Abstract
Background. Invasive salmonelloses are a major cause of morbidity and mortality in Africa, but the incidence and case fatality of each disease vary markedly by region. We aimed to describe the incidence, clinical characteristics, and antimicrobial susceptibility patterns of invasive salmonelloses among children and adults in Kilifi, Kenya.
Methods. We analyzed integrated clinical and laboratory records for patients presenting to the Kilifi County Hospital between 1998 and 2014. We calculated incidence, and summarized clinical features and multidrug resistance.
Results. Nontyphoidal Salmonella (NTS) accounted for 10.8% and 5.8% of bacteremia cases in children and adults, respectively, while Salmonella Typhi accounted for 0.5% and 2.1%, respectively. Among 351 NTS isolates serotyped, 160 (45.6%) were Salmonella Enteritidis and 152 (43.3%) were Salmonella Typhimurium. The incidence of NTS in children aged <5 years was 36.6 per 100 000 person-years, being highest in infants aged <7 days (174/100 000 person-years). The overall incidence of NTS in children varied markedly by location and declined significantly during the study period; the pattern of dominance of the NTS serotypes also shifted from Salmonella Enteritidis to Salmonella Typhimurium. Risk factors for invasive NTS disease were human immunodeficiency virus infection, malaria, and malnutrition; the case fatality ratio was 22.1% (71/321) in children aged <5 years and 36.7% (11/30) in adults. Multidrug resistance was present in 23.9% (84/351) of NTS isolates and 46.2% (12/26) of Salmonella Typhi isolates.
Conclusions. In Kilifi, the incidence of invasive NTS was high, especially among newborn infants, but typhoid fever was uncommon. NTS remains an important cause of bacteremia in children <5 years of age.
Keywords: Salmonella, nontyphoidal, Typhi, incidence, Kenya
Estimates of the burden of invasive Salmonella infections in sub-Saharan Africa are limited by the scarcity of regional data. The global burden of typhoid fever was estimated at 26.9 million cases in 2010 [1], but only Egypt and South Africa contributed to this estimate for the African continent. Within Africa, surveillance from Kenya, Tanzania, Malawi, and South Africa has shown marked regional variation in the incidence and age-specific patterns of typhoid fever [2–4]. Typhoid fever is caused by Salmonella enterica serovar Typhi, whereas nontyphoidal Salmonella (NTS) disease refers to infections caused by Salmonella enterica serovars other than Typhi and Paratyphi A.
The burden of NTS disease in Africa is even less well understood. In high-income countries, NTS causes a self-limiting gastroenteritis and is transmitted though contaminated food. In contrast, infection with NTS in Africa is usually invasive, causing severe life-threatening sepsis. In fact, NTS is a common cause of bacteremia in both children and adults [5, 6]. In a study of community-acquired bloodstream infections across Africa, NTS was the most common pathogenic isolate in adults; in children, it was the second most common, after Streptococcus pneumoniae [6]. Risk factors for invasive NTS (iNTS) disease include malaria infection, human immunodeficiency virus (HIV), and malnutrition [5, 7], all of which have a high prevalence in Africa. Existing estimates of the incidence of iNTS are derived mainly from children and high-risk groups [5, 8–10]. Population-based studies are few and thus incidence estimates are extremely limited. Modeled estimates of iNTS infection, based on extrapolations from these studies, adjusting for the effects of HIV and malaria, suggest an incidence in Africa of 227 cases per 100 000 per year across all age groups, with a case fatality rate of 20% [11].
Although incidence estimates are scarce, NTS repeatedly rank near the top in series of invasive pathogenic bacteria on the continent, suggesting that they are a cause of considerable morbidity and mortality. Vaccines are available to protect against Salmonella Typhi infections and are in development to prevent NTS. The case for vaccine development, and the proper evaluation and deployment of such vaccines in Africa, depends upon a sound understanding of the epidemiology of the disease in different parts of the continent. This study describes the incidence, clinical characteristics, and antimicrobial susceptibility patterns of invasive salmonellosis among children and adults admitted to Kilifi County Hospital in Coastal Kenya.
METHODS
This is a retrospective analysis of integrated demographic, clinical, and microbiological surveillance data accumulated at the Kenya Medical Research Institute (KEMRI)–Wellcome Trust Research Programme over a period of 16 years from August 1998 through December 2014 for children ≤14 years of age, and from January 2007 through December 2014 for adults >14 years of age.
Study Population
The study was undertaken in Kilifi County, a rural area 3 degrees south of the equator on the Indian Ocean coast, typical of much of tropical sub-Saharan Africa. Kilifi County Hospital (KCH) is located at the center of the Kilifi Health and Demographic Surveillance System (KHDSS), which was established in 2000 to monitor births, deaths, in-migration, and out-migration in a population of approximately 280 000 over an area of 891 km2 [12]. Events are monitored at KCH and through routine home visits every 4 months.
Since August 1998, all children (≤14 years of age) admitted to KCH, except those admitted for elective procedures or observations after minor accidents, have been investigated with blood cultures routinely on admission [5]. Full blood counts and microscopy for malaria parasites were also analyzed routinely. Clinical and laboratory data have been systematically recorded on a standard pro forma [13]. Treatment was offered according to local protocols and current World Health Organization recommendations [14].
Meningitis was suspected where 1 or more of the following signs were present: stiff neck, bulging fontanelle, lethargy, loss of consciousness, prostration or history of convulsions (except febrile convulsions), and signs of sepsis in neonates. Lumbar punctures were performed for these cases. Malnutrition was defined as a weight-for-age z score of ≤ − 3. Severe pneumonia was defined as history of cough or difficulty breathing plus lower chest wall indrawing, and very severe pneumonia was defined as cough or difficulty breathing plus either hypoxia, lethargy, loss of consciousness, prostration, or a history of convulsions.
A similar approach for surveillance in adults was established in January 2007. Blood cultures were taken for all patients presenting to the wards with a history of fever, axillary temperature of <36°C or >37.4°C, or signs of focal sepsis. Cerebrospinal fluid (CSF) cultures were taken in patients with any 2 of pyrexia (axillary temperature >37.4°C), meningism (neck stiffness and/or photophobia), or altered mental status.
A case was defined as a patient in whom Salmonella enterica species was cultured from blood or CSF.
Procedures
Pediatric blood samples for bacterial cultures were collected in BACTEC Peds Plus bottles and processed with a BACTEC automated blood culture instrument (Becton Dickinson) for initial detection of bacteria in the blood. In adults, BACTEC Plus Aerobic/F bottles were used. BACTEC-positive samples were subcultured on standard media by routine microbiological techniques. Either biochemical test kits (API, bioMérieux), serological tests, or both were used to confirm suspected pathogens. Good Clinical Laboratory Practice was audited by Qualogy and external quality assurance was provided by the UK National External Quality Assessment service.
The following organisms were considered as contaminants: Bacillus species, coryneforms, Micrococcus species, coagulase-negative Staphylococcus, and viridans group Streptococcus. Salmonella serotypes were determined using the Kauffman–White scheme and commercial antisera. Antimicrobial susceptibilities were determined by broth dilution using Microscan panels (Siemens, Germany), and minimum inhibitory concentrations were interpreted using the latest Clinical and Laboratory Standards Institute guidelines [15]. Organisms that showed intermediate susceptibility results to any of the drugs tested were labeled as nonsusceptible to the drug. Multidrug resistance (MDR) was defined as nonsusceptibility to ≥2 of the following antibiotics: ampicillin, trimethoprim-sulfamethoxazole (cotrimoxazole), and tetracycline.
An automated counter (Beckman Coulter for children, Coulter ACT-5 for adults) measured blood counts and hemoglobin. Blood was examined for malaria parasites by Giemsa-stained thick and thin films at ×1000 magnification. Systematic HIV-1 testing was performed using rapid antibody tests according to national policy, which began in 2007 [16]. Prior to that, HIV testing was undertaken only in those seeking a result. DNA was extracted retrospectively from frozen samples collected at admission by use of Qiagen DNA blood mini-kits (Qiagen, Crawley, United Kingdom) and typed for sickle cell anemia by polymerase chain reaction.
Statistical Analysis
Student t test or Wilcoxon rank-sum test were conducted for normal or nonnormally distributed continuous variables, respectively. We used logistic regression to establish the risk factors for NTS bacteremia compared to other admissions. Variables that were significant (P < .1) in the univariate analysis were included in the multivariable model. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported.
Incidence was calculated among the residents of the KHDSS using the midyear population estimates as the denominator. Age-specific population estimates for the era before the KHDSS began were extrapolated using a log linear model of age-specific data based on subsequent enumerations. We adjusted the incidence to account for the proportion of patients who were eligible for blood cultures but missed them and for the proportion of cultures that were contaminated, assuming the distribution of true pathogens in contaminated vials was represented by the distribution in uncontaminated vials. In adults, we estimated the proportion of the KHDSS that was HIV infected using the population prevalence for Coast Province (4.3%) [17] and used this as a denominator in calculating the incidence of iNTS among HIV-infected individuals.
Hospital-based surveillance has been associated with underascertainment of cases illustrated by distance decay in the incidence of admissions to hospital in patients aged <5 years [18]. We adjusted the incidence of NTS for underascertainment in the number of patients presenting to KCH among children <5 years. We assumed that the true incidence of hospitalization with NTS infection in each location was proportional to the location-specific death rates in the KHDSS and that the location closest to the hospital (Kilifi Township) had complete ascertainment [18]. The analysis was performed using Stata 13 (StataCorp, College Station, Texas).
Ethical Approval
We report results from a series of research studies that were approved by the National Ethical Review Committee of KEMRI (scientific steering committee 1067, 1357, and 1433). Patients were only investigated if we obtained informed written consent either from the patient or, in the case of children, from their parent or guardian.
RESULTS
Bacterial Isolation and Typing
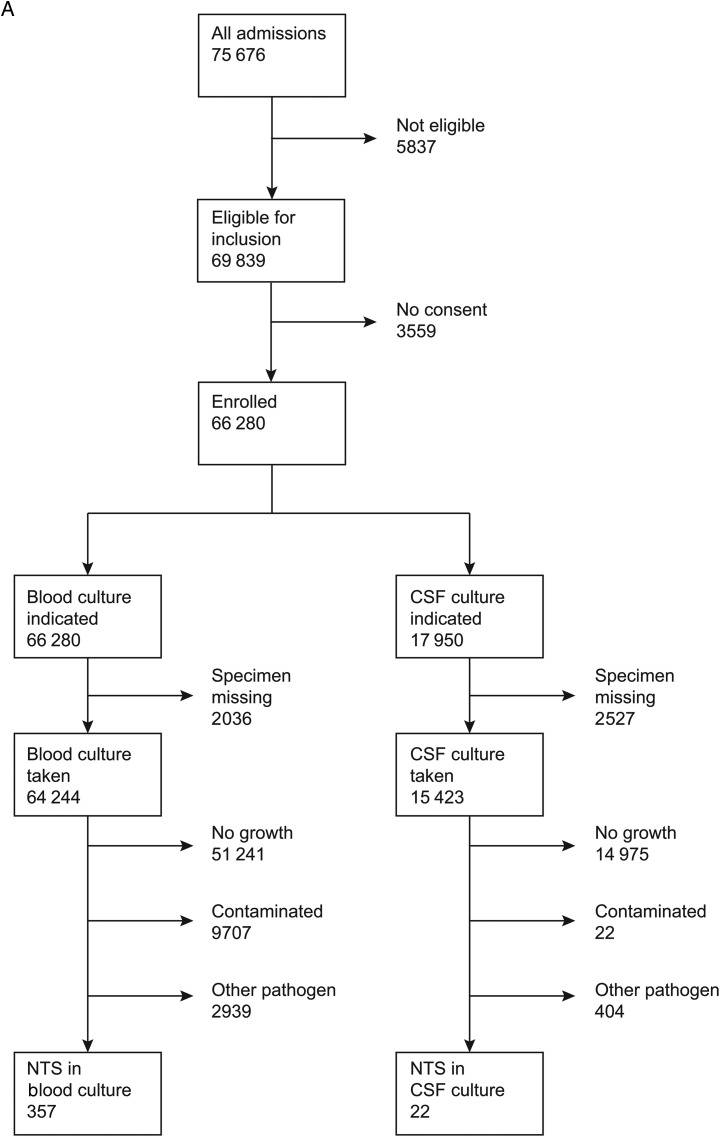
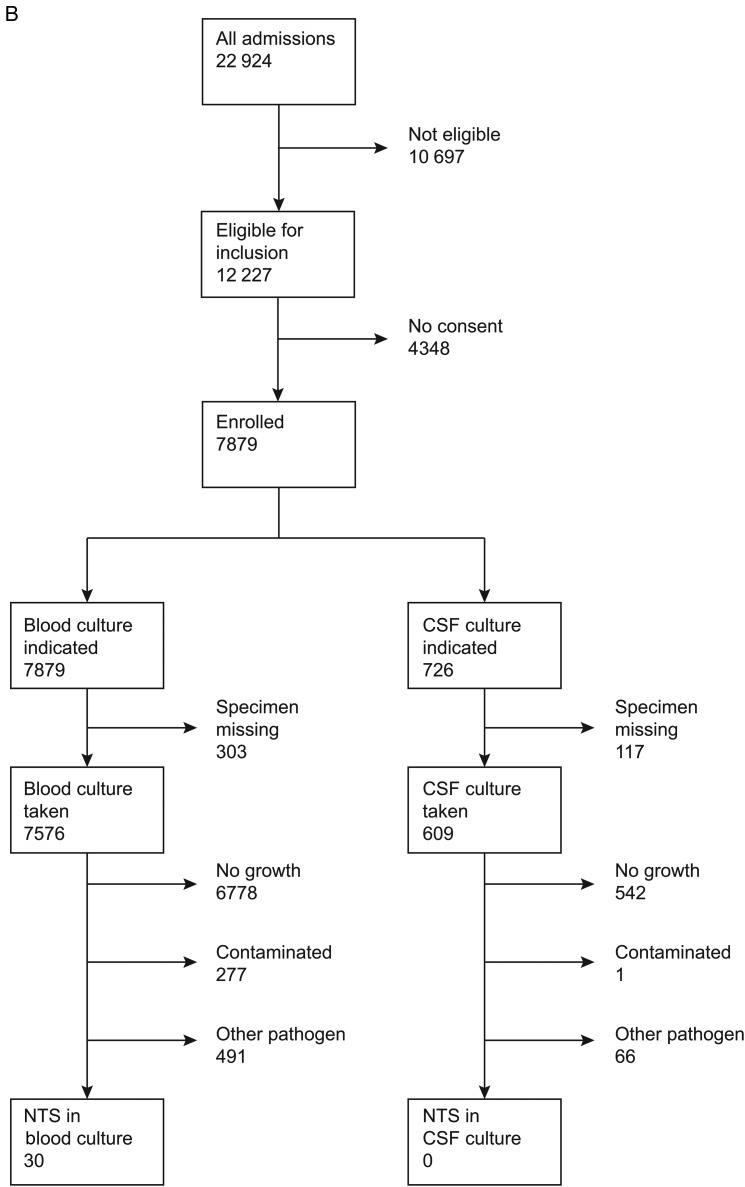
Of 75 676 admissions among children, 3296 had bacteremia of which 357 (10.8%) were NTS and 15 (0.5%) were Salmonella Typhi isolates (Figure 1). Among 22 924 adult admissions, 521 had bacteremia, of which 30 (5.8%) were NTS and 11(2.1%) were Salmonella Typhi isolates. Of 387 isolates of NTS, 351 were serotyped; 160 (45.6%) were Salmonella enterica serovar Enteritidis, 152 (43.3%) were Salmonella enterica serovar Typhimurium, and 39 (11.1%) isolates could not be serotyped using the panel of antisera available. There were no Salmonella Paratyphi organisms isolated. There was 1 case of Salmonella enterica subspecies arizonae that was excluded from this analysis.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram representing flow of patients. A, Children. B, Adults. Abbreviations: CSF, cerebrospinal fluid; NTS, nontyphoidal Salmonella.
Twenty-two isolates of NTS were cultured from CSF samples taken on admission; 8 (36.4%) occurred in neonates <7 days old and all of them occurred in infants. Among these 22 infants, 19 (86.4%) had blood cultures positive for NTS. There were no cases of NTS meningitis in adults. Salmonella Typhi was not isolated from CSF in either children or adults.
Mixed infections with NTS were present in 10 patients, of whom 5 were coinfected with S. pneumoniae; the others had group A Streptococcus (3), Escherichia coli (1), and Pseudomonas species (1).
Age and Sex Distribution
Among children and adults combined, the median age of infection with iNTS was 1 year (interquartile range [IQR], 0–3 years) and with Salmonella Typhi was 8.5 years (IQR, 2–18 years).
Among children, 199 of 357 (55.7%) patients with iNTS and 9 of 15 (60%) with Salmonella Typhi were male. The median age of children with iNTS was 15 months (IQR, 7–28 months) and with Salmonella Typhi was 34 months (IQR, 28–77 months).
Among adults, 11 of 30 (36.7%) patients with iNTS and 8 of 11 (72.7%) with Salmonella Typhi were male. The median age of infection with iNTS and with Salmonella Typhi was 30 years (IQR, 24–42 years) and 22 years (IQR, 18–39 years), respectively. The median age of infection with iNTS did not vary by sex. There were no differences in the serotype distribution across different age groups.
Clinical Characteristics and Risk Factors for Invasive NTS Disease
Among children with iNTS, 261 of 351 (74.4%) presented with fever (axillary temperature >37.4°C), 99 of 352 (28.1%) had Plasmodium falciparum infection on their blood slide, 19 of 268 (7.1%) had sickle cell disease, and 60 of 249 (24.1%) were HIV infected. Significant risk factors were elevated temperature, malnutrition, splenomegaly, and HIV infection (Table 1).
Table 1.
Characteristics of Patients Presenting With Invasive Nontyphoidal Salmonella Disease and Associated Risk Factors in Children and Adults
| Characteristic | NTS Bacteremia, No. Positive/No. Tested (%) |
Other Admissions, No. Positive/ No. Tested (%) |
Unadjusted OR (95% CI) |
P Value | Adjusted OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Children | n = 357 | n = 75 676 | ||||
| Clinical features | ||||||
| Sex, male | 199 (55.7) | 42 495/75 676 (56.4) | 0.96 (.78–1.17) | .667 | ||
| Temperature | ||||||
| Low | 24/351 (6.9) | 11 070/74 361 (14.9) | 0.78 (.49–1.21) | .271 | 0.24 (.07–.80) | .020 |
| Normal | 66/351 (18.8) | 22 792/74 361 (30.7) | ||||
| High | 261/351 (74.4) | 40 499/74 361 (54.5) | 2.17 (1.66–2.82) | <.001 | 2.03 (1.27–3.28) | <.001 |
| Diarrhea | 103/344 (29.9) | 13 739/72 561 (18.9) | 1.88 (1.50–2.36) | <.001 | ||
| Vomiting | 110 (32.0) | 19 121/73 396 (26.1) | 1.33 (1.06–1.67) | .012 | ||
| Malnutrition | 103 (31.4) | 12 619/70 584 (17.9) | 2.16 (1.71–2.71) | <.001 | 2.25 (1.47–3.44) | <.001 |
| Severe pneumonia | 61 (17.1) | 12 563/75 676 (16.8) | ||||
| Very severe | 60 (16.9) | 10 324/75 676 (13.8) | 1.2 (.84–1.71) | .319 | ||
| Pneumonia | ||||||
| Splenomegaly | 40 (19.0) | 3351/56 026 (6.0) | 3.66 (2.60–5.15) | <.001 | 4.61 (2.70–7.86) | <.001 |
| Hepatomegaly | 37 (17.6) | 4133/56 027 (7.4) | 2.67 (1.88–3.79) | <.001 | ||
| Laboratory features | ||||||
| Malaria slide positive | 99/352 (28.1) | 18 645/71 973 (25.9) | 1.08 (.85–1.36) | .532 | ||
| HIV positive | 60/249 (24.1) | 1998/31 077 (6.4) | 4.53 (3.39–6.06) | <.001 | 5.16 (3.29–8.07) | <.001 |
| Adults | n = 30 | n = 22 894 | ||||
| Clinical features | ||||||
| Sex, male | 11 (36.7) | 9846/22 894 (43.0) | 0.77 (.36–1.61) | .485 | ||
| Temperature | ||||||
| Low | 9 (30.0) | 7336/21 339 (34.4) | 1.37 (.54–3.46) | .501 | ||
| Normal | 9 (30.0) | 10 076/21 339 (47.2) | 1 | |||
| High | 12 (40.0) | 3927/21 339 (18.4) | 3.42 (1.44–8.13) | .005 | ||
| Diarrhea | 14 (46.7) | 1604/21 541 (7.4) | 10.88 (5.30–22.32) | <.001 | 4.34 (1.88–10.02) | .001 |
| Vomiting | 12 (40.0) | 2501/21 542 (11.6) | 5.08 (2.44–10.55) | <.001 | ||
| Splenomegaly | 2 (6.7) | 217/21 527 (1.0) | 7.01 (1.66–29.63) | .008 | ||
| Hepatomegaly | 5 (16.7) | 762/21 528 (3.5) | 5.45 (2.08–14.28) | .001 | ||
| Laboratory features | ||||||
| Malaria slide positive | 0/24 (0) | 251/7771 (3.2) | 1.00 (1.00–1.00) | |||
| HIV positive | 21/23 (91.3) | 2583/9351 (27.6) | 27.51 (6.45–117.42) | <.001 | 20.41 (4.67–89.21) | <.001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NTS, nontyphoidal Salmonella; OR, odds ratio.
Among 30 adults with NTS bacteremia, only 12 (40.0%) presented with a febrile illness and none were infected with P. falciparum. HIV infection was present in 21 of the 23 (91.3%) patients tested. In the final model, significant risk factors for iNTS infection were HIV (OR, 20.4 [95% CI, 4.67–89.21]) and diarrhea (OR, 4.34 [95% CI, 1.88–10.02]).
Recurrent infection was observed in only 1 female HIV-infected adult admitted with NTS bacteremia, 6 months after the first admission. The serotype isolated was Salmonella Typhimurium in both admissions.
Incidence
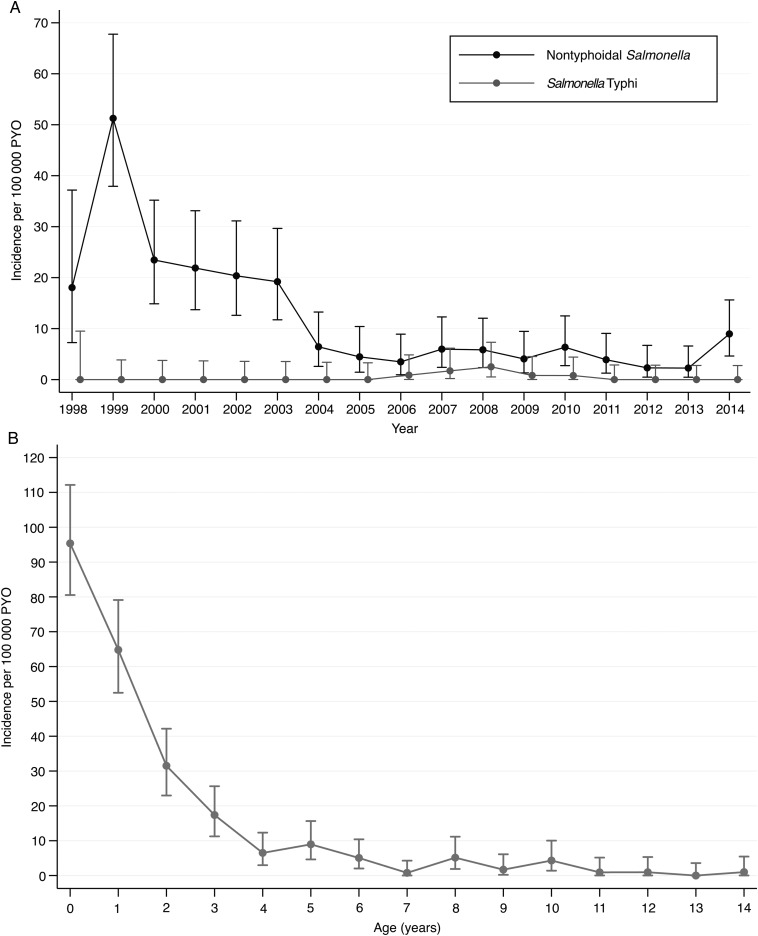
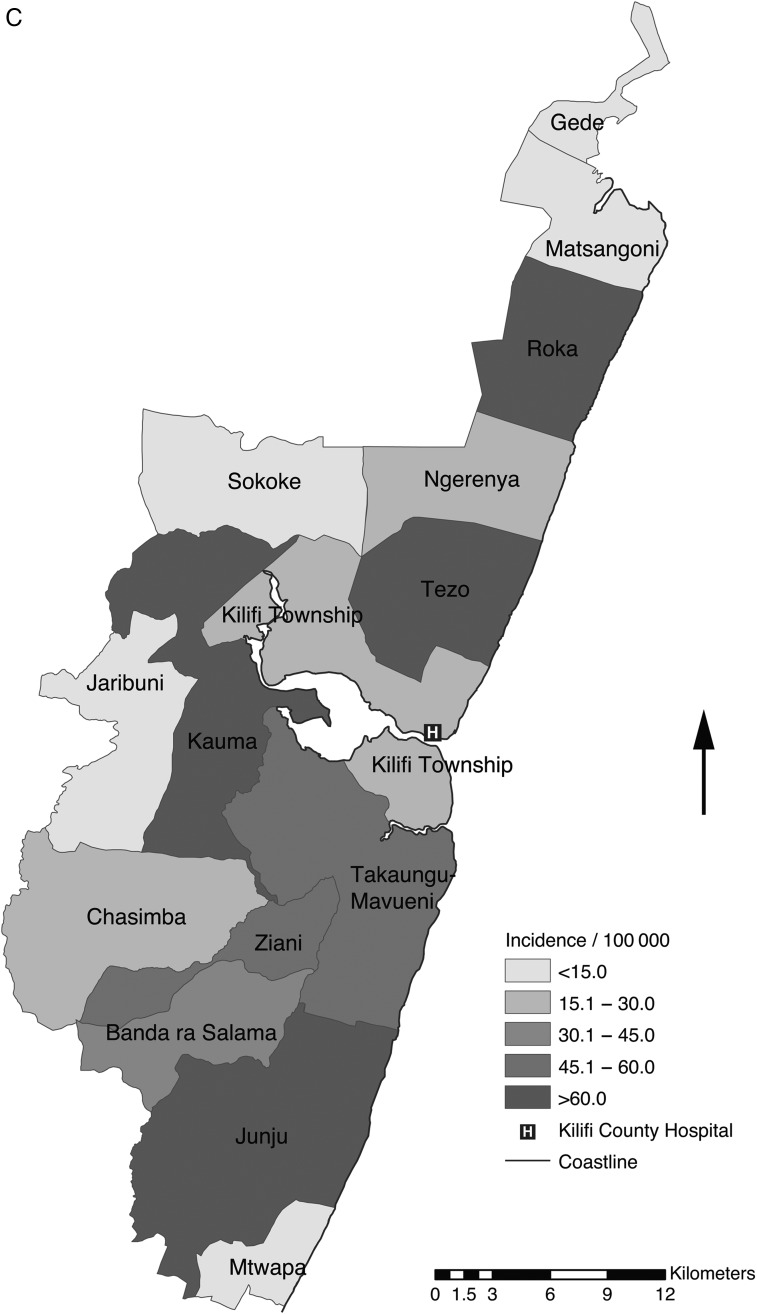
There were 219 cases of iNTS and 13 cases of Salmonella Typhi occurring in residents of the KHDSS, representing 57% and 50% of all hospitalized cases, respectively. The incidence of iNTS among children aged <5 years was 25.6 per 100 000 person-years of observation (PYO). The rate adjusted for missing specimens and contaminated cultures was 32.6 (95% CI, 28.1–37.7) and the rate adjusted, in addition, for access to care was 36.4 per 100 000 PYO (Table 2). The incidence varied by age (Figure 2B) but was highest among newborn infants <7 days old at 174.4 per 100 000 PYO (crude incidence, 136.6 per 100 000 PYO). Variation across the locations of the KHDSS was marked (range, 4.8–69.8 per 100 000 PYO), with the highest rates from locations south of KCH (Figure 2C). By Poisson regression, the incidence of iNTS among children aged <5 years declined, on average, by 16% in each year of the study period (incidence rate ratio [IRR], 0.84 [95% CI, .81–.86]) whereas the incidence of Salmonella Typhi bacteremia remained constant (IRR, 1.05 [95% CI, .90–1.22]). In children, the incidence of Salmonella Typhimurium and Salmonella Enteritidis in children declined by 14% (IRR, 0.86 [95% CI, .81–.90]) and 21% (IRR, 0.79 [95% CI, .74–.83]) per year, respectively (Supplementary Figure 1).
Table 2.
Crude and Adjusted Incidence Rates and Case Fatality Rate of Invasive Nontyphoidal Salmonella Disease Across Different Age Groups
| Age Group, y |
Cases | Deaths | CFR (%) | Cases in KHDSS |
Person-years of Observation |
Crude Incidence (95% CI) |
Adjusted Incidencea (95% CI) |
Access-Adjusted Incidence (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 0–4 | 321 | 71 | 22 | 186 | 726 881 | 25.6 (22.0–29.5) | 32.6 (28.1–37.7) | 36.4 (35.6–37.1) |
| 5–14 | 36 | 3 | 8.3 | 22 | 1 174 129 | 1.9 (1.17–2.84) | 2.4 (1.49–3.62) | … |
| ≥15 | 30 | 11 | 37 | 11 | 1 056 038 | 1.0 (.52–1.86) | 1.7 (.87–3.11) | … |
Incidence rates are given per 100 000 person-years.
Abbreviations: CFR, case fatality rate; CI, confidence interval; KHDSS, Kilifi Health and Demographic Surveillance System.
a Adjustment based on missed blood cultures and contamination rate per age group.
Figure 2.
A, Crude incidence (and 95% confidence intervals [CIs]) of nontyphoidal (NTS) bacteremia among children (<15 years) across the study period. B, Crude age-incidence curve (and 95% CIs) of NTS bacteremia among children.
Figure 2 continued. C, Map of the Kilifi Health and Demographic Surveillance System showing geographical distribution of the access-adjusted rates of NTS bacteremia among children aged <5 years. Abbreviation: PYO, person-years of observation.
In adults, the adjusted incidence of iNTS was 1.7 per 100 000 PYO. Among HIV-infected individuals, the crude incidence of iNTS was 13.2 per 100 000 PYO compared with a crude incidence of 0.1 per 100 000 PYO among HIV-uninfected individuals (IRR, 133.5 [95% CIs, 16.2–6142.4]). The incidence of iNTS in adults did not vary significantly by time. For Salmonella Typhi infections, the incidence among children and adults was 0.4 and 0.5 per 100 000 PYO, respectively.
Antimicrobial Susceptibility
Among 351 NTS isolates, in vitro resistance to ampicillin, trimethoprim-sulfamethoxazole, tetracycline, and cefotaxime was observed in 98 (27.9%), 86 (24.5%), 35 (10.0%), and 21 (6.0%), respectively (Supplementary Table 1). Eighty-four (23.9%) of the NTS isolates were MDR. Of the 21 patients showing nonsusceptibility to third-generation cephalosporins, 8 (38.1%) died during the admission.
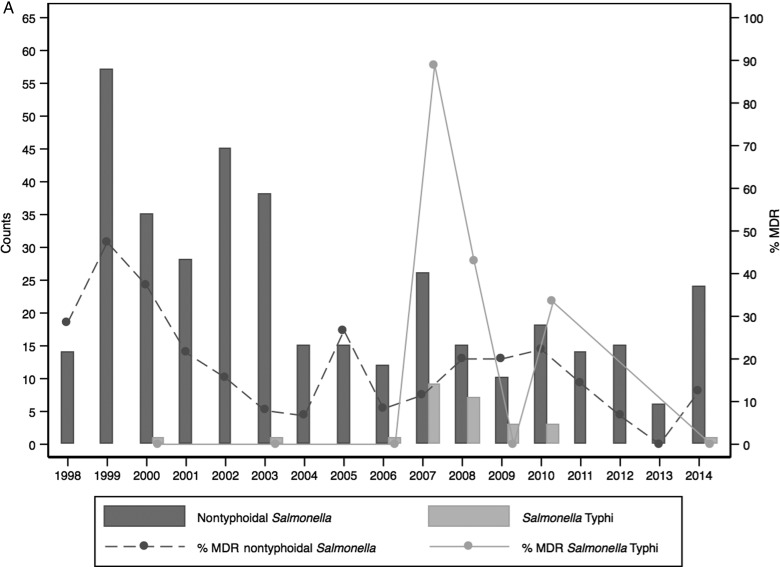
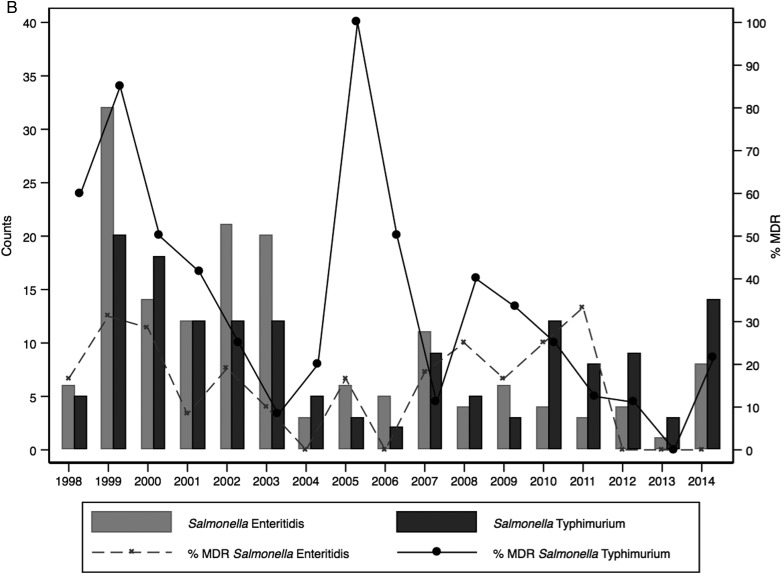
Among the NTS serotypes, resistance to individual drugs was mainly driven by Salmonella Typhimurium, which also had a higher percentage of MDR isolates (55/152 [36.2%]). Twenty of the 21 isolates showing nonsusceptibility to third-generation cephalosporins were Salmonella Typhimurium. Multidrug resistance in iNTS serotypes showed temporal variability with several peaks across the years. A peak in MDR Salmonella Typhimurium in 2005 was followed sequentially by a shift in dominance over Salmonella Enteritidis (Figure 3). Peaks in MDR isolates of Salmonella Enteritidis were seen over time with no apparent change in the proportion of isolates in subsequent years.
Figure 3.
The relationship between multidrug resistance (MDR) and the number of nontyphoidal Salmonella (NTS) and Salmonella Typhi (A) or the number of NTS isolates per serotype (B) among children, August 1998–2014.
Of Salmonella Typhi, 12 of 26 (46.2%) were MDR. Eight (66.7%) of these MDR isolates were isolated in 1 year (2007). Salmonella Typhi isolates were all susceptible to cefotaxime.
Case Fatality Ratio (CFR)
Among 321 cases of iNTS disease in children aged <5 years, 71 (22.1%) died in hospital (Table 2). The CFR for newborn infants <7 days old was 57.1% (4/7) and for NTS meningitis was 45.5% (10/22). Among 10 children with mixed infections of NTS and another pathogen, 6 died in hospital. Among adult cases of iNTS, 11 of 30 (36.7%) died in hospital. Among 26 patients of all ages with Salmonella Typhi bacteremia, 1 (3.8%) adult died.
DISCUSSION
We describe the characteristics of 387 cases of iNTS disease and 26 cases of Salmonella Typhi diagnosed in a rural hospital in Kenya. The importance of NTS as a cause of bacteremia in children has been previously described in this setting and other countries in Africa [5, 6, 10, 19]. Newborns and infants are those most susceptible to iNTS [5, 20] and in our study had the highest CFRs; more than half of all infected newborn infants died during the hospital admission.
Our results show a decrease in the incidence of iNTS over time in children. This is most likely associated with the decline in malaria infections over the same period [21]. The association of NTS with recent or current malaria has been repeatedly documented in children [22–24]. In our analysis, current malaria infection was not significantly associated with iNTS infection but splenomegaly, a marker of recent malaria infection, was. Malnutrition and HIV infection were independent risk factors of iNTS infection.
In previous studies, sickle cell anemia has been shown to be strongly associated with iNTS disease [25]. Among our pediatric cases with iNTS, the prevalence of sickle cell anemia was 7.1% compared with a prevalence of 1% in a birth cohort of >10 000 children recruited during the same period in Kilifi and investigated in infancy [21]. None of the cases of Salmonella Typhi tested had sickle cell anemia.
Among adults, HIV was the principal risk factor for iNTS disease. The incidence of iNTS among HIV-infected patients was 133 times higher than among HIV-uninfected individuals. In an area with a HIV prevalence of 4.3%, this is highly significant. Among adults, iNTS disease was more common in females than in males, reflecting the higher prevalence in women (6.1%) than in men (2.6%) in Kenya [17]. None of the adult cases had a positive malaria slide, although the adult surveillance was only established in 2007 when the prevalence of malaria in Kilifi had already fallen substantially [21].
Only 1 adult case of Salmonella Typhi infection was HIV infected. Similar low prevalence of HIV in cases of Salmonella Typhi has been shown in studies from Tanzania [26, 27]. The incidence of Salmonella Typhi across all age groups was 0.5 per 100 000 PYO, which yielded insufficient cases in which to examine risk factors. In addition, the CFR for typhoid fever in hospital was low at 1 in 26 (3.8%). Although the majority of the Salmonella Typhi cases reported here were in children (58%), there were no deaths in children. Culture-confirmed Salmonella Typhi infections in Africa are less common than NTS infections but are especially associated with urban areas with poor hygiene and sanitation [2, 6]. Salmonella Typhi infections commonly occur in epidemics [28, 29]. In our study, we retrospectively identified a small outbreak of MDR Salmonella Typhi that occurred in 2007–2008 that was associated with 1 death.
Previous reports from Kilifi have shown higher susceptibility to amoxicillin and cotrimoxazole among iNTS isolates compared with Escherichia coli and other gram-negative bacilli by Etest [30]. This general susceptibility to common antibiotics was seen in our study, with the prevalence of MDR declining over time. The majority of the MDR isolates were Salmonella Typhimurium. These could be the distinct genotype ST313 that has been identified among MDR Salmonella Typhimurium causing invasive disease in Kenya and Malawi [31]; unfortunately, because we were unable to do multilocus sequence typing, this cannot be confirmed. The presence of resistance to third-generation cephalosporins among iNTS is alarming and we intend to characterize these isolates further. In low-income countries, extended MDR NTS infections have limited options for alternative therapy, especially among those who are HIV infected [32], and are associated with high mortality.
With incomplete access to care and low blood culture sensitivity for iNTS, the incidence at population level is difficult to measure and is often underestimated as a consequence. We show a marked variation in the incidence by location, with the locations further from KCH having the lowest incidence. However, after adjusting for access to care, the incidence estimate for iNTS admissions among <5-year-olds increased by only 12%, suggesting that for a disease as severe and potentially fatal as iNTS, the barriers to hospital admission are comparatively small.
We have reported one of the longest series of continuous surveillance for invasive bacterial disease in sub-Saharan Africa using consistent demographic, clinical, and laboratory methods. In a rural setting with a high burden of malaria initially and a low prevalence of HIV infection, we have observed a high incidence of iNTS disease among children and a low incidence of typhoid fever. The burden of iNTS in children has declined in synchrony with the dramatic reduction in malaria parasitemia in the study area. However, over the whole period it was the second commonest cause of invasive bacterial infection and had a CFR of 23% in children <5 years of age. It is an especially fatal disease in newborn infants. Theories linking malaria to iNTS disease in children do not readily explain the high risk of disease in neonates who do not often get malaria. As pneumococcal disease is brought under control by vaccination, invasive NTS disease may become the most important cause of sickness and death due to invasive bacterial infections in children in Kilifi, and a common disease of adults. Public health measures to control NTS disease and its transmission, possibly including effective vaccination, are likely to improve child and adult health in the region.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Christopher Nyundo for assisting with the GIS mapping; Ngure Kagia for assisting with the MIC work; the nursing, clinical, and clerical staff of Kilifi County Hospital; and the patients and their families. This article was published with the approval of the KEMRI Director.
Financial support. The work was supported by the Wellcome Trust through core support to the KEMRI–Wellcome Trust Research Programme and training and clinical research fellowships (103951 to A. O. E., 053439 and 083579 to J. A. B., 091758 to T. N. W., 098532 to J. A. G. S.), by Gavi, the Vaccine Alliance, through support for bacteremia surveillance within a study of pneumococcal vaccine. The publication was supported by the Bill & Melinda Gates Foundation (grant OPP1125993).
Supplement sponsorship. This article appeared as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. S. C. M. has received grants from the Bill & Melinda Gates Foundation. A. O. E. has received institutional grant funding from the Wellcome Trust and Gavi. J. A. B. and T. N. W. have received institutional grant funding from the Wellcome Trust. J. A. G. S. has received institutional grant funding from the Wellcome Trust and Gavi. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Breiman RF, Cosmas L, Njuguna H et al. . Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 2012; 7:e29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 2012; 379:2489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabu C, Breiman RF, Ochieng B et al. . Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One 2012; 7:e31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkley JA, Lowe BS, Mwangi I et al. . Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 6.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morpeth SC, Ramadhani HO, Crump JA. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 2009; 49:606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilks CF. Acute bacterial infections and HIV disease. Br Med Bull 1998; 54:383–93. [DOI] [PubMed] [Google Scholar]
- 9.Watera C, Nakiyingi J, Miiro G et al. . 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 2004; 18:1210–3. [DOI] [PubMed] [Google Scholar]
- 10.Sigauque B, Roca A, Mandomando I et al. . Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 2009; 28:108–13. [DOI] [PubMed] [Google Scholar]
- 11.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis 2015; 21:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JA, Bauni E, Moisi JC et al. . Profile: the Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol 2012; 41:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkley JA, Ross A, Mwangi I et al. . Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 2003; 326:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Pocket book of hospital care for children. 2nd ed. Guidelines for the management of common childhood illnesses. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standard Institute. Performance standards for antimicrobial disk susceptibility tests. CLSI document M100-S25. Wayne, PA: CLSI, 2015. [Google Scholar]
- 16.Ministry of Public Health and Sanitation Kenya. National guidelines for HIV testing and counselling in Kenya, 2008. Available at: http://www.who.int/hiv/topics/vct/policy/KenyaGuidelines_Final2009.pdf Accessed 5 June 2015.
- 17.National AIDS/STI Control Program. Kenya AIDS indicator survey: final report, 2012. Available at: http://www.nacc.or.ke/images/documents/KAIS-2012.pdf Accessed 5 June 2015.
- 18.Moisi JC, Nokes DJ, Gatakaa H et al. . Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ 2011; 89:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 2000; 19:312–8. [DOI] [PubMed] [Google Scholar]
- 20.Mandomando I, Macete E, Sigauque B et al. . Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 2009; 14:1467–74. [DOI] [PubMed] [Google Scholar]
- 21.Scott JA, Berkley JA, Mwangi I et al. . Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet 2011; 378:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biggs HM, Lester R, Nadjm B et al. . Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis 2014; 58:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J 2006; 25:230–6. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie G, Ceesay SJ, Hill PC et al. . A decline in the incidence of invasive non-typhoidal Salmonella infection in The Gambia temporally associated with a decline in malaria infection. PLoS One 2010; 5:e10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams TN, Uyoga S, Macharia A et al. . Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet 2009; 374:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crump JA, Ramadhani HO, Morrissey AB et al. . Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health 2011; 16:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crump JA, Ramadhani HO, Morrissey AB et al. . Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 2011; 52:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutterloh E, Likaka A, Sejvar J et al. . Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis 2012; 54:1100–6. [DOI] [PubMed] [Google Scholar]
- 29.Neil KP, Sodha SV, Lukwago L et al. . A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese District, Uganda, 2008–2009. Clin Infect Dis 2012; 54:1091–9. [DOI] [PubMed] [Google Scholar]
- 30.Bejon P, Mwangi I, Ngetsa C et al. . Invasive gram-negative bacilli are frequently resistant to standard antibiotics for children admitted to hospital in Kilifi, Kenya. J Antimicrob Chemother 2005; 56:232–5. [DOI] [PubMed] [Google Scholar]
- 31.Kingsley RA, Msefula CL, Thomson NR et al. . Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 2009; 19:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feasey NA, Cain AK, Msefula CL et al. . Drug resistance in Salmonella enterica ser. Typhimurium bloodstream infection, Malawi. Emerg Infect Dis 2014; 20:1957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.