Abstract
Background. In 2002, following establishment of a clinical microbiology laboratory in the government hospital that admits children with severe illnesses in Bamako, Mali, surveillance to identify pathogens causing invasive bacterial infections (septicemia, bacteremia, meningitis, etc) was initiated.
Methods. Parents/guardians of children aged <16 years admitted to l'Hôpital Gabriel Touré with high fever or clinical syndromes compatible with focal invasive bacterial disease were asked for consent to culture their child's blood/body fluid. Standard bacteriologic techniques speciated isolates; Salmonella serovars were determined.
Results. From July 2002 through June 2014, 687 nontyphoidal Salmonella (NTS) isolates were obtained from 667 children; 667 yielded a single serovar and 20 grew 2 Salmonella serovars, 1 being NTS. Four serovars accounted for 87% of the 687 NTS isolates, including Salmonella Enteritidis (n = 244 [35.5%]), Salmonella Typhimurium (n = 221 [32.2%]), I:4,[5],12:i:- (n = 42 [6.1%]), and Salmonella Dublin (n = 89 [13.0%]). Of 553 patients with invasive NTS from whom 1 of the 4 predominant serovars was isolated in pure culture, 448 (81.0%) were aged <5 years and case fatality was 20.3%; Salmonella Enteritidis case fatality (27.8%) was higher than for other serovars (P = .0009). NTS disease showed a seasonal peak following the rainy season and into the cool, dry season. Since 2010, Salmonella Enteritidis cases have risen and Salmonella Typhimurium fallen.
Conclusions. NTS has become the predominant invasive pathogen as Haemophilus influenzae type b and pneumococcal vaccine use in Mali has diminished invasive disease due to those pathogens. The age distribution and limited serovars involved make control of NTS disease by vaccines epidemiologically feasible, if products under development prove safe and efficacious.
Keywords: nontyphoidal Salmonella, surveillance, invasive bacterial infections, Mali, emerging infections
At the turn of the millennium, the infant mortality rate in Mali was 141 per 1000 and the under-5 mortality rate was 231 per 1000 live births, among the highest in the world [1]. The 1998 national census showed that 1.02 million of Mali's 9.8 million inhabitants lived in the capital, Bamako (10.4%), and the 2009 census recorded that 1.8 million of Mali's total population of 14.5 million (12.4%) lived in Bamako, where 1 large government hospital, l'Hôpital Gabriel Touré (HGT), admitted severely ill children with nonsurgical presentations. Seventy percent of pediatric admissions to HGT throughout the year 2000 were children whose admitting diagnosis indicated suspicion of an infectious process [2], and malaria was the most frequent clinical diagnosis, even during low malaria season. In 2000, the Global Alliance for Vaccines and Immunization (GAVI; now called “Gavi, the Vaccine Alliance”) was launched with funding to allow (through a formal application process) the introduction of new vaccines such as Haemophilus influenzae type b (Hib) conjugate vaccine into the Expanded Programme on Immunization (EPI) for the entire birth cohort of eligible countries for 5 years. However, no clinical microbiology laboratory existed in HGT to determine the number of patients with invasive bacterial infections such as Hib to help Malian public health leaders assess the utility of introducing Hib conjugate vaccine and to assist clinicians in managing patients.
In 2001, the Ministry of Health of Mali and the University of Maryland School of Medicine's Center for Vaccine Development (CVD) formally established the Centre pour le Développement des Vaccins du Mali (CVD-Mali) as a joint venture to identify the burden of vaccine-preventable infections in the Malian population, introduce emerging new vaccines into the EPI and monitor their impact, and test relevant candidate vaccines in clinical trials in Mali. As part of the scope of work, CVD and CVD-Mali established a clinical microbiology laboratory in HGT and arranged intensive training for multiple Malian bacteriologists both in Baltimore and on-site in Bamako.
Beginning in July 2002, routine clinical and bacteriological surveillance for invasive bacterial infections was initiated on all children <16 years of age admitted to HGT with fever or with clinical suspicion of invasive bacterial disease (eg, meningitis, septic arthritis). This surveillance documented that 3 major bacterial infections were responsible for most invasive disease, including Hib [3], Streptococcus pneumoniae (ie, pneumococcus) [4], and nontyphoidal Salmonella (NTS; defined as Salmonella enterica serovars other than Typhi or Paratyphi A or B) [5, 6]. Whereas documenting the importance of invasive infections caused by Hib, S. pneumoniae, and Neisseria meningitidis in Bamako was anticipated, the observation that NTS constituted the third most common pathogen was a surprise. The importance of NTS as a cause of invasive disease has been reported from Rwanda [7], Kenya [8, 9], Malawi [10, 11], The Gambia [12], and Mozambique [13], as well as Mali [6]. The Rwanda, Kenya, Malawi, and Mozambique data derive from populations where the human immunodeficiency virus (HIV) prevalence was high or moderately high, whereas The Gambia and Mali reports represent data from populations with low HIV prevalence.
This paper reviews the microbiologic aspects of the Malian NTS isolates, presents salient epidemiologic features, provides details on the high case fatality observed in children with invasive NTS in Mali, and assesses the relevance of this information for guiding the development of candidate vaccines to prevent invasive NTS disease in children in sub-Saharan Africa.
METHODS
Clinical Features
Systematic prospective surveillance for invasive bacterial disease among children <16 years of age with clinical microbiologic support for bacteriologic confirmation began on 1 July 2002. A systematic retrospective survey of hospital admissions to HGT that was performed prior to the initiation of the prospective surveillance revealed that approximately 70% of all pediatric nonsurgical admissions to HGT were children with high fever or exhibiting clinical signs and symptoms compatible with an invasive bacterial infection [2]. Because bacteriologic cultures had not previously been a routine diagnostic test available to Malian children prior to 2002, the collection and culturing of blood and of clinically relevant body fluids (eg, cerebrospinal, synovial, pleural, and peritoneal fluids) was considered to be clinical research. Accordingly, a clinical research protocol detailing the proposed study to measure disease burden and the consent form were reviewed by the Ethics Committee of the Faculté de Medécine de Pharmacie et d'Odontostomatologie, University of Mali, and by the Institutional Review Board of the University of Maryland, Baltimore. Eligible children were 0–15 years of age with high fever (axillary temperature ≥39°C) or with a clinical syndrome compatible with invasive bacterial disease (meningitis, pneumonia, empyema, soft tissue infections, septic arthritis, peritonitis, pericarditis, sepsis, and enteric fever). CVD-Mali staff in the pediatric emergency department within HGT determined each child's eligibility by inspecting clinical and demographic data and recorded the child's axillary temperature. Parents or guardians of eligible children were asked to have their child participate in the surveillance and the rationale, procedures, risks, and benefits of participation were explained. Consent was sought from parents/guardians of every child; assent of the child was also required for enrollment of children ≥13 years of age. Because of the low literacy rate in Mali (approximately 30%), audiotapes of the consent form were available in 6 local languages. Two to 5 mL of blood or body fluid was collected for culture and inoculated into flasks of Bactec Ped Plus/F medium (BD, Sparks, Maryland) for automated culture.
A case of invasive NTS disease was defined as a child with clinical illness compatible with invasive bacterial disease leading to collection of cultures (high fever or clinical suspicion of focal infection) from whom an appropriate culture grew NTS.
Epidemiologic Characteristics
During the period of surveillance, HGT was the only tertiary care hospital for children with serious medical conditions that also had clinical microbiology capability. A second hospital in Bamako, l'Hôpital du Mali, with 150 beds (including some pediatric beds), was opened in September 2010 on the Rive Droite, on the other side of the Niger River from HGT (Rive Gauche). This hospital, intended to serve the rapidly growing population in new neighborhoods on Rive Droite, began providing care for children with acute illnesses in late 2010. However, this new hospital does not have clinical microbiology capability. Cases analyzed in this report include children from the 6 Communes (boroughs) of Bamako and from Koulikoro, a small city 57 km away that is connected to Bamako by a major road with many forms of transport (prompting many parents who perceive their child to be seriously ill to take them directly to HGT rather than to healthcare facilities in Koulikoro).
Bacteriologic Findings
Upon arrival in the clinical microbiology laboratory, the Bactec Peds Plus/F flasks were inserted into a Bactec 9050 machine. Bottles showing bacterial growth were examined by Gram stain and subcultures were made onto blood agar, chocolate agar, and MacConkey agar plates (bioMérieux, Lyon, France). Clinical specimens of body fluids received were also inoculated directly onto plates containing blood agar, chocolate agar, or MacConkey agar (bioMérieux, Lyon, France). Lactose-negative colonies on MacConkey agar underwent Gram staining and testing for oxidase activity. Oxidase-negative colonies were inoculated into API 20E strips (bioMérieux, Marcy-l’Étoile, France). Isolates identified as Salmonella were serogrouped and serotyped by the multiplex polymerase chain reaction method of Tennant et al [6] and Levy et al [5] (however, from 2002 to 2008 while molecular typing methods were being developed, typing was also performed with antisera as previously described) [5]. Salmonella isolates were frozen in trypticase soy broth supplemented with 15% glycerol at −86°C and shipped to the Molecular Diagnostic and Microbiology Section of the CVD in Baltimore, which served as a reference laboratory to confirm the identity of the isolates. The Kirby-Bauer standardized disk diffusion method was used to determine antimicrobial susceptibility of NTS. Antimicrobial disks containing 10 µg ampicillin, 30 µg ceftriaxone, 30 µg chloramphenicol, and 1.25 µg/23.75 µg trimethoprim-sulfamethoxazole (TMP-SMX) were placed onto a Mueller-Hinton agar plate that had been swabbed with a standardized NTS suspension. Following incubation, the agar plates were examined, and zones of inhibition diameters surrounding the disks were measured.
RESULTS
In the 12-year period spanning 1 July 2002 through 30 June 2014, 47 243 children <16 years of age were admitted to HGT, of whom 29 821 (63%) had a clinical presentation making them eligible for blood/body fluid cultures; parents/guardians of 26 126 (87.6%) of the eligible children consented for their child to have specimens collected for culture. In total, 4691 (18.0%) of the cultured specimens grew a pathogen, of which 837 (17.8%) were invasive Salmonella strains (687 NTS [14.6%] and 150 typhoidal Salmonella [3.2%]) isolated from 805 children. Of the 805 children with invasive Salmonella, cultures of 774 yielded a single Salmonella serovar, 30 grew 2 different serovars, and 1 child's culture had 3 distinct serovars. The typhoidal serovar isolates included 148 Salmonella Typhi and 2 Salmonella Paratyphi A.
The 687 NTS isolates were obtained from 667 children, of whom 636 children yielded a single serovar of NTS, whereas the cultures of 31 other children yielded 51 NTS isolates in mixed infections consisting of 2 different NTS serovars or a mix of NTS and typhoidal serovars. The 687 NTS isolates included 623 isolated solely from blood culture, 53 from cerebrospinal fluid, and 11 from other normally sterile body fluids such as synovial and peritoneal fluids.
Serovar Distribution
Of the 837 invasive Salmonella strains, we isolated 148 S. Typhi and 2 S. Paratyphi A, and 687 NTS strains. Notably, 87.9% of all isolates (604 of 687) from invasive NTS cases were due to the following serovars: Salmonella Typhimurium (221/687 [32.2%]); I:4,[5],12:i:-, which is genetically closely related to Salmonella Typhimurium (42/687 [6.1%]) [14, 15]; I:4,[5],12:NM (NM, nonmotile; 8/687 [1.2%]); Salmonella Enteritidis (244/687 [35.5%]); and Salmonella Dublin (89/687 [13.0%]) (Figure 1). The remaining 12% of isolates included 21 Salmonella Stanleyville (1,4,[5],12,[27]:z4,z23:1,2]) isolates and 35 group B, C, D, F, and G strains of other serovars, as well as 27 strains that did not belong to any of these serogroups.
Figure 1.
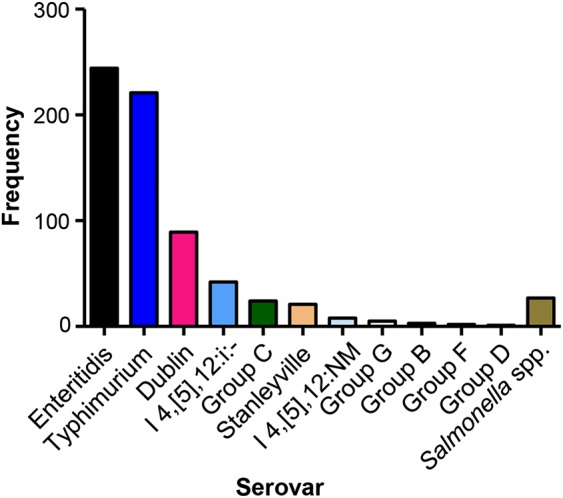
Serovar distribution of the 687 nontyphoidal Salmonella isolates obtained from 667 children.
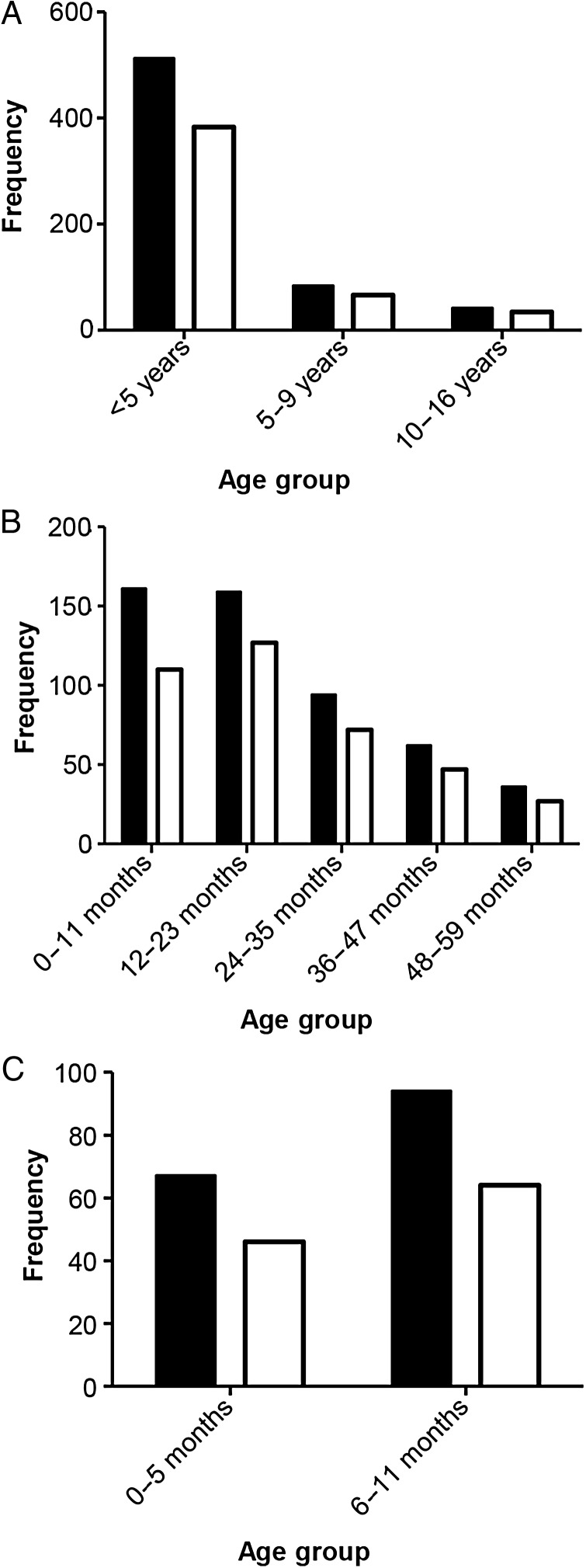
Age Distribution of Cases
The age distribution of the 636 invasive NTS cases whose cultures yielded a single NTS serovar is shown in Figure 2. The burden of this disease is borne by young children, as 80.5% of all cases were <5 years of age and 65.1% of cases were <36 months of age. Notably, within the first year of life the burden was higher in the 6- to 11-month age group (n = 94) than among infants aged <6 months (n = 67).
Figure 2.
Age distribution of the 636 pediatric cases of invasive nontyphoidal Salmonella (NTS) whose cultures yielded a pure culture of a single NTS serovar. The age distributions of the remaining 31 children with invasive NTS whose cultures yielded >1 Salmonella serovar are not shown. NTS (black) and Typhimurium, I:4,[5],12:i:-, I:4,[5],12:NM and Enteritidis combined (white).
Antibiotic Resistance
Table 1 displays the patterns of resistance of NTS isolates to an array of antimicrobials that were clinically relevant in Mali during the period of surveillance. Among Salmonella Typhimurium, I:4,[5],12:i:-, and I:4,[5],12:NM strains tested, 94%–100% were resistant to ampicillin, 88%–98% to chloramphenicol, and 88%–100% to TMP-SMX. Of the Salmonella Enteritidis isolates, 93% showed resistance to ampicillin, 40% to chloramphenicol, and 56% to TMP-SMX. Resistance to ceftriaxone was manifest in <13% of the Salmonella Typhimurium, I:4,[5],12:i:-, and I:4,[5],12:NM strains and only 18% of Salmonella Enteritidis isolates. In contrast to the resistance in these serovars, the Salmonella Dublin and Salmonella Stanleyville isolates were 92%–100% sensitive to all these antibiotics.
Table 1.
Patterns of Resistance to Several Clinically Relevant Antibiotics Among a Sample of Nontyphoidal Salmonella Isolates of the Most Common Serovars Prevalent in Mali From Pediatric Cases of Invasive Disease, 2002–2014
| Serovar | No. of Strains Tested |
Ampicillin |
Chloramphenicol |
Ceftriaxone |
TMP-SMX |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | S | % R | R | S | % R | R | S | % R | R | S | % R | ||
| Typhimurium | 178 | 167 | 11 | 93.8 | 162 | 16 | 91.0 | 0 | 178 | 0.0 | 164 | 14 | 92.1 |
| Enteritidis | 55 | 51 | 4 | 92.7 | 22 | 33 | 40.0 | 10 | 45 | 18.2 | 31 | 24 | 56.4 |
| Dublin | 48 | 4 | 44 | 8.3 | 3 | 45 | 6.3 | 1 | 47 | 2.1 | 4 | 44 | 8.3 |
| I:4,[5],12:i:- | 41 | 41 | 0 | 100.0 | 40 | 1 | 97.6 | 0 | 41 | 0.0 | 41 | 0 | 100.0 |
| Stanleyville | 21 | 0 | 21 | 0.0 | 0 | 21 | 0.0 | 0 | 21 | 0.0 | 0 | 21 | 0.0 |
| I:4,[5],12:NM | 8 | 8 | 0 | 100.0 | 7 | 1 | 87.5 | 1 | 7 | 12.5 | 7 | 1 | 87.5 |
Abbreviations: R, resistant; S, sensitive; TMP-SMX, trimethoprim-sulfamethoxazole.
Case Fatality
The case fatality rates among children <16 years of age with invasive clinical disease due to single infections with the predominant NTS serovars are shown in Table 2 (data from Salmonella Typhimurium; monophasic and nonmotile variants are combined). Invasive NTS disease was associated with a high case fatality rate not just in infants but throughout the first 5 years of life and even among children 5–9 years of age. The overall case fatality rate for children <60 months of age infected with Salmonella Enteritidis (27.8%) was significantly higher than children infected with Salmonella Typhimurium or the monophasic and nonmotile variants (P = .0018) and this was consistently observed at every year of age through the first 5 years of life. Of the 36 cases of invasive NTS due to these serovars in children 10–15 years of age, 5 were fatal (13.9%). Of the 50 cases of meningitis due to NTS, 11 (22%) were fatal.
Table 2.
Case Fatality Rates of the Predominant Serovars of Nontyphoidal Salmonella Associated With Invasive Clinical Disease, by Age Among Children <16 Years of Age
| Age Group |
Salmonella Typhimurium (and I:4,[5],12:i:- and I:4,[5],12:NM) |
Salmonella Enteritidis |
Salmonella Dublin |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Deathsa | CFR | Cases | Deaths | CFR | Cases | Deaths | CFR | |
| 0–11 mo | 53 | 8 | 15.1% | 57 | 19 | 33.3% | 22 | 3 | 13.6% |
| 12–23 mo | 66 | 8 | 12.1% | 61 | 13 | 21.3% | 15 | 2 | 13.3% |
| 24–35 mo | 28 | 5 | 17.9% | 44 | 11 | 25.0% | 14 | 3 | 21.4% |
| 36–47 mo | 25 | 4 | 16.0% | 22 | 8 | 36.4% | 10 | 1 | 10.0% |
| 48–59 mo | 15 | 2 | 13.3% | 3 | 12 | 25.0% | 4 | 1 | 25.0% |
| <60 mo | 187 | 27 | 14.4%b | 196 | 54 | 27.8%b | 65 | 10 | 15.4% |
| 5–9 y | 30 | 3 | 10.0% | 36 | 5 | 13.9% | 10 | 1 | 10% |
| 10–15 y | 27 | 4 | 14.8% | 0 | 7 | 0% | 2 | 1 | |
Data are presented as No. unless otherwise specified.
Abbreviation: CFR, case fatality rate.
a Includes 5 deaths due to I:4,[5]:12:i:-.
b The overall CFR of Salmonella Enteritidis cases (54/196) is significantly higher than the CFR of Salmonella Typhimurium cases (P = .0018).
Seasonality
Although cases of pediatric invasive NTS disease were admitted to HGT during all 12 months of the year, including during March–June (hot, mostly dry season), July–October (rainy season), and November–February (cool dry season), there was nevertheless a clear seasonality visible (Figure 3). The peak of admissions to hospital for invasive NTS cases occurred in the latter part of the rainy season (September and October) and in the first part of the cool, dry season. Both Salmonella Enteritidis and Salmonella Typhimurium and the related monophasic variant (I:4,[5],12:i:-) and nonmotile variant (I:4,[5],12:NM) exhibited identical seasonality.
Figure 3.
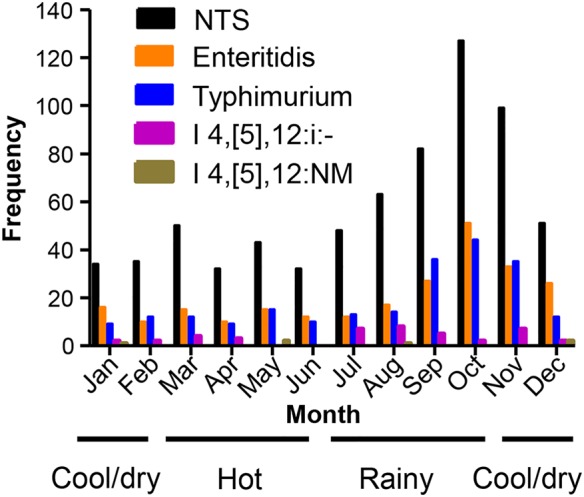
Seasonality of the nontyphoidal Salmonella (NTS) cases: Salmonella Typhimurium, I:4,[5],12:i:-, I:4,[5],12:NM, and Salmonella Enteritidis.
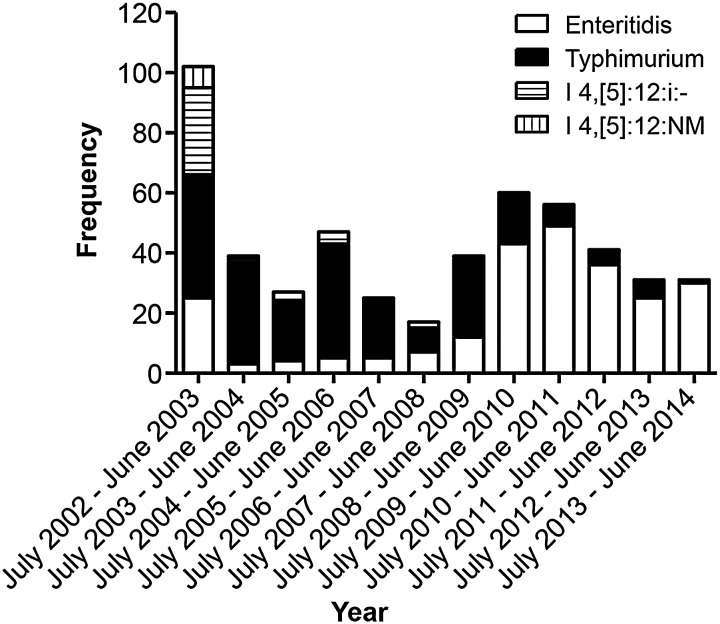
Secular Trends and Emerging Clones
The proportion of invasive NTS disease due to Salmonella Enteritidis vs Salmonella Typhimurium (plus I:4,[5],12:i:- and I:4,[5],12:NM) is shown in Figure 4 for the 12-year period. Whereas there were cases of Salmonella Enteritidis disease every year since surveillance began, in 2010 there was a marked upsurge in the number of Salmonella Enteritidis cases and a fall in the number of Salmonella Typhimurium cases.
Figure 4.
Frequency of Salmonella Typhimurium, I:4,[5],12:i:-, I:4,[5],12:NM, and Salmonella Enteritidis cases by year over the 12-year study period.
DISCUSSION
The original goal of establishing clinical microbiology laboratory–supported surveillance in HGT was to quantify the burden of invasive disease due to Hib and pneumococci to take a decision on the advisability of introducing Hib (and subsequently) pneumococcal conjugate vaccines into the EPI and, more broadly, to investigate the etiology of invasive bacterial infections in Mali. As anticipated, enormous burdens of invasive Hib and pneumococcal disease were documented [3, 4]. A somewhat unexpected finding was the relative importance of NTS as a cause of invasive bacterial disease, ranking third to Hib and pneumococci in the number of cases in the early years but approaching pneumococci with respect to mortality. It is the high case fatality rate of invasive NTS disease in Mali (Table 2) that has galvanized interest in potential control measures to address this major cause of postneonatal young child mortality.
Two observations allude to the virulence of NTS pathogens in the Malian pediatric host. These include the recognition that fatal disease is common beyond infancy and the consistent observation that Salmonella Enteritidis cases exhibit higher lethality than other serovars (Table 2). The explanation for the exceptional virulence of Salmonella Enteritidis is unclear, although genomic investigations have detected a distinct novel Malian clone that has emerged (S. M. Tennant and K. Bornstein, unpublished data), and laboratory studies of pathogenesis currently under way may yield clues. Most Salmonella Typhimuirum and I:4,[5],12:i:- isolates in Mali belong to multilocus sequence type ST313 clones that have spread throughout sub-Saharan Africa [16].
The 12-year surveillance period allowed secular changes to be detected in the frequency of isolation of NTS serovars from cases of invasive disease. During the first few years of surveillance, Salmonella Stanleyville, a serovar rarely isolated in North America and Europe, was isolated from 20 children with invasive disease in Mali. Since 2010, a distinct clone of Salmonella Enteritidis has emerged in Mali and has eclipsed Salmonella Typhimurium as the predominant serovar, as simultaneously the number of annual Salmonella Typhimurium isolates has decreased. It is unclear if the new Salmonella Enteritidis clone offers some competitive advantage within the reservoir of infection or in vehicles of transmission that allows it to displace Salmonella Typhimurium. This constitutes a fascinating question for a multidisciplinary research team of epidemiologists, environmental microbiologists, and genomicists to address.
The Malian burden data corroborate reports from multiple other sources in sub-Saharan Africa, indicating the relative importance of NTS as a cause of invasive bacterial disease. Some of these reports are population-based studies, thereby allowing incidence rates to be calculated [8, 9, 12, 13], whereas others [7, 10, 17], like ours, are hospital-based and show the relative burden of invasive NTS disease vs other pathogens such as Hib and S. pneumoniae. The population-based surveillance systems that have accurate demographic data from defined populations have documented incidence rates of invasive NTS disease similar to the incidence of invasive pneumococcal disease.
The Malian public health authorities are motivated to undertake interventions to diminish the reservoir and the modes of transmission of NTS disease. However, as in other sites in sub-Saharan Africa, neither the identity of the main reservoir of infections nor the modes of transmission have been elucidated [18]. Because of the paucity of fundamental epidemiologic data that could allow suppression of the reservoir of infection and interruption of modes of transmission by eliminating NTS-contaminated vehicles, interest has lately focused on the possibility of use of vaccines to prevent invasive NTS disease, if such vaccines were to become available.
Several features of the epidemiology of NTS disease in Mali support the notion of the utility of vaccination as an intervention. First is that a mere 4 serovars (2 of which are highly related, Salmonella Typhimurium and the monophasic I:4,[5],12:i:-strains) account for 87% of all invasive NTS disease in children. Thus, a bivalent or trivalent vaccine could provide broad coverage. Second, the burden of severe disease clusters between 6 months and 59 months of age. This age-specific disease incidence burden may allow immunization of young infants through the EPI with an effective vaccine to prevent illnesses in late infancy and in toddlers and preschool-aged children. Promising conjugate and other parenteral vaccines and attenuated live oral NTS vaccines for children have been developed that are moving through preclinical testing towards early-phase clinical trials [19–21].
Notes
Acknowledgments. We acknowledge the assistance and cooperation of physicians and nurses working on the Pediatric Service of l'Hôpital Gabriel Touré and in the Clinical Microbiology Laboratory and the support staff of the CVD-Mali.
Disclaimer. The findings and conclusions contained in this work are those of the authors and do not necessarily reflect positions or policies of the funding agencies.
Financial support. This work was supported by the Bill & Melinda Gates Foundation; the Rockefeller Foundation; and the Optimus Foundation.
Supplement sponsorship. This article appeared as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. Two of the authors (M. M. L. and S. M. T.) are recipients of grants from the Wellcome Trust (M. M. L.) and the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (S. M. T. and M. M. L.) that support the development of vaccines to prevent invasive disease caused by NTS and are inventors on a patent titled “Broad Spectrum Vaccine Against Non-Typhoidal Salmonella” (US patent number 9 050 283). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.United Nations Children's Fund State of the world's children 2003. In: Bellamy C, ed. New York: UNICEF, 2015. [Google Scholar]
- 2.Campbell JD, Sow S, Levine MM, Kotloff KL. The causes of hospital admission and death among children in Bamako, Mali. J Trop Pediatr 2004; 50:158–63. [DOI] [PubMed] [Google Scholar]
- 3.Sow SO, Diallo S, Campbell JD et al. Burden of invasive disease caused by Haemophilus influenzae type b in Bamako, Mali: impetus for routine infant immunization with conjugate vaccine. Pediatr Infect Dis J 2005; 24:533–7. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JD, Kotloff KL, Sow SO et al. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. Pediatr Infect Dis J 2004; 23:642–9. [DOI] [PubMed] [Google Scholar]
- 5.Levy H, Diallo S, Tennant SM et al. A PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol 2008; 46:1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tennant SM, Diallo S, Levy H et al. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 2010; 4:e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepage P, Bogaerts J, Van Goethem C et al. Community-acquired bacteraemia in African children. Lancet 1987; 1:1458–61. [DOI] [PubMed] [Google Scholar]
- 8.Berkley JA, Lowe BS, Mwangi I et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 9.Tabu C, Breiman RF, Ochieng B et al. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One 2012; 7:e31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 2000; 19:312–8. [DOI] [PubMed] [Google Scholar]
- 11.Molyneux EM, Walsh AL, Malenga G, Rogerson S, Molyneux ME. Salmonella meningitis in children in Blantyre, Malawi, 1996–1999. Ann Trop Paediatr 2000; 20:41–4. [DOI] [PubMed] [Google Scholar]
- 12.Enwere G, Biney E, Cheung YB et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J 2006; 25:700–5. [DOI] [PubMed] [Google Scholar]
- 13.Mandomando I, Macete E, Sigauque B et al. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 2009; 14:1467–74. [DOI] [PubMed] [Google Scholar]
- 14.Ido N, Lee K, Iwabuchi K et al. Characteristics of Salmonella enterica serovar 4,[5],12:i:- as a monophasic variant of serovar Typhimurium. PLoS One 2014; 9:e104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boland C, Bertrand S, Mattheus W et al. Extensive genetic variability linked to IS26 insertions in the fljB promoter region of atypical monophasic variants of Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 2015; 81:3169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okoro CK, Kingsley RA, Connor TR et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 2012; 44:1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 2006; 6:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariuki S, Revathi G, Kariuki N et al. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol 2006; 55(pt 5):585–91. [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Tennant SM, Wang JY et al. Salmonella Enteritidis Core-O Polysaccharide (COPS) conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with Salmonella Enteritidis. Infect Immun 2011; 79:4240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tennant SM, Wang JY, Galen JE et al. Engineering and pre-clinical evaluation of attenuated non-typhoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun 2011; 79:4175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon R, Wang JY, Boyd MA et al. Sustained protection in mice immunized with fractional doses of Salmonella Enteritidis Core and O polysaccharide-flagellin glycoconjugates. PLoS One 2013; 8:e64680. [DOI] [PMC free article] [PubMed] [Google Scholar]