Abstract
Background. Etiologic agents of childhood bacteremia remain poorly defined in Nigeria. The absence of such data promotes indiscriminate use of antibiotics and delays implementation of appropriate preventive strategies.
Methods. We established diagnostic laboratories for bacteremia surveillance at regional sites in central and northwest Nigeria. Acutely ill children aged <5 years with clinically suspected bacteremia were evaluated at rural and urban clinical facilities in the Federal Capital Territory, central region and in Kano, northwest Nigeria. Blood was cultured using the automated Bactec incubator system.
Results. Between September 2008 and April 2015, we screened 10 133 children. Clinically significant bacteremia was detected in 609 of 4051 (15%) in the northwest and 457 of 6082 (7.5%) in the central region. Across both regions, Salmonella species account for 24%–59.8% of bacteremias and are the commonest cause of childhood bacteremia, with a predominance of Salmonella enterica serovar Typhi. The prevalence of resistance to ampicillin, chloramphenicol, and cotrimoxazole was 38.11%, with regional differences in susceptibility to different antibiotics but high prevalence of resistance to readily available oral antibiotics.
Conclusions. Salmonella Typhi is the leading cause of childhood bacteremia in central Nigeria. Expanded surveillance is planned to define the dynamics of transmission. The high prevalence of multidrug-resistant strains calls for improvement in environmental sanitation in the long term and vaccination in the short term.
Keywords: Salmonellae, bacteremia, children, Nigeria, antibiotic resistance
Data from Nigeria on the etiologic agents of bacteremia in children have been scanty and largely based on outdated blood culture methods, thus posing a challenge for data comparison with other countries in sub-Saharan Africa where there have been several etiologic studies of childhood bacteremia utilizing modern culture techniques [1–3].
In general, in Nigeria, febrile illnesses in children are presumed to be caused by malaria. If fever persists following an empiric course of antimalarials, it is assumed to be due to “typhoid fever” but without blood culture confirmation [4].
In our initial pilot report from the central region [5], the dominance of Salmonella enterica serovar Typhi infections in children at this location may not be applicable to other parts of the country, given the unique infrastructure that consists of informal settlements around the better structured but less affordable settlements in Abuja city. Thus, to evaluate this initial observation further, we established a similar surveillance platform in Kano, northwest Nigeria, a location further north than Abuja, with different climatic conditions, population, and lifestyle. The primary aim of this study was to describe the etiologic agents of bacteremia in children aged <5 years.
METHODS
We performed blood culture for the evaluation of febrile children aged 0–60 months at 2 regional locations in central and north-central Nigeria.
Setting
Nigeria is the most populated country in sub-Saharan Africa, with a population of almost 170 million [6]. The Federal Capital Territory (FCT) has a land area of 8000 km2. Abuja is a “planned” city, as it was mainly built in the 1980s, and officially became Nigeria's capital in 1991, replacing the previous capital in Lagos. In 2006 the population was estimated at 1.7 million, but may currently be about 5.7 million [6]. The FCT continues to experience a huge population growth; it has been reported that some areas around Abuja have been growing at an annual rate of 20%–30% [6]. The rapid spread of squatter settlements and shanty towns in and around the city limits is believed to be an important contributor to this rapid growth. There is perennial malaria transmission, mostly due to Plasmodium falciparum, and human immunodeficiency virus (HIV) prevalence of 7.5% among pregnant women attending antenatal clinics [7].
Kano is the capital of Kano state in northwest Nigeria and one of the oldest cities in the country. Although largely urban, Kano is not as cosmopolitan as Abuja but is more densely populated. According to 2006 census figures, Kano state has a population of 9.38 million [8]. It is recognized as one of the fastest-growing cities in Nigeria, with a population density of about 1000 inhabitants per km2. The dry season lasts from November to April [9]. The entire state is within the meningococcal disease belt. Malaria transmission is seasonal [8], and HIV prevalence among women attending antenatal clinic is 1.3% [7].
Enrollment Sites
The enrollment sites at FCT are as previously described [5]. In Kano we enrolled children from Aminu Kano Teaching Hospital, Hasiya Bayero Pediatric Hospital, and Murtala Specialist Hospital. While Aminu Kano Teaching Hospital serves as a tertiary referral center, the other 2 facilities provide primary and secondary care services. The combined outpatient attendance for children at these 3 facilities is about 1000 daily.
Participant Enrollment
The criteria for enrollment are as previously described [5]. In brief, children aged <5 years who presented to an enrollment facility with fever of >3 days in addition to prostration, convulsion, refusal to feed, diarrhea, or altered consciousness were eligible. Informed consent was obtained from the parent or guardian. A physician administered a detailed questionnaire to obtain information on clinical history and physical examination findings. Appropriate clinical specimens were then obtained, and these included 1–3 mL of blood for culture using the vacutainer set after cleaning the skin with alcohol swabs. The specimen was collected directly into the Bactec 9050 culture bottle and promptly delivered to the regional processing laboratory within 4 hours of collection.
Blood Culture Processing
Blood sampling and processing were as previously described [5]. In brief, we utilized only aerobic blood culture bottles and held cultures in the Bactec 9050 incubator for a maximum of 5 days. Bacteria were identified by a combination of morphology and biochemical tests. For Enterobacteriaceae, we used the API 20E system (bioMérieux, France). Antibiotic susceptibility assessments were determined by Kirby-Bauer disk diffusion test using standard interpretative criteria and using antibiotic disks for locally available antibiotics for the immediate management of patients at the field sites. Bacterial isolates were stored in skim milk at –70°C and further characterized at the clinical microbiology laboratory of the University of Nebraska Medical Center (UNMC) using the Epsilometer method.
Microbiology
Bacteremia was defined as the isolation of at least 1 noncontaminant bacteria from the admission blood culture. Coagulase-negative staphylococci, Bacillus species, Corynebacterium species, or micrococci were classified as contaminants. Streptococcus viridians isolates were considered significant when isolated from children with underlying risk factors for S. viridians infection, such as congenital heart defect.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed at the UNMC microbiology laboratory using the Epsilometer test (Etest, bioMérieux) methodology according to standard methods. Minimum inhibitory concentration (MIC) values were interpreted according to Clinical and Laboratory Standards Institute (CLSI) standards [10]. Due to the lack of CLSI standards, a streptomycin MIC of ≥16 was considered resistant in these studies. Breakpoints for the interpretation of susceptibility for the different antibiotics tested are summarized in Supplementary Table 1.
Salmonella Serotyping
Salmonellae isolates were identified to the serotype level using the Bioplex 200 (Bio-Rad) as previously described using the Centers for Disease Control and Prevention standard Salmonella molecular serotyping protocol [11–13].
Data Collection and Analysis
Clinical information was collected using a structured questionnaire after obtaining a signed informed consent from the parent or guardian.
Study data were collected and managed using REDCap electronic data capture tools hosted at the UNMC [14]. IBM SPSS for statistics was used for data analysis. Dichotomous variables were compared between groups using a χ2 test or χ2 test for trend where appropriate.
Ethics Statement
This study was approved by the ethics committees of the FCT, National Hospital Abuja, Zankli Medical Center, Federal Medical Center Keffi, Aminu Kano Teaching Hospital, and UNMC, Omaha Institutional Review Board.
RESULTS
Overall, we screened 10 133 children for bacteremia. At the FCT location, we screened 6082 children with Salmonella Typhi bacteremia, nontyphoidal salmonellae (NTS), and other forms of bacteremia, with a mean age of 32.01, 21.62, and 8.92 months, respectively. In Kano, the total number of children screened from January 2014 until March 2015 was 4051; detailed demographic data were only available on children screened from September 2014 (n = 1944). Of these, the mean age of those with Salmonella Typhi bacteremia was 31.62 months, whereas the mean age of those with NTS and other forms of bacteremia was 25.62 and 19.26 months, respectively (Table 1).
Table 1.
Distribution of Blood Culture Positivity Rates by Age in Kano, Northwest Nigeria
| Age | Salmonella Typhi | NTS | Other Bacteria | Total With Positive Culture (%) | No. of Children Screened |
|---|---|---|---|---|---|
| 0–12 mo | 34 | 19 | 205 | 258 (11.04) | 734 |
| 13–24 mo | 91 | 27 | 72 | 190 (20.29) | 587 |
| 25–36 mo | 59 | 10 | 26 | 95 (28.44) | 280 |
| 37–48 mo | 49 | 13 | 21 | 83 (33.33) | 180 |
| 49–60 mo | 47 | 4 | 9 | 60 (30.40) | 163 |
| Total | 280 | 73 | 333 | 686 (19.48) | 1944 |
Abbreviation: NTS, nontyphoidal Salmonella.
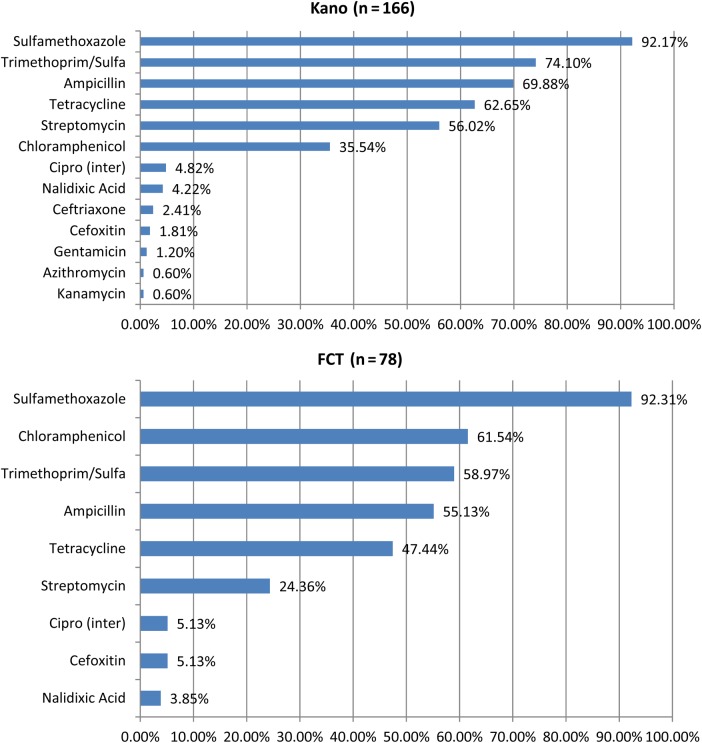
At the central location, FCT, we enrolled 6082 children between June 2012 and March 2015, of whom 457 of 6082 (7.5%) had clinically significant bacteremia. Of these, 110 of 457 (24%) had invasive salmonellosis, consisting of Salmonella Typhi in 84 cases and NTS in 26 cases (Table 2). Culture positivity rates by site for clinically significant bacteria agents are summarized in Figure 1. Enrollment rates were not uniform throughout the period of surveillance due to health workers’ strike action, lack of hospital beds, and civil insurgency in Kano. An estimate for the daily enrollment rate by site is provided in Supplementary Table 1.
Table 2.
Distribution of Blood Culture Positivity Rate by Age in Federal Capital Territory, Central Nigeria
| Age | Salmonella Typhi | NTS | Other Bacteria | Total With Positive Culture (%) | No. of Children Screened |
|---|---|---|---|---|---|
| 0–12 mo | 12 | 11 | 262 | 285 (8.06) | 3537 |
| 13–24 mo | 17 | 6 | 47 | 70 (6.22) | 1126 |
| 25–36 mo | 21 | 4 | 19 | 44 (6.43) | 684 |
| 37–48 mo | 22 | 5 | 12 | 39 (9.44) | 413 |
| 49–60 mo | 12 | 0 | 7 | 19 (5.90) | 322 |
| Total | 84 | 26 | 347 | 457 (7.51) | 6082 |
Abbreviation: NTS, nontyphoidal Salmonella.
Figure 1.
Culture positivity rates by site for clinically significant bacterial agents. Abbreviations: Cipro (inter), ciprofloxacin intermediate resistance; FCT, Federal Capital Territory; Sulfa, sulfamethoxazole.
In Kano, at the northwest location, we screened 4051 children during the period January 2014 to April 2015. Of these, 609 of 4051 (15%) had clinically significant bacteremia. Salmonellae accounted for 364 of 609 (59.8%) cases, consisting of 296 Salmonella Typhi and 68 cases of NTS. Detailed demographic analysis in the report for Kano covers only the period of September 2014–March 2015, during which 1944 children were screened. When stratified by age, the highest blood culture positivity rate was recorded among those aged 37–48 months (Tables 1 and 2). At both locations, Salmonella Typhi bacteremia was more common in older children (mean age, 32 months) or NTS (mean age, 21.6 months) compared with other types of bacteremia (mean age, 8.9 months; Table 3).
Table 3.
Distribution of Nontyphoidal Salmonella Serovars in Nigeria
| Serovar | No. |
|---|---|
| Salmonella Typhimurium | 45 |
| Salmonella Enteritidis | 39 |
| Salmonella Durban | 1 |
| Salmonella Virchow | 1 |
| Salmonella Dublin | 5 |
| Salmonella Abony | 1 |
| Salmonella Brendeney | 1 |
| Salmonella Pasing | 1 |
| Salmonella Poona | 3 |
| Salmonella Colindale | 1 |
| Salmonella Galiema | 1 |
| Salmonella spp | 1 |
| Total | 100 |
Seasonality of Bacteremia
In Kano, there were 327 children with bacteremia during the months of April–September (rainy season) and 447 during the months of October–March 2014 (dry season). Of these, 118 of 327 (36.09%) had Salmonella Typhi bacteremia; 32 of 327 (9.8%) and 175 of 327 (53.25%) had NTS and other forms of bacteremia, respectively. During the dry season, 215 of 447 (48.1%) had Salmonella Typhi, 50 of 447 (11.2%) had NTS, and 181 of 447 (40.5%) had other forms of bacteremia. Using a Pearson χ2 test, there is a significant increase in distribution of cases of typhoid fever during the dry season (P < .001) and higher prevalence of other forms of bacteremia during the rainy season in Kano (P < .001). In FCT, although the duration of surveillance was longer than in Kano, the frequency of cases by seasonal distribution is too scanty for a meaningful interpretation.
NTS Serovars
The distribution of Salmonella serovars is summarized in Table 3. Overall, the most prevalent serovars were Typhimurium and Enteritidis, accounting for 44% and 38%, respectively.
Distribution of Antibiotic Resistance
Antibiotic susceptibility testing was performed on 244 isolates obtained over the period November 2011–March 2013 from Kano and June 2012–March 2013 from the FCT. The majority of Salmonella isolates were resistant to sulfamethoxazole (225 of 244 [92.21%]), whereas none of the isolates were resistant to ciprofloxacin or imipenem. The proportion of isolates that were resistant to chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole (TMP-SMX), classified as multidrug resistant, varied by year and by location. At the FCT, this was 75% in 2009 and 40% in 2012, whereas in Kano it was 18.2% in 2013 and 38.5% in 2014. As a proportion, NTS isolates were 20.14% more resistant than Salmonella Typhi isolates, and the difference in multidrug resistance distribution between NTS and S. Typhi was significant (P = .002).
Resistance Patterns for NTS
Compared with NTS isolates from Abuja, NTS isolates from Kano had a higher proportion resistant with significant differences in distribution to ampicillin (+28.17%; P < .003), cefoxitin (+9.33%; P = .033), TMP-SMX (+23.21%; P = .008), and tetracycline (+24.8%; P = .026).
Resistance Patterns for S. Typhi Isolates
Salmonella Typhi isolates from Kano had a higher proportion resistant with significant differences in distribution to streptomycin (+46%; P < .001), whereas those from Abuja had a higher proportion resistant with significant differences in distribution to chloramphenicol (+33.53%; P < .001).
Mortality
We have did not establish mortality surveillance until very recently due to the logistics of ensuring follow-up of participants from a very wide geographic area and very limited inpatient facilities. Mortality from inpatient surveillance in Kano during the period December 2014–March 2015 was 29. Overall, 13 of 29 deaths were in children aged <1 year. Of these 29 deaths, 15 had positive blood cultures, consisting of Salmonella Typhi (n = 5), Streptococcus pneumoniae (n = 4), α-hemolytic Streptococcus (n = 2), and Escherichia coli, Haemophilus influenzae, Salmonella group B, and Klebsiella pneumoniae (n = 1 each). In 14 cases, the blood culture did not yield any growth. During this limited period of death surveillance, only inpatient fatalities were documented. Pathogen-specific mortality for Salmonella Typhi was 3.16%.
DISCUSSION
To our knowledge, this report provides bacteremia surveillance data on the largest population from Nigeria to date. In addition, it provides a regional comparison of invasive Salmonella isolates at locations with a different population mix, geographic location, and perhaps healthcare-seeking behavior. Overall, the pattern of invasive salmonellosis differs from reports from other countries in sub-Saharan Africa, where NTS is more prevalent than serovar Typhi [1–3].
Although it is widely believed that typhoid fever is endemic in Nigeria, the burden of disease has not been objectively ascertained, as most healthcare facilities are not equipped with a diagnostic microbiology laboratory and evaluation with a blood culture is not normally performed.
Etiologic diagnosis improves the quality of clinical management, and, particularly with invasive salmonellosis, it is important to differentiate between typhoid fever and NTS. From a public health perspective, the implication for patient management with typhoid fever should prompt contact tracing to prevent further spread of the infection and careful patient evaluation and follow-up for delayed complications such as terminal ileal perforation, relapse, and neuropsychiatric complications. There are several reports of terminal ileal perforation from Nigeria; whether the population is unduly susceptible or the incidence is unusually high in this setting warrants a closer evaluation. Mortality associated with this complication ranges from 13% at tertiary referral centers [15, 16] to as high as 70% at primary or secondary facilities [17]. Although these complications are not associated with NTS, diagnosis with NTS should prompt screening for underlying host risk factors such as HIV infection, particularly in older children. Defining the burden of both NTS and typhoidal disease is important as both conditions are potentially vaccine preventable.
Guiding Treatment Options
A pragmatic approach has been adopted in clinical practice in Nigeria and in most developing countries to use parenteral ceftriaxone for the empiric management of any bacteremic syndrome and with good clinical outcome in most cases. However, defining the etiologic agent of infection is critical to improving the quality of care, promoting antibiotic stewardship, and monitoring public health. The invasive isolates from our study were uniformly susceptible to ceftriaxone but the limited susceptibility to oral alternatives except for ciprofloxacin is concerning. The continued use of ceftriaxone for empiric management could portend serious clinical challenges in the near future for a number of reasons. First, as a broad-spectrum agent, it is commonly associated with a high incidence of extended-spectrum β-lactamase (ESBL) enzyme production among the Enterobacteriaceae [17, 18]. The prevalence of ESBLs in this population from Nigeria is currently unknown. Second, it is available only in a parenteral formulation; however, most patients are hospitalized for parenteral treatment only for as long as they can afford the cost of hospitalization and not necessarily for the optimal duration required for cure. Although there may be a place for outpatient daily injections with ceftriaxone, the efficacy of this approach and the optimal duration required is not known [19].
A more convenient oral option, ciprofloxacin, has been adopted recently and is gaining wide application. It is cheaper than ceftriaxone and, based on the data presented in this manuscript, most isolates are susceptible. The potential impact of the widespread use of ciprofloxacin will require continued surveillance for the emergence of resistance among Enterobacteriaceae, including Salmonella and Mycobacterium tuberculosis, another prevalent disease in this setting for which the quinolones are reserved as second-line treatment for multidrug-resistant infection. In our study, although all isolates were susceptible to ciprofloxacin, approximately 5% at each site were intermediate and approximately 4% of isolates at both sites were resistant to nalidixic acid, suggesting that resistance to ciprofloxacin is emerging.
Difference in Antibiotic Resistance Patterns
NTS and Salmonella Typhi are known to have distinct characteristics in their biologic properties, mode of transmission, and pathogenesis [20, 21]. Thus, while their contribution to disease may be different in several countries, the difference in epidemiology of disease within Nigeria is less well described. It is not obvious why the prevalence of drug-resistant invasive salmonellosis is higher in Kano than it is in Abuja. Because it appears this is unlikely to be primarily driven by the prevalence of nonprescription antibiotic use, we are in the process of evaluating the role of host risk factors such as sickle cell disease, HIV, and malnutrition, all of which are conditions known to be associated with frequent antibiotic use. Furthermore, it has been well described that a large proportion of the outpatient antibiotics sold in unregulated local pharmacies are either fake or outdated, making it hard to truly establish the prevalence of antimicrobial consumption [22, 23]. We are also undertaking detailed molecular characterization of these isolates.
The diversity in geography, climatic conditions, ethnic groups, and cultural practices in Nigeria provides a unique platform for comprehensive studies of the ecoepidemiologic factors that affect disease transmission and the identification of reservoirs for these infections. Such studies will provide critical data that will fill current knowledge gaps and optimize the deployment of the new-generation typhoid vaccines and those in development for invasive NTS disease.
Study Limitations
This study was not primarily designed to evaluate the burden of invasive salmonellosis but rather for the evaluation of invasive pneumococcal disease in children. Thus, the study entry criteria were optimized for high yield of invasive pneumococcal disease and not Salmonella infections. We were unable to accurately and systematically document patient blood volume that was cultured by weighing the blood culture bottles. However, clinicians always ensured a minimum of 1 mL of blood, measured by the syringe that was inoculated into the culture bottle. Only children aged <5 years were enrolled in the study. Collection of data from the rest of the population will be desirable to provide more comprehensive disease burden data. We also preferentially enrolled sicker children who required hospitalization rather than those who were managed as outpatients. Even then, due to lack of adequate bed space, several of these patients were not hospitalized. Thus, the current estimate from this surveillance likely underestimates the true burden of Salmonella bacteremia in young children and does not provide any data in older children or in adults. We were unable to establish outcome surveillance until recently at the location in the northwest, due to logistic reasons. Future studies based on these preliminary observations must include children and adults and preferably in the context of a defined population, to allow for generation of true disease incidence estimates. In addition, population-based prospective surveillance for terminal ileal perforation should be incorporated in such surveillance to provide a clearer contextual perspective of the incidence of this complication and also mortality.
In conclusion, this study provides the largest surveillance data to date on the burden of typhoid fever in children in Nigeria, utilizing the gold standard of blood culture. The high prevalence of culture-confirmed disease is of public health concern and should prompt additional studies on the dynamics of disease transmission, so that effective preventive measures can be implemented.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful for the institutional support from administration of local participating sites (University of Abuja Teaching Hospital Gwagwalada, National Hospital Abuja, Nyanya General Hospital, Federal Medical Center Keffi, Aminu Kano Teaching Hospital, Murtala Mohammed Specialist Hospital, and Hasiya Bayero Pediatric Hospitals in Kano). Finally, we are grateful to the parents and guardians of the children who contributed clinical samples to this project.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award number R01AI097493); the Bill & Melinda Gates Foundation (grant number OPP1034619); and internal grants from the University of Nebraska Medical Center.
Supplement sponsorship. This article was published as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Enwere G, Biney E, Cheung YB et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–59 months in The Gambia. Pediatr Infect Dis J 2006; 25:700–5. [DOI] [PubMed] [Google Scholar]
- 2.Berkley JA, Lowe BS, Mwangi I et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 3.Isendahl J, Manjuba C, Rodrigues A et al. Prevalence of community-acquired bacteraemia in Guinea-Bissau: an observational study. BMC Infect Dis 2014; 14:3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arulogun OS, Adeniyi JD, Asa S, Adegbenro CA. Why actions for early treatment of febrile illnesses in children are delayed by caregivers. Int Q Community Health Educ 2011–2012; 32:219–31. [DOI] [PubMed] [Google Scholar]
- 5.Obaro S, Lawson L, Essen U et al. Community acquired bacteremia in young children from central Nigeria—a pilot study. BMC Infect Dis 2011; 11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuja, the Beautiful Capital city (21 January 2011). Available at: http://9ja-land.blogspot.com/2010/05/abuja-beautiful-capital-city.html Accessed 9 May 2015.
- 7.Federal Republic of Nigeria, National Agency for the Control of AIDS, Global AIDS Response Country Progress Report 2014. Available at: http://www.unaids.org/sites/default/files/country/documents/NGA_narrative_report_2014.pdf Accessed 11 May 2015.
- 8.Olayemi IK, Ande AT, Ayanwale AV et al. Seasonal trends in epidemiological and entomological profiles of malaria transmission in north central Nigeria. Pak J Biol Sci 2011; 14:293–9. [DOI] [PubMed] [Google Scholar]
- 9.Federal Republic of Nigeria, National Bureau of Statistics 2006 population census, Kano state statistical table: 2010. Available at: www.nigriast.gov.ng. Accessed 12 May 2015.
- 10.Clinical and Laboratory Standards Institute. M100-S24: performance standards for antimicrobial susceptibility testing; 24th informational supplement. Wayne, PA: CLSI, 2014. [Google Scholar]
- 11.CDC standard protocol: molecular determination of serotype in Salmonella version 2.0, 2011. Available at: www.cifor.us/clearinghouse/getpdf.cfm?id=235. Accessed 12 May 2015.
- 12.Fitzgerald C, Collins M, van Duyne S et al. Multiplex, bead-based suspension array for molecular determination of common Salmonella serogroups. J Clin Microbiol 2007; 45: 3323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera-León S, McQuiston JR, Usera MA et al. Multiplex PCR for distinguishing the most common phase-1 flagellar antigens of Salmonella spp. J Clin Microbiol 2004; 42: 2581–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edino ST, Mohammed AZ, Uba AF et al. Typhoid enteric perforation in north western Nigeria. Niger J Med 2004; 13:345–9. [PubMed] [Google Scholar]
- 16.Edino ST, Yakubu AA, Mohammed AZ, Abubakar IS. Prognostic factors in typhoid ileal perforation: a prospective study of 53 cases. J Natl Med Assoc 2007; 99:1042–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Mogasale V, Desai SN, Mogasale VV, Park JK, Ochiai RL, Wierzba TF. Case fatality rate and length of hospital stay among patients with typhoid intestinal perforation in developing countries: a systematic literature review. PLoS One 2014; 9:e93784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson DL, Bonomo RA. Extended-spectrum b-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum β-lactamases. Clin Microbiol Infect 2008; 14(suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 20.Butler T. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin Microbiol Infect 2011; 17:959–63. [DOI] [PubMed] [Google Scholar]
- 21.Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol 2013; 764:13–26. [DOI] [PubMed] [Google Scholar]
- 22.Baiyewu L. (2 September 2012). Fake antibiotics malaria drugs most common - NAFDAC. Punch. Available at: http://www.punchng.com/news/fake-antibiotics-malaria-drugs-most-common-nafdac/. Accessed 7 September 2015. [Google Scholar]
- 23.Nigerian Pharmacies Selling Fake Antibiotics Shut Down (18 January 2011). Available at: http://www.safemedicines.org/2011/01/nigerian-pharmacies-selling-fake-antibiotics-shut-down.html Accessed 13 May 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.