here, in the PT vs. rest contrast, activation was lowest in the schizophrenia group, intermediate in the FDR group and highest in the healthy control group. Although these differences were highlighted with a motor sequence less complex than the FEP, the finding suggests that at least part of the pathophysiological substrate that underlies motor difficulties in psychosis may be linked to a genetic susceptibility to schizophrenia. Indeed, deficits in motor skills have been reported extensively in individuals at risk of schizophrenia because of either genetic loading or prodromal features (Blanchard et al., 2010; Erlenmeyer-Kimling et al., 2000).
There are some limitations in this study that should be considered. First, the clinical sample size was relatively small. However, the merit of the present sample is that it included both first-episode schizophrenia patients and their non-psychotic siblings. Secondly, the use of PPI analysis may not fully detect the connectivity changes related to the performance of the FEP task. However, PPI is an appropriate method to test the relationship between two simple mental activities (Friston et al., 1997). Other more sophisticated approaches, such as dynamic casual modeling, may help identify further subtle connectivity changes in the performance of the FEP task. Thirdly, the use of the screen to synchronize the motor actions might induce the activation of mirror neurons. However, the contrasts between any two of the three motor actions (FEP, PT, and PS) should minimize this effect by subtraction methods. Finally, we should also consider the validity of our task, since previous subtraction analyses of the FEP task did not elicit activation of the frontal cortex. However, the fact that the left frontal parietal region was significantly activated when participants performed the PT, PS and FEP tasks with their right hand suggests that this hand movement imaging task was valid.
Notwithstanding these limitations, our findings suggest that the FEP task may be a useful endophenotype of schizophrenia, as it fulfills the criteria of being familial and shows a dose–response relationship with genetic susceptibility to schizophrenia. Previous study had demonstrated that patients with schizophrenia showed aberrant brain activation in the premotor area after a week of motor training (Kodama et al., 2001). Given this temporal stability of NSS, the fact that this is an imaging endophenotype rather than a simple behavioral rating arguably enhances its precision. This task is also simpler to carry out when compared to a full NSS scale.
We believe that our findings add valuable information to the understanding of the origin and underlying neural mechanism of motor coordination signs. Future studies should recruit a larger sample of first-episode, preferably medication-naïve schizophrenia patients for further validation of our findings.
Contributors
Raymond CK Chan designed the study, interpreted the data and wrote the manuscript; J Huang and Y Wang analyzed the data; Y Y Lai and Q Zhao administered the clinical and neuropsychological tests; N Hong, David Shum, Eric FC Cheung, X Yu, and P Dazzan commented significantly on the first draft of the manuscript. All authors contributed to and approved the final text.
Conflict of interest
The authors declare that they have no conflict of interest.
Role of funding source
The funding agents had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Acknowledgments
This work was supported by a grant from the Outstanding Young Investigator Award of the National Science Fund China (81088001), the Beijing Training Project for the Leading Talents in S & T (Z151100000315020), the Key Laboratory of Mental Health, Institute of Psychology, and the CAS/SAFEA International Partnership Program for Creative Research Teams (Y2CX131003) and a grant from the National Science Fund China (31100747).
References
- APA . Diagnostic and Statistical Manual of Mental Disorders. fourth edition. APA; Washington, D.C.: 1994. [Google Scholar]
- Barch D.M., Moore H., Nee D.E., Manoach D.S., Luck S.J. CNTRICS imaging biomarkers selection: working memory. Schizophr. Bull. 2012;38(1):43–52. doi: 10.1093/schbul/sbr160. 22080498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard M.M., Jacobson S., Clarke M.C., Connor D., Kelleher I., Garavan H.…Cannon M. Language, motor and speed of processing deficits in adolescents with subclinical psychotic symptoms. Schizophr. Res. 2010;123(1):71–76. doi: 10.1016/j.schres.2010.05.028. 20580205 [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Bertolino A., Mattay V.S., Langheim F.J.P., Duyn J., Coppola R.…Weinberger D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. 11053229 [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Di X., McAlonan G.M., Gong Q.Y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull. 2011;37(1):177–188. doi: 10.1093/schbul/sbp073. 19633214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.C.K., Gottesman I.I. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci. Biobehav. Rev. 2008;32(5):957–971. doi: 10.1016/j.neubiorev.2008.01.005. 18462797 [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Rao H., Chen E.E.H., Ye B.B., Zhang C. The neural basis of motor sequencing: an fMRI study of healthy subjects. Neurosci. Lett. 2006;398(3):189–194. doi: 10.1016/j.neulet.2006.01.014. 16469446 [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Wang Y., Wang L., Chen E.Y.H., Manschreck T.C., Li Z.J.…Gong Q.Y. Neurological soft signs and their relationships to neurocognitive functions: a re-visit with the structural equation modeling design. PLOS ONE. 2009;4(12):e8469. doi: 10.1371/journal.pone.0008469. 20041110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.C.K., Xu T., Heinrichs R.W., Yu Y., Gong Q.Y. Neurological soft signs in non-psychotic first-degree relatives of patients with schizophrenia: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2010;34(6):889–896. doi: 10.1016/j.neubiorev.2009.11.012. 19925825 [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Xu T., Heinrichs R.W., Yu Y., Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr. Bull. 2010;36(6):1089–1104. doi: 10.1093/schbul/sbp011. 19377058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley N.A., Mechelli A., Scott J., Carletti F., Fox P.T., McGuire P., Bullmore E.T. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137(8):2382–2395. doi: 10.1093/brain/awu132. 25057133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzan P., Morgan K.D., Orr K.G., Hutchinson G., Chitnis X., Suckling J.…Murray R.M. The structural brain correlates of neurological soft signs in AE SOP first-episode psychoses study. Brain. 2004;127(1):143–153. doi: 10.1093/brain/awh015. 14570821 [DOI] [PubMed] [Google Scholar]
- Egan M.F., Hyde T.M., Bonomo J.B., Mattay V.S., Bigelow L.B., Goldberg T.E., Weinberger D.R. Relative risk of neurological signs in siblings of patients with schizophrenia. Am. J. Psychiatry. 2001;158(11):1827–1834. doi: 10.1176/appi.ajp.158.11.1827. 11691688 [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L., Rock D., Roberts S.A., Janal M., Kestenbaum C., Cornblatt B.…Gottesman I.I. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-risk Project. Am. J. Psychiatry. 2000;157(9):1416–1422. doi: 10.1176/appi.ajp.157.9.1416. 10964857 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. 9344826 [DOI] [PubMed] [Google Scholar]
- Gay O., Plaze M., Oppenheim C., Mouchet-Mages S., Gaillard R., Olié J.P.…Cachia A. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr. Bull. 2013;39(4):820–829. doi: 10.1093/schbul/sbs083. 22892556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion D., Goldberger C., Olie J.P., Lôo H., Krebs M.O. Neurological and morphological anomalies and the genetic liability to schizophrenia: a composite phenotype. Schizophr. Res. 2004;67(1):23–31. doi: 10.1016/s0920-9964(03)00099-9. 14741321 [DOI] [PubMed] [Google Scholar]
- Heuser M., Thomann P.A., Essig M., Bachmann S., Schröder J. Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res. 2011;192(2):69–76. doi: 10.1016/j.pscychresns.2010.11.009. 21498055 [DOI] [PubMed] [Google Scholar]
- Ho B.C., Mola C., Andreasen N.C. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol. Psychiatry. 2004;55(12):1146–1153. doi: 10.1016/j.biopsych.2004.02.020. 15184033 [DOI] [PubMed] [Google Scholar]
- Hyde T.M., Goldberg T.E., Egan M.F., Lener M.C., Weinberger D.R. Frontal release signs and cognition in people with schizophrenia, their siblings and healthy controls. Br. J. Psychiatry. 2007;191:120–125. doi: 10.1192/bjp.bp.106.026773. 17666495 [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Sanders R.D., Sweeney J.A., Diwadkar V.A., Goldstein G., Pettegrew J.W., Schooler N.R. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am. J. Psychiatry. 2003;160(7):1298–1304. doi: 10.1176/appi.ajp.160.7.1298. 12832245 [DOI] [PubMed] [Google Scholar]
- Kodama S., Fukuzako H., Fukuzako T., Kiura T., Nozoe S., Hashiguchi T.…Nakajo M. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychol. Med. 2001;31(6):1079–1088. doi: 10.1017/s0033291701004196. 11513375 [DOI] [PubMed] [Google Scholar]
- Lawrie S.M., Byrne M., Miller P., Hodges A., Clafferty R.A., Cunningham Owens D.G.C., Johnstone E.C. Neurodevelopmental indices and the development of psychotic symptoms in subjects at high risk of schizophrenia. Br. J. Psychiatry. 2001;178:524–530. doi: 10.1192/bjp.178.6.524. 11388968 [DOI] [PubMed] [Google Scholar]
- Luria A.R. Higher Cortical Functions in Man. Basic Books; New York: 1966. [Google Scholar]
- Mouchet-Mages S., Rodrigo S., Cachia A., Mouaffak F., Olie J.P., Meder J.F.…Krebs M.O. Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr. Scand. 2011;123(6):451–458. doi: 10.1111/j.1600-0447.2010.01667.x. 21219267 [DOI] [PubMed] [Google Scholar]
- Perry W., Heaton R.K., Potterat E., Roebuck T., Minassian A., Braff D.L. Working memory in schizophrenia: transient “online” storage versus executive functioning. Schizophr. Bull. 2001;27(1):157–176. doi: 10.1093/oxfordjournals.schbul.a006854. 11215544 [DOI] [PubMed] [Google Scholar]
- Rao H., Di X., Chan R.C.K., Ding Y., Ye B., Gao D. A regulation role of the prefrontal cortex in the fist–edge–palm task: evidence from functional connectivity analysis. Neuroimage. 2008;41(4):1345–1351. doi: 10.1016/j.neuroimage.2008.04.026. 18495496 [DOI] [PubMed] [Google Scholar]
- Schröder J., Essig M., Baudendistel K., Jahn T., Gerdsen I., Stockert A.…Knopp M.V. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9(1):81–87. doi: 10.1006/nimg.1998.0387. 9918729 [DOI] [PubMed] [Google Scholar]
- Schröder J., Wenz F., Schad L.R., Baudendistel K., Knopp M.V. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167(2):197–201. doi: 10.1192/bjp.167.2.197. 7582669 [DOI] [PubMed] [Google Scholar]
- Thomann P.A., Roebel M., Dos Santos V., Bachmann S., Essig M., Schröder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173(2):83–87. doi: 10.1016/j.pscychresns.2008.07.006. 19540731 [DOI] [PubMed] [Google Scholar]
- Thomann P.A., Wüstenberg T., Dos Santos V.D., Bachmann S., Essig M., Schröder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol. Med. 2009;39(3):371–379. doi: 10.1017/S0033291708003656. 18578894 [DOI] [PubMed] [Google Scholar]
- Tsuang M.T., Faraone S.V. The concept of target features in schizophrenia research. Acta Psychiatr. Scand. 1999;99:2–11. doi: 10.1111/j.1600-0447.1999.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Tsuang M.T., Gilbertson M.W., Faraone S.V. The genetics of schizophrenia. Current knowledge and future directions. Schizophr. Res. 1991;4(2):157–171. doi: 10.1016/0920-9964(91)90031-l. 2039759 [DOI] [PubMed] [Google Scholar]
- Umetsu A., Okuda J., Fujii T., Tsukiura T., Nagasaka T., Yanagawa I.…Yamadori A. Brain activation during the fist–edge–palm test: a functional MRI study. Neuroimage. 2002;17(1):385–392. doi: 10.1006/nimg.2002.1218. 12482091 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Li Z., Huang J., Yan C., Dazzan P., Pantelis C.…Chan R.C.K. Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophr. Bull. 2014;40(3):626–641. doi: 10.1093/schbul/sbt063. 23671197 [DOI] [PMC free article] [PubMed] [Google Scholar]