Abstract
Local control remains a major issue for patients with unresectable, locally advanced pancreatic cancer (LAPC). The role of radiation therapy in the management of LAPC represents an area of some controversy. Stereotactic body radiotherapy is an emerging treatment option for LAPC as it can provide a therapeutic benefit with significant advantages for patients’ quality of life over standard conventional chemoradiation. The objective of this review is to present the rationale for stereotactic body radiotherapy in LAPC, as well as to discuss the potential limitations and caveats of the currently available studies.
Keywords: Pancreatic cancer, Stereotactic body radiotherapy, Local control
1. Introduction
Pancreatic cancer remains a highly lethal disease with 5-year survival rates of approximately 6%.1,2 It is the second most common gastrointestinal malignancy and the fourth leading cause of cancer related deaths in men and women of all age groups in developed countries. Despite advances in multimodality treatments, the incidence rate of pancreatic cancer still approximates its mortality rate. In 2014, the incidence of pancreatic cancer was estimated at 46,420 with over 38,000 deaths.1 Surgical resection is the only potentially curative option for managing pancreatic cancer. Unfortunately, fewer than 20% are eligible and of those, 30–50% are found to be unresectable intra-operatively.3 Patients with locally advanced, unresectable pancreatic cancer comprises a group of patients with intermediate prognosis between resectable and metastatic disease. They require non-surgical treatment and are committed to non-curative options with chemotherapy and conventional fractionated radiotherapy over 2–6 weeks. Local failure remains a major component of disease progression, which often can lead to symptoms of pain, obstruction, and other morbidities that can considerably decrease quality of life. In a recent autopsy series, it was shown that 30% of patient with pancreatic cancer died with locally destructive disease and only minimal systemic disease.4 Improved methods of controlling the primary cancer are warranted.
2. Criteria for resection and staging of pancreatic tumors
The National Comprehensive Cancer Network (NCCN) guidelines define clear criteria to describe tumor resectability so as to improve patient selection for surgery and improve the likelihood of attaining negative surgical margins.5 Following staging by CT or MRI (and EUS/ERCP in some cases), liver function tests, and chest imaging, disease is classified as: (1) resectable; (2) borderline resectable (i.e., tumors that are involved with nearby structures so as to be neither clearly resectable nor clearly unresectable); (3) locally advanced unresectable (i.e., tumors that are involved with nearby structures to an extent that renders them unresectable despite the absence of evidence of metastatic disease); or (4) disseminated (i.e., distant metastases or metastases to lymph nodes beyond the field of resection). The distinction between borderline and locally advanced designations is based on the likelihood of attaining a margin negative (R0) resection; however, this may vary depending on surgical technique and institutional practices. In fact, recent advances in surgical techniques now allow for resection of selected patients with tumors involving the superior mesenteric vein and performing venous reconstructions, thus, making what might be called a borderline tumor at one institution a resectable one in the hands of a surgeon who performs vein grafts.6 Locally advanced tumors considered to be truly unresectable demonstrate the following: greater than 180° superior mesenteric artery encasement, celiac or inferior vena cava abutment, unreconstructible superior mesenteric vein or portal vein, or aortic invasion or occlusion. In this chapter, we will focus on the use of stereotactic body radiotherapy in the setting of locally advanced pancreatic cancer (LAPC).
3. Role of radiotherapy for locally advanced pancreatic cancer
Clinical trials have demonstrated conflicting results regarding the role of radiotherapy in the treatment of LAPC (Table 1). Nonetheless, the rationale for chemoradiation treatment (CRT) for patients with LAPC is based on the role it plays as the only option for local therapy in the setting of unresectable disease. Serial studies of the Gastrointestinal Tumor Study Group (GITSG) with patients with LAPC have shown that combined-modality therapy is superior to either optimal radiotherapy or chemotherapy alone. In 1981, GITSG randomized 194 patients with histologically confirmed locally unresectable adenocarcinoma of the pancreas to therapy 60 Gy alone, to 40 Gy+ 5-fluorouracil (5-FU), and to 60 Gy plus 5-FU. Radiotherapy was delivered as a split-course of 2 weeks on 1 week off. Both 5-FU-containing treatment regimens produced a highly significant survival improvement when compared with radiation alone. The 1-year overall survival was 40% in the combined regimens group compared with 10% in the radiation-only group. Survival differences between 40 Gy plus 5-FU and 60 Gy plus 5-FU were not significant with an overall median survival of 10 months. Twenty-six percent of patients in each of the combined modality treatment arm experienced local failure plus distant metastases at first progression. Although this study is criticized for using split-course radiotherapy and for having small numbers, it had nevertheless established a role for combined modality therapy in the management of locally advanced pancreatic cancer.7
Table 1.
Prospective randomized trials on radiation therapy for unresectable LAPC – clinical outcomes of patients enrolled onto the CRT arm.
| Study | Patients in the CRT arm, n | RT dose (in Gy) | Grade 3+ GI toxicities (unless otherwise specified) | Local control | Median OS (months) |
|---|---|---|---|---|---|
| GITSG (1981)7 | 83 | Moderate-dose (40 Gy) split-course radiation + 5-FU | On patient had nonfatal gastrointestinal hemorrhage during 5-FU plus radiation therapy and one patient had lethal GI bleeding 18 days following completion of 4000 rads plus 5-FU | 26% of patients in each arm experienced local failure plus distant metastases at first progression | 10 |
| 86 | High-dose (60 Gy) split-course radiation + 5-FU | 1% in the 60 Gy + 5-FU experienced local failure only at first progression | |||
| GITSG (1988)8 | 24 | 54 Gy radiotherapy (1.8 Gy daily, 5 days/week) + SMF chemotherapy | 2 (10%) | Progression appearing first in the pancreas was noted in 10 patients. | 10 |
| ECOG (2011)9 | 34 | 50.4 Gy radiotherapy (1.8 Gy daily, 5 days/week) + gemcitabine | Grades 4 and 5 toxicities 41% | Local recurrences were at the documented first site of metastasis in four patients. | 11.1 |
| GERCOR (2008)10 | 72 | Induction chemotherapy followed by 55 Gy radiotherapy (1.8 Gy daily, 5 days/week) + 5-FU | Not assessed | Not assessed | 15 |
| FFCD/SFRO (2008)11 | 59 | 60 Gy radiotherapy (2 Gy daily, 5 days/week) + infusional 5-FU and intermittent cisplatin followed by maintenance gemcitabine | During induction: 36% During maintenance: 32% |
Not assessed | 8.6 |
| GERCOR LAP 07 (2014)12,13 | 133 | Induction gemcitabine and erlotinib followed by 54 Gy radiotherapy + capecitabine | Not available | 66% at the time of analysis. | 15.2 |
This study was followed by another GITSG prospective trial that compared the survival of patients treated with multidrug chemotherapy [streptozocin, mitomycin, and 5-fluorouracil (SMF)] versus radiation combined with 5-fluorouracil followed by the same three-drug SMF combination. This study showed an improvement in overall survival following the combined-modality treatment program (41% at 1 year) compared with SMF chemotherapy alone (19% at 1 year). The first site of progression was local in 10 patients in each arm.8
Likewise, the Eastern Cooperative Oncology Group (ECOG) 4201 study revealed an increase in overall survival with CRT. This study evaluated the role of radiation therapy with concurrent gemcitabine (arm B) compared with gemcitabine alone (arm A) in patients with LAPC. Overall survival was 9.2 months and 11.1 months in the gemcitabine-alone group and the CRT group, respectively. Overall grades 3 and 4 toxicities were common in both arms (77% versus 79%), but as expected, grades 4 and 5 toxicities were greater in arm B (41%) versus arm A (9%). One grade 5 toxicity occurred in each arm (arm A, cardiac; arm B, acute respiratory distress syndrome). Local recurrences were at the documented first site of metastasis in 11 and 4 patients in arms A and B, respectively (not statistically significant).9
A combined analysis of the Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR) trials also confirmed an increase in overall survival with CRT versus chemotherapy alone with a median overall survival of 15.0 and 11.7 months, respectively. Treatment-related toxicities and local control in the CRT arm were not assessed.10
Conversely, the Fédération Francophone de Cancérologie Digestive and Société Francophone de Radiothérapie Oncologique (FFCD-SFRO) study reported diminished overall survival and increased toxicity with CRT. In this study, patients were randomly assigned to either chemotherapy and high-dose radiotherapy group (60 Gy, 2 Gy/fraction; concomitant 5-fluorouracil infusion and cisplatin) or gemcitabine alone. Maintenance gemcitabine was given in both arms until disease progression or toxicity. This intensive combined CRT was more toxic and less effective than gemcitabine alone. Overall survival was shorter in the CRT than in the gemcitabine arm (median survival 8.6 and 13 months, respectively), likely related to the inability to deliver adequate systemic therapy in the high-dose radiotherapy group due to increased toxicity with this regimen. Local control was not assessed in this study.11
Recently, the phase III GERCOR LAP 07 study reported no significant improvement in overall survival with the addition of CRT. In this study, LAPC patients were first randomized to gemcitabine or gemcitabine plus erlotinib. Patients with controlled disease after 4 months of chemotherapy were then randomized to two additional months of chemotherapy or CRT with 54 Gy and concurrent capecitabine. The overall survival was not significantly different between the two arms (15.2 versus 16.5 months).12
A total of 238 patients were found to have progression of disease, in 96 patients (50.5%) first site of progression was locoregional and in 97 patients (49.5%) it was distant metastatic disease. In the CRT arm, patients had significantly less local tumor progression compared to the chemotherapy arm (34% versus 65%, p < 0.0001). Median time without treatment (i.e., reintroduction of chemotherapy) was longer in the CRT arm compared to the chemotherapy arm (159 versus 96 days, respectively, p = 0.05). Therefore, the conclusion was that even though the OS was not improved in the CRT arm, patients with nonprogressive LAPC after 4 months of induction chemotherapy had a longer time without treatment in the CRT arm with significantly less local tumor progression which could translate into a better quality of life.13
Given the relatively poor outcomes with conventionally fractionated chemoradiation for patients with LAPC, there has been an effort to combine more aggressive chemotherapy regimens with intensified radiotherapy techniques. Investigators from the University of Michigan conducted a phase I dose escalation trial among 37 patients with unresectable or incompletely resected pancreatic cancer to evaluate the role of radiation dose intensification.14 Radiation dose escalation was achieved by increasing the fraction size, keeping the duration of radiotherapy at 3 weeks. In this first study, the radiation fields were planned with three-dimensional radiotherapy planning, and covered only the gross target volume with a 1-cm margin (i.e., no elective nodal radiotherapy). Doses ranged from 24 to 42 Gy (1.6–2.8-Gy fractions). Standard dose gemcitabine was administered concurrently with the radiation. At the final planned dose level (42 Gy in 2.8-Gy fractions), dose-limiting gastrointestinal toxicity was noted in two of six assessable patients. With a median follow-up of 22 months, median survival was 11.6 months and local progression was noted in 7, regional progression in 3, and distant progression in 25 of 37 patients. The reduction in the field size did not appear to result in excess locoregional failure.14 Subsequently, this group reported the phase I/II study of 55 Gy in 25 fractions and demonstrated a 2-year freedom from local progression of 59% and median survival of 14.8 months.15
Given the low rate of nodal progression in the University of Michigan trial, the use of more focal radiotherapy addressing just the primary tumor was attempted. The rationale for continued study of radiation dose escalation of the gross tumor volume is underscored by the importance of preventing local tumor progression which is the cause of significant morbidity and pain for patients with LAPC. Previously, the use of focal, high-dose radiotherapy for pancreatic cancer had been hampered by both the close proximity of adjacent highly radiosensitive critical organs as well as tumor motion due to respiration. With the introduction of newer techniques to deliver focal radiotherapy more accurately and methods to account for organ motion, stereotactic body radiotherapy has been an emerging option for patients with LAPC.
4. Clinical outcomes in patients treated with SBRT
Multiple single-institution studies have looked at the role of SBRT in the setting of LAPC and suggested an overall dose–response effect and increased local control (Table 2). The first clinical report on stereotactic radiosurgery in patients with LAPC was published in 2004 from the Stanford University. Fifteen patients were enrolled on this phase II dose escalation study. Patients received a single dose of radiotherapy consisting of either 15 Gy, 20 Gy, or 25 Gy delivered using the CyberKnife Robotic radiosurgery System (Accuray, Sunnyvale, CA). No grade 3 or higher acute GI toxicity was observed. In the six evaluable patients treated with 25 Gy, the median overall survival was 8 months with a median follow-up time of 4.5 months. All of these patients had local control of their pancreatic tumors until death or at last follow-up and developed distant metastases as the site of first progression.16 The same group then conducted a phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by an SBRT boost in patients with LAPC. In this prospective study, 19 patients were treated with 45 Gy intensity modulated radiotherapy (IMRT) to the tumor and regional lymph nodes with concurrent 5-fluorouracil followed by a single dose of 25 Gy SBRT boost to the primary tumor. Sixteen patients completed the planned treatment. Local control rates were still high (94%) but the median overall survival was shorter (8.3 months) and an increased rate of acute grade 3 GI toxicity was noted (12.5%).17
Table 2.
Stereotactic body radiation therapy for locally advanced pancreatic cancer.
| Study | Patients, n | SBRT dose (in Gy) | Grade 3+ GI toxicities (unless other wise specified) | Local control at 1 year | Median OS (months) | Median follow-up (months) |
|---|---|---|---|---|---|---|
| Koong et al.16 | 15 | 15, 20, or 25 Gy × 1 | 0 | 100% | 11 | 5 |
| Koong et al.17 | 19 | 45 Gy IMRT followed by 25 Gy × 1 boost | 2 (12.5%) | 94% | 8.3 | 6 |
| Hoyer et al.18 | 22 | 15Gy × 3 | 79% acute grade 2+ | 57% | 5.4 | Not available |
| Schellenberg et al.19 | 16 | 25 Gy × 1 after induction gemcitabine + post-SBRT gemcitabine | 1 (6%) acute 2 (13%) late |
100% | 11.4 | 9.1 for all patients; 22.3 for living patients |
| Schellenberg et al.20 | 20 | 25 Gy × 1 after induction gemcitabine + post-SBRT gemcitabine | 0 acute 1 (5%) late |
94% | 11.8 | 4.3 |
| Herman et al.21 | 49 | 6.6 Gy × 5 after induction gemcitabine | 1 (2%) acute 3 (6%) late |
78% | 13.9 | 13.9 |
| Mahadevan et al.24 | 36 | 8, 10, or 12 Gy × 3 followed by adjuvant gemcitabine | 5 (14%) | 78% | 14.3 | 24 |
| Mahadevan et al.25 | 39 | 8–12 Gy × 3 after induction gemcitabine | 0 acute 3 (9%) late |
85% | 20 | 21 |
| Gurka et al.26 | 10 | 5 Gy × 5 with concurrent gemcitabine | 0 | 40% | 12.2 | Not available |
| Polistina et al.28 | 23 | 10 Gy × 3 with induction and concurrent gemcitabine, ± surgery, ± maintenance chemotherapy | 0 | 82.6% | 10.6 | 9 |
Conversely, a phase II study from Denmark showed that SBRT for LAPC is associated with poor outcome and high rates of toxicity. In this study, 22 patients were treated with 15 Gy × 3 fractions. Two patients (9%) had a partial response to CRT, while the remaining had no change or progression or progression of disease after treatment. The progression free survival after 1 year was 9% and the median overall survival was 5.4 months. With five local failures within 6 months after treatment, the actuarial local control rate was 57%. Progression to toxicity grade 2 or higher was observed in 79% of the patients. Four patients suffered from severe mucositis or ulceration of the stomach or duodenum and one of the patients had a non-fatal ulcer perforation of the stomach.18 The median volume treated by this group was 136cc, whereas the median volume treated by the Stanford group was 41cc.20 In addition, the planning target volume (PTV) was encompassed by the 67% isodose surface. This was thought to account for the differences outcomes between the Stanford and the Danish groups.
After demonstrating that a single fraction of SBRT using small margins could be delivered safely and was capable of producing local control rates higher than 90%, the Stanford group incorporated SBRT with chemotherapy using gemcitabine. In a first study published in 2008, 16 patients were included in this study and received one 3-week cycle of gemcitabine followed by SBRT delivered using the Cyberknife. Gemcitabine was resumed 2 weeks after SBRT and was continued until progression or dose limiting toxicity. The most common acute toxicities were pain and gastritis symptoms, experienced by 19% of patients (n = 3). Of these three patients, two patients had grade 2 toxicity and one patient experienced acute grade 3 gastrointestinal toxicity; late grade 3+ gastrointestinal toxicity was more common, with five ulcers (grade 2), one duodenal stenosis (grade 3), and one duodenal perforation (grade 4). Freedom from local progression was 100% at 1 year. The median time to progression was 9.7 months, and the median overall survival was 11.4 months.19 The same group published in 2011 a phase II trial looking at the toxicity, local control, and overall survival in patients treated with sequential gemcitabine and linear accelerator-based SBRT rather than the CyberKnife. Twenty patients completed SBRT and a median of five cycles of gemcitabine. No acute grade 3 or greater GI toxicity was observed. Late grade 3 or greater toxicities occurred in one patient (5%) and consisted of a duodenal perforation (grade 4).20 Three patients (15%) developed ulcers (grade 2) that were medically managed.
To reduce the late GI toxicity, the subsequent study was designed using a hypofractionated course of radiotherapy (6.6 Gy × 5 fractions) integrated with gemcitabine chemotherapy. Investigators from Stanford University, Johns Hopkins University and Memorial Sloan-Kettering Cancer center (MSKCC) conducted a phase II multi-institutional study to determine whether gemcitabine with fractionated SBRT results in equivalent freedom from local progression and acceptable late GI toxicity when compared to prior trials of gemcitabine with single-fraction SBRT in patients with LAPC. Forty-nine patients received gemcitabine followed by SBRT (33·0 Gy in 5 fractions). Results from this study show a reduced rate of grade 2+ toxicities. Of 49 patients analyzed, 1 (2%) experienced an acute grade 4 duodenal ulcer. Late GI grade ≥2 toxicities occurred in five patients. Median OS was 13·9 months and 1-year freedom from local progression was 78%.21
However, SBRT may be more effective at doses higher than 6.6 Gy per fraction. Studies by Fuks and Kolesnick have demonstrated that high-dose (>8 Gy) per fraction rapidly activates the cell membrane acid sphingomyelinase ASMase that hydrolyses sphingomyelin to generate the proapoptotic second messenger ceramide, thus initiating transmembrane signaling of apoptosis.22 Endothelial cells are 20-fold enriched in secretory ASMase compared with any other cell in the body and are particularly sensitive to radiation-induced apoptosis in vitro and in vivo via the ASMase pathway.23 High-dose radiation treatment appears to induce primarily sublethal lesions in tumor cells that become lethal due to apoptotic microvascular dysfunction. The proposed mechanism of tumor cell death related to microvascular damage may overcome the apparent radioresistance of pancreatic cancer evidenced by the poor local control with conventional RT. Based on these considerations, MSKCC is currently leading a dose escalation trial to evaluate the safety and feasibility of SBRT delivered in three fractions for patients with LAPC who have received induction FOLFIRINOX or gemcitabine/Abraxane chemotherapy. The primary objective is to determine the maximum tolerated dose of three-fraction SBRT starting at 9 Gy for patients with LAPC after 4 months of induction chemotherapy. This would allow for better integration of radiotherapy with more effective chemotherapy regimens. Other groups in the United States have also looked into the role of SBRT in the setting of LAPC. Mahadevan et al. explored the safety and effectiveness of a 3-day course of hypofractionated SBRT followed by gemcitabine in this population. Thirty-six patients were treated with 24–36 Gy in three fractions followed by 6 months of gemcitabine chemotherapy. The local control rate was 78% with low rates of grade 3+ toxicities (14%). Median survival was 14.3 months.24 In a subsequent study, the same group looked into induction gemcitabine followed by SBRT in patients with LAPC. Thirty-nine patients received gemcitabine followed by 24–36 Gy in three fractions. The local control rate was 85% and median survival 20 months. Late Grade III toxicities such as GI bleeding and obstruction were observed in 9% (3/39) of patients. No acute grade 3+ toxicity was observed.25
A more recent small study from Georgetown University investigated the safety of SBRT when integrated in a gemcitabine chemotherapy regimen. Ten patients received six cycles of gemcitabine. During week 4 of cycle 1, patients received SBRT (25 Gy delivered in five consecutive daily fractions of 5 Gy prescribed to the 75–83% isodose line. The median progression free and overall survivals were 6.8 months and 12.2 months, respectively. Local control was poor as six patients experienced local progression before death.26 An Italian study reported early clinical experience in SBRT delivered using volumetric intensity modulated arc therapy with RapidArc in patients with primary or metastatic tumors at abdominal sites. Thirty-seven patients were treated using RA. Of these, nine had pancreatic cancer. Dose prescription varied from 45 to 75 Gy to the Clinical Target Volume in 3–6 fractions. In this study, SBRT was associated with good early clinical results in terms of local control rate and acute toxicity profile.27
The same Italian group then explored the option of using neoadjuvant chemoradiotherapy (gemcitabine plus SBRT) and subsequent surgical exploration in patients with LAPC. Twenty-three patients with histologically confirmed LAPC underwent SBRT. Radiotherapy (30 Gy) was delivered in three fractions. No grade 2 or higher acute or late toxicity was observed. The overall local response rate was 82.6% (14 partial responses, 2 complete responses, and 3 had stable disease). Median survival was 10.6 months and 8% of patients became resectable.28 Numerous other published trials have also looked at the role of SBRT in the treatment of locally advanced pancreatic cancer.29–36 In Table 1, we present a selected number of studies addressing this topic.
5. Technical considerations for SBRT in pancreatic cancer
SBRT is an emerging treatment option for LAPC as it can provide significant advantages for patients’ quality of life and potential therapeutic benefit. Nonetheless, SBRT can be technically challenging as it requires a high degree of confidence in tumor location. High quality diagnostic and near real-time imaging studies are currently used for accurate treatment delivery and precise assessment of physiologic tumor motion. SBRT relies upon implantation of gold fiducial markers into the pancreatic tumors for targeting purposes. At our institution, these markers are placed under CT guidance and are radiographically visualized by kilovoltage X-rays. Because these seeds can migrate several mm in the first few days after implantation, it is important to wait approximately 5 days before performing the treatment planning scans. For each patient, a custom-made immobilization device is constructed at the simulation session to hold the body in a reproducible position during treatment. With patients in the treatment position, a PET-CT scan is performed to obtain accurate co-registration of the pancreatic tumor with the areas of hypermetabolic activity. This will allow for an accurate identification of the gross tumor volume (GTV). In addition, respiratory gating scans are used to correct for respiratory related pancreatic tumor movement. Previous fluoroscopy studies have shown that pancreatic cancers move with respiration in the inferior and superior directions.37 Respiratory gating allows for reduction in the treatment margin and the consequent radiation exposure to normal tissues. The purpose of respiratory gating is to define treatment margins to account for motion, select appropriate gate intervals for gated treatment and limit treatment-related toxicities.38 Alternatively, abdominal compression using a pneumatic abdominal compression belt developed in-house can be used to reduce respiratory motion of patients undergoing SBRT for LAPC.39
Treatment is delivered using intensity-modulated radiation therapy (IMRT), which allows selective delivery of high doses of radiation to the region of interest with steep dose gradients at the transition to adjacent normal tissues (Fig. 1). Stringent dosimetric parameters must be applied, paying close attention to the spatial arrangement of adjacent normal tissues. The Stanford group reported on the dosimetric determinants of duodenal toxicity with single-fraction SBRT. Seventy-three patients with LAPC were treated with a single fraction of 25 Gy. Dose–volume histogram endpoints evaluated include V5 (volume of duodenum that received 5 Gy), V10, V15, V20, V25, and Dmax (maximum dose to 1 cm3). Normal tissue complication probability (NTCP) was evaluated with a Lyman model. Keeping V15 < 9.1 cm3, or V20 < 3.3 cm3, reduced the 12-month toxicity rate from 52% to 11%. Dmax also correlated significantly with duodenal toxicity. With Dmax < 23 Gy, the 12-month toxicity rate decreased from 49% to 12% (p = 0.004). The PTV did not correlate with duodenal toxicity.40 At our institution, when treating to 33 Gy given in five consecutive fractions (6.6 Gy per fraction), we typically use the following dose constraints to normal structures: Proximal duodenum, stomach, small bowel: 9cc < 15 Gy, 33cc < 20 Gy, 1cc < 33 Gy; liver: 50% < 12 Gy; combined kidneys: 75% < 12 Gy and spinal cord: 1cc < 8 Gy.41 The majority of the tumors that we treat are less than 75cc. Assessing response after SBRT is particularly difficult because of post-therapy inflammatory changes that can obscure the interpretation of tumor size by conventional CT scans. Metabolic imaging with FDG-Pet before and after treatment is a complementary method for determining response to radiation therapy.
Fig. 1.
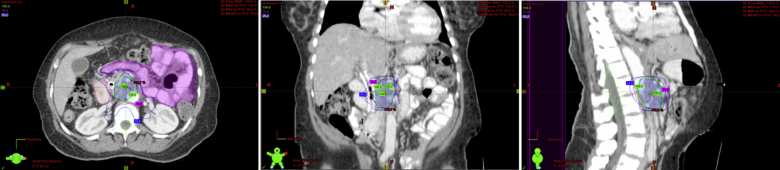
Representative axial, coronal and sagittal views of isodose lines.
6. Conclusion and future directions
The local-regional effects of the spread of pancreatic cancer to adjacent organs and to regional lymphatics often produce much of the morbidity and mortality related to the progression of disease. While palliative surgical bypass can alleviate the obstructive symptoms and jaundice related to biliary ductal involvement, local progression of the tumor in the retroperitoneum is accountable for severe pain and deterioration of quality of life. Clinical trials have demonstrated conflicting results in terms of improvement in overall survival with conventional CRT in the setting of LAPC. In addition, most prospective randomized trials of locally advanced pancreatic cancer have reported relatively low local control rates. Nonetheless, it was recently suggested that locoregional treatment with CRT can improve local control which could translate into a better quality of life. Most locally advanced diseases cannot be satisfactorily controlled by a conventional course of fractionated radiotherapy. SBRT takes advantage of the technologic advancements in image guidance and radiation dose delivery to direct ablative doses to tumors with acceptable toxicity that was not previously achievable with conventional techniques. SBRT s particularly appealing for LAPC, as it may deliver a potentially more effective, focally ablative therapy over a short period; versus 5–6 weeks of conventional RT. Radiation dose-escalation is critical in this setting as it can help prevent local tumor progression. This innovative therapy has potential to improve quality of life in patients with poor outcome, by controlling their disease locally. SBRT however failed to show an improvement in overall survival as distant metastases still account for the vast majority of disease-related mortality. SBRT can be easily integrated into a regimen of aggressive chemotherapy, preventing unnecessary delays or discontinuation of effective chemotherapy regimens during more conventionally fractionated radiotherapy. The ultimate goal of SBRT would be conversion to resectability and hope for cure in patients who are enrolled on a non-curative treatment regimen as they are not amenable to surgery. Moreover, additional multi-institutional studies are warranted to establish the role of SBRT as a comparable noninvasive alternative to surgery. To date, there is no established standard for the SBRT dose and fractionation schedule for LAPC. Toxicity is a major problem because of the proximity of the pancreas to the duodenum and the stomach. Most commonly reported toxicities include enteritis, gastritis, ulcer, or fistula. Careful selection of cases is essential to achieve disease control and to minimize treatment related acute and late toxicity. Prospective studies for dose escalation and possible use of radiosensitizers are essential to determine optimum radiation schedules and to integrate the use with systemic chemotherapy programs. Finally, better defining the genomic landscape of pancreatic cancer and identifying targetable oncogenes will likely prove to be the most promising advance in the management of this disease. Recently, patterns of chromosomal structural variation were used to classify pancreatic ductal adenocarcinoma into four subtypes with possible clinical utility: stable, locally rearranged, scattered and unstable.42 Several studies have also suggested that the tumor suppressor gene DPC4 (SMAD-4) is correlated with patterns of failure and can be used as a potential prognostic biomarker, identifying patients with more locally destructive (SMAD-4 intact) versus metastatic (SMAD4 mutated) phenotypes. SMAD-4 status has also been correlated with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. The impact of SMAD-4 status in patients with locally advanced pancreatic cancer is being evaluated prospectively in an ongoing cooperative group trial, Radiation Therapy Oncology Group (RTOG) 1201 in which patients will be stratified by SMAD-4 status.43
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Carla Hajj, Email: hajjc@mskcc.org.
Karyn A. Goodman, Email: goodmank@mskcc.org.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(January–February (1)):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ma J., Siegel R., Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. J Natl Cancer Inst. 2013;105(November (22)):1694–1700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 3.Kelsen D.P., Portenoy R., Thaler H., Tao Y., Brennan M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122(1):53–59. doi: 10.1016/s0039-6060(97)90264-6. [DOI] [PubMed] [Google Scholar]
- 4.Iacobuzio-Donahue C.A., Fu B., Yachida S. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tempero M.A., Malafa M.P., Behrman S.W. Pancreatic adenocarcinoma, version 2.2014. J Natl Compr Cancer Netw. 2014;12(August (8)):1083–1093. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 6.Wayne J.D., Abdalla E.K., Wolff R.A. Localized adenocarcinoma of the pancreas: the rationale for preoperative chemoradiation. Oncologist. 2002;7:34–45. doi: 10.1634/theoncologist.7-1-34. [DOI] [PubMed] [Google Scholar]
- 7.Moertel C.G., Frytak S., Hahn R.G. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer. 1981;48(October (8)):1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 9.Loehrer P.J., Sr., Feng Y., Cardenes H. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huguet F., André T., Hammel P. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 11.Chauffert B., Mornex F., Bonnetain F. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Ng J., Allendorf J., Saif M.W. Locally advanced pancreatic adenocarcinoma: are we making progress? Highlights from the “2011 ASCO Annual Meeting”. Chicago, IL, USA; June 3–7, 2011. JOP. 2011;12(4):347–350. [PubMed] [Google Scholar]
- 13.Huguet F., Hammel P., Vernerey D. Abstract. ASCO annual meeting. 2014. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. [Google Scholar]
- 14.McGinn C.J., Zalupski M.M., Shureiqi I. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19(22):4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Josef E., Schipper M., Francis I.R. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDRG) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84(December (5)):1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koong A.C., Le Q.T., Ho A. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Koong A.C., Christofferson E., Le Q.T. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer M., Roed H., Sengelov L. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Schellenberg D., Goodman K.A., Lee F. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [Epub 2008 Apr 18] [DOI] [PubMed] [Google Scholar]
- 20.Schellenberg D., Kim J., Christman-Skieller C. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Herman J., Chang D.T., Goodman K.A. Phase II multi-institutional trial evaluating gemcitabine and stereotactic body radiation therapy for locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolesnick R., Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 23.Fuks Z., Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Mahadevan A., Jain S., Goldstein M. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [Epub 2010 Feb 18] [DOI] [PubMed] [Google Scholar]
- 25.Mahadevan A., Miksad R., Goldstein M. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(November (4)):e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [Epub 2011 Jun 12] [DOI] [PubMed] [Google Scholar]
- 26.Gurka M.K., Collins S.P., Slack R. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8(March):44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scorsetti M., Bignardi M., Alongi F. Stereotactic body radiation therapy for abdominal targets using volumetric intensity modulated arc therapy with RapidArc: feasibility and clinical preliminary results. Acta Oncol. 2011;50(May (4)):528–538. doi: 10.3109/0284186X.2011.558522. [Epub 2011 Feb 21] [DOI] [PubMed] [Google Scholar]
- 28.Polistina F., Costantin G., Casamassima F. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(August (8)):2092–2101. doi: 10.1245/s10434-010-1019-y. [Epub 2010 Mar 12] [DOI] [PubMed] [Google Scholar]
- 29.Chang D.T., Schellenberg D., Shen J. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 30.Didolkar M.S., Coleman C.W., Brenner M.J. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547–1559. doi: 10.1007/s11605-010-1323-7. [DOI] [PubMed] [Google Scholar]
- 31.Rwigema J.C., Heron D.E., Parikh S.D. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer. 2012;43:70–76. doi: 10.1007/s12029-010-9203-7. [DOI] [PubMed] [Google Scholar]
- 32.Rwigema J.C., Parikh S.D., Heron D.E. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 33.Chuong M.D., Springett G.M., Freilich J.M. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]; Tozzi A., Comito T., Alongi F. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyal K., Einstein D., Ibarra R.A. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174:319–325. doi: 10.1016/j.jss.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scorsetti M., Bignardi M., Alongi F. Stereotactic body radiation therapy for abdominal targets using volumetric intensity modulated arc therapy with rapid arc: feasibility and clinical preliminary results. Acta Oncol. 2011;50:528–538. doi: 10.3109/0284186X.2011.558522. [DOI] [PubMed] [Google Scholar]
- 36.Lominska C.E., Unger K., Nasr N.M. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol. 2012;7:74. doi: 10.1186/1748-717X-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy M.J., Martin D., Whyte R. The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002;53(2):475–482. doi: 10.1016/s0360-3016(01)02822-x. [DOI] [PubMed] [Google Scholar]
- 38.Santoro J.P., Yorke E., Goodman K.A. From phase-based to displacement-based gating: a software tool to facilitate respiration-gated radiation treatment. J Appl Clin Med Phys. 2009;10(October (4)):2982. doi: 10.1120/jacmp.v10i4.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovelock D.M., Zatcky J., Goodman K. The effectiveness of a pneumatic compression belt in reducing respiratory motion of abdominal tumors in patients undergoing stereotactic body radiotherapy. Technol Cancer Res Treat. 2014;13(June (3)):259–267. doi: 10.7785/tcrt.2012.500379. [Epub 2013 Nov 4] [DOI] [PubMed] [Google Scholar]
- 40.Murphy J.D., Christman-Skieller C., Kim J. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(December (5)):1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [Epub 2010 Apr 14] [DOI] [PubMed] [Google Scholar]
- 41.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(April (7)):1128–1137. doi: 10.1002/cncr.29161. [Epub 2014 Dec 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waddell N., Pajic M., Patch A.M. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(February (7540)):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman J.M., Fan K.Y., Wild A.T. Correlation of Smad4 status with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2013;87(November (3)):458–459. doi: 10.1016/j.ijrobp.2013.06.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]


