Abstract
Treatment delays in completing radiotherapy (RT) for many neoplasms are a major problem affecting treatment outcome, as increasingly shown in the literature. Overall treatment time (OTT) could be a critical predictor of local tumor control and/or survival. In an attempt to establish a protocol for managing delays during RT, especially for heavily overloaded units, we have extensively reviewed the available literature on head and neck cancer. We confirmed a large deleterious effect of prolonged OTT on both local control and survival of these patients.
Keywords: Radiotherapy, Overall treatment time, Delays, Local control, Survival
1. Introduction
The time factor is a key element in radiation oncology.1 Furthermore, the importance of delays during a course of radiotherapy (RT) has been emphasized in recent decades, and different recommendations on the delay-compensation options have been published.2–5 Fast tumor cell repopulation has been suggested as the main reason why prolonging overall treatment time (OTT) negatively affects local control (LC) and overall survival (OS) in many human tumors.6 However, there is still a considerable lack of high-level evidence supporting this observation.
The aim of the present review is both to provide an insight into the problem and to establish an evidence-based protocol to practically address delays in OTT in RT, especially in centers with a considerable workload. To do so, first, we conducted an extensive bibliographic search for articles with the highest level of evidence available. Next, we developed a protocol focusing on different tumor sites and histological groups depending on the observed level of evidence. By summarizing the available evidence, we tried to identify which tumors and situations need to be prioritized for further compensation, so that RT units with excessive workload can at least compensate in the most urgent cases.
The present work focuses exclusively on head and neck cancer (H&NC), by far the most extensively studied tumor site.
2. Materials and methods
An extensive bibliographic search was undertaken focusing on the possible relationship between prolongation of OTT and loco-regional control (LRC) or overall survival (OS) in H&NC. In this sense, we consider ‘Significant’ any study showing a significant adverse relationship between prolongation of OTT and LRC and/or OS, even if the relationship is for LC only, but not for regional control or vice versa, or if it includes survival types other than OS. If no significant adverse relationship is seen, the report is considered ‘Non-significant’ (NS).
We searched several databases, including MEDLINE, using free terms/MESH terms in the Title/Abstract which included “tumoral repopulation”, “radiotherapy delays”, “treatment interruptions”, “overall treatment time”, “time factor”, “compensation maneuvers”, and “time relationship”. After identifying original, full-text articles, we also searched their reference lists. In an attempt to avoid time period bias (e.g., old techniques such as orthovoltage, inadequate doses, and pre-CT era of treatment planning), we arbitrarily chose 1980 as the earliest valid date. We included, however, only one article addressing orthovoltage,7 since it was one of the most cited works in the search.
Assuming that most of the information included was retrospective, we paid special attention to the quality of the reported data by carefully dividing the articles into those based on univariate analysis (UN-An) or multivariate (MV-An) analysis. Thus, we categorized each article as MV-An when this was explicitly stated by the authors or determined unequivocally from the data. Any other situation was categorized as UN-An. When only the abstract was available, we generally considered these cases as UN-An.
Anatomical subsites were considered ‘mixed’ when they included at least two predominant subsites. Stage was classed as stage I–II, III–IV, or mixed. However, in a series where a subsite or stage was clearly marginal with respect to sample size, we included it in the most representative group.
Some authors have calculated the loss of LRC in terms of a percentage lost per day or week, often indicating the median or mean/average ± the range. To simplify the matter, we considered only the median or mean/average values. In addition, Fowler et al.8 calculated the percentage LCR lost per day or week when the authors of original papers had not done so, and recalculated it from authors that had done so. We included both calculated and recalculated values, the latter indicated as the original author with the added term “as in Fowler ‘92”.
Some authors estimated the required daily dose increase to offset the loss of LRC resulting from delays in RT (again, with a median or mean/average value ± range), although the approaches used were very heterogeneous, as follows:
-
1.
The raw ‘γ/α’ factor, applicable when using very small fractions.
-
2.
Dprolif, the tradeoff dose per day when a fraction size of 2 Gy (or other) is used.
-
3.
The most heterogeneous group, including different definitions of the previous two, as ‘dose lost’, ‘extra dose’, ‘significant repopulation more than…’, ‘mean time factor’, ‘proliferation rate’, and many others (for simplicity, grouped under the term ‘k factor’)
Undoubtedly, since the third form refers to one of the first two, without specifying exactly which one, we preferred to group them together, even at the risk of overlap.
Similarly, Withers et al.9 calculated the average ‘k factor’ from data sets when the original studies had not provided it and recalculated it from studies which had previously calculated it. Again, we included all the available calculated values, and, as above, indicated the author of the original paper with the added term “as in Withers ‘88”.
While investigating whether prolonged OTT negatively affected both LRC and OS in patients receiving RT for H&NC, we partially set aside those reports that show what happens when OTT is shortened, since this was not envisaged as the main objective of this study. Therefore, articles about accelerated fractionation (AC-Fx) schemes were not included in the present work, unless when within the previously mentioned search strategy limits. Such articles were taken into account for comparison purposes and to ‘complete the picture’ in Section 4.
3. Results
Of sixty-five articles considered eligible for this study, 58 contained original data and 4 were pooled-data analyses of previously published series. The other three articles comprised an editorial, a commentary, and a literature review. Therefore, our study is based on 62 studies with a median of 465 patients per article (range, 42–4668). The larynx was the most frequently reported anatomical site (26/62, 41.9%), followed by mixed sites (e.g. two or more different sites; 24/62, 38.7%).
While most reports are from retrospective series, 12 reports included data from prospective studies. Except for two AC-Fx reports, all of them are Significant. The 4 pooled-data reports are also Significant.
Table 1 shows the main results of the review. Practically all the subgroups are Significant, both in terms of LRC and OS. However, reports of AC-Fx seem to worsen the results. In terms of LRC, the worsening is only about 2%, but the difference in OS exceeds 20%, because five out of six OS-NS reports correspond to AC-Fx schemes.
Table 1.
Loco-regional control and survival by type of analyses and other main factors.
| Anatomical site | Stage | Treatment intention | Significant-NSe/no. of total reports [references] | LRCa [references] |
SVb [references] |
Comments [references] | ||
|---|---|---|---|---|---|---|---|---|
| Mv-Anc | Un-And | Mv-Anc | Un-And | |||||
| Larynx | I–II | Radical | Significant: 9/12 [10–18] NS: 3/12 [19–21] |
Significant: 7/9 [10–14,16,18] NS: 2/9 [20,21] |
Significant: 2/3 [15,17] NS: 1/3 [19] |
Significant: 2/3 [14,16] NS: 1/3 [21] |
NS: 1/1 [19] | LRCa, p = 0.08 for stage I [19] Not significant for OSf or DFSg [21] |
| III–IV | Radical | Significant: 1/1 [7] | Significant: 1/1 [7] | |||||
| Mixed stages | Radical | Significant: 12/13 [22–33] NS: 1/13 [34] |
Significant: 7/8 [22,23,27–30,32] NS: 1/8 [34] |
Significant: 5/5 [24–26,31,33] | Significant: 1/1 [28] | Significant: 1/1 [24] | Larynx and hypopharynx ± larynx. Included here for majority of larynx patients [23] LRCa, p = 0.08 [34] |
|
| Total larynx | Significant: 22/26 (84.6%) | Significant: 14/17 (82.3%) | Significant: 8/9 (88.9%) | Significant: 3/4 (75%) | Significant: 1/2 (50%) | Total Significant LRCa: 22/26 (84.6%) Total Significant SVb: 4/6 (66.7%) |
||
| Mixed location | III–IV | Radical | Significant: 3/3 [35–37] | Significant: 2/2 [35,37] | Significant: 1/1 [36] | NS: 1/1 [36] | Hyperfractionation and Accelerat. Concom. boost fractionat. arms: trend toward improved DFSg (p = 0.067 and p = 0.054, respect.). OSf NSe [36] | |
| Adjuvant | Significant: 1/2 [38] NS: 1/2 [39] |
Significant: 1/1 [38] | NS: 1/1 [39] | NS: 1/1 [38] | NS: 1/1 [39] | Tpot showed correlation with DFSg (↓ 14%), but not in MV-Anc [38] High-risk patients, Acceler. Fraction. trends for > LRCa (p = 0.11) and for > OSf (p = 0.08) [39] |
||
| Mixed stages | Radical | Significant: 13/13 [8,9,40–50] | Significant: 5/5 [43,44,47,48,50] | Significant: 7/7 [8,9,40–42,45,49] | Significant: 4/5 [43,44,46,50] NS: 1/6 [48] |
Significant: 1/1 [40] | LRCa Significant in Ni+ (p = 0.07–0.08 in Tj3-4). OSf Significant in Stage III-IV [40] LRCa Significant in advan. stages. CSSh, p = 0.06 [48] Significant for LRCa (any T, not Ni), preservation of voice and CSSh. OSf not improved [50] |
|
| Adjuvant | Significant: 2/3 [51,52] NS: 1/3 [53] |
Significant: 2/2 [51,52] | NS: 1/1 [53] | Significant: 1/1 [51] | ||||
| Mixed | Significant: 2/2 [54,55] | Significant: 1/1 [55] | Significant: 1/1 [54] | SEER–Medicare linked database. OSf Significant for laryngeal cancer, trend for salivary gland (p = 0.06). Remaining locations not significant, ‘attributable to smaller sample sizes’ [55] | ||||
| Total mixed location | Significant: 21/23 (91.3%) | Significant: 10/10 (100%) | Significant: 8/10 (80%) | Significant: 6/8 (75%) | Significant: 2/4 (50%) | Total Significant LRCa: 18/20 (90%) Total Significant SVb: 8/12 (66.7%) |
||
| Naso-pharynx | Mixed stages | Radical | Significant: 2/3 (66.6%) [56,57] NS: 1/3 [58] |
Significant: 1/2 (50%) [57] NS: 1/2 [58] |
Significant: 1/1 (100%) [56] | Significant: 1/1 (100%) [57] | Nodal control, p = 0.06 [58] | |
| Oro-pharynx | Mixed stages | Radical | Significant: 7/7 (100%) [59–65] | Significant: 6/6 (100%) [60–65] | Significant: 1/1 (100%) [59] | Significant: 1/1 (100%) [65] | Significant: 1/1 (100%) [63] | |
| Oral cavity | I–II | Radical | Significant: 1/1 (100%) [66] | Significant: 1/1 (100%) [66] | ||||
| Mixed stages | Adjuvant | NS: 1/1 (100%) [67] | NS: 1/1 (100%) [67] | LRCa for OTTk <52 days (R0k, p = 0.11), < 46 days (R1l, p = 0.18) [67] | ||||
| Total | Significant: 53/61 (86.9%) | Significant: 31/35 (88.6%) | Significant: 19/23 (82.6%) | Significant: 11/14 (78.6%) | Significant: 4/7 (57.1%) | Total Significant LRCa: 50/58 (86.2%) Total Significant SVb: 15/21 (71.4%)* |
||
| Total without AFx | Significant: 48/54 (88.9%) | Significant: 28/31 (90.3%) | Significant: 18/21 (85.7%) | Significant: 10/10 (100%) | Significant: 4/5 (80%) | Total Significant LRCa: 47/53 (88.7%) Total Significant SVb: 14/15 (93.3%)* |
||
LCR, loco-regional control.
SV, survival (any kind, see Section 2).
Mv-An, multivariate analysis.
Un-An, univariate analysis.
NS, not significant.
OS, overall survival.
DFS, disease-free survival.
CSS, cancer-specific survival.
N, N-stage.
T, T-stage.
OTT, overall treatment time. kR0, complete surgical resection.
R1, incomplete surgical resection (microscopic disease).
Mostly referred to OS.
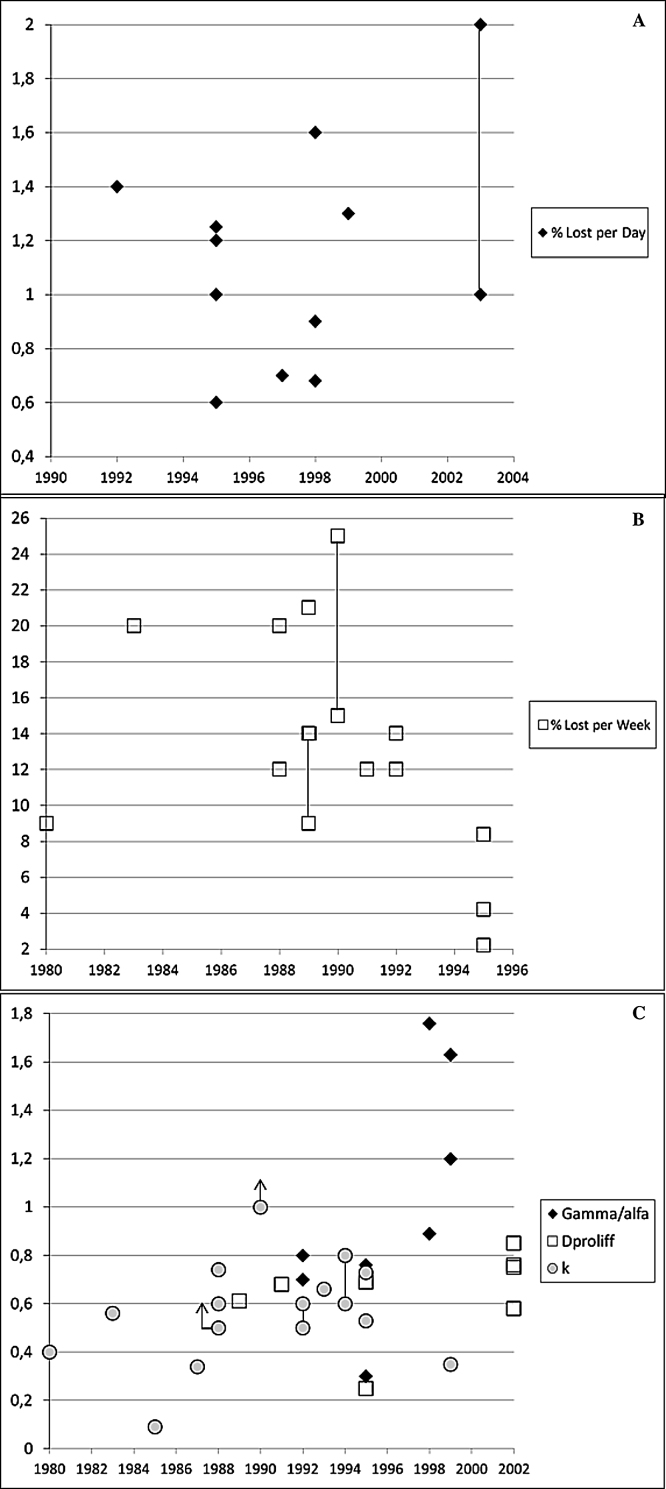
Table 2 details the daily and weekly loss of LRC, OS, and the dose/time factor; it also outlines a series of conclusions and recommendations summarized from the literature, as well as other relevant details (discussed below). These data were used to construct Fig. 1 using the horizontal axis as the date of publication of the original article (even when recalculated by others [see Table 2]). The figure shows a wide dispersion of values, with an average loss of LRC that ranged from 1–1.2% per day to 12–14% per week. An average dose/time factor of approximately 0.6–0.8 Gy/day can also be set, taking into account the different forms of the factor itself (as outlined in the previous section). An average lag can be established at 3–4 weeks.
Table 2.
| Anatomical site | Loss of LRCa [references] | Loss of survival | Time factor (Gy/d) and lag (Tkc, weeks) | Comments |
|---|---|---|---|---|
| Mixed Location | Daily (%) 1–2 [52] Weekly (%) 9 (as in Fowler’92) [40]; 12 (as in Fowler’92) [43]; 14 [8]; 21 (as in Fowler’92) [51]; 15–25 (as in Fowler’92) [42] |
3-year OSd: ↓ 9–13% [43,44] 5-year OSd: ↓ 10–43% [40,46,51,54] 5-year CSSe: ↓ 20% [51] ↓ 30% median OSd in larynx (19% for salivary gland, p = 0.06) [55] 5-year DSSf: ↓7% [50] (*) |
γ/α2Gy (Dprolif)g: 0.612 [41]; 0.58 (during gap, ≈0.76; during days with RT, 0.2; during weeks 3–4, 0.85; and > 4 weeks, 0.75) [49] Kh: 0.6, >1, 0.66 [9,42,45] Tkc: 4 wks ± 1 [9]; lag not possible < 2 weeks [41]; lag 29 days [45]; ≥2 weeks [49] |
LRCa: Try to maintain OTTk < 45–50 days [35,41]. In any case, avoid OTTk ≥60 days (LRCa and/or OSd) [37,43] Delays ≥5 days must be avoided (LRCa and/or OSd) [44,46,52] No prognostic significance: No. of consecutive treatment days missed [43] and gap position [44,52]. In adjuvant radiotherapy, may be significant early gaps in R0l and late gaps in R1m pts and total n° of gaps in both [52]. No adverse effect if break in the first 3 weeks [54] TCPn significantly correlated with OTTk and gap duration [49] Impossible to establish if weekends scored as gaps is important [49] Induction chemo not negate to avoid interruptions [46] OTTk protraction significantly affected LRCa only in G1-2 tumors [47], especially in laryngeal subgroup [50] (*) ↓ 12% preservation of voice in laryngeal cancer [50] |
| Larynx | Daily (%) 0.68 [29]; 0.9/1.6 (*) [28]; 1.2 (*) [23]; 1.3 [17]; 1.4 (as in Cox’92) [22] Weekly (%) 2.2 [32]; 8.4 (*) [23]; 12 (as in Fowler’92) [26]; 12 (as in Fowler’92) [22]; 20 (as in Fowler’92) [7]; 5–12 TCP [15] |
5-year OSd: ↓ 33% [16] 5-year DSSf: ↓ 17% [16] 5-year DFPo: ↓ 15–20% [28] |
γ/αi: 0.7 (adjusted for stage and subsite: 0.8) [22]; 0.76 [23]; 0.89 [29]; 1.2/1.63 (***) [27]; 1.76/2.69 (**) [28] γ/α2Gy (Dprolif)g: 0.69 [23] γ/α2.5Gy (Dprolif)j: 0.64 (adjusted for stage and subsite: 0.73) [22] Kh: 0.35 [17]; 0.4 [0.74 as in Withers’88) [19]; 0.56 (as in Withers’88) [7]; >0.5 [26]; 0.5–0.6 [31]; 0.6–0.8 [25]; 0.8 [15] Tkc: Lag ≤ 3 weeks [31]; 20 days [25]; 32/34 days (***) [27] |
LRCa in T1-2N0 glottis cancer: Try to maintain OTTk < 40–47 days [10,16–18]. In any case, avoid OTTk > 60 days [33]. LRCa in any other site: Try to maintain OTTk < 45–50 days [7,11,33] For OTTk schemes ≤4 weeks, try to avoid delays > 4 days [24] A single gap is potentially damaging for LRCa, especially for T1N0 tumors [28]. It may be related to gap position (in the first 19 days of radiotherapy or >29 days, but not between [30]; >28 days in T2N0 [12]) or maybe not: Any gap results in the same amount of OTTk prolongation, and, hence, OTTk is more important than the position of the gap [28,29]. In any case, avoid gaps ≥3 days for LRCa and OSd [12,28] (*) ↓ 0.6%/day and 4.2%/week LRCa in hypopharynx ± larynx patients. γ/αi for hypopharynx, 0.3 Gy/d (Dproliff, 0.25 Gy/d) [23] (**) ↓ LCp 0.9%/day for OTTk extension as a whole and 1.6% for gaps ≥ 3 days; γ/αi, 1.76 or 2.69, depending on the mathematical model used [28] (***) γ/αi 1.2 or 1.63, and Tkc, 32 or 34 days, when 4 or 3 centers, respectively, are analyzed [27] The hazard rate for LCRa failure ↑ 0.067%/day of interruption [18] One NSq study showed a ↓ 12%/week LRCa (as in Fowler’92) [19] NSq lag phase; however, best estimate of Tkc is 21 days [15] |
| Nasopharynx | The hazard rate for DFSr failure ↑ 2.9%/day of interruption [57] | Kh: 0.09 (as in Withers’88) [56] | An OTTk delay ≥ 3 weeks is significantly deleterious [56] The position of the gap (beginning or end) is NSq[57] The hazard rate for LCRa failure ↑ 3.3%/day of interruption [57] LRCa: One NSq study showed a ↓ 5.9%/day [58] |
|
| Oropharynx | Daily (%) 0.7 [65]; 1/1.25 (*) [64]; 1 TCP [62] Weekly (%) 14 (as in Fowler’92) [61]; 20 (as in Fowler’92) [60] |
OSd: ↓ 17% [63] 5-y OSd: ↓ 19–21% [65] |
γ/α2Gy (Dprolif)g: 0.68 [62] Kh: 0.34 (as in Withers’88) [59]; 0.53/0.73(*) [64] |
For LRCa, avoid OTTk > 50–55 days [61–63] If ERTs + BTt, avoid OTTk >7 weeks and interval RTEs-BTt > 20 days for LRCa and 5-years OSd [65] G-1 tumors may perform worse than G-2/3 tumors [61,62] (*) LRCa lost of 1% or 1.25% per day for T2N+ or T3N+ tumors, respectively. Kh: 0.53 or 0.73, for an assumed non-significant lag of 30 days or without lag, respectively [64] |
| Oral cavity | Weekly (%) 9–14 (as in Fowler’92) [66] |
Try to keep OTTk ≤40 days [66] | ||
LRC, loco-regional control.
SV, survival (any kind, see Section 2).
Tk, time-lag to kick off accelerated proliferation of tumor clonogens.
OS, overall survival.
CSS, cancer-specific survival.
DSS, disease-specific survival.
γ/α2Gy (Dprolif). (See Section 2).
K. (See Section 2).
γ/α. (See Section 2).
γ/α2.5Gy (Dprolif). (See Section 2).
OTT, overall treatment time.
R0, complete surgical resection.
R1, incomplete surgical resection (microscopic disease).
TCP, tumoral control probability.
DFP: disease-free progression.
LC: Local control.
NS: non significant.
DFS: disease-free survival.
ERT, external radiotherapy.
BT, brachytherapy.
Fig. 1.
(Panel A) Daily loss of LRC (%). (Panel B) Weekly loss of LRC (%). One NS report showed a loss in LRC of 12% per week (Budihna,19 as in Fowler '92), not included in the figure. (Panel C) Time factor. From this figure, one value from Table 2 has been removed, γ/α: 2·69 Gy/d, considered to be unrealistic by the author.28 (Panels A, B, and C) A line connecting two symbols represents a range between both symbols, rather than a single value, as published by corresponding authors (see Table 2). (Panel C) A symbol with an up-arrow represents a value ‘greater than’ that indicated by the symbol, as published by the corresponding authors (see Table 2).
Table 3 summarizes the 58 articles with original data divided into three groups according to the RT scheme applied. There are 8 AC-Fx articles, while the continuous hyperfractionated accelerated radiation therapy (CHART) trial from Dische et al.68 was removed from all tables because of its extremely accelerated scheme. Total dose reduction was almost 20% with respect to conventional fractionation and OTT was less than two weeks. There were no statistically significant differences between the arms with respect to LCR or OS, although a non-significant trend toward improved LC was observed, with an estimated 12% reduction in the risk of recurrence (more pronounced in laryngeal cancer). This trend was interpreted by the authors as evidence that avoiding accelerated tumor repopulation when using such a shortened OTT counterbalances the reduction in total dose. Therefore, we believe the CHART trial cannot be considered Significant or NS and excluded it from the analysis.
Table 3.
Results according to the radiotherapy scheme used.
| RTa scheme (number of papers) | Significant–non-significantb/total no. of reports [references] |
Notes [references] |
|---|---|---|
| Split-course (11) | Significant: 9/11 (81.8%) [7,26,32,40,47,51,57,59,62] NS: 2/11 (18.2%) [19,34] |
All reports have mixed split-course and continuous RTa patients, except [26], in which patients are treated only with split-course and then compared with historical continuous RTa series. Data from previous randomized trials DAHANCA 2 and 5 (Phase-III trials, both) [47] |
| Accelerated fractionation (7) | Significant: 5/7 (71.4%) [36,38,48,50,60] NS: 2/7 (28.6%) [21,39] |
Simple randomization (70 pts) [38] All Phase-III trials [21,36,39,48,50] |
| Other than before* (39) | Significant: 39/43 (90.7%) [8–18,22–25,27–31,33,35,37,41–46,49,52,54–56,61,63–66] NS: 4/43 (9.3%) [20,53,58,67] |
Data from RTOG 79-13 and 79-15 (both Phase III, hyperfraction.) [43] Data from RTOG 83-13 (randomized Phase I-II trial, hyperfraction). [44] Data from multicenter prospective randomized clinical trials [15] Data from 2 randomized trials from the British Institute of Radiology fractionation study [23] Prospective non-randomized single-arm study [46] SEERc – Medicare linked database analysis [55] Pooled-data reports [8,9,27,45] |
RT, radiotherapy
NS, non-significant.
SEER: Surveillance, Epidemiology, and End Results.
Mostly maintain the overall treatment time within limits that can be considered standard.
4. Discussion
To the best of our knowledge, this is the largest and most comprehensive bibliographic review to date on negative outcome of H&NC in patients treated with RT when OTT is prolonged. We found only a few published reviews in our search,2–5,69 which all seem to be quite less extensive than ours.
The current explanation is based on accelerated proliferation of clonogenic tumor cells during the course of RT as a response to therapy-induced injury and subsequent tumor debulking. This may be of special concern in rapidly repopulating tumors, that is, in those with high labeling indexes (LI) and effective doubling times of up to five days.6,70,71 Other existing hypothesis include repair aspects.72
Withers et al.9 provided the first clinical evidence that if the OTT was prolonged, the observed LRC loss would have required an average increase of 0.6 Gy/day to compensate for it. In addition, there was a lag of about 4 ± 1 weeks for the hypothetical accelerated repopulation to be triggered. The review by Fowler et al.8 revealed a median value of 14% of LRC loss per week of extra overall time.
In the present review (Table 1), a strong negative relationship between OTT prolongation and LRC and/or OS is shown. Most of the reports performed a MV_An and their results were consistently Significant in practically all the subgroups analyzed; most articles that included data from prospective or pooled-data studies were also Significant.
Fig. 1 shows an average loss in LRC that ranged from 1–1.2% per day to 12–14% per week, while dose/time factor ranged between 0.5 and 0.9 Gy/day and with greater accuracy of 0.6–0.8 Gy/day. The most likely average lag period seems to be around 3–4 weeks (21–28 days), and it seems unlikely that accelerated repopulation begins in the first two weeks of RT (<15 days) or after the fifth week (>35 days). Our study results are very similar to those reported by Withers et al.9 and Fowler et al.8 and are consistent with in vitro experimental data.71
By eliminating the AC-Fx articles (Tables 1 and 3) the total number of Significant results relating to LRC was improved by about 2%, and the OS results by more than 20%. Although intriguing at first glance, when compared with the results of two meta-analyses published in 2006,73,74 both works demonstrated that LRC improves significantly with hyperfractionated schemes and AC-Fx based on continuous radiation schedules without compromising the total dose, while OS was not improved with any AC-Fx schemes (while hyperfractionation was). An excess of RT-associated deaths seems to be the most likely cause in that improvement in LRC did not translate into improved OS.
Furthermore, most time factor calculations were made under the assumption that the accelerated proliferation rate of tumor clonogens was constant, although such a pattern of repopulation may not be absolutely correct.71 Clonogens may begin with accelerated proliferation soon after the start of RT or do so at the end; their rate can increase as treatment proceeds, decreases, or levels off. Given that few data have been published about the heterogeneity of tumor clonogen repopulation in human tumors, some tumors may even present more efficient repopulation and others may present similar histology but less efficient repopulation.
An interesting aspect of the current study is the attempt to elucidate which is more important: the position of the gap, the total number of gaps, or the number of consecutive treatment days missed. Of the nine reports that addressed these questions, six concluded that gap position and number of consecutive treatment days missed seemed not to matter while total delay in OTT actually did,28,29,43,49,52,57 although no consensus can be established. As highlighted,28 two reports30,54 showed that the number of patients with gaps (more than 50%) and/or the total length of the gaps were quite large (up to 1–2 weeks). Therefore, the position of the gap may exercise an effect only when the delay is long.
One additional aspect deserves further comment. In their study of 1124 patients, Tarnawski et al.49 calculated a dose/time factor during gaps of 0.75–0.77 Gy/day, which decreased during the treatment days to 0.2–0.35 Gy/day. The difference was statistically significant. The explanation was that if not all tumor clonogens were sterilized by the daily treatment fraction and the predominant effect of irradiation for those surviving clonogens was mitotic delay, after a recovery period, this delay would have finished and the surviving clonogens could have become available for repopulation. Consequently, clonogen repopulation in tumors is not significantly prevented when the interfraction intervals are longer than 24 h, whereas during shorter interfraction intervals (≤24 h), there may not be enough time to overcome mitotic delay and initiate rapid repopulation. This, in turn, may suggest that the effect of delivering a second daily fraction of irradiation twice a week to compensate for a gap may differ somewhat from delivering these fractions on Saturday and Sunday, which has obvious implications if compensation maneuvers are to be used. For similar reasons, eliminating weekend breaks may increase tumor control rates more than previously expected. However, it was impossible to establish whether scoring weekends as gaps was an important aspect of the analysis.
Both statistically significant47,50,62 and non-statistically significant trends61,68 negative relationship were found between the extension of OTT and tumor grade, so that only well- and/or moderately differentiated tumors—but not poorly differentiated ones—would be affected by prolonged OTT. The main hypothesis for this observation47,50 is that the mechanism of repopulation in squamous H&NC is similar to the response in the normal mucosa from where the tumor originated; therefore, the primary tumor responds to RT in a similar way to tumors in the epithelium. In order to do so, the tumor needs to have a functional mechanism capable of regeneration, which most likely happens in well-differentiated tumors. Furthermore, this reaction might be controlled by signaling from the surrounding normal mucosa (i.e., high expression of epidermal growth factor, which is also linked to repopulation); therefore, this response would only be seen in primary tumors and not in nodal metastases, a hypothesis that is consistent with some clinical observations,50,68 although not always.47
Grading on its own is not an ideal predictive assay; however, in view of its reported prognostic significance, it could be usefully combined with other biological measurements to determine individual RT scheduling.75 Thus, well-differentiated tumors with high cell loss factors (high LI) would benefit from accelerated regimens (lower total dose), whereas poorly differentiated tumors with low LI would benefit from conventional protracted regimens (high total dose).
It seems unlikely that chemotherapy (CHT) does not favor accelerated repopulation in the same way as RT.9,71 Budach et al.74 found that while a statistically significant survival benefit of 12.0 months and an absolute survival gain of 13–15% at two years was seen for simultaneous RT-CHT in studies that did not significantly prolong OTT, a smaller (but still significant) survival advantage of 7.9 months was seen with prolonged OTT ≥1 week (11% loss in LRC for a prolongation of the OTT of around 17 days). It indicated that tumor cell repopulation is still a problem, even when RT-CHT is used. In this review, we have tried to shed some light on this issue; however, the vast majority of reports used only RT, and very few used induction CHT in a small part of their patients. Anyway, the only articles that investigate this problem37,46 concluded that ‘the use of induction CHT does not negate the need to avoid treatment interruptions’.
On the other hand, the equivalent radiation effect of the concomitant CHT in H&NC RT-CHT has been estimated to about 7.2 Gy (in 2 Gy fractions),76,77 which would explain (partially, at least) the improvement in LRC (and maybe in OS) observed in meta-analysis.74 If we consider a time factor average of 0.6–0.8 Gy lost per day of delay, as our results show, then a delay of 10 days in a RT-CHT treatment would entail the loss of almost all of the CHT-presumed biological improvement. This, in turn, highlights the importance of avoiding OTT prolongations even when CHT is used.
Finally, most of the previous evidence was from retrospective studies. Hence, the results can be affected by numerous known (and unknown) biases, and the validity of their results would be questionable according to the principles of Evidence-Based Medicine (EBM). However, these principles were intended for evaluation of the benefits of a therapeutic intervention, but not for the evaluation of the risks of exposure to potentially avoidable hazards.5 A different approach is necessary, that is, one much closer to the approach generally used in evaluating environmental hazards, which means that it is not possible to undertake randomized clinical trials to evaluate potential harm to patients for obvious ethical reasons.
It is certainly arguable that the published randomized trials of AC-Fx, with the two meta-analyses, are themselves a very clear source of first-level evidence. If so, such meta-analyses (and the findings of the present work confirm it), clearly demonstrated that the time factor definitely affected LRC, but without much influence on OS. However, despite the meta-analytic approach, the considerable heterogeneity of the respective study populations can make the outcomes worse than expected; this observation has a radiobiological basis71 and would be a valid explanation for the lack of effect on OS. If so, retrospective studies could provide best available evidence, and an extensive review such as the present one is a valuable source on which to base our decision of when and how delays in RT should be compensated.
The therapeutic window in H&NC has widened in the last decade owing to rapid technological advances in RT.78,79 It is of vital importance to prevent, if possible, any part of that gain from being lost by OTT delays.
5. Conclusions
From our comprehensive review one can perhaps draw the following conclusions:
-
(1)
A strong Significant relationship between OTT delay and LRC (86.2%) and/or OS (71.4%) exists, with most studies providing a MV_An.
-
(2)
Delays in RT may result in an average loss of LRC ranging from as low as 1.2% per day to as high as 12–14% per week.
-
(3)
A daily dose increase of about 0.6–0.8 Gy/d would be required to compensate for the extra time occurring during the prolonged OTT.
-
(4)
An average lag period of around 3–4 weeks seems to be necessary for a kick-off of fast repopulation, unlikely to occur during the first two weeks of RT.
-
(5)
The AC-Fx group worsened these figures mainly at the expense of OS. Excluding this group improved the OS results by >20%, possibly related to the excess deaths associated with radiotherapy toxicity.
-
(6)
Total days of OTT prolongation seem to be what really matters if prolongation is short (e.g., ≤one week). Gap position and the number of missed consecutive treatment days seem to have no prognostic significance, except, perhaps, in longer extensions (≥one week). Prolongations ≥3 days in total, irrespective of the position, should be discouraged, anyway.
-
(7)
Only well- and/or moderately differentiated tumors seem to be affected by RT delays, although not the poorly differentiated ones, and mainly depending on the primary tumor (not for lymph node metastases)
-
(8)
Although no strong evidence exists, the use of CHT does not seem to eliminate the negative consequences of treatment interruptions.
-
(9)
The principles of EBM are not suitable for evaluating the effect of time extension on RT outcomes. A different approach, closer to that generally used in evaluating environmental hazards, seems to be justified. Therefore, the results of retrospective studies may well be the best evidence available.
In addition to the present one, a second manuscript will include the remaining tumors/sites other than H&NC, while the third one will contain an evidence-based compensations maneuvers protocol for OTT delays.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgments
We thank to Dra. Maia Ddzhugashvili for critical review of the manuscript, and to Mr. Thomas O’Boyle (whose fees were paid by IMO-Group) for writing assistance.
Contributor Information
José A. González Ferreira, Email: ferreira68@yahoo.es.
Javier Jaén Olasolo, Email: jjaen2008@gmail.com.
Ignacio Azinovic, Email: iazinovic@grupoimo.com.
Branislav Jeremic, Email: nebareje@gmail.com.
References
- 1.Hall E.J., Giaccia A.J. Time, dose and fractionation in radiotherapy. In: Hall E.J., Giaccia A.J., editors. Radiobiology for the radiologist. 6th ed. Lippincott Williams; Philadelphia, PA: 2006. pp. 378–397. [Google Scholar]
- 2.Hendry J.H., Bentzen S.M., Dale R.G. A modelled comparison of the effects of using different ways to compensate for missed treatment days in radiotherapy. Clin Oncol (R Coll Radiol) 1996;8:297–307. doi: 10.1016/s0936-6555(05)80715-0. [DOI] [PubMed] [Google Scholar]
- 3.Dale R.G., Hendry J.H., Jones B., Robertson A.G., Deehan C., Sinclair J.A. Practical methods for compensating for missed treatment days in radiotherapy, with particular reference to head and neck schedules. Clin Oncol (R Coll Radiol) 2002;14:382–393. doi: 10.1053/clon.2002.0111. [DOI] [PubMed] [Google Scholar]
- 4.Bese N.S., Hendry J., Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Board of Faculty of Clinical Oncology. The Royal College of Radiologists . 3rd ed. The Royal College of Radiologists; 2008. The timely delivery of radical radiotherapy: standards and guidelines for the management of unscheduled treatment interruptions. Available at: https://www.rcr.ac.uk/docs/oncology/pdf/BFCO%2808%296_Interruptions.pdf [accessed 17.04.13] [Google Scholar]
- 6.Tubiana M. Repopulation in human tumors. A biological background for fractionation in radiotherapy. Acta Oncol. 1988;27:83–88. doi: 10.3109/02841868809090328. [DOI] [PubMed] [Google Scholar]
- 7.Maciejewski B., Preuss-Bayer G., Trott K.R. The influence of the number of fractions and of overall treatment time on local control and late complication rate in squamous cell carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1983;9:321–328. doi: 10.1016/0360-3016(83)90290-0. [DOI] [PubMed] [Google Scholar]
- 8.Fowler J.F., Lindstrom M.J. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys. 1992;23:457–467. doi: 10.1016/0360-3016(92)90768-d. [DOI] [PubMed] [Google Scholar]
- 9.Withers H.R., Taylor J.M., Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 10.Chera B.S., Amdur R.J., Morris C.G., Kirwan J.M., Mendenhall W.M. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:461–466. doi: 10.1016/j.ijrobp.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 11.Fein D.A., Lee W.R., Hanlon A.L. Do overall treatment time, field size, and treatment energy influence local control of T1-T2 squamous cell carcinomas of the glottic larynx? Int J Radiat Oncol Biol Phys. 1996;34:823–831. doi: 10.1016/0360-3016(95)02205-8. [DOI] [PubMed] [Google Scholar]
- 12.Groome P.A., O'Sullivan B., Mackillop W.J. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer: population-based outcomes study supporting need for intensified treatment schedules. Int J Radiat Oncol Biol Phys. 2006;64:1002–1012. doi: 10.1016/j.ijrobp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Le Q.T., Fu K.K., Kroll S. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:115–126. doi: 10.1016/s0360-3016(97)00284-8. [DOI] [PubMed] [Google Scholar]
- 14.Mendenhall W.M., Amdur R.J., Morris C.G., Hinerman R.W. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–4036. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 15.Roberts S.A., Hendry J.H., Brewster A.E., Slevin N.J. The influence of radiotherapy treatment time on the control of laryngeal cancer: a direct analysis of data from two British Institute of Radiology trials to calculate the lag period and the time factor. Br J Radiol. 1994;67:790–794. doi: 10.1259/0007-1285-67-800-790. [DOI] [PubMed] [Google Scholar]
- 16.Rudoltz M.S., Benammar A., Mohiuddin M. Prognostic factors for local control and survival in T1 squamous cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys. 1993;26:767–772. doi: 10.1016/0360-3016(93)90490-m. [DOI] [PubMed] [Google Scholar]
- 17.Skladowski K., Tarnawski R., Maciejewski B., Wygoda A., Slosarek K. Clinical radiobiology of glottic T1 squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:101–106. doi: 10.1016/s0360-3016(98)00375-7. [DOI] [PubMed] [Google Scholar]
- 18.van der Voet J.C.M., Keus R.B., Hart A.A., Hilgers F.J.M., Bartelink H. The impact of treatment time and smoking on local control and complications in T1 glottic cancer. Int J Radiat Oncol Biol Phys. 1998;42:247–255. doi: 10.1016/s0360-3016(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 19.Budihna M., Skrk J., Smid L., Furlan L. Tumor cell repopulation in the rest interval of split-course radiation treatment. Strahlentherapie. 1980;156:402–408. [PubMed] [Google Scholar]
- 20.Burke L.S., Greven K.M., McGuirt W.T., Case D., Hoen H.M., Raben M. Definitive radiotherapy for early glottic carcinoma: prognostic factors and implications for treatment. Int J Radiat Oncol Biol Phys. 1997;38:37–42. doi: 10.1016/s0360-3016(96)00613-x. [DOI] [PubMed] [Google Scholar]
- 21.Hliniak A., Gwiazdowska B., Szutkowski Z. A multicentre randomized/controlled trial of a conventional versus modestly accelerated radiotherapy in the laryngeal cancer: influence of a 1 week shortening overall time. Radiother Oncol. 2002;62:1–10. doi: 10.1016/s0167-8140(01)00494-7. [DOI] [PubMed] [Google Scholar]
- 22.Barton M.B., Keane T.J., Gadalla T., Maki E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother Oncol. 1992;23:137–143. doi: 10.1016/0167-8140(92)90323-m. [DOI] [PubMed] [Google Scholar]
- 23.Chappell R., Nondahl D.M., Rezvani M., Fowler J.F. Further analysis of radiobiological parameters from the First and Second British Institute of Radiology randomized studies of larynx/pharynx radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33:509–518. doi: 10.1016/0360-3016(95)00133-j. [DOI] [PubMed] [Google Scholar]
- 24.Duncan W., MacDougall R.H., Kerr G.R., Downing D. Adverse effect of treatment gaps in the outcome of radiotherapy for laryngeal cancer. Radiother Oncol. 1996;41:203–207. doi: 10.1016/s0167-8140(96)01838-5. [DOI] [PubMed] [Google Scholar]
- 25.Hendry J.H., Roberts S.A., Slevin N.J., Keane T.J., Barton M.B., Agren-Cronqvist A. Influence of radiotherapy treatment time on control of laryngeal cancer: comparisons between centres in Manchester, UK and Toronto, Canada. Radiother Oncol. 1994;31:14–22. doi: 10.1016/0167-8140(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 26.Overgaard J., Hjelm-Hansen M., Johansen L.V., Andersen A.P. Comparison of conventional and split-course radiotherapy as primary treatment in carcinoma of the larynx. Acta Oncol. 1988;27:147–152. doi: 10.3109/02841868809090334. [DOI] [PubMed] [Google Scholar]
- 27.Roberts S.A., Hendry J.H. Time factors in larynx tumor radiotherapy: lag times and intertumor heterogeneity in clinical datasets from four centers. Int J Radiat Oncol Biol Phys. 1999;45:1247–1257. doi: 10.1016/s0360-3016(99)00320-x. [DOI] [PubMed] [Google Scholar]
- 28.Robertson A.G., Robertson C., Perone C. Effect of gap length and position on results of treatment of cancer of the larynx in Scotland by radiotherapy: a linear quadratic analysis. Radiother Oncol. 1998;48:165–173. doi: 10.1016/s0167-8140(98)00038-3. [DOI] [PubMed] [Google Scholar]
- 29.Robertson C., Robertson A.G., Hendry J.H. Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys. 1998;40:319–329. doi: 10.1016/s0360-3016(97)00716-5. [DOI] [PubMed] [Google Scholar]
- 30.Skladowski K., Law M.G., Maciejewski B., Steel G.G. Planned and unplanned gaps in radiotherapy: the importance of gap position and gap duration. Radiother Oncol. 1994;30:109–120. doi: 10.1016/0167-8140(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 31.Slevin N.J., Hendry J.H., Roberts S.A., Agren-Cronqvist A. The effect of increasing the treatment time beyond three weeks on the control of T2 and T3 laryngeal cancer using radiotherapy. Radiother Oncol. 1992;24:215–220. doi: 10.1016/0167-8140(92)90226-k. [DOI] [PubMed] [Google Scholar]
- 32.Van den Bogaert W., Van der Leest A., Rijnders A., Delaere P., Thames H., van der Schueren E. Does tumor control decrease by prolonging overall treatment time or interrupting treatment in laryngeal cancer? Radiother Oncol. 1995;36:177–182. doi: 10.1016/0167-8140(95)01597-a. [DOI] [PubMed] [Google Scholar]
- 33.Wang C.C., Efird J.T. Does prolonged treatment course adversely affect local control of carcinoma of the larynx? Int J Radiat Oncol Biol Phys. 1994;29:657–660. doi: 10.1016/0360-3016(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 34.Hoekstra C.J., Levendag P.C., van Putten W.L. Squamous cell carcinoma of the supraglottic larynx without clinically detectable lymph node metastases: problem of local relapse and influence of overall treatment time. Int J Radiat Oncol Biol Phys. 1990;18:13–21. doi: 10.1016/0360-3016(90)90261-h. [DOI] [PubMed] [Google Scholar]
- 35.Bataini J.P., Bernier J., Asselain B. Primary radiotherapy of squamous cell carcinoma of the oropharynx and pharyngolarynx: tentative multivariate modelling system to predict the radiocurability of neck nodes. Int J Radiat Oncol Biol Phys. 1988;14:635–642. doi: 10.1016/0360-3016(88)90083-1. [DOI] [PubMed] [Google Scholar]
- 36.Fu K.K., Pajak T.F., Trotti A. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs I.C., Le Q., Terris D.J. The influence of overall radiation time (ORT) in patients treated with chemoradiotherapy for squamous cancer of the head and neck. Int J Radiat Oncol Biol Phys. 1999;45(Suppl. 3):376. [Google Scholar]
- 38.Awwad H.K., Lotayef M., Shouman T. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. Br J Cancer. 2002;86:517–523. doi: 10.1038/sj.bjc.6600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang K.K., Trotti A., Brown B.W. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 40.Parsons J.T., Bova F.J., Million R.R. A re-evaluation of split-course technique for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1980;6:1645–1652. doi: 10.1016/0360-3016(80)90246-1. [DOI] [PubMed] [Google Scholar]
- 41.Maciejewski B., Withers H.R., Taylor J.M., Hliniak A. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx: tumor dose-response and repopulation. Int J Radiat Oncol Biol Phys. 1989;16:831–843. doi: 10.1016/0360-3016(89)90503-8. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J.M., Withers H.R., Mendenhall W.M. Dose-time considerations of head and neck squamous cell carcinomas treated with irradiation. Radiother Oncol. 1990;17:95–102. doi: 10.1016/0167-8140(90)90096-f. [DOI] [PubMed] [Google Scholar]
- 43.Pajak T.F., Laramore G.E., Marcial V.A. Elapsed treatment days – a critical item for radiotherapy quality control review in head and neck trials: RTOG report. Int J Radiat Oncol Biol Phys. 1991;20:13–20. doi: 10.1016/0360-3016(91)90132-n. [DOI] [PubMed] [Google Scholar]
- 44.Cox J.D., Pajak T.F., Marcial V.A. Interruptions adversely affect local control and survival with hyperfractionated radiation therapy of carcinomas of the upper respiratory and digestive tracts. New evidence for accelerated proliferation from Radiation Therapy Oncology Group Protocol 8313. Cancer. 1992;69:2744–2748. doi: 10.1002/1097-0142(19920601)69:11<2744::aid-cncr2820691119>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 45.Roberts S.A., Hendry J.H. The delay before onset of accelerated tumour cell repopulation during radiotherapy: a direct maximum-likelihood analysis of a collection of worldwide tumour-control data. Radiother Oncol. 1993;29:69–74. doi: 10.1016/0167-8140(93)90175-8. [DOI] [PubMed] [Google Scholar]
- 46.Alden M.E., O’Reilly R.C., Topham A., Dale Lowry L., Brodovsky H., Curran W.J., Jr. Elapsed radiation therapy treatment time as a predictor of survival in patients with advanced head and neck cancer who receive chemotherapy and radiation therapy. Radiology. 1996;201:675–680. doi: 10.1148/radiology.201.3.8939214. [DOI] [PubMed] [Google Scholar]
- 47.Hansen O., Overgaard J., Hansen H.S. Importance of overall treatment time for the outcome of radiotherapy of advanced head and neck carcinoma: dependency on tumor differentiation. Radiother Oncol. 1997;43:47–51. doi: 10.1016/s0167-8140(97)01904-x. [DOI] [PubMed] [Google Scholar]
- 48.Horiot J.C., Bontemps P., Van den Bogaert W. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–121. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 49.Tarnawski R., Fowler J., Skladowski K. How fast is repopulation of tumor cells during the treatment gap? Int J Radiat Oncol Biol Phys. 2002;54:229–236. doi: 10.1016/s0360-3016(02)02936-x. [DOI] [PubMed] [Google Scholar]
- 50.Overgaard J., Hansen H.S., Specht L. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 51.Amdur R.J., Parsons J.T., Mendenhall W.M., Million R.R., Cassisi N.J. Split-course versus continuous-course irradiation in the postoperative setting for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1989;17:279–285. doi: 10.1016/0360-3016(89)90440-9. [DOI] [PubMed] [Google Scholar]
- 52.Suwinski R., Sowa A., Rutkowski T., Wydmanski J., Tarnawski R., Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/s0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 53.Pedraza Muriel V., Guerrero Tejada M.R., Luna del Castillo J.D. Time-dose-response relationships in postoperatively irradiated patients with head and neck squamous cell carcinomas. Radiother Oncol. 2001;60:137–145. doi: 10.1016/s0167-8140(01)00381-4. [DOI] [PubMed] [Google Scholar]
- 54.Herrmann T., Jakubek A., Trott K.R. The importance of the timing of a gap in radiotherapy of squamous cell carcinomas of the head and neck. Strahlenther Onkol. 1994;170:545–549. [PubMed] [Google Scholar]
- 55.Fesinmeyer M.D., Mehta V., Blough D., Tock L., Ramsey S.D. Effect of radiotherapy interruptions on survival in medicare enrollees with local and regional head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;78:675–681. doi: 10.1016/j.ijrobp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Vikram B., Mishra U.B., Strong E.W., Manolatos S. Patterns of failure in carcinoma of the nasopharynx: I. Failure at the primary site. Int J Radiat Oncol Biol Phys. 1985;11:1455–1459. doi: 10.1016/0360-3016(85)90332-3. [DOI] [PubMed] [Google Scholar]
- 57.Kwong D.L., Sham J.S., Chua D.T., Choy D.T., Au G.K., Wu P.M. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:703–710. doi: 10.1016/s0360-3016(97)00339-8. [DOI] [PubMed] [Google Scholar]
- 58.Lee A.W., Chan D.K., Fowler J.F. Effect of time, dose and fractionation on local control of nasopharyngeal carcinoma. Radiother Oncol. 1995;36:24–31. doi: 10.1016/0167-8140(95)01579-6. [DOI] [PubMed] [Google Scholar]
- 59.Gardner K.E., Parsons J.T., Mendenhall W.M., Million R.R., Cassisi N.J. Time-dose relationships for local tumor control and complications following irradiation of squamous cell carcinoma of the base of tongue. Int J Radiat Oncol Biol Phys. 1987;13:507–510. doi: 10.1016/0360-3016(87)90064-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang C.C. Local control of oropharyngeal carcinoma after two accelerated hyperfractionation radiation therapy schemes. Int J Radiat Oncol Biol Phys. 1988;14:1143–1146. doi: 10.1016/0360-3016(88)90390-2. [DOI] [PubMed] [Google Scholar]
- 61.Bataini J.P., Asselain B., Jaulerry C. A multivariate primary tumour control analysis in 465 patients treated by radical radiotherapy for cancer of the tonsillar region: clinical and treatment parameters as prognostic factors. Radiother Oncol. 1989;14:265–277. doi: 10.1016/0167-8140(89)90138-2. [DOI] [PubMed] [Google Scholar]
- 62.Bentzen S.M., Johansen L.V., Overgaard J., Thames H.D. Clinical radiobiology of squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 1991;20:1197–1206. doi: 10.1016/0360-3016(91)90228-v. [DOI] [PubMed] [Google Scholar]
- 63.Pernot M., Malissard L., Hoffstetter S. Influence of tumoral, radiobiological, and general factors on local control and survival of a series of 361 tumors of the velotonsillar area treated by exclusive irradiation (external beam irradiation+brachytherapy or brachytherapy alone) Int J Radiat Oncol Biol Phys. 1994;30:1051–1057. doi: 10.1016/0360-3016(94)90309-3. [DOI] [PubMed] [Google Scholar]
- 64.Withers H.R., Peters L.J., Taylor J.M. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys. 1995;33:549–562. doi: 10.1016/0360-3016(95)00228-Q. [DOI] [PubMed] [Google Scholar]
- 65.Hoffstetter S., Marchal C., Peiffert D. Treatment duration as a prognostic factor for local control and survival in epidermoid carcinomas of the tonsillar region treated by combined external beam irradiation and brachytherapy. Radiother Oncol. 1997;45:141–148. doi: 10.1016/s0167-8140(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 66.Mendenhall W.M., Parsons J.T., Stringer S.P., Cassisi N.J., Million R.R. T2 oral tongue carcinoma treated with radiotherapy: analysis of local control and complications. Radiother Oncol. 1989;16:275–281. doi: 10.1016/0167-8140(89)90039-x. [DOI] [PubMed] [Google Scholar]
- 67.Parsons J.T., Mendenhall W.M., Stringer S.P., Cassisi N.J., Million R.R. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39:137–148. doi: 10.1016/s0360-3016(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 68.Dische S., Saunders M., Barrett A., Harvey A., Gibson D., Parmar M. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44:123–136. doi: 10.1016/s0167-8140(97)00094-7. [DOI] [PubMed] [Google Scholar]
- 69.Russo G., Haddad R., Posner M., Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 70.Fowler J.F. Potential for increasing the differential response between tumors and normal tissues: can proliferation rate be used? Int J Radiat Oncol Biol Phys. 1986;12:641–645. doi: 10.1016/0360-3016(86)90074-x. [DOI] [PubMed] [Google Scholar]
- 71.Trott K.R. Cell repopulation and overall treatment time. Int J Radiat Oncol Biol Phys. 1990;19:1071–1075. doi: 10.1016/0360-3016(90)90036-j. [DOI] [PubMed] [Google Scholar]
- 72.Thames H.D., Bentzen S.M. Time factor for tonsillar carcinoma. Int J Radiat Oncol Biol Phys. 1995;33:755–758. doi: 10.1016/0360-3016(95)02124-T. [DOI] [PubMed] [Google Scholar]
- 73.Bourhis J., Overgaard J., Audry H. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 74.Budach W., Hehr T., Budach V., Belka C., Dietz K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer. 2006;6:28. doi: 10.1186/1471-2407-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slevin N.J., West C.M., Wilson G.D., Hendry J.H. The potential benefit from individualised radiotherapy scheduling for head and neck tumours on the basis of both histological grade and kinetics. Radiother Oncol. 1999;51:109–111. doi: 10.1016/s0167-8140(99)00067-5. [DOI] [PubMed] [Google Scholar]
- 76.Kasibhatla M., Kirkpatrick J.P., Brizel D.M. How much radiation is the chemotherapy worth in advanced head and neck cancer? Int J Radiat Oncol Biol Phys. 2007;68(5):1491–1495. doi: 10.1016/j.ijrobp.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 77.Fowler J.F. Correction to Kasibhatla et al. How much radiation is the chemotherapy worth in advanced head and neck cancer? Int J Radiat Oncol Biol Phys. 2008;71(2):326–329. doi: 10.1016/j.ijrobp.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Millan J., Romero Fernández J., Medina Carmona J.A. Current status of IMRT in head and neck cancer. Rep Pract Oncol Radiother. 2013;18(6):371–375. doi: 10.1016/j.rpor.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pérez Romasanta L.A., García Velloso M.J., López Medina A. Functional imaging in radiation therapy planning for head and neck cancer. Rep Pract Oncol Radiother. 2013;18(6):376–382. doi: 10.1016/j.rpor.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]