Abstract
Aims and objectives
To compare dosimetrically the manual optimisation with IPSA using dose volume histograms (DVH) among patients treated for carcinoma of cervix with intracavitary brachytherapy.
Background
With the advent of advanced imaging modalities, there has been a shift from conventional X-ray based planning to three-dimensional planning. Manual optimisation is widely used across various institutions but it is time consuming and operator dependant. Inverse planning simulated annealing (IPSA) is now available in various brachytherapy planning systems. But there is a paucity of studies comparing manual optimisation and IPSA in treatment of carcinoma cervix with intracavitary brachytherapy and hence this study.
Materials and methods
Fifteen consecutive patients treated between December 2013 and March 2014 with intracavitary brachytherapy for carcinoma of cervix were selected for this study. All patients were initially treated with external beam radiotherapy followed by intracavitary brachytherapy. The DVH was evaluated and compared between manually optimised plans and IPSA in the same set of patients.
Results
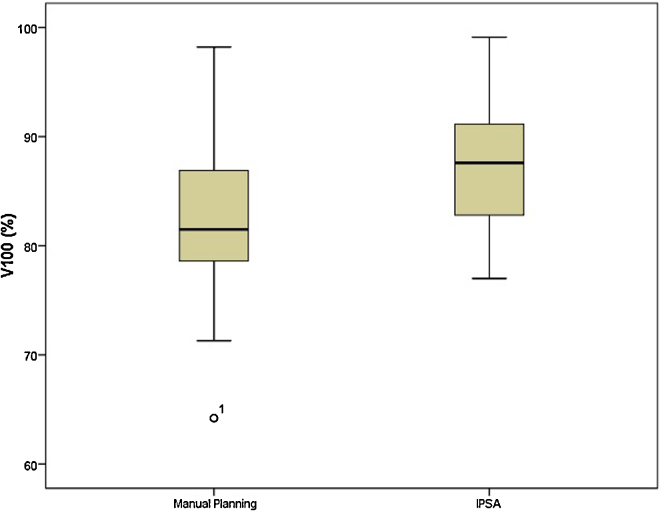
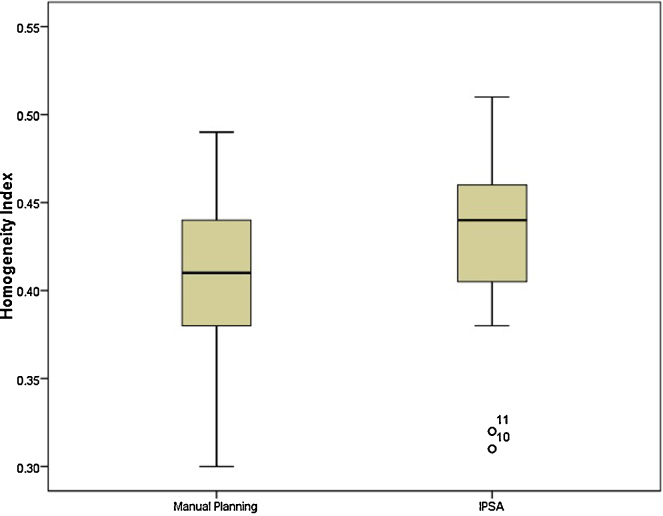
There was a significant improvement in the HRCTV coverage, mean V100 of 87.75% and 82.37% (p = 0.001) and conformity index 0.67 and 0.6 (p = 0.007) for plans generated using IPSA and manual optimisation, respectively. Homogeneity index and dose to the OARs remained similar between the two groups.
Conclusion
The use of inverse planning in intracavitary brachytherapy of cervix has shown a significant improvement in the target volume coverage when compared with manual planning.
Keywords: Carcinoma cervix, Manual Optimisation, Inverse planning, High dose rate
1. Background
Brachytherapy has been a standard component of definitive radiation therapy for cervical cancer since shortly after the discovery of radium. Intracavitary brachytherapy is the most commonly used brachytherapy technique in patients with cervical cancer. With advancements in dose optimisation and with the advent of advanced imaging modalities, there have been various evolutions of this technique overcoming a number of limitations.
With the use of computerised tomography scanning (CT scan), three-dimensional planning is feasible by direct visualisation of target volumes and critical structures with respect to the applicators. Customised dose distributions are now possible that may improve local control and reduce complications when compared to conventional two-dimensional brachytherapy planning.
With advances in imaging, the optimisation methods also have improved. Manual optimisation is a trial and error method where the planner keeps changing the dwell weights until an optimal solution is obtained. It allows the user to change the dwell weights manually or mouse drag the isodose lines such that the target coverage is adequate with maximal sparing of organ at risk (OAR). It is time consuming and planner dependant.
The recent advance in optimisation is the use of inverse planning simulated annealing (IPSA), which is based on a mathematical algorithm where clinical objectives are defined as mathematical equations. The optimal solution is obtained through iterative process by minimising the objective function.
In many centres, including our institution, the CT scan is shared with a radiology department. In a high volume centre like ours, there is usually a simulation delay of 3–4 h from the time of applicator placement to the time when first fraction of brachytherapy is delivered. This also includes 30–45 min of planning if manual optimisation is used. If the plans generated by IPSA are found to be comparable with manual optimisation plans, this could potentially reduce the time required for brachytherapy planning and could lead to earlier delivery of the next fraction.
2. Aim
To compare dosimetrically, manual optimisation with inverse planning simulated annealing using dose volume histograms (DVH) among patients treated for carcinoma of cervix with intracavitary brachytherapy.
3. Materials and methods
Data set of 15 consecutive patients treated between December 2013 and March 2014 with intracavitary brachytherapy for carcinoma of cervix was selected for this dosimetric study. All fifteen patients belonged to the International Federation of Gynecology and Obstetrics (FIGO) stage II. All patients were initially treated with external beam radiotherapy to a dose of 45 Gy in 1.8 Gy per fraction. After a gap of 7–10 days this was followed by intracavitary brachytherapy to a dose of 26 Gy in four equally divided fractions over two days with a minimum interfraction duration of 6 h. CT scan compatible tandem and ovoids were inserted after spinal anaesthesia with aseptic precautions after examination under anaesthesia. Image acquisition for treatment planning was done after the insertion of applicators using axial CT scans of 3 mm slice thickness. The images were transferred to Brachytherapy planning system (HDR plus v3.0) which also has the ability to perform IPSA in addition to manual optimisation. Contouring of High Risk Clinical Target Volume (HRCTV), Intermediate risk Clinical Target Volume and OARs were according to GEC-ESTRO guidelines1 and Viswanathan et al.2 Brachytherapy was delivered using BEBIG Brachytherapy system (Eckert & Ziegler) using Cobalt-60 sources.
High dose rate (HDR) brachytherapy system became available in our department from December 2013 and all patients treated between December 2013 and March 2014 was planned using the manual optimisation technique. Initially, a uniform dwell-time of 1 s was prescribed to all activated portions of the applicator. The active dwell weights were changed by clicking the isodose line and mouse dragging it to the desired location to cover the HRCTV. Alternately, the dwell weights of those positions contributing to dose to the OARs were reduced. This was done repeatedly until a satisfactory plan was obtained with respect to HRCTV coverage and OARs. Emphasis was put on limiting the dose to the OARs (D2cc rectum: 4 Gy, D2cc bladder: 5 Gy) while trying to adequately cover the HRCTV. The dose volume parameters were noted.
To compare IPSA with manual optimisation, inverse planning was done for the same fifteen patients who were treated previously after manual optimisation. Dose constraints were set to OARs and HRCTV. IPSA was used to generate an inverse plan, which identifies the combination of dwell times that best conforms to dose constraints of HRCTV and OARs. No manual optimisation was allowed. The relative weightage and dose constraints were changed until an optimal plan was obtained that meets the dose objective parameters of both target volume and OARs.
The following dose volume parameters were compared between the two optimisation methods: (1) D100 and D90 for the minimum doses to 100% and 90% volumes of HRCTV;(2) V100, V150, V200 and V300 for the volumes of HRCTV enclosed by 100, 150, 200 and 300% of the prescribed dose; (3) volumes covered by 100% (VPD) and 200% (V2PD) of the prescription dose; (4) D2cc bladder – maximal dose received by 2cc of bladder; (5) D2cc rectum – maximal dose received by 2cc of rectum; (6) conformity index (COIN); and (7) homogeneity index (HI).
COIN and HI were calculated using the formula:
- The coefficient c1 describes how accurately the target volume is covered by a reference dose Dref, i.e. PD.
where Vref,TV is the volume of a reference dose covering the target volume and TV is the target volume. The ideal value of c1 is equal to 1. - The coefficient c2 is also a measure of how accurately the target volume is covered by the reference dose Dref. Moreover, it measures how much of normal tissue volume outside the target volume receives the reference dose Dref
The volume Vref is the volume of reference dose. - The last coefficient, c3, evaluates how much irradiation is received by OARs. It is defined as:
where VOAR is the volume of OAR and VOARcrit is the volume of OAR receiving critical dose.
These parameters were compared between the two groups using a paired t test. Wilcoxon signed rank test was used for the comparison between two groups for parameters not following normal distribution. The level of significance was set at 0.05.
4. Results
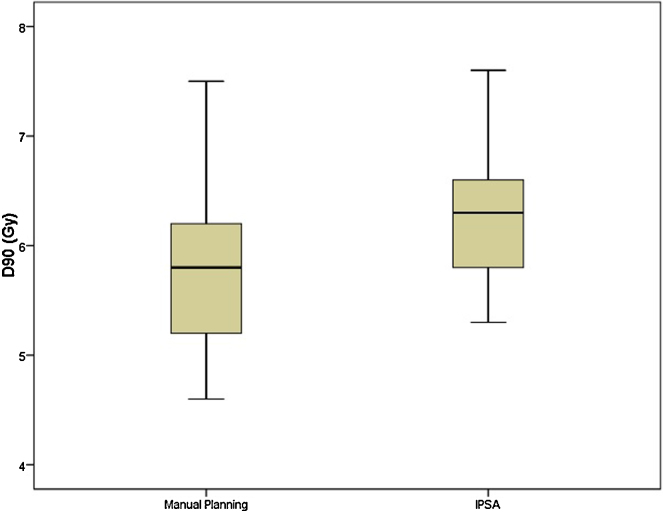
The mean volume of HRCTV was 57.9cc. The coverage of HRCTV with IPSA was significantly better than manual optimisation plans (Fig. 1; mean V100 of 87.75% and 82.37%; p = 0.001). Mean D90 dose was also significantly better with IPSA compared with manual optimisation (Fig. 2; mean D90 of 6.34 Gy and 5.86 Gy; p = 0.001). Mean volume receiving 150% of dose was 50.49% and 49.19% in IPSA and manual optimisation arm, respectively (p = 0.38). This was reflected in HI. Plans generated by IPSA showed a trend towards homogeneity when compared with manual optimisation (Fig. 3; HI of 0.42 and 0.40; p = 0.67). Also, the plans generated by IPSA were more conformal than manual planning (COIN of 0.67 and 0.6; p = 0.007). The values of V200 and V300 were similar between both arms (p = 0.460 and p = 0.865). The VPD and V2PD were also similar between both arms (p = 0.2 and p = 0.73).
Fig. 1.
Comparison of V100 between manual planning and IPSA.
Fig. 2.
Comparison of D90 between manual planning and IPSA.
Fig. 3.
Comparison of Homogeneity index between manual planning and IPSA.
The dose to the D2cc of the bladder and D2cc of the rectum were not statistically significant between the two groups (D2cc bladder mean dose – 4.58 Gy and 4.58 Gy; p = 0.54 and D2cc rectum mean dose – 3.92 Gy and 4 Gy; p = 0.96).
The planning time for IPSA was about 5 min (including changing constraints and re-evaluating the dose volume parameters to arrive at the optimal plan), whereas optimisation time for manual optimisation was about 30–45 min.
5. Discussion
Brachytherapy has been an integral component of therapy for carcinoma of cervix for over 100 years. Several studies have shown ICRU prescription points to be inaccurate estimations of bladder and rectal maximum doses, and volume based therapy recommended by GEC-ESTRO is now practised in the majority of centres.3,4 One of the key advantages with HDR brachytherapy over low dose rate brachytherapy is optimisation of doses to target volume and OARs. The aim of optimisation in cervical cancer brachytherapy is to achieve a typical pear shaped distribution with specific pattern of high dose regions with maximal target volume coverage and minimal dose to the OARs. This kind of dose distribution would result in high local control and minimal side effects.
Manual optimisation is the one which is frequently used in many institutions. Inverse planning algorithms are already implemented into external beam radiation therapy and the main benefits are the improvement in target coverage and sufficient sparing of OAR, reduction in planning time and good reproducibility. In brachytherapy, the use of IPSA is still in the infancy stage. For brachytherapy, IPSA should result in a faster and more reproducible planning process, with better target coverage and organ sparing, especially in cases where complex topography and application techniques are involved. There is a paucity of studies comparing IPSA and manual optimisation in intracavitary brachytherapy and the majority of the studies which have compared the use of IPSA in brachytherapy are for prostate cancer and interstitial gynaecological implants.5–8 In a study by Dewitt et al.,9 the authors found with the use of IPSA that there was a significant reduction in the dose to OARs with no significant improvement in target volume coverage. In our institution, where we put emphasis on limiting the dose to the OARs, the use of IPSA resulted in superior plans in terms of HRCTV coverage while achieving the dose constraints for the rectum and the bladder. Similar results were obtained in a study by Trnková et al.10 where plans generated by IPSA were superior to manual optimisation.
Limited studies are available regarding the outcomes of patients treated using IPSA. In a study done by Kim et al.,11 the locoregional control and overall survival for locally advanced cervical cancer at two years were 91% and 86%, respectively, assuring that IPSA is a safe technique even in brachytherapy. But this was a retrospective study and attempt to compare it with manual optimisation was not made.
During IPSA, caution is needed regarding the spatial distribution and volume of high dose regions to avoid unexpected high dose regions around a single applicator. When plan evaluation is based only on the parameters proposed by GEC-ESTRO recommendations, important changes at the high-dose level may be overlooked. At first sight, IPSA generated plans can look better or the same as a manually optimised plan, although high-dose regions may have changed unacceptably in form and location. But in our study, we found that IPSA plans showed a trend towards better homogeneity when compared with manual optimisation and the volumes of V150, V200, V300 were similar between the two groups.
Objective evaluation was also carried out using COIN. Though not intended for intracavitary brachytherapy, this index can serve as a relative parameter to distinguish between the two 3D plans by taking into account the coverage of HRCTV and high doses inside and outside the target volume. In the present study, plans generated using IPSA had a significantly better COIN compared to manual planning.
The volume of tissue covered by 100% (VPD) and 200% (V2PD) was found to be indicators of toxicity in some reports.12,13 In the present study, VPD and V2PD were similar between the two groups.
Another important aspect of treatment planning is the time required to create a clinically acceptable plan. As an institutional protocol, we deliver four fractions of HDR intracavitary brachytherapy over two days with a minimum interfraction time interval of 6 h. Though not objectively measured in this study, the planning time when using IPSA was considerably shorter than manual optimisation. This would be advantageous in delivering the first fraction in the morning earlier thereby leading to earlier delivery of the next fraction.
Advantage of this study is that the HRCTV coverage and doses to OARs by using IPSA were calculated for patients who were previously planned and treated using manual optimisation. This eliminates the possibility of selection bias, since proper applicator positioning is considerably more important in achieving a better coverage than optimisation.
Pitfall of this study is it being only a dosimetric study and that clinical outcomes in terms of local control and toxicity were not measured, although, theoretically, improved target volume coverage should result in a better local control.
6. Conclusion
The use of inverse planning in intracavitary brachytherapy of cervix has shown a significant improvement in the target volume coverage and conformity index when compared with manual planning, while the volume of high dose region and doses to organ at risk remained similar between the two groups. Further clinical studies are essential to conclude whether this improvement in target volume coverage would lead to improved local control.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Pötter R., Haie-Meder C., Van Limbergen E. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan A.N., Dimopoulos J., Kirisits C. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Vishwanathan A.N., Cormack R., Holloway C.L. Magnetic resonance guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys. 2006;66:91–99. doi: 10.1016/j.ijrobp.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K., Yamazaki H., Takenaka T. A dose volume analysis of magnetic resonance imaging-aided high-dose-rate image-based interstitial brachytherapy for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2009;77:765–772. doi: 10.1016/j.ijrobp.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Lessard E., Hsu I.C., Pouliot J. Inverse planning for interstitial gynecologic template brachytherapy: truly anatomy-based planning. Int J Radiat Oncol Biol Phys. 2002;54:1–9. doi: 10.1016/s0360-3016(02)03802-6. [DOI] [PubMed] [Google Scholar]
- 6.Lessard E., Pouliot J. Inverse planning anatomy-based dose optimization for HDR brachytherapy of the prostate using fast simulated annealing algorithm and dedicated objective function. Med Phys. 2001;28:773–779. doi: 10.1118/1.1368127. [DOI] [PubMed] [Google Scholar]
- 7.Lachance B., Nadeau D., Lessard E. Early clinical experience with anatomy-based inverse planning dose optimization for HDR boost of the prostate. Int J Radiat Oncol Biol Phys. 2002;54:86–100. doi: 10.1016/s0360-3016(02)02897-3. [DOI] [PubMed] [Google Scholar]
- 8.Hsu I.C., Lessard E., Weinberg V. Comparison of inverse planning simulated annealing and geometrical optimization for prostate high dose rate brachytherapy. Brachytherapy. 2004;3:147–152. doi: 10.1016/j.brachy.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Dewitt K.D., Hsu I.C., Speight J. 3D inverse treatment planning for the tandem and ovoid applicator in cervical cancer. Int J Radiat Oncol Biol Phys. 2005;63:1270–1274. doi: 10.1016/j.ijrobp.2005.07.972. [DOI] [PubMed] [Google Scholar]
- 10.Trnková P., Baltas D., Karabis A. A detailed dosimetric comparison between manual and inverse plans in HDR intracavitary/interstitial cervical cancer brachytherapy. J Contemp Brachyther. 2010;2(4):163–170. doi: 10.5114/jcb.2010.19497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D.H., Wang-Chesebro A., Weinberg V. High-dose rate brachytherapy using inverse planning simulated annealing for locoregionally advanced cervical cancer: a clinical report with 2-year follow-up. Int J Radiat Oncol Biol Phys. 2009;75:1329–1334. doi: 10.1016/j.ijrobp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Crook J.M., Esche B.A., Chaplain G. Dose-volume analysis and the prevention of radiation sequelae in cervical cancer. Radiother Oncol. 1987;8:321–332. doi: 10.1016/s0167-8140(87)80182-2. [DOI] [PubMed] [Google Scholar]
- 13.Barillot I., Horiot J., Maingon P. Impact on treatment outcome and late effects of customized treatment planning in cervix carcinomas: baseline results to compare new strategies. Int J Radiat Oncol Biol Phys. 2000;48:189–200. doi: 10.1016/s0360-3016(00)00556-3. [DOI] [PubMed] [Google Scholar]