Abstract
To investigate whether novel pathways of vitamin D3 (D3) and 7-dehydrocholesterol (7DHC) metabolism initiated by CYP11A1 and previously characterized in vitro, occur in vivo, we analyzed samples of human serum and epidermis, and pig adrenals for the presence of intermediates and products of these pathways. We extracted human epidermis from 13 individuals and sera from 13 individuals and analyzed them by LC/qTOF-MS alongside the corresponding standards. Pig adrenal glands were also analyzed for these steroids and secosteroids. Epidermal, serum and adrenal samples showed the presence of D3 hydroxy-derivatives corresponding to 20(OH)D3, 22(OH)D3, 25(OH)D3, 1,25(OH)2D3, 20,22(OH)2D3, 20,23(OH)2D3, 20,24(OH)2D3, 20,25(OH)2D3, 20,26(OH)2D3, 1,20,23(OH)3D3 and 17,20,23(OH)3D3, plus 1,20(OH)2D3 which was detectable only in the epidermis. Serum concentrations of 20(OH)D3 and 22(OH)D3 were only 30- and 15-fold lower than 25(OH)D3, respectively, and at levels above those required for biological activity as measured in vitro. We also detected 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3 in the adrenals. Products of CYP11A1 action on 7DHC, namely 22(OH)7DHC, 20,22(OH)27DHC and 7-dehydropregnenolone were also detected in serum, epidermis and the adrenal. Thus, we have detected novel CYP11A1-derived secosteroids in the skin, serum and adrenal gland and based on their concentrations and biological activity suggest that they act as hormones in vivo.
Vitamin D3 (D3) is a product of the photochemical transformation of 7-dehydrocholesterol (7DHC, precursor to cholesterol) after the absorption of ultraviolet B (UVB) energy (280–320 nm) by the unsaturated B ring of the sterol1,2. In humans, >95% of systemic D3 is produced in the outer layer of the skin, the epidermis, where it serves as a prohormone for conversion to the biologically active 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3)2,3. The D3, formed in the epidermis or provided by the diet, is transported to the liver where it is hydroxylated at C25 by CYP27A1 or CYP2R1 to form 25(OH)D3. The 25(OH)D3 enters the circulation and is then hydroxylated at C1α either in the kidney or peripheral tissues expressing CYP27B1, to form 1,25(OH)2D34,5,6,7. This process, D3 → 25(OH)D3 → 1,25(OH)2D3, also operates locally in the epidermis2,8,9. 1,25(OH)2D3, in addition to regulating calcium metabolism, has important pleiotropic effects that include stimulation of differentiation and inhibition of proliferation of cells of different lineage, anti-cancerogenic effects, stimulation of innate- and inhibition of adaptive- immunity, inhibition of inflammation as well as several other endocrine and developmental effects4,10,11,12,13,14. 1,25(OH)2D3 is inactivated by sequential oxidations and shortening of its side chain which starts with hydroxylation at C24, all catalyzed by CYP24A15,7,15,16,17.
The traditionally recognised function of CYP11A1 is the catalysis of the sequential hydroxylation of cholesterol (C) followed by cleavage of the side chain to produce pregnenolone (P): C → 22(OH)C → 20,22(OH)2C → P18,19. Recently, evidence has been provided that 7-dehydrocholesterol (7DHC), ergosterol and vitamins D3 and D2 also serve as substrates for CYP11A120,21,22,23,24,25. 7DHC is a slightly better substrate for CYP11A1 than cholesterol and its side chain cleavage follows a similar sequence producing 7-dehydropregnenolone (7DHP): 7DHC → 22(OH)7DHC → 20,22(OH)27DHC → 7DHP (reviewed in26). In contrast, CYP11A1-mediated metabolism of vitamin D involves sequential hydroxylations that start predominantly at C20 or C22, but do not lead to the cleavage of the side chain. Rather, several hydroxy-derivatives of D3 are produced: D3 → 20(OH)D3 + 22(OH)D3 → (OH)nD3 (reviewed in26,27). The major product of this pathway, 20OH)D3, can also serve as a substrate for CYP24A1 and CYP27A1, with CYP24A1 hydroxylating it at C24 or C25 and CYP27A1 hydroxylating it at C25 or C2628,29,30. The products of the above reactions can be further hydroxylated at C1α, by CYP27B131,32 with the exception of 17,20,23(OH)3D3 which is the final product of CYP11A1 action on D325,26.
Products of the novel CYP11A1-initiated secosteroidal pathways exert anti-proliferative, pro-differentiation, and anti-inflammatory effects on cultured skin cells, comparable or better than those of 1,25(OH)2D333,34,35,36,37,38,39,40,41. They show antifibrotic activities, both in vitro37,38,39 and in vivo, on bleomycin induced scleroderma39 and also shows anti-cancer activities that are dependent on the cell-type and lineage38,40,41,42,43,44. The novel hydroxy-derivatives of D3 exert these phenotypic effects acting as biased agonists on the vitamin D receptors (VDR)27,45 or as reverse agonists on retinoic orphan acid receptors (ROR) α and γ46. Thus, we have discovered novel secosteroidal pathways initiated by CYP11A1 and modified by other CYPs for which the intermediates and/or products display biological activity26,27.
The major product of CYP11A1 action on D3, 20(OH)D3, is noncalcemic at extremely high doses: 30–60 μg/kg in mice40,42,47 and 3 μg/kg (the highest dose tested to date) in rats44, without any signs of toxicity. Therefore 20(OH)D3, and perhaps its metabolites, are excellent candidates for primary or adjuvant therapy of hyperproliferative or inflammatory disorders27.
The current challenge is to define whether the novel secosteroids are produced in vivo from endogenous D3 and can therefore be defined as natural products. Our initial studies using cultured keratinocytes and colon cells, as well as fragments of adrenal glands and placentae, clearly demonstrated that addition of exogenous vitamin D or 7DHC to incubation media results in their transformation to the secosteroidal derivatives in a dose-dependent manner that requires CYP11A148,49,50,51. The above considerations have mandated the testing of whether these compounds are indeed produced in vivo from their accumulation in human skin and sera, or in adrenal glands where expression of CYP11A1 is high. In this paper we report for the first time that many of hydroxy-derivatives of vitamin D and products of 7DHC metabolism resulting from the action of CYP11A1, are detectable in the human epidermis and serum and in pig adrenal glands by liquid chromatography-mass spectrometry (LC-MS).
Results and Discussion
An overview
To investigate whether the novel pathways of 7DHC (provitamin D3) and D3 metabolism initiated by CYP11A1, defined from in vitro studies26,27, may also be functional in vivo, we extracted human epidermis from samples obtained from 13 individuals including 6 African-Americans (AA) and 7 Caucasians (C), and sera from 13 individuals (12C and 1 Hispanic), and analyzed them by LC/qTOF-MS alongside the corresponding standards. These measurements were supplemented by analysis of the pig adrenal gland, an organ expressing high levels of CYP11A1.
In vivo detection of 7DHC metabolites
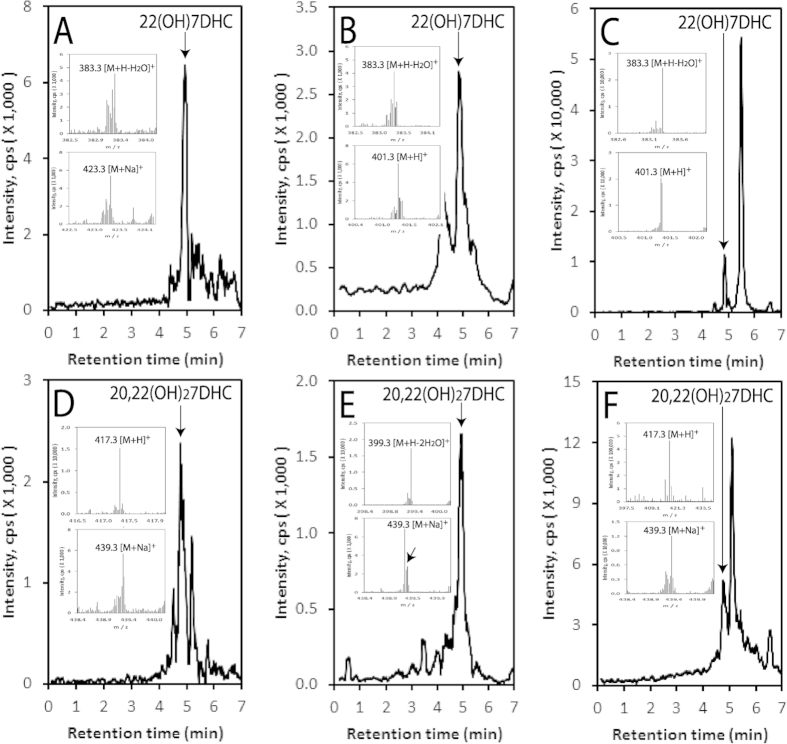
The analyses of extracted samples using LC/qTOF-MS demonstrate that endogenous 7DHC (Fig. 1A–C) is present in human serum, human epidermis and the pig adrenal gland, as is the product of CYP11A1 action, 7DHP (Fig. 1D–F). Specifically 7DHP-derived species with m/z corresponding to 315.2 [M + H]+ and 337.2 [M + Na]+, and a retention time corresponding to the 7DHP, were detected in the human epidermis. In human serum, 7DHP-derived species displayed m/z values of 315.2 [M+H]+ and 279.2 [M + H − H2O]+, while in pig adrenals m/z values were 279.2 [M + H − H2O]+, 315.2 [M+H]+ and 337.2 [M + Na]+ ] (Fig. 1D–F). The estimated concentration of 7DHP in the epidermis was 36.84 ± 17.14 ng/mg protein and there were no differences in its concentration in relation to sex, age or racial background. These samples were also analyzed for the presence of 22(OH)7DHC and 20,22(OH)27DHC, intermediates in the conversion of 7DHC to 7DHP. Again species with m/z and RT corresponding to the 22(OH)7DHC and 20,22(OH)27DHC standards were clearly present in the pig adrenal and human epidermis and serum (Fig. 2). For 22(OH)7DHC m/z values of 401.3 [M + H]+, 383.3 [M + H − H2O]+ and/or 423.3 [M + Na]+ were observed while for 20,22(OH)27DHC the expected m/z values of 417.3 [M + H]+, 399.3 [M + H − H2O]+ and/or 439.3 [M + Na]+ were seen. These data substantiate the results of our previous in vitro and ex-vivo assays 20,21,48,49 and provide important documentation that 7DHC can be metabolized to 7DHP by CYP11A1 in vivo , in a sequential manner: (7DHC → 22(OH)7DHC → 20,22(OH)27DHC), with local (epidermis, adrenal gland) and systemic (serum) accumulation of the intermediates and products of the pathway. A future challenge will be to determine whether 7DHP is further metabolized by other steroidogenic enzymes which are expressed in the skin (reviewed in52) and whether these ∆7-sterols/steroids undergo UVB-induced transformation to the corresponding secosteroids, as suggested by in vitro studies38,53,54,55.
Figure 1. Detection of 7DHC and 7DHP in the human epidermis, human serum and pig adrenals.
Extracted ion chromatograms (EIC) are shown for the human epidermis (A,D), human serum (B,E) and the pig adrenal (C,F) using m/z = 367.2 [M + H − H2O]+ for 7DHC (A–C) and m/z = 337.2 [M + Na]+ (D), 279.3 [M + H-2H2O]+ (E) or 315.2 = [M + H]+ (F) for 7DHP. Arrows indicate the retention times of the corresponding standards. Inserts for panels (A–C) show the mass spectra corresponding in retention time of 7DHC while inserts of panels (D–F) panels show the mass spectra corresponding to the retention time of 7DHP. Note that the UPLC conditions were different for (F) where a longer column was used compared to the other panels (see Materials and Methods).
Figure 2. Detection of 22(OH)7DHC and 20,22(OH)27DHC in the pig adrenal, human epidermis and human serum.
The LC-MS spectra were measured on fractions with retention times corresponding to either 22(OH)7DHC or 20,22(OH)27DHC that were pre-purified on a Waters C18 column (250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water (40–100%) (see Materials and Methods). Extracted ion chromatograms are shown for human epidermis (A,D), serum (B,E) and the pig adrenal (C,F), and were measured using m/z = 383.3 [M + H − H2O]+ (A) or 401.3 [M + H]+ (B,C) for 22(OH)7DHC, and m/z = 439.3 [M+Na]+ (D,E) or m/z = 417.3 [M + H]+ (F) for 20,22(OH)27DHC. Arrows indicate the retention times of the corresponding standards. Inserts show the mass spectra corresponding to the retention time of either 22(OH)7DHC (A–C) or 20,22(OH)27DHC (D–F).
In vivo detection of novel vitamin D3 hydroxyderivatives
Following our in vitro and ex-vivo studies on the transformation of exogenous vitamin D3 to hydroxy-derivatives (with 20(OH)D3 being the major metabolite) by cells and tissues expressing CYP11A150, we tested the human and pig samples for the presence of the novel mono-, di- and tri-hydroxy-derivatives of D3 in comparison to 25(OH)D3 and 1,25(OH)2D3. It should be noted that for the precise detection of vitamin D hydroxy-derivatives, they were first separated on Waters C18 column (250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water (40–100%). Fractions with RTs corresponding to specific standards were then analyzed by UPLC on an Agilent Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm particle size) with a gradient of methanol in water (see Materials and Methods), connected to a Xevo™ G2-S qTOF.
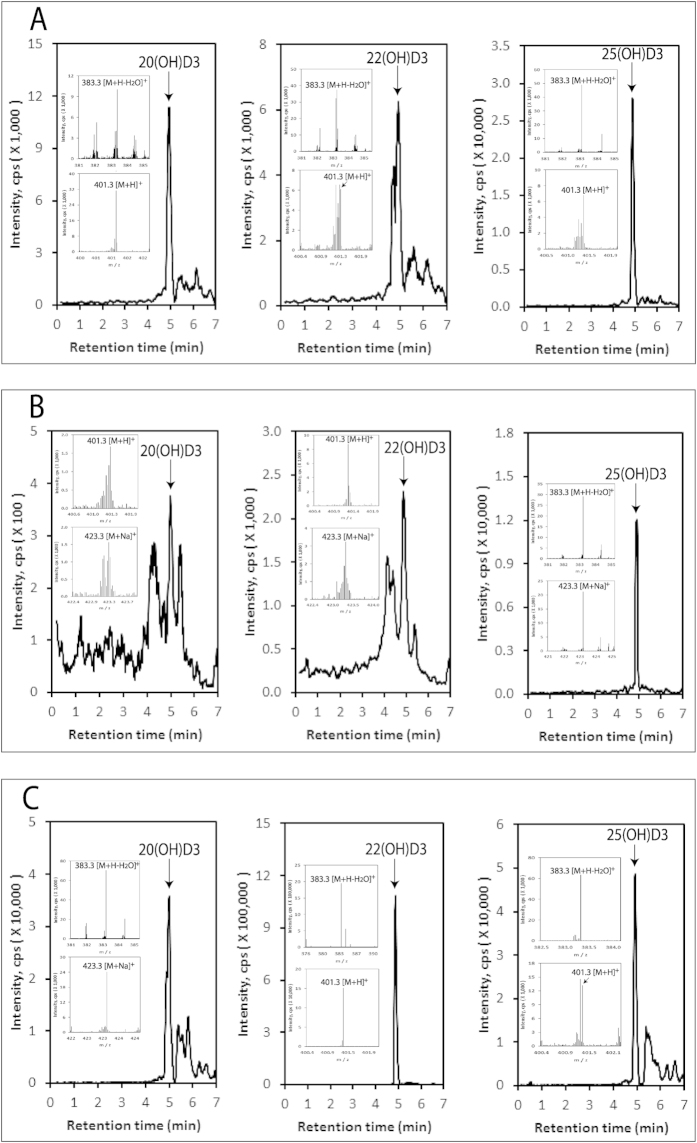
Monohydroxy-D3 species were detected in the epidermis, serum and adrenal glands with the extracted ion chromatogram (EIC) analyzed using m/z = 383.3 [M + H − H2O]+, with retention times corresponding to the 20(OH)D3, 22(OH)D3 and 25(OH)D3 standards (Fig. 3). Additional ions detected at RTs of the corresponding standards included 401.3 [M + H]+ and/or 423.3 [M + Na]+ for the epidermis, serum and adrenals, (Fig. 3, inserts), further supporting the identification of 20(OH)D3, 22(OH)D3 and 25(OH)D3 in these samples. This detection of 20(OH)D3 and 22(OH)D3 in vivo is in agreement with our previous demonstration that cells or tissues expressing CYP11A1 are able to hydroxylate exogenous vitamin D3 in positions C20 or C2250. Quantification of the concentrations of these monohydroxy-D3 metabolites in the epidermis and serum showed that there is a significantly higher concentration of 25(OH)D3 in human serum than 20(OH)D3 and 22(OH)D3 (30 and 15 folds, respectively). The concentration of 25(OH)D3 in the epidermis was lower than that of 20(OH)D3 (Table 1). These differences indicate that the classical pathway of D3 activation (D3 → 25(OH)D3) is the major one with respect to the systemic level of vitamin D metabolites, because of the massive production of 25(OH)D3 in the liver. However, the higher concentration of 20(OH)D3 than 25(OH)D3 in the epidermis may arise from it being produced at a greater rate, or alternatively, it being metabolised more slowly than 25(OH)D3. Both possibilities require further experimental testing using skin organ culture. Preliminary analysis of levels of these metabolites for gender, age and racial group showed no statistical difference in epidermal concentration of either 20(OH)D3 or 25(OH)D3 (Supplemental Fig. 1). Similarly there were no differences for these parameters in human serum except for significantly higher levels of 25(OH)D3 in older individuals than younger ones (Supplemental Fig. 2). The higher levels of 25(OH)D3 in the serum of older subjects than younger ones may result from higher compliance by senior individuals to include an oral supplementation of D3.
Figure 3. Detection of 20(OH)D3, 22(OH)D3 and 25(OH)D3 in the human epidermis, human serum and the pig adrenal gland.
The LC-MS spectra were measured on fractions that were pre-purified on a Waters C18 column (250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water (40–100%) as in Fig. 2. EICs are shown for epidermis (A), serum (B) and the pig adrenal gland (C) and were measured using m/z = 383.3 [M + H − H2O]+ for (A,C) and 25(OH)D3 in (B) or 401.3 [M + H]+ for 20(OH)D3 and 22(OH)D3 in (B). The identified peaks had RTs corresponding to either the 20(OH)D3, 22(OH)D3 or 25(OH)D3 standards, indicated by arrows. The inserts show the mass spectra of samples corresponding to the retention times of the standards.
Table 1. Serum and tissue content of mono-hydroxy D3 metabolites.
| Secosteroid | Epidermis (ng/mgprotein) | Serum (ng/ml) |
|---|---|---|
| 20(OH)D3 | 0.40 ± 0.15 | 1.15 ± 0.20 |
| 22(OH)D3 | ND* | 2.38 ± 0.65 |
| 25(OH)D3 | 0.06 ± 0.02 | 33.58 ± 5.36 |
| D3 | 0.13 ± 0.05 | 2.39 ± 0.34 |
*ND, not done because the 22(OH)D3 signal is buried in the contaminating peaks.
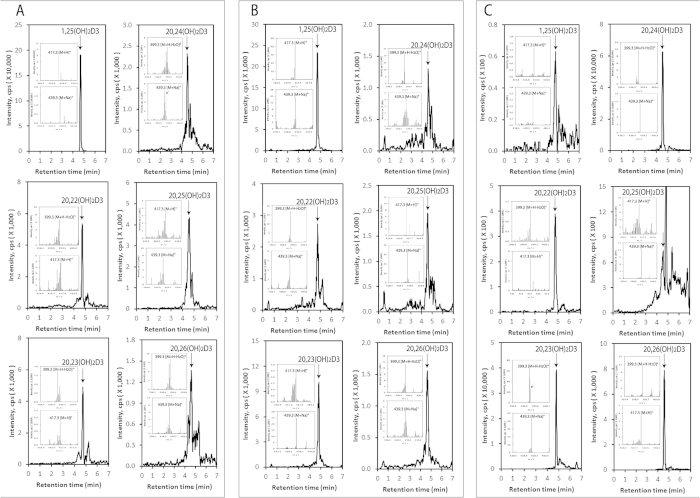
The human and pig samples were further analyzed for the presence of dihydroxyvitamin D3 metabolites. Dihydroxy-D3 species were detected in the epidermis, serum and adrenal glands with the EIC analyzed using m/z = 399.3 [M + H − H2O]+ (Fig. 4). Identified metabolites with retention times corresponding to authentic standards were 1,25(OH)2D3, 1,20(OH)2D3, 20,22(OH)2D3, 20,23(OH)2D3, 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3. 1,20(OH)2D3 was also detected in the epidermis (data not shown) but was below the level of detection in the serum and adrenal. The mass spectra of samples taken at the retention times of the authentic standards (inserts, Fig. 4) showed the expected ions at 417.3 [M + H]+ and 439.3 [M + Na]+ as well as the 399.3 ion, further confirming the identity of products as species of dihydroxyvitamin D3. It should be noted that the pre-purification of the secosteroids in the sample by HPLC prior to LC-MS (see Fig. 4 legend) was required to separate 20,22(OH)2D3 from 20,23(OH)2D3, 20,24(OH)2D3 from 20,25(OH)2D3, and 20,26(OH)2D3 and 1,20(OH)2D3 from the background. We also analyzed adrenal extracts directly by UPLC-MS without this pre-purification step and detected dihydroxy-D3 ions at 417.3 [M + H]+, 399.3 [M + H − H2O]+ and 439.3 [M + Na]+ with RTs corresponding to 1,25(OH)2D3 or 20,22(OH)2D3, 20,23(OH)2D3 or 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3 (not shown), which further confirms the endogenous accumulation of these metabolites in the adrenal gland.
Figure 4. Novel dihydroxyvitamin D3 metabolites can be detected in the human epidermis, human serum and the pig adrenal gland.
The LC-MS spectra were measured on fractions that were pre-purified on Waters C18 column (250 × 4.6 mm, 5 μm particle size) as in Fig. 2. EICs are shown for epidermis (A), serum (B) and the pig adrenal (C) and were measured using m/z = 399.3 [M + H − H2O]+ for 20,22(OH)2D3 and 20,23(OH)2D3 in A, and 1,25(OH)2D3, 20,22OH)2D3, 20,24(HO)2D3 and 20,26(OH)2D3 in C; 417.3 [M + H]+ for 1,25(OH)2D3 in (A) and 20,23(OH)2D3 and 20,25(HO)2D3 in C; 439.3 [M + Na]+ for 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3 in (A,B). Arrows indicate the retention times of the corresponding standards. The mass spectra of samples corresponding to the retention time of each dihydroxyvitamin D3 standard are shown in the inserts.
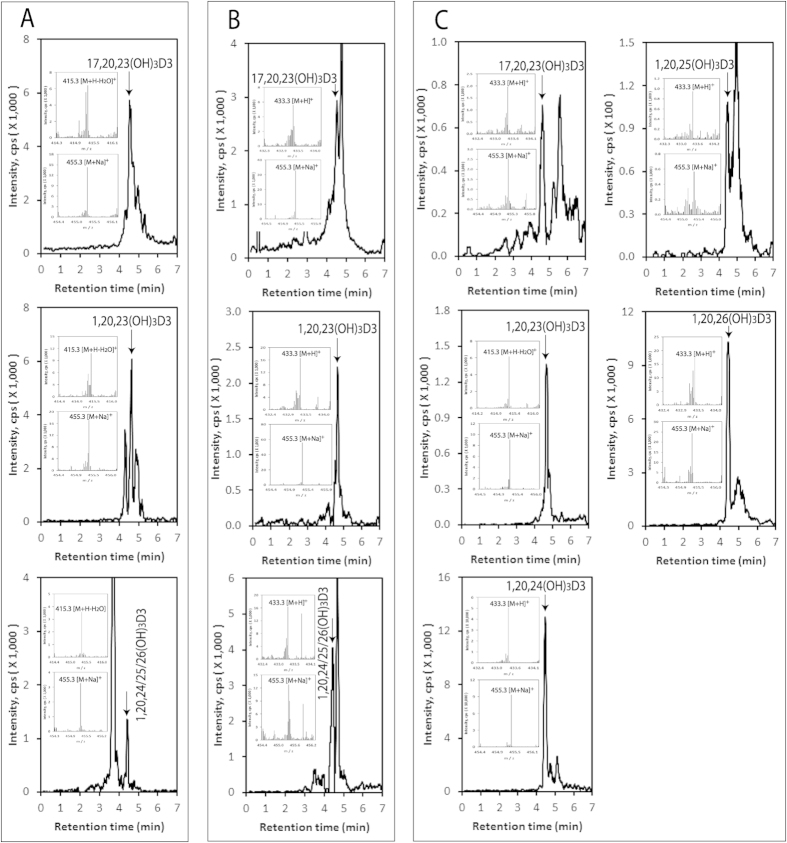
Novel trihydroxy-D3 metabolites were detected in the epidermis, serum and pig adrenal glands with the EICs being measured using m/z = 433.3 [M + H]+ (Fig. 5). Identified trihydroxyvitamin D3 species with retention times corresponding to authentic standards included 17,20,23(OH)3D3 and 1,20,23(OH)3D3. Besides the 433.3 ion, the mass spectra (Fig. 5 inserts) also gave ions with m/z values of 415.3 [M + H − H2O]+ and/or 455.3 [M + Na]+, further supporting their identification as trihydroxyvitamin D3 species. We also detected a peak in the epidermal and serum extracts corresponding to the single RT displayed by 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3 (unseparated by LC under the conditions used (see Materials and Methods)) (Fig. 5A,B, lower panels). Pre- separation of these secosteroids by HPLC on a Waters C18 column (250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water (40–100%) prior to LC-MS provided insufficient sample to detect the individual trihydroxyvitamin D3 species in serum and the epidermis. However, we could detect all three compounds in the adrenal extracts by this procedure (Fig. 5C).
Figure 5. Novel trihydroxy-vitamin D3 metabolites are also present in in the human epidermis, human serum and pig adrenal.
The LC-MS spectra were measured on fractions which were pre-purified on a Waters C18 column (250 × 4.6 mm, 5 μm particle size), as in Fig. 2. EICs are shown for the epidermis (A), serum (B), adrenal (C) and were measured using m/z = 415.3 [M + H − H2O]+ except 17,20,23(OH)3D3 in (C) (455.3 [M + Na]+). Each standard is identified by arrow and the mass spectra of samples corresponding to the retention time of each trihydroxyvitamin D3 standard are shown in the inserts.
The above results show that in addition to classical 25(OH)D3 and 1,25(OH)2D3, CYP11A1-derived monohydroxxy-, dihydroxy- and trihydroxy-D3 metabolites are present in the human epidermis and serum, and in pig adrenals. Detection and quantification of 20(OH)D3 in human serum and the epidermis using LC/qTOF-MS has substantiated our initial finding of the presence of 20(OH)D3 in the serum50 and epidermis56 using LC-MS/MS. Detection of 22(OH)D3 in these samples represents a new finding demonstrating that D3 can be hydroxylated either at C20 or C22 in vivo for local or systemic use, by tissues or organs expressing CYP11A1, consistent with in vitro and ex-vivo data21,26,50,57. While 22(OH)D3 shows significantly lower biological activity than 20(OH)D3 towards skin cells57, its higher concentration in human serum mandates future testing of its phenotypic potency towards other cell types to determine whether it displays a cell-type dependent role in physiology or pathology. Detection of 20,22(OH)2D3, 20,23(OH)2D3, 20,24(OH)2D3, 20,25(OH)2D3, 20,26(OH)2D3, 1,20,23(OH)3D3 and 17,20,23(OH)3D3 in the epidermis and human serum and pig adrenal gland is of great significance, because it shows that in vivo D3 can be hydroxylated in a sequential fashion by CYP11A1 following the sequence: D3 → 20(OH)D3 + 22(OH)D3 → 20,22(OH)2D3 + 20,23(OH)2D3 → 17,20,23(OH)3D3 as predicted theoretically26 and consistent with in vitro data21,25,50,57. Furthermore the hydroxy-derivatives identified illustrate that 20(OH)D3 can be further metabolized by CYP27A1 and CYP24A1, or liver microsomes, as documented in vitro28,29,58. The latter indicates that the following pathway of 20(OH)D3 metabolism occurs in vivo that is independent of CYP11A1: 20(OH)D3 → 20,24(OH)2D3 + 20,25(OH)2D3 + 20,26(OH)2D3. The detection of 1,20(OH)2D3 and 1,20,23(OH)3D3 in some of the samples analyzed demonstrates that CYP11A1-derived D3 hydroxymetabolites are further hydroxylated by CYP27B1, the only enzyme with appreciable 1α–hydroxylase activity, substantiating our previous in vitro31,32 and ex vivo data50. The absence of 1,20(OH)2D3 in the human serum suggests that in vivo, 1α-hydroxylation of 20(OH)D3 occurs predominantly at the local level, which is consistent with low catalytic efficiency of CYP27B1 toward 20(OH)D3 or 20(OH)D232,40,59. We also detected species corresponding to 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3 in the adrenal extracts. These results suggest that products of CYP24A1 or CYP27A1 hydroxylation of 20(OH)D3 may be further hydroxylated at C1α, at least in the adrenal gland, which does expresses CYP27B150. This pathway is consistent with the high catalytic efficiency that purified CYP27B1 displays towards 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D332.
The 1α-hydroxy-derivatives of 20(OH)D3 and 20(OH)D240,60 and of 20,23(OH)2D3 inhibit keratinocyte proliferation31 with similar potency to that of 1,25(OH)2D327. Detection of 17,20,23(OH)3D3 in the epidermis, serum and adrenal glands deserves special attention since it represents the final product of CYP11A1 dependent metabolism of D3 and it is not a substrate for CYP27B132 and is probably poorly metabolized by CYP27A1 and CYP24A126. Of note, preliminary studies demonstrate that 17,20,23(OH)3D3 is biologically active in skin cells27,39,45 suggesting that it may have a role in biological regulation, perhaps exerted in a tissue- and cell lineage- dependent fashion.
Concluding remarks and perspectives
In this study we show for the first time the presence of CYP11A1-derived products of 7DHC metabolism (22(OH)7DHC, 20,22(OH)27DHC and 7DHP) and D3 metabolism (20(OH)D3, 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3 and 17,20,23(OH)3D3) in the human epidermis and serum, and the pig adrenal gland. Our data also indicate that some of the CYP11A1-derived secosteroids can be acted on by CYP27B1 (producing 1,20(OH)2D3 and 1,20,23(OH)3D3) or CYP27A1 and/or CYP24A1 (producing 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3). These findings complement and are consistent with previous in vitro and ex-vivo studies on the CYP11A1-initiated metabolism of D320,23,25,27,28,29,31,32,50,57,58 and 7DHC20,23,48,49 by demonstrating that these pathways do operate in vivo, and they are further modified by other CYPs metabolizing D3 or steroids, as predicted from in vitro analyses26. In the skin, CYP11A1-derived ∆7-sterols/steroids are likely to be transformed into the corresponding secosteroids after exposure to UVB21,37,38, the significance of which represents a future challenge to explore. Importantly, the major metabolites of these novel steroid/secosteroidogenic pathways display anti-proliferative, prodifferentiation and anti-inflammatory activities in a cell-lineage dependent fashion27,33,34,35,37,38,39,41,43,44,48,56,57. Thus these pathways, as well as their intermediates and/or products, are likely play a role in the regulation of physiological process and their related pathology. This is supported by the relatively high concentrations of 22(OH)D3 and 20(OH)D3 in the plasma, levels only 15–30 fold lower than 25(OH)D3 and therefore higher than 1,25(OH)2D3, and at the concentration levels required to see effects in vitro. This further suggests that the measurement of the major CYP11A- derived hydroxy-derivatives of D3 in the human serum may be necessary to fully assess vitamin deficiency or sufficiency, as opposed to a single measurement of 25(OH)D3. Some of these new secosteroids are non-toxic and non-calcemic at relatively high doses, as demonstrated for 20(OH)D342,44,47 and 20,23(OH)2D339, suggesting that they are not only endogenous bioregulators, but also potential therapeutics (primary or adjuvant) for the treatment of inflammatory and/or hyperproliferative skin disorders resistant to 1,25(OH)2D3 action. In conclusion, the detection of novel CYP11A1-derived secosteroids in the skin, adrenal gland and serum in conjunction with their biological activity suggest that they act as hormones in vivo for which the effects would depend on their local and systemic production, and their metabolism.
Materials and Methods
Source of steroids and secosteroids
7DHC, vitamin D3, 25(OH)D3, and 1,25(OH)2D3 were obtained from Sigma-Aldrich (St. Louis, MO). 7DHP was synthesized as described previously38,53, while 22(OH)7DHC and 20,22(OH)27DHC 20(OH)D3 were produced from 7DHC enzymatically using purified bovine CYP11A149. 20(OH)D3, 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3 and 17,20,23(OH)3D3 were produced from vitamin D3 using purified CYP11A1, while 1,20(OH)2D3 was similarly produced from 1α(OH)D323,25,57,60. 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)3D3, were produced from 20(OH)D3 using recombinant CYP27A1 or CYP24A128,29. 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3 were made by by 1α-hydroxylation of either 20,24(OH)2D3, 20,25(OH)2D3 or 20,26(OH)2D3 using recombinant CYP27B132. These compounds were purified by reverse-phase HPLC, and their structures and purities were determined by NMR and mass spectrometry23,25,28,29,32,36,48,49,57,60.
Use of tissues and serum samples
The experiments were performed in accordance with relevant guidelines (see below) and the experiments were approved by the Institutional Review Board (IRB) (Human Subject Assurance Number 00002301) and the Institutional Animal Care and Use Committee (IACUC)(Animal Welfare Assurance Number A3325–01) at the University of Tennessee Health Science Center (UTHSC).
The use of human skin and cells was approved by the IRB at the UTHSC as an exempt protocol #4 (Dr. A. Slominski, P.I.). The protocol was classified for exempt status under 45CFR46.102 (f) in that is does not involve “human subjects” as defined therein. Informed consent is waived in accord with 45CFR46.116(d). The research involved no more than minimal risk, it will not adversely affect the right and welfare of the subjects, and it could practicably be carried out without the waiver. Material in this category consists of tissues that are left over, or in excess of what is needed, for pathological diagnosis or analysis. Condition (4) at 45CFR46.116(d) is not applicable to this study. Human skin samples (n = 13) from both males (n = 7) and females (n = 6), 30 to 90 years old of African-American (AA; n = 6) and Caucasian (C, n = 7) races were obtained from the Regional One Health Center and the Methodist University Hospital, Memphis, TN.
The collection of human serum was approved by the IRB protocol #7526 (Dr. A. Postlethwaite, P.I.). An informed consent was obtained from all subjects involved in this study. Human serum was collected from 13 volunteers (12 Caucasians and 1 Hispanic), 25–61 years old including 10 females and 3 males following protocols described previously50.
Collection of pig adrenals was approved by the UTHSC Institutional Animal Care and Use Committee (IACUC), which has the Animal Welfare Assurance Number A3325–01. Pig adrenals were collected from a female Landrace cross Large White pig, 2 years old following protocols approved by IACUC as described previously50.
Extraction of vitamin D3, 7DHC and their metabolites from tissues and serum
Extraction of D3, 7DHC and their metabolites from tissues and serum followed protocols described previously49,50 with some modifications61. Briefly, the epidermis was separated from dermis and processed as described in61. The epidermal tissue was homogenized in PBS followed by a second homogenization in 75% acetonitrile. After centrifugation the supernatants were filtered and dried. The human serum samples were extracted with methanol:water (9:1) with vortexing. The precipitated proteins were removed by centrifugation, the supernatants filtered using a syringe filter (PES, 0.45 μm, 30 mm; Celltreat, Shirley, MA) and then dried using a speedvac drier (Savant instruments, Inc. Holbrook, NY). Pieces of pig adrenal glands were suspended in PBS and homogenized following the addition of 2.5 volumes of methylene chloride. Samples were centrifuged and the supernatants dried as above. The dried extracts were stored at −80 °C until further analyses.
Detection of vitamin 7DHC and vitamin D3 metabolites
The above extracts were analyzed by liquid chromatography and mass spectrometry (LC-MS) as described previously49,50,51,61 with some modifications. In most cases (as indicated in the Figure legends), to minimize the interference from other molecules present in the samples, we initially pre-purified samples by HPLC using a long column (Waters C18 column, 250 × 4.6 mm, 5 μm particle size). Elution was carried out with a gradient of acetonitrile in water (40–100%) at a flow rate of 0.5 ml/min (15 min), followed by a wash with 100% acetonitrile for 30 min at a flow rate of 0.5 ml/min and for 20 min at a flow rate of 1.5 ml/min. Fractions with retention times (RTs) corresponding to the secosteroids of interest, which were well separated under these conditions (RTs decreasing from mono-, di- to trihydroxylated), were collected separately. These fractions were then subjected to UPLC (Waters ACQUITY I-Class UPLC (ultra-performance liquid chromatography) system (Waters, Milford, USA)) on an Agilent Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm particle size), which was connected to a Xevo™ G2-S qTOF (quadrupole hybrid with orthogonal acceleration time-of-flight) tandem mass spectrometer (Waters, Milford, USA)51,61. For the UPLC, a gradient of methanol in water containing 0.1% formic acid (20–60% for 3 min, 60–100% for 1 min, 100% for 2.1 min, 100–20% for 0.1 min), at flow rate of 0.3 ml/min, was used. Using this short column and under the UPLC conditions employed, these metabolites showed similar but distinct retention times (as indicated in Fig. 2). For 7DHP detection in the skin and serum, the initial HPLC step was omitted and the samples were directly analyzed by LC-MS using the UPLC conditions described above. Similarly, 7DHC and vitamin D3 were directly analyzed by LC-MS but a Waters Atlantis dC18 column (100 × 4.6 mm, 5 μm particle size) was used with a gradient of methanol in water (85–100%) containing 0.1% formic acid for 20 min followed by 100% methanol containing 0.1% formic acid for 10 min, at a flow rate of 0.5 ml/min. These conditions were also used for measuring 7DHP in the pig adrenal (results shown in Fig. 1F).
For MS analysis the scan range was 50 to1000 Da in the positive mode, and all data were collected in the centroid mode. The capillary and cone voltages were 3.0 kV and 30 V, respectively. The desolvation gas was maintained at 1000 L/h at a temperature of 500 °C. The cone gas was 100 L/h with a source temperature of 150 °C. The data acquisition rate was 0.3 s, with a 20 second interval. The lockspray frequency was every 20 s using Leucine Enkephalin solution (100 ng/mL) as the lockspray reference compound (m/z 556.2771) with a flow rate of 5 μL/min. The MS data were collected in the full scan mode with low (6 V) and high (ramp from 20 V to 40 V) collision energy (CE) data channels to get both the parent ions (MS) and the daughter ions (MS/MS). All data were acquired and processed by Waters MassLynx v4.1 software.
For quantification of 20(OH)D3, 22(OH)D3 and 25(OH)D3, LC/qTOF-MS was used. The UPLC of extracts was carried out with a Waters Atlantis dC18 column (100 × 4.6 mm, 5 μm particle size) with a gradient of methanol in water (85–100%) containing 0.1% formic acid for 20 min followed by 100% methanol containing 0.1% formic acid for 10 min, at a flow rate of 0.5 ml/min. An Agilent Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) was used to quantify 7DHP and 17,20,23(OH)3D3 in extracts using a gradient of methanol in water containing 0.1% formic acid (20–60% for 3 min, 60–100% for 1 min, 100% for 2.1 min, 100–20% for 0.1 min), at flow rate of 0.3 ml/min. MS analysis was performed as described above. The concentrations of 7DHP and secosteroids in the epidermis and serum were calculated from MS peak areas in relation to standards curves generated using the corresponding standards at m/z = 315.2 [M + H]+ for 7DHP, 383.3 [M + H − H2O]+ for 20(OH)D3, 22(OH)D3 and 25(OH)D3, and 455.3 [M + Na]+ for 17,20,23(OH)3D3. The values are presented as means ± SE in ng per mg protein or ml serum.
Statistical analysis
Data are presented as means ± SE and were analyzed using the Student’s t-test, using Microsoft Excel and Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted in tables and figures.
Additional Information
How to cite this article: Slominski, A. T. et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 5, 14875; doi: 10.1038/srep14875 (2015).
Supplementary Material
Acknowledgments
This work was supported by NIH grants 2R01AR052190-A6, 1R01AR056666-01A2, 1R21AR0666505-01A1 (ATS), and 1R21AR063242-01A1, 1S10OD010678-01, and 1S10RR026377-01 (WL) and the University of Western Australia (RCT), West Cancer Center Research Support Award (ATS) and UTHSC Dean Funds to ATS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.-K.K. performed experiments, analyzed data and prepared figures, A. S. provided biological material, designed experiment, analyzed data and wrote the manuscript. R.C.T. and W.L. provided synthetic standards, analyzed data and wrote the paper. A. P. collected serum samples, wrote the paper. E.K.Y.T. and E.W.T. provided synthetic standards. All authors reviewed the manuscript.
References
- MacLaughlin J. A., Anderson R. R. & Holick M. F. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 216, 1001–1003 (1982). [DOI] [PubMed] [Google Scholar]
- Holick M. F. Vitamin D: A millenium perspective. J Cell Biochem 88, 296–307, 10.1002/jcb.10338 (2003). [DOI] [PubMed] [Google Scholar]
- Bikle D. D. Vitamin D: an ancient hormone. Experimental Dermatology 20, 7–13 (2011). [DOI] [PubMed] [Google Scholar]
- Holick M. F. Vitamin D deficiency. N Engl J Med 357, 266–281, 10.1056/NEJMra070553 (2007). [DOI] [PubMed] [Google Scholar]
- Jones G., Prosser D. E. & Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55, 13–31, 10.1194/jlr.R031534 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N. et al. Enzymatic properties of human 25-hydroxyvitamin D3 1alpha-hydroxylase coexpression with adrenodoxin and NADPH-adrenodoxin reductase in Escherichia coli. Eur J Biochem 265, 950–956 (1999). [DOI] [PubMed] [Google Scholar]
- Sakaki T., Sawada N., Takeyama K., Kato S. & Inouye K. Enzymatic properties of mouse 25-hydroxyvitamin D3 1 alpha-hydroxylase expressed in Escherichia coli. Eur J Biochem 259, 731–738 (1999). [DOI] [PubMed] [Google Scholar]
- Bikle D. D. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 347, 80–89, 10.1016/j.mce.2011.05.017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B., Genehr T., Knuschke P., Pietzsch J. & Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol 117, 1179–1185, 10.1046/j.0022-202x.2001.01538.x (2001). [DOI] [PubMed] [Google Scholar]
- Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol 7, 337–345, 10.1038/nrendo.2010.226 (2011). [DOI] [PubMed] [Google Scholar]
- Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 76, 315–325, 10.1111/j.1365-2265.2011.04261.x (2012). [DOI] [PubMed] [Google Scholar]
- Plum L. A. & DeLuca H. F. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov 9, 941–955, 10.1038/nrd3318 (2010). [DOI] [PubMed] [Google Scholar]
- Bikle D. D. The vitamin D receptor: a tumor suppressor in skin. Discov Med 11, 7–17 (2011). [PMC free article] [PubMed] [Google Scholar]
- Mason R. S. & Reichrath J. Sunlight vitamin D and skin cancer. Anticancer Agents Med Chem 13, 83–97 (2013). [PubMed] [Google Scholar]
- Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta 1814, 186–199, 10.1016/j.bbapap.2010.06.022 (2011). [DOI] [PubMed] [Google Scholar]
- Tieu E. W., Tang E. K. & Tuckey R. C. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. The FEBS journal 281, 3280–3296, 10.1111/febs.12862 (2014). [DOI] [PubMed] [Google Scholar]
- Sakaki T. et al. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem 267, 6158–6165 (2000). [DOI] [PubMed] [Google Scholar]
- Miller W. L. & Auchus R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews 32, 81–151, 10.1210/er.2010-0013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C. Progesterone synthesis by the human placenta. Placenta 26, 273–281, 10.1016/j.placenta.2004.06.012 (2005). [DOI] [PubMed] [Google Scholar]
- Guryev O., Carvalho R. A., Usanov S., Gilep A. & Estabrook R. W. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA 100, 14754–14759, 10.1073/pnas.2336107100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271, 4178–4188 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol 12, 931–939 (2005). [DOI] [PubMed] [Google Scholar]
- Slominski A. et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272, 4080–4090 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C. et al. Human cytochrome P450scc (CYP11A1) catalyzes epoxide formation with ergosterol. Drug Metab Dispos 40, 436–444, 10.1124/dmd.111.042515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C. et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 275, 2585–2596 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol 151, 25–37, 10.1016/j.jsbmb.2014.11.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol 144PA, 28–39, 10.1016/j.jsbmb.2013.10.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu E. W. et al. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol 129, 163–171, 10.1016/j.jsbmb.2011.11.012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu E. W. et al. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol 84, 1696–1704, 10.1016/j.bcp.2012.09.032 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu E. W. et al. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol 149, 153–165, 10.1016/j.jsbmb.2015.02.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E. K. et al. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos 38, 1553–1559, 10.1124/dmd.110.034389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E. K. et al. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos 41, 1112–1124, 10.1124/dmd.113.050955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B. et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol 128, 2271–2280 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z. et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One 4, e5988 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z., Tuckey R. C., Nguyen M. N., Thorpe E. M. Jr. & Slominski A. T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol 223, 36–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 75, 926–935, 10.1016/j.steroids.2010.05.021 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski M. A. et al. Synthesis and photochemical transformation of 3 beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids 76, 193–203, 10.1016/j.steroids.2010.10.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. et al. Novel vitamin D photoproducts and their precursors in the skin. Dermato-Endocrinology 5, 1–13, 10.4161/derm.23938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. et al. 20S-Hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab 98, E298–303, 10.1210/jc.2012-3074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300, C526–541, 10.1152/ajpcell.00203.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res 32, 3733–3742 (2012). [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res 32, 739–746 (2012). [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z. et al. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer 105, 1874–1884, 10.1038/bjc.2011.458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One 5, e9907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. K. et al. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol 361, 143–152, 10.1016/j.mce.2012.04.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 28, 2775–2789, 10.1096/fj.13-242040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. Novel vitamin d analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res 34, 2153–2163 (2014). [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One 4, e4309, 10.1371/journal.pone.0004309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol 44, 2003–2018, 10.1016/j.biocel.2012.07.027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J 26, 3901–3915, 10.1096/fj.12-208975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol 383, 181–192, 10.1016/j.mce.2013.12.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 137, 107–123, 10.1016/j.jsbmb.2013.02.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski M. A. et al. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci 7, 1570–1576, 10.1039/b809005j (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski M. A. et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3 beta, 17 alpha, 20-triol and their bioactivity in melanoma cells. Steroids 74, 218–228, 10.1016/j.steroids.2008.10.017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Slominski A. T., Miller D. D. & Li W. Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR. Dermatoendocrinol 5, 142–149, 10.4161/derm.24339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T. et al. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol 148, 52–63, 10.1016/j.jsbmb.2015.01.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C. et al. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos 39, 1577–1588, 10.1124/dmd.111.040071 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Slominski A. T. & Tuckey R. C. Metabolism of 20-hydroxyvitamin D3 by mouse liver microsomes. J Steroid Biochem Mol Biol 144 Pt B, 286–293, 10.1016/j.jsbmb.2014.08.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E. K., Voo K. J., Nguyen M. N. & Tuckey R. C. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1alpha-hydroxylase (CYP27B1). J Steroid Biochem Mol Biol 119, 171–179, 10.1016/j.jsbmb.2010.02.022 (2010). [DOI] [PubMed] [Google Scholar]
- Tuckey R. C. et al. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 112, 213–219, 10.1016/j.jsbmb.2008.10.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. K., Lin Z., Tidwell W. J., Li W. & Slominski A. T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol 404, 1–8, 10.1016/j.mce.2014.07.024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.