Abstract
Background. The Malawi Liverpool Wellcome Trust Clinical Research Programme (MLW) has routinely collected specimens for blood culture from febrile patients, and cerebrospinal fluid from patients with suspected meningitis, presenting to Queen Elizabeth Central Hospital (QECH), Blantyre, Malawi, since 1998.
Methods. We present bloodstream infection (BSI) and meningitis surveillance data from 1998 to 2014. Automated blood culture, manual speciation, serotyping, and antimicrobial susceptibility testing were performed at MLW. Population data for minimum-incidence estimates in urban Blantyre were drawn from published estimates.
Results. Between 1998 and 2014, 167 028 blood cultures were taken from adult and pediatric medical patients presenting to QECH; Salmonella Typhi was isolated on 2054 occasions (1.2%) and nontyphoidal Salmonella (NTS) serovars were isolated 10 139 times (6.1%), of which 8017 (79.1%) were Salmonella Typhimurium and 1608 (15.8%) were Salmonella Enteritidis. There were 392 cases of NTS meningitis and 9 cases of Salmonella Typhi meningitis. There have been 3 epidemics of Salmonella BSI in Blantyre; Salmonella Enteritidis from 1999 to 2002, Salmonella Typhimurium from 2002 to 2008, and Salmonella Typhi, which began in 2011 and was ongoing in 2014. Multidrug resistance has emerged in all 3 serovars and is seen in the overwhelming majority of isolates, while resistance to third-generation cephalosporins and fluoroquinolones is currently uncommon but has been identified.
Conclusions. Invasive Salmonella disease in Malawi is dynamic and not clearly attributable to a single risk factor, although all 3 epidemics were associated with multidrug resistance. To inform nonvaccine and vaccine interventions, reservoirs of disease and modes of transmission require further investigation.
Keywords: Salmonella, bloodstream infection, Africa, multi-drug resistance
Malawi is a small (118 000 m2), subtropical country located in sub-Saharan Africa. There is one long rainy season from November to March [1].
It is consistently rated as one of the 10 poorest countries in the world. The estimated mean per capita income in 2013 was $270 and 90% of people live on <$2/day (http://www.worldbank.org/en/country/malawi). The population at the time of the last census in 2008 was approximately 13 million; however, with a fertility rate of 5.3 births per woman, sharp falls in under-5 mortality, and increasing adult life expectancy, this is projected to rise to 16.3 million by 2015 (www.nsomalawi.mw). Blantyre is the principal city in the southern region, with an urban population of approximately 885 000 (www.nsomalawi.mw), and Blantyre district has a population of 1.3 million.
Disease due to serovars of Salmonella is commonly considered to be either typhoidal (also known as enteric fever) due to human-restricted invasive Salmonella enterica serovars Typhi and Paratyphi A, or nontyphoidal (NTS), due to one of the other Salmonella serovars, and commonly associated with self-limiting enterocolitis. Invasive nontyphoidal Salmonella (iNTS) disease was first reported in Blantyre as a cause of life-threatening disease in children in 2000 in association with human immunodeficiency virus (HIV), severe malaria, severe anemia, and malnutrition [2–4]. Subsequently, iNTS disease has been reported in adults in Blantyre in association with advanced HIV infection [2]. The national prevalence of HIV in adults was estimated at 10.3% in 2013 (www.unaids.org), but at 22% in urban Blantyre in 2001, falling to 17.9% in 2010 [5]. There has been a highly successful antiretroviral therapy (ART) program, which has been extensively rolled out since 2005. In 2013, the total number of patients alive on ART in Malawi was 472 865, representing coverage of 83% of patients with a CD4 count ≤350 cells/µL (www.unaids.org).
Malawi is a malaria-endemic country with seasonal peaks; the malaria season commences shortly after the start of the rains and lasts until May/June. All febrile children presenting to Queen Elizabeth Central Hospital (QECH), Blantyre, have a thick film examined for malaria parasites; between 2001 and 2010, 61 320 of 242 953 (25%) patients were positive for Plasmodium falciparum. Numbers of positive slides declined after 2003, and then leveled off up to 2010 [6].
The prevalence of underweight-for-height status in children aged <5 years in Malawi, which was static between 2000 and 2004, has declined from 6% in 2004 [7] to 4% in 2010 [1]. Admissions for severe acute malnutrition also peak during the rainy season, as increased infection risk coincides with the peak of the “hungry season” when household food supplies are running low while the new season's crops are growing.
Older antimicrobials, such as amoxicillin, chloramphenicol, and cotrimoxazole have been widely available in the community for many years. Cotrimoxazole prophylactic therapy is widely used for patients with HIV infection, and sulfonamides have also been extensively used to treat malaria as part of the combination agent sulfadoxine-pyrimethamine since 1993. Ciprofloxacin (2002) and ceftriaxone (2005) were more recently introduced and are more difficult to acquire outside large government hospitals.
METHODS
Study Site
QECH is the largest government hospital in Malawi and serves Blantyre district and the Southern region, providing free healthcare to approximately 10 000 adult (age ≥16 years) and 50 000 pediatric medical inpatients a year, of whom an estimated 70% of adult inpatients are HIV infected [8]. Diagnostic microbiology facilities were provided by the Malawi Liverpool Wellcome Trust Clinical Research Programme (MLW; http://www.mlw.medcol.mw/).
Time Period
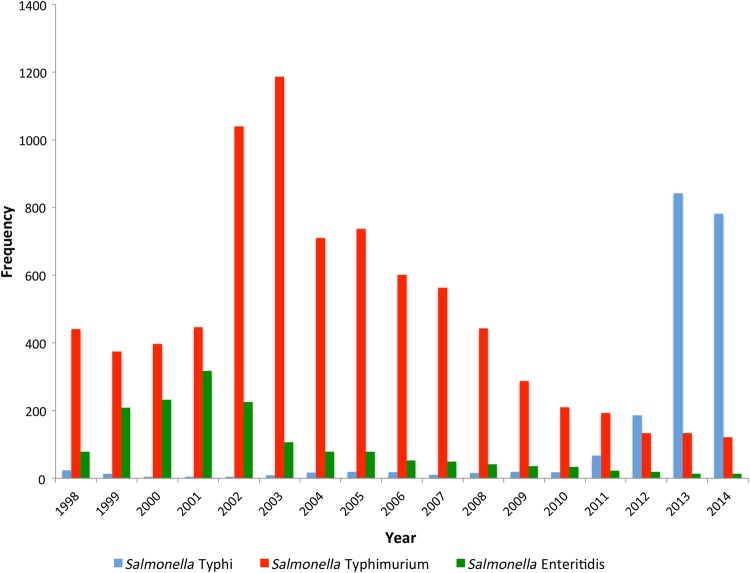
MLW instituted routine blood culture in 1998 and routine cerebrospinal fluid (CSF) culture in 2000. We extracted data from passive hospital-based longitudinal surveillance for bloodstream infection (BSI) from 1998 through 2014 and for meningitis from 2000 through 2014 (Figure 1).
Figure 1.
Temporal trends in Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Typhi bloodstream infection at Queen Elizabeth Central Hospital, Blantyre, Malawi, 1998–2014.
Eligibility Criteria
During the study period, blood cultures were routinely obtained from any adult medical patient with an axillary temperature >37.5°C or clinical suspicion of sepsis. Blood cultures were obtained from febrile children admitted to the hospital who had a thick blood film that was negative for malaria parasites or who were considered to be critically ill regardless of the thick-film result or temperature. CSF was routinely obtained from all patients in whom meningitis was clinically suspected.
Estimation of Denominator Population and Minimum Incidence of Salmonella BSI
Population projections following the 2008 census were used to provide a denominator for minimum incidence calculations (National Statistics Office, Malawi; www.nsomalawi.mw/2008-population-and-housing-census.html). Minimum incidence of invasive Salmonella per 100 000 patients per year was calculated using an estimated sensitivity of blood culture of 50%; therefore, the number of positive blood cultures recorded in each year was doubled to provide a numerator for each of typhoid fever and iNTS disease [9, 10].
Age Distribution
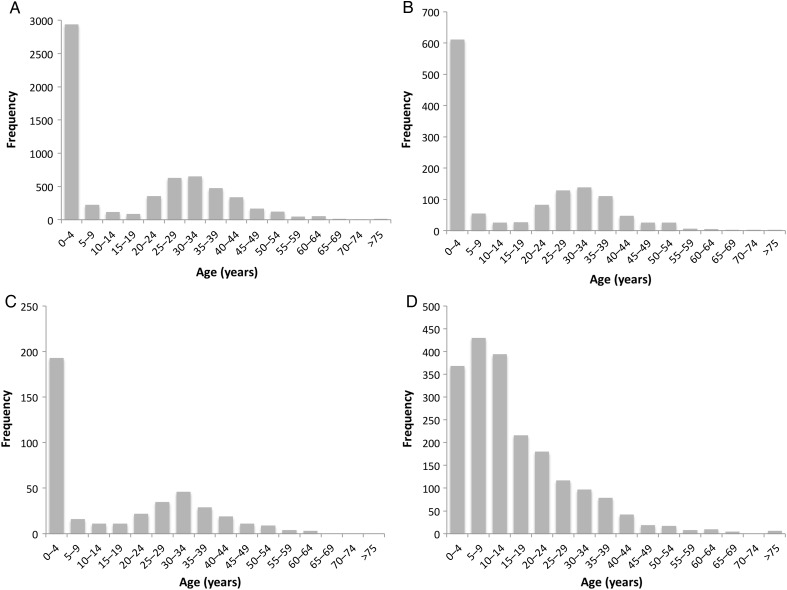
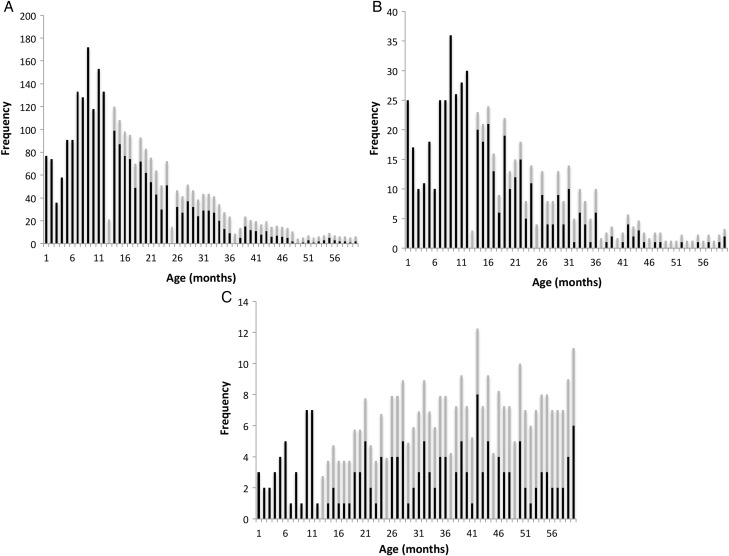
Aggregate age distribution data are reported in 4 categories: Salmonella enterica serovar Typhimurium, Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Typhi, and an aggregate of locally untypable serotypes of Salmonella enterica species. Data for all 4 groups were reported in 5-year blocks for all age groups (Figure 2) and on a monthly basis for children ≤5 years of age for the 3 most common serotypes (Figure 3). Children <1 year of age routinely had age described in months, but where the age of older children was reported in whole years, the total frequency of cases for that age was equally apportioned across that year of life to smooth the graph—that is, if there were 12 children aged 2 years, 1 case was added to the total for each of months 24–35.
Figure 2.
Frequency plots of aggregate age distribution data for Salmonella Typhimurium (A), Salmonella Enteritidis (B), Salmonella species (C), and Salmonella Typhi (D) in 5-year age groups.
Figure 3.
Frequency plots of aggregate age distribution data by month for Salmonella Typhimurium (A), Salmonella Enteritidis (B), and Salmonella Typhi (C) for children <5 years of age. Black bars represent cases where age in months is known and gray bars represent the cases where age is known in years evenly distributed across the year.
The large numbers of Salmonella Typhimurium and Salmonella Typhi permit further analysis of trends in age at presentation. Due to the bimodal age distribution of Salmonella Typhimurium [11, 12], data were analyzed separately for children <16 years of age and adults ≥16 years. As the age distributions for both the children and adults were nonnormal, the Kruskal–Wallis 1-way analysis of variance was used to screen for variation in age; if significant, an appropriate multiple-comparison post hoc test was used to clarify whether there was a significant long-term increasing or decreasing age trend.
Inpatient Case Fatality Data
Patient outcome data were only available for selected periods: 2011–2013 for Salmonella Typhi, 1999, 2000, 2002–2004, and 2009–2010 for iNTS disease in adults, and 1998 and 2006 for iNTS disease in children (Table 1).
Table 1.
Temporal Trends in Isolation of Salmonella and Estimated Minimum Incidence of Salmonella Bloodstream Infection at Queen Elizabeth Central Hospital, Blantyre, Malawi, 1998–2014
| Characteristic | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typhoidal Salmonella isolated, No.a | 24 | 13 | 5 | 4 | 4 | 9 | 17 | 19 | 18 | 10 | 16 | 19 | 18 | 67 | 186 | 843 | 782 |
| Incidence, 100/100 000/yb | 9.1 | 4.9 | 1.9 | 1.4 | 1.4 | 3.1 | 5.7 | 6.2 | 5.7 | 3.1 | 4.8 | 5.5 | 5 | 23.4 | 47.5 | 206.6 | 184.1 |
| Case fatality rate, %c | 3 [20] | 3 [20] | 3 [20] | ||||||||||||||
| Total nontyphoidal Salmonella, No. | 541 | 623 | 686 | 783 | 1381 | 1323 | 876 | 847 | 671 | 629 | 498 | 343 | 264 | 216 | 154 | 148 | 138 |
| Salmonella Typhimurium, No. | 441 | 375 | 397 | 446 | 1040 | 1187 | 710 | 737 | 601 | 563 | 443 | 287 | 209 | 185 | 131 | 129 | 119 |
| Salmonella Enteritidis, No. | 79 | 209 | 232 | 318 | 226 | 106 | 79 | 78 | 53 | 49 | 42 | 36 | 34 | 22 | 19 | 12 | 13 |
| Other Salmonella serovars, No. | 21 | 39 | 57 | 19 | 115 | 30 | 87 | 32 | 17 | 17 | 13 | 20 | 21 | 9 | 4 | 7 | 6 |
| Incidence, 100/100 000/yearb | 103 | 117 | 130 | 137 | 242 | 228 | 147 | 138 | 106 | 97 | 75 | 50 | 37 | 30 | 20 | 19 | 16 |
| Case fatality rate, %c | |||||||||||||||||
| Adults | 29 [18] | 47 [2] | 27 [18] | 19 [18] | 20 [18] | 11 [19] | 11 [19] | ||||||||||
| Children | 23 [3] | 20d | |||||||||||||||
| Malaria prevalence in febrile children, % | 35 | 33 | 24 | 26 | 17 | 20 | 25 | 26 | 25 | 22 | |||||||
| Total No. of blood cultures taken | 8545 | 7823 | 8052 | 7653 | 8978 | 11 174 | 10 653 | 12 933 | 10 123 | 9167 | 8628 | 7991 | 8507 | 9890 | 10 433 | 12 815 | 13 663 |
| Estimated population, Blantyre urban, thousands | 527 | 530 | 526 | 571 | 571 | 580 | 596 | 613 | 632 | 645 | 663 | 692 | 721 | 752 | 783 | 816 | 850 |
a All Salmonella Typhi, no Salmonella Paratyphi detected.
b Assumes sensitivity of blood culture 50%.
c Case fatality rate where available from substudies.
d Previously unpublished data.
Laboratory Methods
Automated blood culture was undertaken at MLW using a standard aerobic bottle (BacT/Alert, bioMérieux, Marcy-L'Etoile, France) since 2000. Prior to this, manual culture was undertaken [13]. CSF samples were cultured at 37°C on sheep blood and chocolate agar for 48 hours under aerobic and microaerophilic conditions. All isolates were identified using standard diagnostic techniques [14]. Salmonellae were identified either by biochemical profile using API 10E or 20E (bioMérieux) or by a combination of their appearance on triple sugar iron agar slant (acid from glucose, no gas, trace or full production of hydrogen sulfide) and a negative urease test. They were serotyped according to the White–Kauffmann–Le Minor scheme by the following antisera: polyvalent O and H, O4, O9, Hd, Hg, Hi, Hm, and Vi antisera (Pro-Lab Diagnostics) [15]. MLW audits blood culture volumes and contamination rates [16] and subscribes to the UK National External Quality Assessment Service (www.ukneqas.org.uk) scheme.
Antimicrobial susceptibility testing was performed by disc diffusion using ampicillin, chloramphenicol, cotrimoxazole, cefpodoxime, and ciprofloxacin discs according to British Society of Antimicrobial Chemotherapy methods and breakpoints. Since 2009, isolates found to be resistant to ciprofloxacin or ceftriaxone by disc testing have had Etests (bioMérieux) performed [17]. Diminished ciprofloxacin susceptibility was classified on the basis of a minimum inhibitory concentration (MIC) between 0.06 mg/L and 1 mg/L) [17]. Isolates were described as “fully susceptible” if susceptible to these 5 antimicrobials and multidrug resistant (MDR) if resistant to ampicillin, cotrimoxazole, and chloramphenicol. Prior to October 2010, these data were entered into ledgers, then double-entered into a validated database. Since then, they have been directly entered into an electronic laboratory information management system.
Ethics Statement
These data were collected as part of routine surveillance and comply with institutional guidelines.
RESULTS
Between January 1998 and December 2014, 167 028 blood cultures were taken from adult and pediatric medical patients presenting to QECH (Figure 1). NTS serovars were isolated 10 139 times, of which 8017 (79.1%) were Salmonella Typhimurium, 1608 (15.8%) were Salmonella Enteritidis, and 514 (5.1%) were not Salmonella Typhimurium or Salmonella Enteritidis and could not be typed further with locally available antisera. Salmonella Typhi was isolated on 2054 occasions, and Salmonella Paratyphi A has not been identified in Blantyre.
During the study period, there were 3 epidemics of Salmonella BSIs, each caused by a different serovar: Salmonella Enteritidis peaked in 2001, Salmonella Typhimurium peaked in 2003, and Salmonella Typhi peaked in 2013. The number of NTS isolated from blood peaked at 1381 cases in 2002 and declined to 138 cases in 2014, resulting in an incidence of iNTS of 242 per 100 000 population in 2002 and 16 per 100 000 population in 2014. The number of Salmonella Typhi isolated from blood peaked at 843 in 2013 for an incidence of 207 per 100 000 population in 2013.
Age Distribution of Salmonella Serovars Isolated From Blood
Supplementary Table 1 and Figure 2 reveal a bimodal age distribution of iNTS disease, which is strikingly similar for Salmonella Typhimurium (Figure 2A), Salmonella Enteritidis (Figure 2B), and Salmonella species (Figure 2C), with peaks under the age of 5 years and between the ages of 20 and 45 years. In contrast, a frequency plot of age distribution of typhoid fever (Figure 2D) reveals that it is most common in children and young adults, with a peak age range of 5–10 years.
Analysis of aggregate age distribution data for children ≤5 years of age (Figure 3) reveals a peak of iNTS disease for both Salmonella Typhimurium and Salmonella Enteritidis in the first 1–2 months of life, prior to a decline at age 2–4 months and then a subsequent climb to a second peak at age 6–12 months (Figure 3A and 3B and Supplementary Table 2). There is no clear trend in the first year of life for Salmonella Typhi (Figure 3C).
The 2 serovars responsible for the largest epidemics were investigated for evidence of a significant change in age over time (Supplementary Table 3, Supplementary Figure 1). As Salmonella Typhimurium clearly has a bimodal age distribution, median pediatric (<16 years) and median adult (≥16 years) ages were evaluated separately. Supplementary Figure 1A reveals that the median age of pediatric iNTS Typhimurium disease has been increasing from a median age of 1.1 years (interquartile range [IQR], 0.7–2.1) at the peak of the epidemic in 2003 to 2.0 (IQR, 1.0–3.0) in 2014. There was evidence of significant variation over time (P < .001), and post hoc testing revealed a clear and significant increasing trend in age over time. In the case of adult iNTS Typhimurium disease (Supplementary Figure 1B), there was a less obvious change over time, and although there was significant variation in this large data set (P < .001) no clear or significant trend was identified. In the case of Salmonella Typhi (Supplementary Figure 1C), there were too few cases to make a meaningful comparison prior to the onset of the epidemic in 2011. Since then, there has been an apparent fall in median age of presentation. Although the overall variation in age across the Salmonella Typhi dataset was significant (P = .12), there was no statistically significant trend either for the periods 1998–2014 or the epidemic years 2011–2014.
Case Fatality
Data were available from 5 published studies and 1 unpublished study on the case fatality rate associated with iNTS disease in adults [2, 18, 19], iNTS disease in children [3], and typhoid fever in both adults and children [20]. Most data were available for adult iNTS disease, which reveals a drop from a peak case fatality rate of 47% observed in 2000, to 11% in 2010 (Table 1). The available data on pediatric case fatality rates are sparser, but suggest a more modest decline from 23% in 2000 to 20% in 2006. Data from the current epidemic of typhoid fever indicate a case fatality rate of 2.5%. The number of annual deaths attributable to iNTS disease and typhoid fever has here been estimated using these hospital case fatality data (Supplementary Table 4) and suggest that in total, blood culture–confirmed iNTS disease caused approximately 2179 inpatient deaths during the study period and typhoid fever caused 51 deaths.
Salmonella Meningitis
In addition to causing BSI, NTS are a common cause of meningitis at QECH (Supplementary Table 5 and Supplementary Figure 2). Between 1998 and 2014, there were 392 cases of culture-confirmed NTS meningitis, 294 (75.0%) due to Salmonella Typhimurium, 73 (18.6%) due to Salmonella Enteritidis, and 25 (6.4%) due to other NTS. Meningitis caused by Salmonella Enteritidis peaked in 2001 and that caused by Salmonella Typhimurium peaked in 2002. In contrast, Salmonella Typhi has been an uncommon cause of meningitis, with only 9 cases of culture-confirmed Salmonella Typhi meningitis.
Nontyphoidal salmonellae were responsible for 23% of cases of culture-confirmed meningitis in children aged 3 months to 5 years between 2000 and 2004, dropping to <10% by 2012; however, they are a less prominent cause of meningitis in older age groups [21]. The case fatality rate is very high in children at 52.3% (average from 1997–2006); however, data are unavailable for adults [22]. Case fatality data are unavailable for the cases of Salmonella Typhi meningitis.
Antimicrobial Resistance
Antimicrobial susceptibility testing was performed on 12 034 Salmonella isolates.
Multidrug Resistance
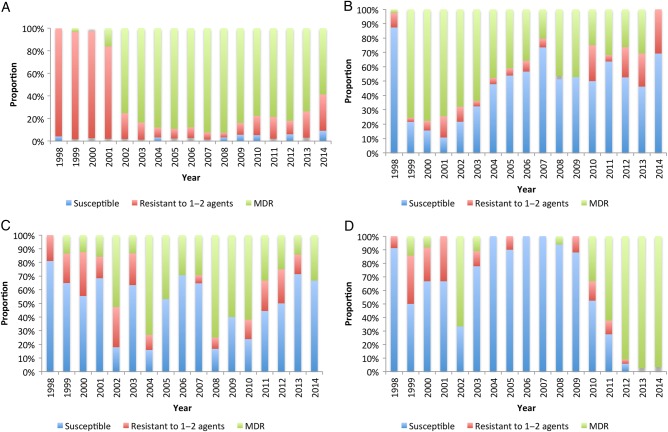
The emergence of all 3 epidemics was strongly associated with the MDR phenotype (Figure 4 and Supplementary Table 6). In the case of Salmonella Enteritidis (Figure 4B), the MDR phenotype subsequently declined from a peak of 78% in 2000, to 0% in 2014, when 0 of 14 isolates were MDR. In the case of Salmonella Typhimurium (Figure 4A), the MDR phenotype became dominant in 2001 (72% isolates) and was sustained until 2008, when 93% isolates were MDR. Since then, the proportion that was MDR has begun to decline slightly; however, as of 2014, the majority (59%) of isolates remained MDR. Strains of Salmonella Typhimurium that were MDR have been found to be part of a novel clade, of multilocus sequence type ST313, with a novel virulence plasmid [23]. In the case of the typhoid fever epidemic (6D), 97% isolates were MDR in 2014. This epidemic is associated with the emergence of an H58-lineage Salmonella Typhi in which the multidrug resistance region has inserted itself into the bacterial chromosome on a transposon [20]. In the case of the presumably diverse group of other Salmonella species, there was no clear trend (Figure 4C) in drug resistance over time. Only a subset of these isolates has been fully serotyped; however, Salmonella Paratyphi has not been detected [18].
Figure 4.
Change in susceptibility pattern to first-line antimicrobial therapy over time for Salmonella Typhimurium (A), Salmonella Enteritidis (B), Salmonella species (C), and Salmonella Typhi (D). Abbreviation: MDR, multidrug resistant.
Resistance to Fluoroquinolones and Third-Generation Cephalosporins
In total, 19 isolates have been reported as having altered susceptibility to ciprofloxacin, but only 2 isolates since 2009, when confirmatory MIC testing was introduced: 1 Salmonella Typhimurium isolate, which was ciprofloxacin resistant, and 1 Salmonella Typhi, which had diminished ciprofloxacin susceptibility confirmed by MIC testing. Sixteen isolates have been reported to be resistant to third-generation cephalosporins, 4 since 2009 (3 Salmonella Typhimurium and 1 Salmonella Enteritidis). In total, 6 isolates were resistant to both ciprofloxacin and ceftriaxone (Supplementary Table 6), 1 of which, an Salmonella Typhimurium isolate from 2009, was further evaluated by whole-genome sequencing and revealed a CTX-M gene on an IncHI2 plasmid found in addition to virulence plasmid pSLT-BT [24].
DISCUSSION
There have been 3 epidemics of Salmonella BSI in Blantyre, Malawi, between 1998 and 2014, all of which were associated with multidrug resistance. These findings are mirrored across sub-Saharan Africa, and pan-continental genomic studies have highlighted the role MDR status has played in facilitating the spread of both MDR Salmonella Typhimurium ST313 and MDR H58-lineage Salmonella Typhi [25, 26]. Although typhoid fever is currently much more commonly diagnosed than iNTS disease, the high case fatality rate for iNTS disease means that all serovars continue to be responsible for a considerable burden of mortality in Blantyre.
We have confirmed the bimodal age distribution of iNTS disease, and additionally demonstrated a relative peak in the first 2 months of life, likely reflecting neonatal or early nosocomial invasive disease. Such data are important for vaccination and prevention strategies. Furthermore, we have demonstrated a gradually increasing median age among children presenting with Salmonella Typhimurium BSI.
MDR typhoid fever and iNTS disease are highly prominent causes of hospital admission in Malawi, and are driving the empiric use of third-generation cephalosporins and ciprofloxacin. Although extended spectrum β-lactamase (ESBL)–producing or fluoroquinolone-resistant isolates remain uncommon, they have been detected. Furthermore, the impact of sustained broad-spectrum antimicrobial use on the antimicrobial susceptibility patterns of other Enterobacteriaceae, Streptococcus pneumoniae, and Staphylococcus aureus is of considerable concern.
Limitations
These data represent passive hospital-based surveillance from a single center in Blantyre; however, as QECH is the only center in Blantyre where effective treatments are freely available, most patients with MDR invasive Salmonella disease are ultimately likely to present to QECH. Some cases will be missed due to deaths in the community, whereas others with drug-susceptible disease may have their disease treated in the community; nonetheless, these are robust estimates of the minimum incidence of invasive Salmonella disease in Blantyre.
CONCLUSIONS
This large longitudinal dataset reveals a dynamic picture of invasive Salmonella disease in Blantyre, which emphasizes the importance of country-level surveillance for severe BSI and the pitfalls of extrapolating data across African settings or between different time periods. Invasive Salmonella disease in Blantyre displays a complex, changing age distribution and epidemic waves, rather than a static endemic picture, which cannot be attributed to a single risk factor. Sequential epidemics have been associated with MDR, and the first signs of the emergence of ESBL and fluoroquinolone resistance have been seen. The MDR phenotype appears to be a major driver of transmission of invasive Salmonella disease, but there is an urgent need to better understand additional factors determining the emergence of different serotypes. There is a particular need for detailed microbiological investigations into potential reservoirs of novel epidemic variants of Salmonella, such as Salmonella Typhimurium ST313, to understand the transmission of these neglected tropical diseases so that effective vaccine and nonvaccine interventions can be designed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the staff and patients of Queen Elizabeth Central Hospital and everyone who contributed to the Malawi Liverpool Wellcome Trust Clinical Research Programme (MLW) diagnostic laboratory.
Financial support. This work was supported by the Wellcome Trust and the Bill & Melinda Gates Foundation. The MLW was supported by the Wellcome Trust Major Overseas Programme (grant number 101113/Z/13/Z). N. A. F. was supported by Wellcome Research Training Fellowship WT092152MA. M. A. G. is supported by an Institutional Strategic Support Fund to the University of Liverpool. Publication of this work was made possible with the support of the Bill & Melinda Gates Foundation (grant OPP1125993).
Supplement sponsorship. This article appeared as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Malawi National Statistical Office. Malawi Demographic and Health Survey 2010. Zomba, Malawi: National Statistical Office, 2011. [Google Scholar]
- 2.Gordon MA, Banda HT, Gondwe M et al. Non-typhoidal Salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 2002; 16:1633–41. [DOI] [PubMed] [Google Scholar]
- 3.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg 2000; 94:310–4. [DOI] [PubMed] [Google Scholar]
- 4.Calis JC, Phiri KS, Faragher EB et al. Severe anemia in Malawian children. N Engl J Med 2008; 358:888–99. [DOI] [PubMed] [Google Scholar]
- 5.MacPherson P, Lalloo DG, Choko AT et al. Suboptimal patterns of provider initiated HIV testing and counselling, antiretroviral therapy eligibility assessment and referral in primary health clinic attendees in Blantyre, Malawi. Trop Med Int Health 2012; 17:507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roca-Feltrer A, Kwizombe CJ, Sanjoaquin MA et al. Lack of decline in childhood malaria, Malawi, 2001–2010. Emerg Infect Dis 2012; 18:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malawi 2000: results from the Demographic and Health Survey. Stud Fam Plann 2003; 34:285–9. [PubMed] [Google Scholar]
- 8.Gould FK, Denning DW, Elliott TS et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012; 67:269–89. [DOI] [PubMed] [Google Scholar]
- 9.Wain J, Diep TS, Ho VA et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MA, Kankwatira AM, Mwafulirwa G et al. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 2010; 50:953–62. [DOI] [PubMed] [Google Scholar]
- 11.Feasey NA, Archer BN, Heyderman RS et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 2010; 16:1448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 2012; 379:2489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon MA, Walsh AL, Chaponda M et al. Bacteraemia and mortality among adult medical admissions in Malawi—predominance of non-typhi salmonellae and Streptococcus pneumoniae. J Infect 2001; 42:44–9. [DOI] [PubMed] [Google Scholar]
- 14.Barrow GI, Feltham RKA. Cowan and Steele's manual for the identification of medical bacteria. Cambridge, UK: Cambridge University Press, 1993. [Google Scholar]
- 15.Kauffmann F. Kauffmann-White scheme. J Hyg 1934; 34:333–50.20475239 [Google Scholar]
- 16.Mtunthama N, Gordon SB, Kusimbwe T, Zijlstra EE, Molyneux ME, French N. Blood culture collection technique and pneumococcal surveillance in Malawi during the four year period 2003–2006: an observational study. BMC Infect Dis 2008; 8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.British Society of Antimicrobial Chemotherapy. BSAC methods for antimicrobial susceptibility testing, 2015. Available at: http://bsac.org.uk/susceptibility/methodologylatestversion/. Accessed 1 June 2015.
- 18.Gordon MA, Graham SM, Walsh AL et al. Epidemics of invasive Salmonella enterica serovar Enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 2008; 46:963–9. [DOI] [PubMed] [Google Scholar]
- 19.Feasey NA, Houston A, Mukaka M et al. A reduction in adult blood stream infection and case fatality at a large African hospital following antiretroviral therapy roll-out. PLoS One 2014; 9:e92226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feasey NA, Gaskell K, Wong V et al. Rapid emergence of multidrug resistant, h58-lineage Salmonella Typhi in Blantyre, Malawi. PLoS Negl Trop Dis 2015; 9:e0003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall EC, Everett DB, Mukaka M et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis 2014; 58:e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molyneux EM, Mankhambo LA, Phiri A et al. The outcome of non-typhoidal Salmonella meningitis in Malawian children, 1997–2006. Ann Trop Paediatr 2009; 29:13–22. [DOI] [PubMed] [Google Scholar]
- 23.Kingsley RA, Msefula CL, Thomson NR et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 2009; 19:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feasey NA, Cain AK, Msefula CL et al. Drug resistance in Salmonella enterica ser. Typhimurium bloodstream infection, Malawi. Emerg Infect Dis 2014; 20:1957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoro CK, Kingsley RA, Connor TR et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 2012; 44:1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VK, Baker S, Pickard DJ et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 2015; 47:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.