Abstract
Different test types lead to different intelligence estimates in autism, as illustrated by the fact that autistic individuals obtain higher scores on the Raven's Progressive Matrices (RSPM) test than they do on the Wechsler IQ, in contrast to relatively similar performance on both tests in non-autistic individuals. However, the cerebral processes underlying these differences are not well understood. This study investigated whether activity in the fluid “reasoning” network, which includes frontal, parietal, temporal and occipital regions, is differently modulated by task complexity in autistic and non-autistic individuals during the RSPM. In this purpose, we used fMRI to study autistic and non-autistic participants solving the 60 RSPM problems focussing on regions and networks involved in reasoning complexity. As complexity increased, activity in the left superior occipital gyrus and the left middle occipital gyrus increased for autistic participants, whereas non-autistic participants showed increased activity in the left middle frontal gyrus and bilateral precuneus. Using psychophysiological interaction analyses (PPI), we then verified in which regions did functional connectivity increase as a function of reasoning complexity. PPI analyses revealed greater connectivity in autistic, compared to non-autistic participants, between the left inferior occipital gyrus and areas in the left superior frontal gyrus, right superior parietal lobe, right middle occipital gyrus and right inferior temporal gyrus. We also observed generally less modulation of the reasoning network as complexity increased in autistic participants. These results suggest that autistic individuals, when confronted with increasing task complexity, rely mainly on visuospatial processes when solving more complex matrices. In addition to the now well-established enhanced activity observed in visual areas in a range of tasks, these results suggest that the enhanced reliance on visual perception has a central role in autistic cognition.
Keywords: Autism, fMRI, Reasoning, Connectivity, PPI, Intelligence
Highlights
-
•
Reasoning network is less modulated by problem complexity in autism.
-
•
Autistic individuals rely more extensively on visuospatial processes to solve complex problems.
-
•
Results support the central role of visual perception in autistic cognition.
1. Introduction
One of the most stable and intriguing properties of autistic intelligence is that the relative level of difficulty of the different tasks used to estimate intelligence is not the same for autistic and non-autistic people. For instance, autistic individuals obtain a better score when evaluated with the Raven's Progressive Matrices (RSPM) test (Raven, 1976) than with the Wechsler IQ test, whereas non-autistic individuals obtain similar scores on both tests (Dawson et al., 2007; Charman et al., 2011; Nader et al., 2014). In parallel, autistic individuals tend to exhibit a relative advantage on visuospatial tasks in comparison to verbal tasks, as reflected by their ability on visual search, embedded figures, Wechsler's Block Design, pattern discrimination and mental imagery tasks (Soulières et al., 2011; Stevenson and Gernsbacher, 2013).
The unique pattern of cognitive performance described above is accompanied by an alteration in underlying patterns of cerebral activity and connectivity (Samson et al., 2012; Tyszka et al., 2014). Stronger recruitment of visual perceptual brain regions can be found in a wide array of tasks using faces, objects and words as stimuli (Samson et al., 2012), including higher order tasks such as fluid reasoning (Sahyoun et al., 2010; Soulières et al., 2009; Yamada et al., 2012). In terms of functional connectivity, initial investigations yielded results supporting widespread long-range underconnectivity and local overconnectivity in autism (Courchesne and Pierce, 2005; Just et al., 2007). More recent studies temper this view, demonstrating that local over-connectivity depends on the specific type of analyses conducted (Vissers et al., 2012) and that perceptual long-range functional connectivity is sometimes stronger in autistic relative to non-autistic participants (Keehn et al., 2013; Leveille et al., 2010; McGrath et al., 2012). White matter microstructural alterations are also found and correlate with autistic signs and symptoms (Gibbard et al., 2013; Ikuta et al., 2014; McFadden and Minshew, 2013), performance in visuospatial tasks (McGrath et al., 2013), and fluid reasoning abilities (Ellmore et al., 2013).
Fluid reasoning relates to the ability to infer logical solutions when solving novel problems (Cattell, 1987). A spatially extended brain network underlies fluid reasoning, with crucial components in prefrontal and parietal regions (Jung and Haier, 2007; Perfetti et al., 2009). Activity in the fronto-parietal reasoning network is modulated by reasoning complexity and is associated with individual reasoning ability levels (Gray et al., 2003; Lee et al., 2006; Perfetti et al., 2009). Increasing reasoning complexity is associated with widespread increases in activity in the reasoning network, with marked increases in dorsolateral prefrontal cortex (Kalbfleisch et al., 2007; Kroger et al., 2002; Wendelken et al., 2008). Higher reasoning abilities are associated with higher activity in frontal and parietal regions (Perfetti, 2009), and particularly in posterior parietal cortex (Lee et al., 2006).
In autistic individuals, solving fluid reasoning problems is accompanied by higher activity in occipital and temporal regions, but lower activity in some frontal (middle frontal gyrus) and parietal regions (precuneus), compared to non-autistic individuals (Soulières et al., 2009), and lower structural connectivity between frontal language areas and temporal regions (Sahyoun et al., 2010; Yamada et al., 2012). In a previous fMRI study (Soulières et al., 2009), we recorded brain activity while participants performed the RSPM, the emblematic fluid reasoning test (see examples in Fig. 1). Autistic participants performed the self-paced RSPM task with an accuracy similar to that of their comparison group, but unexpectedly exhibited a 40% shorter response time. During the resolution of the matrices, left middle occipital gyrus (BA18) was disproportionally engaged in autistic participants, suggesting that autistic reasoning might rely more heavily on the involvement of occipital regions and their associated perceptual processes during fluid reasoning. However, in light of recent findings identifying increased activity in perceptual areas in autistic individuals in a wide range of tasks (see Samson et al., 2012 for a meta-analysis), one can question whether the increased activity in occipital areas during a reasoning task contributes to reasoning processes per se. We inferred that observing connectivity between this region and other elements of the reasoning network that was modulated by problem complexity would provide further evidence that occipital areas genuinely contribute to autistic reasoning.
Fig. 1.
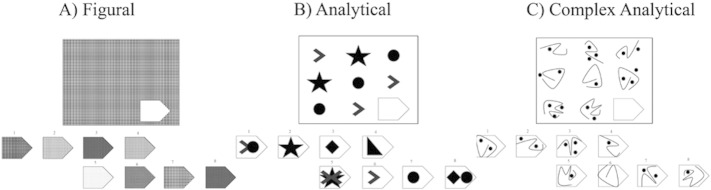
Examples similar to items from the Raven's Standard Progressive Matrices. This fluid reasoning test is composed of 60 matrix problems of increasing complexity. To solve the matrices, participants have to choose among 8 choices the one that best fill in the missing entry (bottom right of the matrix). The three examples represent the three complexity levels included in our study: figural, analytical and complex analytical.
The objective of this study was to characterize how regional cerebral activity and connectivity are modulated by task complexity in autistic individuals during fluid reasoning. We were specifically interested in verifying whether the increased activity previously observed in visuospatial areas in autistic individuals during matrix reasoning would be associated with activity and connectivity modulations according to problem complexity. We therefore conducted psychophysiological interaction analyses (PPI) on data from our previous RSPM study (Soulières et al., 2009), with two sets of seed regions. The first set was based on areas of maximal activity common to both groups while solving the RSPM. Often in studies with clinical samples, the selection of seed regions is based on task-related activity patterns observed in the control group, with those seed regions possibly being slightly displaced from activity peaks in the clinical group. This selection approach could result in a bias towards detecting reduced functional connectivity in the clinical sample. Here, we employed a more neutral approach, selecting seed regions based on the conjunction of task-related activity observed in both groups. Then, using a second set of seed regions based on areas of between-group differences in task-related activity, we more specifically addressed our primary goal to examine the contribution of occipital regions to autistic reasoning.
We predicted that autistic participants would exhibit higher functional connectivity involving the occipital seeds with increasing reasoning complexity, relative to non-autistic participants, as suggested by previous observations of stronger reasoning-related activity and anatomical local connectivity in occipital regions (Sahyoun et al., 2010; Soulières et al., 2009). Given numerous previous findings of lower parieto-frontal functional connectivity in autism and the lower frontal activity seen during reasoning in autistic individuals in our previous study, we also predicted lower functional connectivity between prefrontal and posterior parietal regions during the resolution of the more complex reasoning items in autistic relative to non-autistic participants.
2. Methods
2.1. Participants
MRI data were collected from 15 autistic participants (aged 14–35, M = 22.40, SD = 5.95, 2F) and 18 non-autistic participants (aged 14–36, M = 21.72, SD = 5.20, 3F) (dataset from Soulières et al., 2009; see participant characteristics in Table 1). Participants were matched on age, Wechsler Full-Scale IQ and handedness. Autistic participants received a diagnosis according to DSM-IV criteria and were evaluated with two diagnostic instruments, the Autism Diagnosis Observation Schedule (ADOS-G; Lord et al., 2000) and Autism Diagnostic Interview (ADI-R; Le Couteur et al., 1989), by a multidisciplinary team of expert clinicians. A comparison group of participants self-reporting no psychiatric or neurological conditions were recruited from the local population. Primary exclusion criteria for both groups were uncorrected visual impairment, use of psychoactive or vasoactive medication as well as use of illegal drugs or excessive alcohol consumption. A neurologist reviewed all structural scans to rule out any anatomical abnormality. All participants gave written informed consent to participate. The study was approved by the institutional review boards of Regroupement Neuroimagerie/Québec and Rivière-des-Prairies Hospital.
Table 1.
Participant information.
| AUT | Non-AUT | p | |
|---|---|---|---|
| Sample size (sex) | 15 (2F, 13M) | 18 (3F, 15M) | |
| Age M (SD) | 22.40 (5.95) | 21.72 (5.20) | 0.73 |
| Full scale IQ M (SD) | 100.87 (12.05) | 106.22 (12.97) | 0.23 |
| Verbal IQ M (SD) | 99.20 (14.39) | 110.17 (11.50) | 0.02 |
| Performance IQ M (SD) | 102.80 (11.98) | 100.72 (14.39) | 0.65 |
| Manual preference M (SD) | 67.93 (45.68) | 57.89 (49.15) | 0.55 |
| ADI-R M (cut-off) | |||
| Social | 23.27 (10) | ||
| Communication | 18.47 (8) | ||
| Behavior | 7.00 (3) |
Note. IQ was assessed using Wechsler scales (WAIS-III and WISC-III), manual preference was tested using the Edinburgh Inventory test and autistic traits were evaluated with the Autism Diagnostic Interview-Revised (ADI-R).
2.2. Task
A computerized version of the RSPM test was used (see Fig. 1 for examples similar to the items in the RSPM test). For each of the 60 test items, participants selected the missing entry in a matrix among 8 possible choices, with no time limit. The RSPM task was slightly modified from its original paper version to suit the fMRI environment. First, the two rows of answer choices were shifted horizontally to simplify the mapping between answers and response pads. Participants had to answer by pressing a button with their left (choices 1–4) or right hand (choices 5–8). The number of left hand versus right hand responses was counterbalanced. The item remained visible until an answer was given by the participant, followed by a fixation period varying from 4 to 7 s following an exponential distribution. The second modification made to the original test was to present the 60 items in a randomized order (instead of ascending order of complexity as in the original test), to avoid any presentation order/difficulty confound in the analyses.
2.3. Procedure
Participants first had two practice sessions of a relatively easy pattern matching task sharing the same stimulus presentation specificities as the RSPM task. The first practice session was done while sitting in front of a computer screen, while the second was done in a mock scanner. These practice sessions allowed participants to familiarize themselves with the tasks and fMRI environment. The fMRI testing session took place immediately after completing the mock scanner practice session. The RSPM task took between 14 to 35 min, as the participants were instructed to take as much time as needed to solve each item. Last, a structural MRI scan was acquired. The pattern matching task data were not used in the current study.
2.4. Image acquisition
Images were acquired using a Siemens Trio 3 T scanner with an 8-channel phased-array head coil. Functional data were obtained using an echo planar imaging (EPI) BOLD sequence, using a variable epoch design (48 contiguous slices, 3 mm cubic voxels, TR = 2850 ms, TE = 30 ms, flip angle = 90°, FOV = 192 mm2), and structural data were T1 weighted (MP-RAGE, 176 slices, 1 mm cubic voxels, TR = 2530 ms, TE = 3.48 ms, flip angle = 7°, FOV = 256 mm2).
2.5. Data analysis
2.5.1. Behavioral data
The 60 RSPM test items were divided into 3 complexity levels: figural (16 items), analytical (23 items) and complex analytical (21 items). First, the items were divided into figural versus analytical types based on two previous classifications (Lynn et al., 2004; van der Ven and Ellis, 2000). The figural items can be solved with visual strategies, such as visual pattern completion. Conversely, the analytical and complex analytical items require abstraction and application of one or more rules (Carpenter et al., 1990). The analytical items were then further divided into two complexity levels based on RSPM accuracy data from 26 non-autistic adults in our research database. The complex analytical items required the application of more complex rules and/or a combination of rules and resulted in lower accuracy than the easier analytical items and figural items. Group (Autistic, Non-autistic) × Complexity (Figural, Analytical, Complex analytical) Analysis of Variance (ANOVA) were conducted on accuracy and response times using SPSS (IBM Corp. (2012) version 21.0).
2.5.2. Image analysis
Images were preprocessed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) following the same procedure as in Soulières et al. (2009). Statistical modeling and visualization were done with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and MRIcron (Rorden and Brett, 2000).
2.5.2.1. Preprocessing
Slice timing was corrected to the middle slice of the volume, and then a two-pass realignment involving first registration to the first image and then to the mean of the realigned images, followed by reslicing with 4th degree b-spline interpolation. Realigned images were spatially normalized using the SPM5 EPI template. Eight mm full-width at half maximum FWHM source image smoothing was applied and images were resampled to 2 × 2 × 2 mm. Finally, normalized EPI images were smoothed using an isotropic Gaussian smoothing kernel with FWHM of 9 × 9 × 9 mm, to compensate for residual individual and group anatomical differences.
2.5.2.1.1. Eye and head movement
To verify the presence of atypical oculomotor movement sometimes reported in autism we compared saccade density during the reasoning task in the two groups. We extracted the time course of the BOLD contrast signal activity within two 12.5 mm spherical ROI (one for each eye), for each participant. An estimate of the net saccade density during the RSPM was obtained by computing the standard deviation of the activity time course, and compared between groups with a t test.
Head motion data (head translation and rotation estimates) was extracted during the preprocessing realignment and included as covariates in the first-level models. Moreover, as to insure that there was no group difference in head movement, head motion data was directly compared between groups. Mean displacement rate (mm/s) and rotation rate (degrees/s), as well as peak-to-peak translation (x, y, z) and rotation (pitch, roll, yaw) for each axis, were computed and compared with independent samples t tests.
2.5.2.2. Statistical modeling
For each participant, a first-level general linear model was generated with three task regressors (figural, analytical and complex analytical), using all trials (correct and incorrect), and six motion estimate regressors. Individual contrasts for each of the three levels of complexity were used in the subsequent second-level model. Within and between-group mixed-effect analyses on the Complexity factor (figural < analytical < complex analytical) were computed using a threshold of p < 0.001 uncorrected with extent threshold K = 50 contiguous voxels. Additional analyses were conducted in each group to verify the influence of response times on task-related activity, by entering response times as a covariate in a t-test.
2.5.2.2.1. Selection of seed regions
Two sets of seed regions were selected for connectivity analyses. A first set of seed regions was derived from a conjunction analysis of the overall RSPM task-related activity across all levels of complexity, allowing identification of areas of activity common to both groups. Using conjunction analysis for seed selection ensured having high levels of activity in both groups in order to guide the seed selection for the connectivity analyses. Coordinates of local task-related maximal activity were identified in each of the frontal, parietal and occipital lobes. These seeds were located in left inferior occipital gyrus (BA18), left superior parietal lobule (BA7) and right inferior frontal gyrus (BA9).
A second set of seed regions was selected using the results of the between-group contrast analysis exploring the complexity factor, in order to represent areas maximizing activity differences between the two groups. Peak coordinates of clusters more active in autistic participants (autistic > non-autistic), as well as in non-autistic participants (non-autistic > autistic), were identified on the complexity contrast (Figural < Analytical < Complex Analytical), yielding seeds in the left superior occipital gyrus, left middle frontal gyrus and left precuneus.
2.5.2.3. Psychophysiological interaction analyses (PPI)
The modulation of functional connectivity by task complexity was assessed using a PPI approach (Friston, 2011; Friston et al., 1997; Gitelman et al., 2003). Using a 6 mm radius sphere around each of the seeds, eigenvariates (reflecting the mean activity of voxels showing maximum variance within the sphere) were extracted for each participant, while adjusting for the effects of interest. Each extracted time series was then entered in a PPI analysis model in order to form the interaction between the seed region activity and task complexity (Figural < Analytical < Complex Analytical). This step created 3 vectors: the interaction term, the volume of interest (VOI) eigenvariate and the relative complexity contrast vector. First-level analyses were then performed using the movement parameters and the 3 vectors. Finally, 6 s-level factorial models, one per seed region, were computed to illustrate the interaction between functional connectivity involving the seed regions and task complexity.
3. Results
3.1. Behavioral data
The Group × Complexity ANOVA on accuracy revealed a significant main effect of Complexity, F(2, 90) = 63.019, p < 0.001, but no significant effect of Group, F(1, 90) = 0.964, p = 0.329, and no significant interaction, F(2, 90) = 0.133, p = 0.876. Post-hoc comparisons confirmed a lower accuracy for complex analytical (58.25%) problems than for figural (90.35%) and analytical problems (83.50%), both p < 0.001. The Group × Complexity ANOVA on response times revealed a significant main effect of Group, F(1, 90) = 14.047, p < 0.001, and of Complexity, F(2, 90) = 55.185, p < 0.001, with no significant interaction, F(2, 90) = 2.242, p = 0.112. Autistic participants (12.52 s) were significantly faster than non-autistic participants (17.29 s) by an average of 4.77 s. Response times were longer for complex analytical (23.87 s) than for analytical problems (13.03 s), p < 0.001, and analytical than figural problems (7.82 s), p = 0.002 (see Table 2).
Table 2.
Performance on the RSPM task.
| AUT | Non-AUT | p | |
|---|---|---|---|
| RSPM task (60 items) | |||
| Percent correct | 78.57 (19.36) | 76.16 (17.16) | 0.520 |
| RT | 12.52 (6.89) | 17.29 (10.79) | 0.010 |
| Figural (16 items) | |||
| Percent correct | 92.26 (11.54) | 88.43 (10.75) | 0.340 |
| RT | 6.89 (1.97) | 8.75 (2.57) | 0.033 |
| Analytical (23 items) | |||
| Percent correct | 84.82 (9.04) | 82.18 (8.30) | 0.403 |
| RT | 10.98 (3.79) | 15.08 (5.73) | 0.022 |
| Complex analytical (21 items) | |||
| Percent correct | 58.63 (17.18) | 57.87 (13.47) | 0.889 |
| RT | 19.69 (6.28) | 28.05 (11.09) | 0.017 |
Note. Performance on the Raven's Standard Progressive Matrices (RSPM) expressed in percentage of correct answers and in response time (RT; s).
3.2. fMRI data
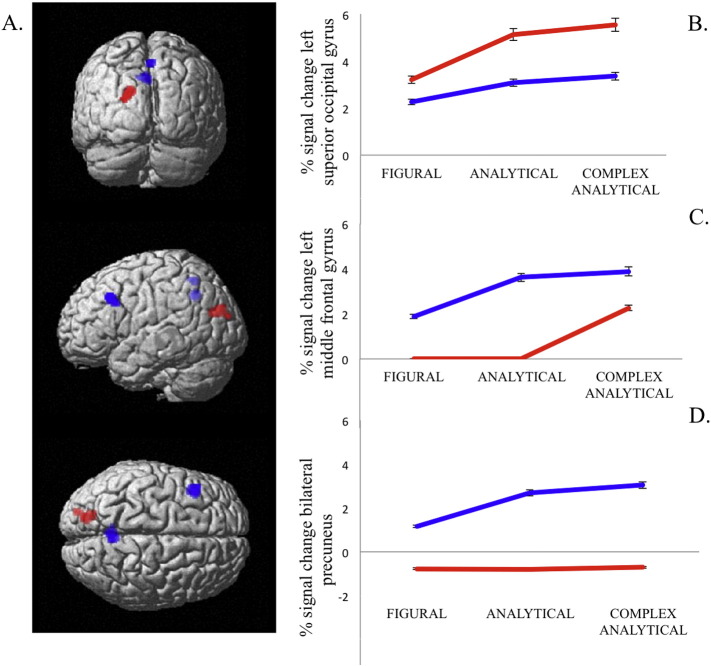
A linear contrast examining reasoning complexity (Figural < Analytical < Complex Analytical) revealed an extended network involving frontal, parietal, occipital, cerebellar and basal ganglia regions, all associated with increasing reasoning complexity (Table 3). Entering response times as a covariate in within-group analyses did not result in any significant changes in the findings. Significant between-group differences in the effects of complexity on task-related activity were revealed in several areas. First, increased complexity-related activity was observed in the left superior occipital gyrus and the left middle occipital gyrus for autistic participants relative to non-autistic participants. Second, non-autistic participants showed increased activity in the left middle frontal gyrus (MFG) and bilateral precuneus, relative to autistic participants (Fig. 2). Fig. 2C and D further illustrates that left MFG was only recruited for complex analytical problems in autistic participants, and that precuneus was not significantly recruited during the task in the autistic group. These results suggest a stronger reliance on occipital areas for autistic participants as task complexity increased.
Table 3.
Statistical modeling results using a complexity contrast (figural < analytical < complex analytical), p < 0.001 unc, K = 50 contiguous voxels.
| Brain region | BA | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|---|
| T scores | X | Y | Z | T scores | X | Y | Z | ||
| Autistic group | |||||||||
| Frontal lobe | |||||||||
| Inferior frontal gyrus | 44 | 7.36 | 50 | 10 | 30* | ||||
| 45 | 7.89 | −44 | 28 | 28* | |||||
| 48 | 7.77 | 40 | 32 | 24* | |||||
| Precentral gyrus | 44 | 7.83 | −42 | 4 | 34* | ||||
| Middle frontal gyrus | 6 | 7.33 | −26 | 0 | 56* | 6.34 | 30 | 0 | 56* |
| Parietal lobe | |||||||||
| Superior parietal lobule | 7 | 10.25 | −24 | −64 | 50* | ||||
| Temporal lobe | |||||||||
| Fusiform gyrus | 19 | 8.41 | 36 | −76 | −14* | ||||
| Occipital lobe | |||||||||
| Inferior occipital gyrus | 18 | 13.82 | −28 | −90 | −6* | 14.43 | 28 | −94 | −4* |
| Middle occipital gyrus | 18 | 8.62 | 34 | −86 | 12* | ||||
| 19 | 10.10 | −26 | −72 | 34* | |||||
| Insula | |||||||||
| Insula | 47 | 5.86 | −28 | 22 | −4* | ||||
| Sub-solar | 47 | 5.00 | 30 | 24 | 0* | ||||
| Subcortical | |||||||||
| Red nucleus | 0 | 4.89 | −4 | −24 | −10* | ||||
| Cerebellum | |||||||||
| Cerebellum | 0 | 6.27 | −6 | −74 | −26* | 6.53 | 10 | −74 | −26* |
| Lobule VIIa | 0 | 4.22 | 34 | −66 | −50* | ||||
| Cerebellar tonsil | 0 | 4.16 | 2 | −54 | −38 | ||||
| Non-autistic group | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 0 | 4.74 | 36 | 60 | 8 | ||||
| 8 | 9.51 | 30 | 8 | 56* | |||||
| 44 | 11.08 | −48 | 24 | 34* | |||||
| 45 | 7.13 | 50 | 28 | 34 | |||||
| Inferior frontal gyrus | 44 | 8.33 | 46 | 8 | 28* | ||||
| 47 | 5.91 | 30 | 26 | −2* | |||||
| 48 | 9.59 | −48 | 26 | 24* | |||||
| 48 | 10.61 | −44 | 16 | 30* | |||||
| Parietal lobe | |||||||||
| Superior parietal lobule | 7 | 10.69 | 18 | −66 | 58 | ||||
| Occipital lobe | |||||||||
| Inferior occipital gyrus | 18 | 18.14 | 28 | −94 | −4* | ||||
| 19 | 15.26 | −32 | −88 | −10* | |||||
| Insula | |||||||||
| Sub-solar | 47 | 7.08 | −28 | 26 | −2* | ||||
| Subcortical | |||||||||
| Pallidum | 0 | 5.11 | 16 | −2 | 0 | ||||
| Caudate nucleus | 0 | 5.06 | −10 | 8 | 12* | ||||
| Globus pallidus | 0 | 4.77 | −16 | −2 | 0 | ||||
| Red nucleus | 0 | 4.05 | −4 | −22 | −10 | 3.67 | 6 | −22 | −8 |
| AUT > non-AUT | |||||||||
| Occipital lobe | |||||||||
| Superior occipital gyrus | 19 | 3.96 | −16 | −82 | 26 | ||||
| Middle occipital gyrus | 18 | 3.53 | −22 | −90 | 20 | ||||
| Non-AUT > AUT | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 9 | 4.44 | −42 | 20 | 34 | ||||
| Parietal lobe | |||||||||
| Precuneus |
0 | 4.10 | −2 | −58 | 38 | ||||
| 7 | 4.07 | 2 | −56 | 54 | |||||
Clusters significant at p < 0.05, FWE corrected.
Fig. 2.
Complexity contrast. A. Non-autistic > autistic contrast (in blue): increased activity in left middle frontal gyrus and bilateral precuneus as complexity increased. AUT > non-AUT contrast (in red): increased activity in the left superior occipital gyrus and the left middle occipital gyrus as complexity increased (p < 0.001 unc, K = 50 contiguous voxels). B, C, D. Percent signal change in the three regions of between-group differences displayed for each task complexity level, in the autistic group (in red) and non-autistic group (in blue).
3.2.1. Eye and head movement
The eye movement analysis showed similar levels of net saccade density during the RSPM task between the autistic participants (3.96) and non-autistic participants (4.44; t = 0.89, p = 0.38).
Head motion analyses did not reveal any significant between-group differences. Similar mean displacement rates were observed in autistic (0.032 mm/s) and non-autistic participants (0.038 mm/s; t = 0.72, p = 0.48). Similar mean rotation rates were observed in the two groups (autistic group: 0.025°/s; non-autistic group: 0.036°/s; t = 1.28, p = 0.21). No significant differences were observed in the peak-to-peak translations or rotations (all p > 0.05).
3.3. PPI analyses
3.3.1. Conjunction seeds
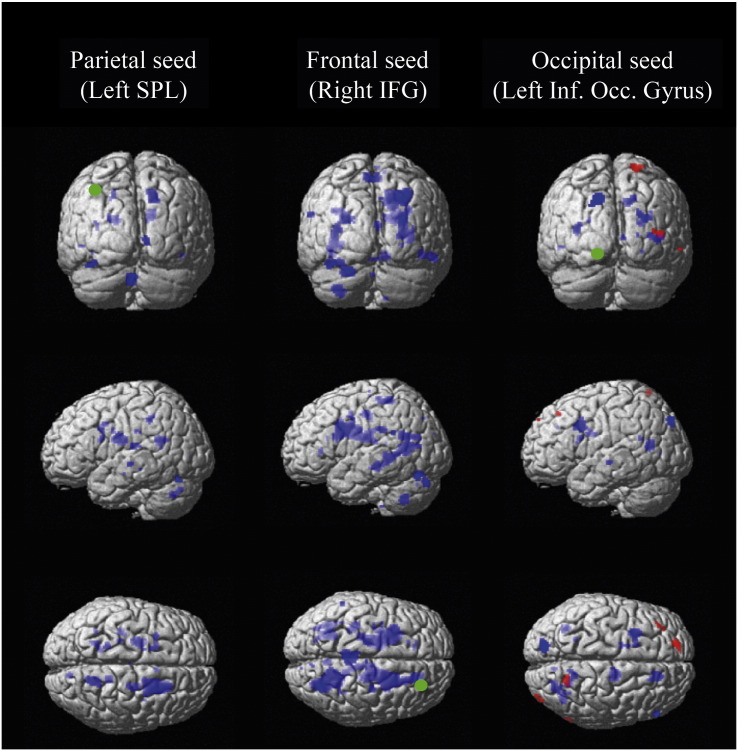
Conjunction seeds (right IFG (BA9), left SPL (BA7) and left inferior occipital gyrus (BA18)) represent the common areas of activity in both groups during the RSPM task. Their associated time series were used in PPI analyses in order to assess how these key fluid reasoning regions interact with other brain regions as complexity increases. Within-group analyses in the autistic group revealed an interaction between the frontal seed (right IFG) and the left IFG. The occipital seed showed an interaction with frontal, temporal, occipital, parietal regions as well as the cerebellum. Moreover, the parietal seed interacted with the left IFG, left IPL, left angular gyrus, right postcentral gyrus and left cerebellum. The within-group analyses in the non-autistic group showed interaction between the 3 seeds and multiple regions in frontal, parietal, temporal and occipital areas. A between-group contrast (AUT > non-AUT) showed that autistic participants had greater connectivity than non-autistic participants (p < 0.001, unc.) between the occipital seed (left inferior occipital gyrus) and areas in the left superior frontal gyrus, right SPL, right middle occipital gyrus and right inferior temporal gyrus. In contrast, non-autistic participants had greater connectivity than autistic participants between all 3 seeds and multiple other regions involved in reasoning (frontal, parietal, temporal and occipital regions) as complexity increased (Table 4). Overall, non-autistic participants exhibited more complexity-related connectivity than autistic participants in the selected frontal, parietal and occipital seeds (Fig. 3), demonstrating that autistic individuals exhibit reduced modulation of connectivity by reasoning complexity in those areas.
Table 4.
PPI analyses Based on common areas of activity (conjunction seeds) (p < 0.001 unc.).
| Brain region | BA | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|---|
| T scores | X | Y | Z | T scores | X | Y | Z | ||
| Right inferior frontal gyrus seed (52 10 28) | |||||||||
| Non-AUT > AUT | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 44 | 5.10 | −32 | 10 | 34 | ||||
| Superior frontal gyrus | 32 | 4.23 | 18 | 32 | 34 | ||||
| Precentral gyrus | 6 | 3.78 | −38 | −4 | 40 | 4.05 | 32 | −16 | 46 |
| 6 | 3.46 | −36 | −12 | 44 | |||||
| SMA | 4 | 3.55 | 10 | −20 | 60 | ||||
| 4 | 3.42 | 2 | −20 | 70 | |||||
| Paracentral lobule | 4 | 3.73 | −6 | −30 | 60 | ||||
| Parietal lobe | |||||||||
| Precuneus | 5 | 3.82 | −6 | −42 | 60 | ||||
| 0 | 3.78 | 4 | −38 | 58 | |||||
| Supramarginal gyrus | 42 | 3.68 | −58 | −44 | 24 | ||||
| Temporal lobe | |||||||||
| Middle temporal gyrus | 20 | 3.88 | 54 | −36 | −14 | ||||
| Fusiform gyrus | 19 | 3.71 | −32 | −70 | −18 | ||||
| Occipital lobe | |||||||||
| Calcarine gyrus | 17 | 3.78 | 4 | −60 | 16 | ||||
| Cuneus | 7 | 3.42 | 6 | −78 | 40 | ||||
| 18 | 3.40 | 10 | −88 | 24 | |||||
| Insula | |||||||||
| Posterior cingulate cortex | 0 | 3.61 | −6 | −38 | 14 | ||||
| Cerebellum | |||||||||
| Lobule VIIa | 0 | 5.07 | −32 | −56 | −44 | ||||
| 0 | 4.68 | −24 | −80 | −26 | |||||
| 0 | 3.94 | −40 | −70 | −20 | |||||
| Lobule V | 0 | 3.95 | 12 | −40 | −12 | ||||
| 0 | 3.91 | 14 | −32 | −14 | |||||
| Cerebellar vermis Lobule VIIb | 0 | 3.47 | 0 | −70 | −30 | ||||
| Subcortical | |||||||||
| Caudate nucleus | 0 | 5.90 | 20 | 0 | 24* | ||||
| 0 | 5.54 | 16 | −8 | 22 | |||||
| 0 | 5.34 | 18 | −16 | 22 | |||||
| Hippocampus | 20 | 4.23 | 28 | −18 | −16 | ||||
| Parahippocampal gyrus | 28 | 3.82 | 16 | 0 | −24 | ||||
| AUT > non-AUT | |||||||||
| None | |||||||||
| Left superior parietal lobule seed (−34 −46 50) | |||||||||
| AUT < non-AUT | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 44 | 3.96 | −30 | 10 | 32 | ||||
| Superior frontal gyrus | 6 | 3.62 | 24 | −10 | 58 | ||||
| Temporal lobe | |||||||||
| Middle temporal gyrus | 48 | 4.05 | −48 | −20 | −4 | ||||
| Occipital lobe | |||||||||
| Calcarine gyrus | 18 | 3.67 | 10 | −78 | 2 | ||||
| Insula | |||||||||
| Sub-lobar | 48 | 3.89 | −32 | −24 | 14 | ||||
| Subcortical | |||||||||
| Hippocampus | 20 | 3.55 | 32 | −8 | −18 | ||||
| Cerebellum | |||||||||
| Lobule VIIa | 0 | 3.87 | −40 | −70 | −22 | ||||
| 0 | 3.82 | −4 | −64 | −36 | |||||
| Cerebelar vermis Lobule VIIb | 0 | 3.58 | 0 | −70 | −30 | ||||
| AUT > non-AUT | |||||||||
| None | |||||||||
| Left inferior occipital gyrus seed (−32 −86 −10) | |||||||||
| AUT < non-AUT | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 44 | 4.72 | −32 | 10 | 34 | ||||
| Inferior frontal gyrus | 45 | 3.76 | 56 | 32 | 12 | ||||
| Parietal lobe | |||||||||
| Precuneus | 18 | 3.64 | 24 | −60 | 20 | ||||
| Temporal lobe | |||||||||
| Middle temporal gyrus | 48 | 3.78 | −48 | −20 | −6 | ||||
| Occipital lobe | |||||||||
| Cuneus | 19 | 4.25 | −16 | −84 | 38 | ||||
| Middle occipital gyrus | 19 | 4.18 | 30 | −72 | 22 | ||||
| Superior occipital gyrus | 19 | 3.72 | 22 | −76 | 26 | ||||
| Calcarine gyrus | 18 | 3.46 | 10 | −70 | 4 | ||||
| 17 | 3.46 | 8 | −74 | 4 | |||||
| 19 | 3.40 | 22 | −72 | 8 | |||||
| Lingual gyrus | 19 | 3.40 | 26 | −54 | −8 | ||||
| Subcortical | |||||||||
| Caudate nucleus | 0 | 4.01 | −18 | 10 | 22 | ||||
| 0 | 3.53 | −16 | 14 | 16 | |||||
| AUT > non-AUT | |||||||||
| Frontal lobe | |||||||||
| Superior frontal gyrus | 9 | 4.65 | −18 | 54 | 42 | ||||
| 9 | 4.39 | −10 | 58 | 42 | |||||
| Middle frontal gyrus | 9 | 3.65 | −36 | 36 | 46 | ||||
| 9 | 3.64 | −34 | 40 | 44 | |||||
| Temporal lobe | |||||||||
| Inferior temporal gyrus | 37 | 3.66 | 62 | −58 | −6 | ||||
| Parietal lobe | |||||||||
| Superior parietal lobule | 7 | 4.15 | 20 | −62 | 70 | ||||
| Occipital | |||||||||
| Middle occipital gyrus | 0 | 4.14 | 44 | −88 | 8 | ||||
Clusters significant at p < 0.05, FWE corrected.
Fig. 3.
PPI analyses based on common areas of activity (conjunction seeds). Non-autistic > autistic contrast (in blue): As complexity increased, non-autistic participants exhibited a wider increase in functional connectivity with other elements of the reasoning network for the 3 regions of interest, in comparison with autistic participants. Autistic > non-autistic contrast (in red): As complexity increased, autistic participants showed a higher functional connectivity than non-autistic participants only for the occipital seed, for which they displayed an increase in connectivity between the left inferior occipital gyrus (seed) and areas in the left superior frontal gyrus, right superior parietal lobule, right middle occipital gyrus and right inferior temporal gyrus (p < 0.001, unc.). Green spots indicate seeds.
3.3.2. Maximum difference seeds
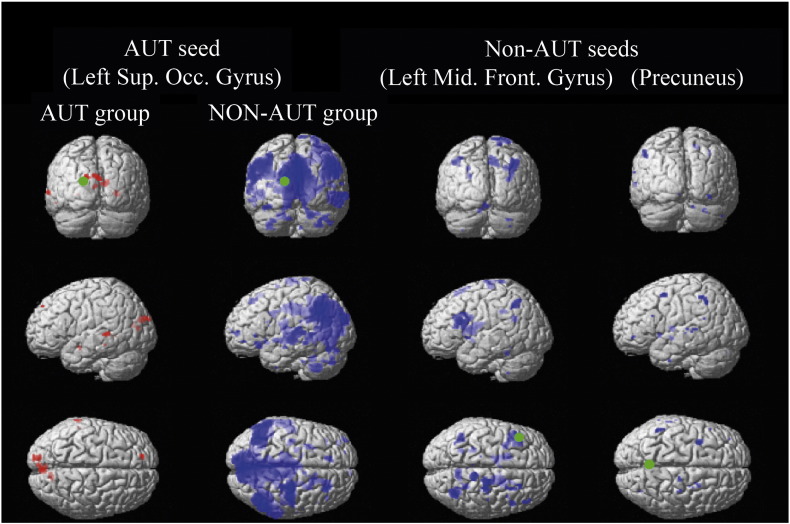
In order to explore group-specific patterns of connectivity in relation to complexity, maximum difference seeds were used to identify the key areas associated with reasoning complexity that are unique to each group in within-group analyses. In the autistic group, reasoning complexity modulated connectivity between the occipital seed (left superior occipital gyrus) and circumscribed frontal, temporal, occipital and subcortical regions (Table 5). However no interaction involving the frontal and precuneus seeds were seen. In the non-autistic group, reasoning complexity modulated connectivity between each of the 3 seeds and an extended network of cortical and subcortical regions (Fig. 4). This second set of PPI analyses also suggests less extended network interactions involved in reasoning in autistic individuals relative to non-autistic individuals.
Table 5.
PPI analyses Based on areas of maximal between-group differences in activity (maximum difference seeds) (p < 0.001 unc.).
| Brain region | BA | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|---|
| T scores | X | Y | Z | T scores | X | Y | Z | ||
| Left superior occipital gyrus seed (−16 −82 26) | |||||||||
| AUT group | |||||||||
| Frontal lobe | |||||||||
| Superior frontal gyrus | 9 | 4.56 | −18 | 50 | 42 | ||||
| Temporal lobe | |||||||||
| Middle temporal gyrus | 22 | 5.02 | −68 | −38 | 4 | ||||
| 20 | 4.02 | −56 | −36 | −12 | |||||
| 21 | 3.85 | −56 | −2 | −18 | |||||
| Superior temporal gyrus | 21 | 4.05 | −56 | 0 | −14 | ||||
| Occipital lobe | |||||||||
| Lingual gyrus | 19 | 3.88 | 22 | −50 | 2 | ||||
| Calcarine gyrus | 17 | 4.35 | −2 | −80 | 10 | 4.06 | 6 | −76 | 16 |
| 18 | 4.64 | 14 | −74 | 16 | |||||
| Superior occipital gyrus | 18 | 4.62 | −14 | −98 | 24 | ||||
| 18 | 4.51 | −16 | −88 | 24 | |||||
| Cuneus | 18 | 4.14 | −4 | −92 | 26 | 4.16 | 4 | −88 | 22 |
| Subcortical | |||||||||
| Parahippocampal gyrus | 37 | 3.89 | −26 | −50 | 0 | ||||
| 19 | 5.56 | 28 | −56 | 0 | |||||
| Non-AUT group | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 10 | 4.10 | −38 | 56 | 4 | ||||
| 9 | 4.31 | 26 | 30 | 38 | |||||
| 4.22 | 30 | 28 | 48 | ||||||
| Precentral gyrus | 6 | 5.88 | 24 | −16 | 78 | ||||
| Postcentral gyrus | 4 | 5.72 | 42 | −20 | 46 | ||||
| 4 | 5.20 | 10 | −38 | 80 | |||||
| Superior frontal gyrus | 9 | 4.43 | −12 | 34 | 46 | ||||
| 8 | 3.85 | 16 | 24 | 56 | |||||
| 11 | 3.71 | −22 | 54 | 4 | |||||
| Inferior frontal gyrus | 38 | 4.21 | −40 | 24 | −14 | ||||
| 45 | 4.02 | 42 | 30 | 2 | |||||
| 47 | 4.09 | −36 | 30 | 0 | 3.66 | 48 | 18 | −14 | |
| 47 | 3.67 | −32 | 32 | 0 | |||||
| SMA | 6 | 4.17 | 8 | 0 | 68 | ||||
| Middle orbital gyrus | 47 | 3.94 | −34 | 44 | −8 | ||||
| Middle cingulate cortex | 24 | 3.92 | 8 | 16 | 30 | ||||
| 0 | 3.82 | 12 | 2 | 36 | |||||
| 0 | 3.77 | 12 | 6 | 36 | |||||
| Superior medial gyrus | 10 | 3.83 | 8 | 62 | 4 | ||||
| 10 | 3.67 | 10 | 64 | 8 | |||||
| Temporal lobe | |||||||||
| Middle temporal gyrus | 48 | 7.15 | −48 | −20 | −6* | ||||
| 21 | 5.56 | 66 | −40 | −4 | |||||
| 21 | 4.11 | −62 | −20 | −12 | 5.44 | 56 | −38 | −8 | |
| 21 | 3.71 | −58 | −26 | −8 | |||||
| 22 | 3.93 | 64 | −48 | 12 | |||||
| 20 | 4.02 | −58 | −38 | −10 | |||||
| 22 | 3.93 | −54 | −10 | −12 | |||||
| 21 | 3.66 | −52 | −28 | −8 | |||||
| Superior temporal gyrus | 22 | 4.89 | −64 | −22 | 4 | ||||
| 41 | 4.02 | 48 | −32 | 10 | |||||
| 48 | 4.63 | −38 | −26 | 6 | |||||
| 20 | 3.79 | 40 | 10 | −28 | |||||
| 22 | 3.68 | −66 | −40 | 18 | 3.75 | 56 | −2 | −6 | |
| 22 | 3.67 | 62 | −10 | −2 | |||||
| Fusiform gyrus | 19 | 4.22 | −38 | −70 | −18 | ||||
| Heschl's gyrus | 41 | 4.21 | 36 | −32 | 12 | ||||
| Inferior temporal gyrus | 20 | 3.82 | 52 | −12 | −28 | ||||
| 20 | 3.72 | 48 | −12 | −30 | |||||
| Parietal lobe | |||||||||
| Angular gyrus | 39 | 7.40 | −44 | −64 | 34* | ||||
| Middle cingulate cortex | 5 | 3.73 | −16 | −34 | 44 | ||||
| Supramarginal gyrus | 42 | 3.71 | −66 | −32 | 20 | ||||
| Occipital lobe | |||||||||
| Calcarine gyrus | 17 | 7.63 | 8 | −66 | 14* | ||||
| Subcortical | |||||||||
| Thalamus | 0 | 4.86 | −12 | −12 | 6 | 5.84 | 20 | −14 | 10 |
| 0 | 3.66 | −18 | −12 | −2 | |||||
| Putamen | 0 | 4.64 | −30 | −12 | −4 | ||||
| Amygdala | 34 | 3.80 | 28 | 2 | −16 | ||||
| Cerebellum | |||||||||
| Lobule VIIa | 0 | 5.45 | −36 | −48 | −46 | 4.50 | 46 | −50 | −40 |
| 0 | 4.68 | −30 | −72 | −38 | 4.16 | 36 | −72 | −44 | |
| 0 | 4.31 | −16 | −74 | −30 | 4.06 | 32 | −76 | −38 | |
| 0 | 3.69 | 40 | −70 | −36 | |||||
| 0 | 4.75 | 28 | −56 | −46 | |||||
| Lobule VI | 0 | 4.22 | −22 | −50 | −30 | 4.52 | 28 | −62 | −28 |
| 0 | 3.70 | −18 | −52 | −22 | 3.67 | 44 | −70 | −36 | |
| 0 | 3.66 | −30 | −36 | −36 | |||||
| Lobule IX | 0 | 4.23 | 6 | −50 | −48 | ||||
| Lobule V | 0 | 3.91 | −26 | −34 | −34 | ||||
| 0 | 3.68 | −12 | −50 | −20 | |||||
| Left middle frontal gyrus seed (−42 20 34) | |||||||||
| AUT group | |||||||||
| None | |||||||||
| Non-AUT group | |||||||||
| Frontal lobe | |||||||||
| Precentral gyrus | 6 | 5.67 | 16 | −20 | 72 | ||||
| 6 | 5.65 | 28 | −22 | 76 | |||||
| 4 | 3.69 | 44 | −18 | 52 | |||||
| Inferior frontal gyrus | 44 | 5.23 | −44 | 20 | 22 | 4.07 | 48 | 14 | 32 |
| 44 | 4.32 | −34 | 24 | 28 | 4.00 | 40 | 8 | 30 | |
| Superior frontal gyrus | 6 | 4.59 | 26 | 22 | 62 | ||||
| 6 | 3.86 | 22 | 6 | 46 | |||||
| 6 | 3.79 | 28 | 8 | 70 | |||||
| Middle frontal gyrus | 45 | 4.13 | 46 | 34 | 28 | ||||
| 45 | 3.69 | 46 | 40 | 22 | |||||
| Paracentral lobule | 4 | 3.66 | −16 | −28 | 80 | ||||
| Parietal lobe | |||||||||
| Angular gyrus | 7 | 5.53 | 38 | −66 | 42 | ||||
| Postcentral gyrus | 4 | 5.57 | 12 | −36 | 78 | ||||
| Precuneus | 0 | 5.57 | 12 | −58 | 42 | ||||
| Inferior parietal lobule | 7 | 3.84 | −36 | −62 | 52 | ||||
| 40 | 3.68 | −38 | −50 | 40 | |||||
| 40 | 4.25 | −44 | −54 | 44 | |||||
| Subcortical | |||||||||
| Caudate nucleus | 0 | 5.69 | −12 | −4 | 18 | 6.73 | 20 | −14 | 20 |
| 0 | 5.10 | 20 | 2 | 18 | |||||
| Pallidum | 0 | 4.45 | −14 | 4 | −2 | ||||
| 25 | 3.78 | −12 | 0 | −6 | |||||
| Putamen | 48 | 3.80 | 30 | −18 | 8 | ||||
| Cerebellum | |||||||||
| Lobule I−IV | 0 | 4.43 | −2 | −46 | −14 | ||||
| 0 | 3.87 | −10 | −44 | −24 | |||||
| Lobule VI | 0 | 4.34 | 28 | −62 | −34 | ||||
| Lobule VIIa | 0 | 3.69 | 30 | −76 | −44 | ||||
| Lobule VIIIa | 0 | 3.68 | −34 | −46 | −50 | ||||
| Left precuneus seed (−2 −58 38) | |||||||||
| AUT group | |||||||||
| None | |||||||||
| Non-AUT group | |||||||||
| Frontal lobe | |||||||||
| Middle frontal gyrus | 6 | 4.48 | −34 | 4 | 54 | ||||
| Superior frontal gyrus | 6 | 4.05 | 26 | 4 | 46 | ||||
| Inferior frontal gyrus | 48 | 3.70 | −52 | 20 | 24 | ||||
| Middle orbital gyrus | 47 | 3.69 | −38 | 46 | −6 | ||||
| Parietal lobe | |||||||||
| Inferior parietal lobule | 40 | 4.10 | −52 | −44 | 50 | ||||
| 40 | 3.98 | −50 | −50 | 44 | |||||
| Temporal lobe | |||||||||
| Fusiform gyrus | 20 | 4.06 | −28 | −2 | −38 | ||||
| 19 | 4.22 | 34 | −72 | −12 | |||||
| Superior temporal gyrus | 20 | 3.79 | 42 | 12 | −26 | ||||
| 22 | 3.91 | −64 | −26 | 6 | |||||
| 22 | 3.66 | −64 | −48 | 20 | |||||
| Middle temporal gyrus | 22 | 3.90 | −62 | −38 | 6 | ||||
| 21 | 3.67 | −62 | −32 | 2 | |||||
| Subcortical | |||||||||
| Pallidum | 25 | 5.44 | 12 | 4 | −6 | ||||
| 0 | 3.72 | 22 | −8 | 0 | |||||
| Putamen | 0 | 3.85 | −12 | 10 | −4 | 4.24 | 18 | 12 | 0 |
| Thalamus | 0 | 4.36 | 22 | −18 | 2 | ||||
| 0 | 3.87 | 16 | −22 | 0 | |||||
| 0 | 3.77 | 8 | −24 | −2 | |||||
| Cerebellum | |||||||||
| Lobule VIIa | 0 | 3.85 | 50 | −52 | −40 | ||||
| 0 | 3.84 | 30 | −62 | −34 | |||||
| 0 | 3.69 | 12 | −78 | −26 | |||||
| Lobule VI | 0 | 3.81 | 26 | −60 | −32 | ||||
| 0 | 3.71 | 28 | −64 | −30 | |||||
| Lobule V | 0 | 3.68 | −6 | −50 | −10 | ||||
| Lobule I−IV | 0 | 3.65 | 14 | −38 | −28 | ||||
Fig. 4.
PPI analyses based on areas of maximal between-group differences in activity (maximum difference seeds). Non-autistic participants (in blue) demonstrated a wide connectivity with other elements of the reasoning network for the three seeds. Autistic participants (in red) only showed functional connectivity for the occipital seed, which exhibited an interaction with frontal, temporal, occipital and sub-cortical areas (p < 0.001, unc.). Green spots indicate seeds.
4. Discussion
Our objective was to investigate how task complexity modulates the level of activity and connectivity among brain regions during fluid reasoning in autism. While both groups showed similar accuracy in solving RSPM problems, autistic participants performed faster than non-autistic participants at all complexity levels, as we previously reported in a study identifying occipital cortex as being more active in autistic individuals during matrix reasoning (Soulières et al., 2009). Here we demonstrated that this increased activity was actually modulated by reasoning complexity. Moreover, while autistic participants exhibited less modulation of fronto-parietal activity and connectivity as reasoning complexity increased, connectivity involving occipital areas was more consistently modulated by task complexity in autistic than non-autistic participants. Occipital regions are not only known for their involvement in visuospatial processes, but also in the identification of correct answers with abstract rules (Skosnik et al., 2002), number subtraction and addition (Benn et al., 2012). The greater use of these regions as complexity increases in a reasoning task has an equivalent in typical individuals, albeit to a lesser extent (Goel, 2007; Soulières et al., 2009; Yamada et al., 2012).
In contrast, compared to autistic participants, non-autistic participants more strongly engaged a combination of regions related to verbal and visuospatial processing as task complexity increased. The left middle frontal gyrus is known to be involved in inner speech (Geva et al., 2011; Jones and Fernyhough, 2007), working memory (Liakakis et al., 2011) and other verbal processes (Prado et al., 2013). The precuneus in both hemispheres is involved in visuospatial imagery, episodic memory retrieval and self-referential processing (Cavanna and Trimble, 2006). In this group an occipital to frontal/parietal activation shift is seen when participants inhibit perceptual information to engage in logical reasoning, and by extension with increasing reasoning complexity (Houde et al., 2000). The present results suggest that this shift might not be as visible in autistic individuals, as perceptual processes able to support this type of reasoning are engaged more strongly. Thus, we suggest that autistic individuals rely more specifically on visuospatial processes to resolve complex matrices whereas non-autistic individuals rely more prominently on a combination of verbal and visuospatial processes.
Our hypothesis that autistic participants would exhibit higher cortical connectivity in the posterior parts of the reasoning network as task complexity increased, compared to non-autistic participants, was partially confirmed. Indeed, a stronger long-range functional connectivity was identified in autistic participants, relative to non-autistic participants, between the left inferior occipital gyrus and the left superior frontal gyrus and the right SPL as task complexity increased. The possibility of enhanced connectivity between visual associative areas and other parts of the brain, even the most distant, has been raised by several prior studies (Leveille et al., 2010; Keehn et al., 2013; Shen et al., 2012; Supekar et al., 2013), views at odds with dominant accounts of reduced long-range connectivity (Dichter, 2012; Just et al., 2012). In combination with the demonstration of enhanced activity associated with visual processing in many contexts in autism (Samson et al., 2012), we believe that autism is characterized, not only by enhanced perceptual performance, but also by enhanced role of perceptual processing in higher order cognitive processes (Mottron et al., 2013).
By contrast, none of the analyses involving seeds in prefrontal cortex revealed increased functional connectivity in autistic participants relative to non-autistic participants. To the contrary, autistic participants generally exhibited less connectivity in the anterior elements of the network, a finding also reported by previous studies (Sahyoun et al., 2010; Yamada et al., 2012). McGrath and colleagues also obtained similar results in a mental rotation task, as they observed a general decrease in functional connectivity as task complexity increased in autistic individuals, the visual cortex being the only exception to this decrease (McGrath et al., 2012). This decreased modulation of the fronto-parietal network could suggest that autistic individuals are less influenced by task complexity than non-autistic individuals, as they showed less change in the network's connectivity as complexity increased, while maintaining equal accuracy. Considering that both groups showed similar accuracy, and autistic individuals responded more quickly during the task, we cannot conclude that our results are best interpreted as “altered” network activity in the network as suggested by Yamada et al. (2012). Rather, they may reflect lower complexity-induced modulation of the network in autistic individuals, with potential positive consequences on performance.
As the enhanced perceptual functioning (EPF) model suggests, autistic cognition is, among other elements, characterized by a stronger engagement of perceptual processes and a different equation between neuronal engagement and task difficulty (Mottron et al., 2013). Our results of a stronger engagement of visuospatial processes and lower modulation of the reasoning network as complexity increases during reasoning directly contribute to this model and add to the body of work describing a stronger engagement of perceptual processes in higher cognition in autistic individuals.
4.1. Limitations and future directions
This study focussed on the influence of visuospatial processes on fluid reasoning in autistic individuals and may not reflect all the cognitive process involved in other aspects of intelligence. Also, we chose to use a liberal threshold (p < 0.001 uncorrected) in order to observe the full extent of between-group differences in the cerebral activity and connectivity underlying reasoning, as a more stringent threshold may smooth out some interesting differences. Finally, our sample was composed of participants with measured intelligence in the normal range only; hence, these results may or may not apply to the whole spectrum of autistic intelligence.
Nevertheless, these results provide us with a better understanding of the atypical, yet not dysfunctional, reasoning abilities of autistic individuals (Dawson et al., 2007). Indeed, the relative advantage of autistic individuals at the RSPM might be linked to a more efficient recruitment of posterior brain regions analyzing the visuospatial information provided in the problems. Further studies could explore the effect of complexity in other domains of autistic cognition, as well as how the modulation of cognitive processes in relation to complexity occurs in autistic individuals with lower reasoning abilities. This could lead to the application of these findings to optimizing learning and work environments for autistic individuals.
References
- Benn Y., Zheng Y., Wilkinson I.D., Siegal M., Varley R. Language in calculation: a core mechanism? Neuropsychologia. 2012;50(1):1–10. doi: 10.1016/j.neuropsychologia.2011.09.045. 22079204 [DOI] [PubMed] [Google Scholar]
- Carpenter P.A., Just M.A., Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven progressive matrices test. Psychol. Rev. 1990;97(3):404–431. 2381998 [PubMed] [Google Scholar]
- Cattell R.B. North-Holland; Oxford, England: 1987. Intelligence: Its Structure, Growth and Action. [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Charman T., Jones C.R., Pickles A., Simonoff E., Baird G., Happé F. Defining the cognitive phenotype of autism. Brain Res. 2011;1380:10–21. doi: 10.1016/j.brainres.2010.10.075. 21029728 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. 15831407 [DOI] [PubMed] [Google Scholar]
- Dawson M., Soulieres I., Ann Gernsbacher M., Mottron L. The level and nature of autistic intelligence. Psychol. Sci. 2007;18(8):657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S. Functional magnetic resonance imaging of autism spectrum disorders. Dial. Clin. Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmore T.M., Li H., Xue Z., Wong S.T.C., Frye R.E. Tract-based spatial statistics reveal altered relationship between non-verbal reasoning abilities and white matter integrity in autism spectrum disorder. J. Int. Neuropsychol. Soc. 2013;19(06):723–728. doi: 10.1017/S1355617713000325. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Functional and effective connectivity: a review. Brain Connectivity. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Geva S., Jones P.S., Crinion J.T., Price C.J., Baron J.-C., Warburton E.A. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain. 2011;134(10):3071–3082. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbard C.R., Ren J., Seunarine K.K., Clayden J.D., Skuse D.H., Clark C.A. White matter microstructure correlates with autism trait severity in a combined clinical-control sample of high-functioning adults. Neuroimage Clin. 2013;3:106–114. doi: 10.1016/j.nicl.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goel V. Anatomy of deductive reasoning. Trends Cogn. Sci. 2007;11(10):435–441. doi: 10.1016/j.tics.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Gray J.R., Chabris C.F., Braver T.S. Neural mechanisms of general fluid intelligence. Nat. Neurosci. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Houdé O., Zago L., Mellet E., Moutier S., Pineau A., Mazoyer B., Tzourio-Mazoyer N. Shifting from the perceptual brain to the logical brain: the neural impact of cognitive inhibition training. J. Cogn. Neurosci. 2000;12(5):721–728. doi: 10.1162/089892900562525. [DOI] [PubMed] [Google Scholar]
- Ikuta T., Shafritz K.M., Bregman J., Peters B.D., Gruner P., Malhotra A.K., Szeszko P.R. Abnormal cingulum bundle development in autism: a probabilistic tractography study. Psychiatry Res. Neuroimag. 2014;221(1):63–68. doi: 10.1016/j.pscychresns.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.R., Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin. Psychol. Rev. 2007;27(2):140–154. doi: 10.1016/j.cpr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Jung R.E., Haier R.J. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 2007;30(02):135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Keller T.A., Malave V.L., Kana R.K., Varma S. Autism as a neural systems disorder: a theory of frontal–posterior underconnectivity. Neurosci. Biobehav. Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbfleisch M.L., Van Meter J.W., Zeffiro T.A. The influences of task difficulty and response correctness on neural systems supporting fluid reasoning. Cogn Neurodyn. 2007;1(1):71–84. doi: 10.1007/s11571-006-9007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B., Shih P., Brenner L.A., Townsend J., Müller R.A. Functional connectivity for an “island of sparing” in autism spectrum disorder: an fMRI study of visual search. Hum. Brain Mapp. 2013;34(10):2524–2537. doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger J.K., Sabb F.W., Fales C.L., Bookheimer S.Y., Cohen M.S., Holyoak K.J. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb. Cortex. 2002;12(5):477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Le Couteur A., Rutter M., Lord C., Rios P., Robertson S., Holdgrafer M., McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J. Autism Dev. Disord. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Choi Y.Y., Gray J.R., Cho S.H., Chae J.H., Lee S., Kim K. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29(2):578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Léveillé C., Barbeau E.B., Bolduc C., Limoges É, Berthiaume C., Chevrier É.…Godbout R. Enhanced connectivity between visual cortex and other regions of the brain in autism: a REM sleep EEG coherence study. Autism Res. 2010;3(5):280–285. doi: 10.1002/aur.155. [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. Diversity of the inferior frontal gyrus — a meta-analysis of neuroimaging studies. Behav. Brain Res. 2011;225(1):341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C.…Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lynn R., Allik J., Irwing P. Sex differences on three factors identified in Raven's Standard Progressive Matrices. Intelligence. 2004;32(4):411–424. [Google Scholar]
- McFadden K., Minshew N.J. Evidence for dysregulation of axonal growth and guidance in the etiology of ASD. Front. Hum. Neurosci. 2013;7:671. doi: 10.3389/fnhum.2013.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Johnson K., Ecker C., O'Hanlon E., Gill M., Gallagher L., Garavan H. Atypical visuospatial processing in autism: insights from functional connectivity analysis. Autism Res. 2012;5(5):314–330. doi: 10.1002/aur.1245. [DOI] [PubMed] [Google Scholar]
- McGrath J., Johnson K., O'Hanlon E., Garavan H., Leemans A., Gallagher L. Abnormal functional connectivity during visuospatial processing is associated with disrupted organisation of white matter in autism. Front. Hum. Neurosci. 2013;7:434. doi: 10.3389/fnhum.2013.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L., Bouvet L., Bonnel A., Samson F., Burack J.A., Dawson M., Heaton P. Veridical mapping in the development of exceptional autistic abilities. Neurosc. Biobehav. Rev. 2013;37(2):209–228. doi: 10.1016/j.neubiorev.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Nader A.M., Courchesne V., Dawson M., Soulières I. Does WISC-IV underestimate the intelligence of autistic children? J. Autism Dev. Disord. 2014 doi: 10.1007/s10803-014-2270-z. [DOI] [PubMed] [Google Scholar]
- Perfetti B., Saggino A., Ferretti A., Caulo M., Romani G.L., Onofrj M. Differential patterns of cortical activation as a function of fluid reasoning complexity. Hum. Brain Mapp. 2009;30(2):497–510. doi: 10.1002/hbm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Booth J.R. Fractionating the neural substrates of transitive reasoning: task-dependent contributions of spatial and verbal representations. Cereb. Cortex. 2013;23(3):499–507. doi: 10.1093/cercor/bhr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. The Psychological Corporation; Toronto, Canada: 1976. Raven Progressive Matrices. [Google Scholar]
- Rorden C., Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Soulières I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F., Mottron L., Soulières I., Zeffiro T.A. Enhanced visual functioning in autism: an ALE meta-analysis. Hum. Brain Mapp. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.D., Shih P., Öttl B., Keehn B., Leyden K.M., Gaffrey M.S., Müller R.A. Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: evidence from functional and effective connectivity. Neuroimage. 2012;62(3):1780–1791. doi: 10.1016/j.neuroimage.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik P.D., Mirza F., Gitelman D.R., Parrish T.B., Mesulam M.M., Reber P.J. Neural correlates of artificial grammar learning. Neuroimage. 2002;17(3):1306–1314. doi: 10.1006/nimg.2002.1291. [DOI] [PubMed] [Google Scholar]
- Soulières I., Dawson M., Samson F., Barbeau E.B., Sahyoun C.P., Strangman G.E.…Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Hum. Brain Mapp. 2009;30(12):4082–4107. doi: 10.1002/hbm.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulières I., Zeffiro T.A., Girard M.L., Mottron L. Enhanced mental image mapping in autism. Neuropsychologia. 2011;49(5):848–857. doi: 10.1016/j.neuropsychologia.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Stevenson J.L., Gernsbacher M.A. Abstract spatial reasoning as an autistic strength. PLOS One. 2013;8(3):e59329. doi: 10.1371/journal.pone.0059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Khouzam A., Phillips J., Gaillard W.D., Kenworthy L.E.…Menon V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka J.M., Kennedy D.P., Paul L.K., Adolphs R. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb. Cortex. 2014;24(7):1894–1905. doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven A.H.G.S., Ellis J.L. A Rasch analysis of Raven's Standard Progressive Matrices. Pers. Individ. Dif. 2000;29(1):45–64. [Google Scholar]
- Vissers M.E., Cohen M.X., Geurts H.M. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci. Biobehav. Rev. 2012;36(1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wendelken C., Nakhabenko D., Donohue S.E., Carter C.S., Bunge S.A. “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J. Cogn. Neurosci. 2008;20(4):682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ohta H., Watanabe H., Kanai C., Tani M., Ohno T.…Hashimoto R. Functional alterations in neural substrates of geometric reasoning in adults with high-functioning autism. PLOS One. 2012;7(8):e43220. doi: 10.1371/journal.pone.0043220. [DOI] [PMC free article] [PubMed] [Google Scholar]