Abstract
Background
Dietary antioxidants can inhibit reactions accompanying neurodegeneration, and thus prevent cognitive impairment. We describe associations of dietary antioxidants with cognitive function in a large biracial population, while testing moderation by sex, race and age and mediation by depressive symptoms.
Methods
This was a cross-sectional analysis of 1,274 adults (541 men and 733 women) aged 30–64y at baseline (Mean±SD: 47.5±9.3) in the Healthy Aging in Neighborhoods of Diversity Across the Lifespan Study (HANDLS), Baltimore city, MD. Cognitive performance in the domains of memory, language/verbal, attention, spatial, psychomotor speed, executive function, and global mental status were assessed. The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) scale was used to measure depressive symptoms. Dietary intake was assessed with two 24-hr recalls, estimating daily consumption of total carotenoids, vitamins A, C and E, per 1,000 kcal.
Results
Among key findings, one standard deviation (SD~2.02 mg/1,000kcal) higher vitamin E was associated with a higher score on verbal memory, immediate recall, (β=+0.64±0.19, p=0.001) and better language/verbal fluency performance (β=+0.53±0.16, p=0.001), particularly among the younger age group. Women with higher vitamin E intake (β=+0.68±0.21, p=0.001) had better performance on a psychomotor speed test. The vitamin E-verbal memory association was partially mediated by depressive symptoms (proportion mediated=13–16%).
Conclusions
In sum, future cohort studies and dietary interventions should focus on associations of dietary vitamin E with cognitive decline, specifically for domains of verbal memory, verbal fluency and psychomotor speed.
Keywords: Antioxidants, cognitive function, depressive symptoms, midlife
INTRODUCTION
Impaired cognitive function, a major cause for functional disability in old age, leads to loss of independence ascribed mostly to age-related dementing illnesses, most commonly, Alzheimer’s Disease (AD). With the rise in the proportion of people over 65 years of age in the United States and elsewhere, it is expected that AD will quadruple in prevalence by 2050 to more than 100 million people worldwide, when 1 in 85 will be living with AD (1–5). However, efforts are currently under way to uncover modifiable risk factors, including dietary patterns, nutrient and antioxidant intakes, that might reduce the prevalence of cognitive impairment, dementing illnesses and AD.
Several findings suggest oxidative stress plays an important role in neurodegenerative processes accompanying cognitive impairment and dementia, particularly AD. The brain is particularly vulnerable to reactive oxygen species (ROS) as its metabolism accounts for approximately 20% of all oxygen consumption within the body. (6). The exposure of ROS has been shown to result in oxidative modification of DNA in brain tissue that in some cases has been shown to accumulate due to reductions of DNA repair. (7–9) Oxidative stress among AD patients is marked by increased antioxidant brain levels, acting as free radical scavengers. (10) In vitro studies suggest that exogenous antioxidants may reduce β-amyloids toxicity in AD patients’ brains (10–12). Dietary antioxidants, mainly β-carotene (as well as other carotenoids), vitamin C and vitamin E, were shown to inhibit lipid peroxidation (6), the production of ROS (13), apoptosis (13), and oxidative damage to protein (14) and DNA (15). It is hypothesized that dietary antioxidants can potentially improve middle-aged adults’ cognitive performance and ultimately delay onset of AD in older age.
However, many previous studies used global cognition tests (16–20) or assessed dementia/AD diagnosis in older adults (21–24), and did not examine the association of antioxidants with various cognitive function domains in middle-age adults. It is important to test whether dietary intakes of carotenoids, vitamins A, C and E are differentially associated with areas of cognition among middle-aged US adults and to examine whether associations differ by sex and race. In addition, although many recent studies have found a protective effect of antioxidants against depressive symptoms (25, 26), and depressive symptoms have been directly related to poor cognitive functioning in middle-age (27–30), no study has assessed the mediating or moderating roles of depressive symptoms in association with antioxidants and middle-aged adults’ cognitive functioning. Indeed, dietary antioxidants may affect cognition directly through reducing oxidative stress at the neuronal level in areas of the brain that relate to memory and other cognitive domains or indirectly by affecting oxidative stress in areas that were linked to depressive symptoms which in turn can affect cognitive performance. Associations between antioxidants and cognition were shown to be significant only for specific cognitive domains in some studies (31–33) while other studies have shown that those associations were restricted to specific socio-demographic groups or genotypes in others (34–36). Moreover, the bioavailability of many nutrients, including antioxidants, is highly dependent socio-demographic variables and thus the variable recommendations for different age and sex groups. (37–40). Therefore, it is hypothesized that sex, race and age may moderate the association between antioxidants and cognition while depressive symptoms may act as a mediating factor.
In this report we describe the adjusted associations between dietary antioxidants and cognitive function in various cognitive domains in a large biracial population, while examining socio-demographic differences in those associations and the mediating role of depressive symptoms.
METHODS
Database and study sample
The HANDLS study, an ongoing prospective cohort study, recruited a representative sample of African Americans and whites (30–64 years old) at baseline, living in Baltimore, Maryland, using an area probability sampling design of 13 census segments. The initial phase of HANDLS involved screening, recruitment and household interviews, while phase 2 included examinations in mobile Medical Research Vehicles (MRV). (41) Initiated in 2004, HANDLS completed baseline data in 2009 and recently completed the first follow-up wave data. The protocol of this study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences. The present study uses baseline HANDLS cohort cross-sectional data (i.e. phases 1 and 2).
Of 3,720 participants sampled in phase 1 (Sample 1), complete phase 2 examination data at baseline was available for 2,802 (Sample 2a). This study included only participants with two days of dietary recall and CES-D data (n=1,739; Sample 2b). Notable income level and sex differences between Sample 2b and Sample 1 were found (51.9% above poverty, 43.2% are men in Sample 2b vs. 64.8% above poverty and 47.1% are men in remaining subjects in Sample 1), with participants in Sample 2b having higher African-American representation (i.e. 67.7% vs. 55.9%). Sample size of participants with complete and reliable cognitive tests (main outcome) as well as predictor/covariate variables was 1,274 participants (Sample 3). Sample 3 did not differ from the remaining group of Sample 1 participants on sex, age, race or poverty/income ratio distribution.
Cognitive assessment
Nine cognitive tests resulting in 13 test scores that cover 7 domains (Global, attention, learning/memory, executive function, visuo-spatial/visuo-construction ability, psychomotor speed, language/verbal) were outcomes: the Mini-Mental State Examination (MMSE), the California Verbal Learning Test immediate free recall, List A (CLVT-List A) and Delayed Free Recall (CVLT-DFR), Digit Span Forward and Backwards tests (DS-F and DS-B), the Benton Visual Retention Test (BVRT), Animal Fluency test (AF), Brief Test of Attention (BTA), Trailmaking test, parts A and B (Trails A and B), Clock Drawing Test (CDT), Card Rotations (CR) and Identical Pictures (IP) (See Supplemental Digital Content 1 for full tests and score descriptions). Participants ability to undergo informed consent was evaluated through probing for protocol comprehension. Although formal dementia diagnoses were not performed, participants were administered mental status tests, which they completed at adequate levels indicative of normal cognition. Low mental status performance was consistently due to poor literacy skills with no signs of dementia.
For those participants unable to understand a test for cognitive reasons, scores were set to the total sample maximum or minimum, corresponding to the poorest cognitive performance. Scores were considered unreliable and set to missing if participants had sensory problems that precluded them from reliably completing the test.
Dietary assessment
Trained interviewers administered two 24-hr dietary recalls using the U.S. Department of Agriculture’s (USDA) Automated Multiple Pass Method (AMPM), a standardized 5-step process validated for protein, carbohydrate, fat and energy intakes in obese and non-obese individuals (42–44). A database converted grams of USDA food codes into nutrients consumed per day (45). The average of the two recalls was considered after nutrient intakes were summed for each individual per recall day.
Four exposures of interest were investigated: Vitamins A, C and E, divided by energy intake and expressed as retinol equivalent, RE/1,000 kcal/d, mg/1,000 kcal/d, and mg/1,000 kcal/d, respectively, and the sum of five carotenoids (α-carotene, β-carotene, lutein+zeaxanthin, β-cryptoxanthin and lycopene) termed “total carotenoids” and expressed as µg per 1,000 kcal of dietary intake per day.
Depressive symptom assessment
The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) scale was used to measure baseline depressive symptoms emphasizing affective and depressed mood (46). CES-D total score was used in all analyses. CES-D was previously shown to have an invariant factor structure between The National Health and Nutrition Examination Survey I and HANDLS data, with four distinct components emerging in both surveys (47).
Covariates
Socio-demographic, lifestyle and health-related potential confounders
The socio-demographic and lifestyle factors age, sex, race/ethnicity (White vs. African American), marital status (married vs. unmarried), completed years of education (<High School (HS); HS and >HS), poverty income ratio (PIR<125% for “poor”), measured body mass index (kg/m2), lifetime drugs use (opiates, marijuana or cocaine” vs. not), and smoking status (0: “never or former smoker” and 1 “current smoker” were included in our analyses as potential confounders.. The Wide Range Achievement Test Letter and Word Reading (WRAT) total score (48) was added to multivariate models as a literacy measure.
Dietary potential confounders
Potential confounding by nutrients formerly linked to cognitive performance among other health outcomes was also adjusted for in multivariate models. These nutrients, expressed as per 1000 kcal of energy intake and entered into models as standardized z-scores, included specific B-vitamins (B-6, B-12 and folate) (49–59) and n-3 highly unsaturated fatty acids (% of energy) (17, 60–69). To emulate a multivariate nutrient density model (70), total energy intake was included as a potentially confounding variable.
Statistical Analyses
Stata release 13.0 was used. (71) First, two-sided independent-samples t-tests compared means across binary variables, whereas χ2 test was conducted to examine relationships between categorical variables. Second, multiple ordinary least square (OLS) models were conducted to evaluate independent predictors of each dietary antioxidant exposure. Four exposures of interest included: total dietary intakes of total carotenoids, vitamin A, vitamin C and vitamin E (α-tocopherol), divided by total energy intake and multiplied by a factor of 1000 (i.e. per 1000 kcal). Third, multiple OLS (most outcomes) and Poisson (MMSE total error count) regression models were conducted to determine the association between dietary antioxidants and individual cognitive scores. In this main part of the analyses, dietary antioxidant exposures were expressed as standardized z-score and interpreted as a 1 SD increase in their value. In all multivariate analyses, adjustment was made on other socio-demographic, lifestyle and health-related, and selected dietary covariates. Key findings regarding significant covariates predicting each of the cognitive test score outcomes in those model were also presented. Sex, race and age group (<median vs. ≥median) were considered as potential effect modifiers in the associations between antioxidants and cognitive test scores. In a separate model, 2-way interaction terms between each antioxidant and each of the putative effect modifiers were added and tested for significance, while retaining the main effects.
Each of the 13 cognitive variables was considered as an endogenous variable that was potentially associated with both CES-D total score and dietary antioxidants. To test mediation, two methods were used. First, structural equations models (SEM) were carried out where antioxidants, socio-demographic, lifestyle and health-related factors were exogenous to CES-D and each of the three domain scores separately (See equations 1.1–1.4, 2 and 3).
| Eq. 1.1–1.4 |
| Eq. 2. |
| Eq. 3. |
Where X is the main dietary exposure variable (each antioxidant per 1,000 kcal, z-score), i ranges from 1 to 4, j is the number of covariate terms included, CS stands for cognitive score with l ranging from 1 to 13 and Zj is a vector of socio-demographic, lifestyle, health-related and dietary exogenous variables. Assuming additivity between each antioxidant exposure and the CES-D score, a mediation proportion (MP) was computed as the percent of total effect of each antioxidant on each cognitive test that is indirectly explained through CES-D: Mediation Proportion (MP)=(indirect effect)*100/(total effect). Based on Eq. 1.1–1.4, 2 and 3: α31=direct effect; α21×α32=indirect effect; total effect= α31 + α21×α32. (72, 73) The significance of the mediation proportion was ascertained using the Sobel-Goodman (S-G) test, with a type I error 0.05. (74) Details about this method are discussed elsewhere. (75)
Second, when relaxing the assumption of addivitiy (RAA) between each antoxidant exposure and the CES-D score by including an interaction term, we further computed four estimates with their SEE and p-values, namely the controlled direct effect (CDE), the natural direct effect (NDE), the natural indirect effect (NIE) and the marginal total effect (MTE). Details about this latter approach are provided elsewhere. (76) The CDE is the effect of setting X to 1 versus 0 (i.e. 1 SD higher than the mean vs. the mean) while controlling M to some defined reference value m. In this case, M is the continuous CES-D score which is set at a value close to the mean, namely 11.0. The NDE is the same setting of the exposure X, but this time M (CES-D score) is set not to a single pre-defined value m, but instead a value that is potentially distinct for every person in the data set. It is the value that m would have taken at the referent value of the exposure (in this case, the exposure level that is at the mean). The NIE is the outcome contrast observed when holding exposure constant at the mean, and contrasting two different M values: the value of the CES-D score that would be observed for that person under the X value of the mean and the value of CES-D that would be observed for that person under the 1 SD higher X value. The total effect is the sum of the NIE and the NDE. It is the total effect of varying X by 1 SD, irrespective of M (or the CES-D score). (76) Using NIE and MTE, a mediation proportion can be estimated as MP = (NIE*100)/MTE. (77) In all three approaches (i.e. SEM, S-G test and RAA), only results with significant total effects of antioxidants vs. cognitive tests, controlling for the other covariates, were assessed for mediation.
Potential selection bias in OLS, logistic and Poisson regression models was accounted for using a two-stage Heckman selection model (78), in which an inverse mills ratio was added to all equations and models after predicting the probability of being selected conditional on baseline pseudo-complete (N~3, 720) socio-demographic variables such as age, sex, marital status and smoking status (79).
In all analyses, a type I error of 0.05, while p<0.10 was considered significant for interaction terms, prior to correction for multiple testing. Using a familywise Bonferroni procedure, we corrected for multiple testing taking into account only cognitive tests, assuming that hormonal exposures are linked to separate substantive hypotheses. (80) Therefore, for main effects, p<0.004 was deemed significant for cognitive test performance vs. antioxidants hypotheses (13 test scores) but a type I error of 0.05 was used for all other hypotheses. Due to their lower statistical power compared to main effects, interaction terms had critical p-values reduced to 0.05.
RESULTS
Study characteristics by sex, race and age group (<median=48y vs. ≥median) are shown in Table 1. Women (vs. men), African-Americans (vs. Whites) and younger participants (vs. older) were more likely to be below poverty. African-Americans and younger participants had on average lower educational attainment and literacy compared to Whites and older participants, respectively. Men and younger participants were more likely to be current smokers than women and older participants, respectively. Similarly, African-Americans, men and younger participatns had a higher prevalence of illicit drug ever use compared to Whites, women and older participants, respectively. Average BMI was higher among women than men. Men, Whites and younger individuals were more likely to be married compared to women, African-Americans and older inidividuals, respectively.
Table 1.
Selected study participant characteristics by sex and race/ethnicity (n=1,274); HANDLS study1
| All | Men | Women | Whites | African- Americans |
Age<48y | Age≥48 | P2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Men vs. women |
Whites vs. African- Americans |
Age<48 vs.≥48 |
||||||||
| N | 1,274 | 541 | 733 | 513 | 761 | 621 | 653 | |||
| Age | 47.5±9.3 | 47.8±9.3 | 47.3±9.5 | 47.5±9.5 | 47.5±9.1 | 39.5±5.2 | 55.1±4.8 | 0.36 | 1.00 | <0.001 |
| Men, % | 42.5 | __ | __ | 40.3 | 43.9 | 40.1 | 44.7 | __ | 0.21 | 0.10 |
| African-American, % | 59.7 | 61.7 | 58.2 | __ | __ | 60.0 | 59.6 | 0.21 | __ | 0.90 |
| Married, % | 53.3 | 60.6 | 47.9 | 60.3 | 48.6 | 58.6 | 48.1 | <0.001 | <0.001 | <0.001 |
| Education, % | 0.44 | <0.001 | 0.005 | |||||||
| <HS | 6.4 | 7.4 | 5.7 | 8.9 | 4.7 | 5.0 | 7.8 | |||
| HS | 58.8 | 57.5 | 59.8 | 49.1 | 65.3 | 63.1 | 54.7 | |||
| >HS | 34.8 | 35.1 | 34.5 | 41.9 | 30.0 | 31.9 | 37.5 | |||
| Literacy (WRAT score) | 42.5±7.8 | 42.4±8.5 | 42.6±7.2 | 45.5±7.3 | 40.5±7.4 | 42.7±7.8 | 42.3±7.8 | 0.71 | <0.001 | 0.31 |
| PIR <125%, % | 48.7 | 43.6 | 52.5 | 36.6 | 56.9 | 52.7 | 45.0 | 0.002 | <0.001 | 0.006 |
| Current smoking status, % | 0.001 | 0.31 | 0.002 | |||||||
| Currently smoking | 43.5 | 49.3 | 39.2 | 40.9 | 45.1 | 44.6 | 41.3 | |||
| Missing | 9.3 | 8.7 | 9.7 | 9.9 | 8.8 | 5.6 | 4.3 | |||
| Ever use of illicit drugs, % | <0.001 | 0.034 | <0.001 | |||||||
| Used any type | 58.9 | 67.8 | 52.4 | 54.6 | 61.9 | 63.3 | 54.8 | |||
| Missing | 7.6 | 7.2 | 7.9 | 8.4 | 7.1 | 9.2 | 6.1 | |||
| Body mass index, kg.m−2 | 29.7±7.7 | 27.9±6.0 | 31.1±8.5 | 29.9±7.7 | 29.6±7.7 | 29.4±7.7 | 30.1±7.3 | <0.001 | 0.58 | 0.09 |
| Dietary intake, per 1,000 kcal/d | ||||||||||
| Total carotenoids, mg | 4.09±4.83 | 3.78±4.61 | 4.31±4.98 | 4.51±5.12 | 3.81±4.61 | 4.04±4.99 | 4.13±4.68 | 0.05 | 0.011 | 0.74 |
| Vitamin A, RE | 344±541 | 312±508 | 367±564 | 330±253 | 353±669 | 326±586 | 360±496 | 0.07 | 0.45 | 0.26 |
| Vitamin C, mg | 39.3±40.4 | 36.5±37.5 | 41.4±42.3 | 35.3±35.6 | 42.1±43.2 | 39.9±39.2 | 39.36±41.51 | 0.030 | 0.003 | 0.77 |
| Vitamin E, mg | 3.34±2.02 | 3.08±1.62 | 3.54±2.25 | 3.59±2.07 | 3.17±1.97 | 3.24±1.81 | 3.44±2.19 | <0.001 | <0.001 | 0.07 |
| Vitamin B-6, mg | 0.92±0.47 | 0.93±0.45 | 0.92±0.48 | 0.95±0.53 | 0.91±0.42 | 0.89±0.43 | 0.95±0.50 | 0.77 | 0.12 | 0.033 |
| Vitamin B-12, mg | 3.19±5.64 | 3.18±5.74 | 3.20±5.57 | 2.91±3.25 | 3.38±6.79 | 3.19±6.3 | 3.19±4.95 | 0.97 | 0.15 | 0.99 |
| Folate, mg | 187±102 | 182±103 | 191±102 | 205±111 | 175±95 | 179±93 | 194±110 | 0.09 | <0.001 | 0.011 |
| n-3 HUFA, % energy | 0.10±0.27 | 0.09±0.32 | 0.11±0.21 | 0.07±0.13 | 0.13±0.32 | 0.10±0.31 | 0.10±0.21 | 0.27 | <0.001 | 0.99 |
| Depressive symptoms | ||||||||||
| CES-D score | 11.2±8.0 | 10.3±7.2 | 11.9±8.9 | 11.2±8.4 | 11.2±7.7 | 11.5±7.8 | 10.9±8.2 | <0.001 | 0.95 | 0.13 |
| Cognitive function test scores | ||||||||||
| MMSE, error count | 2.16±2.06 | 2.36±2.23 | 2.02±1.92 | 1.72±2.01 | 2.45±2.05 | 2.01±1.90 | 2.29±2.19 | 0.003 | <0.001 | 0.018 |
| CVLT, List A | 25.1±6.7 | 23.7±6.4 | 26.2±6.8 | 27.2±7.2 | 23.7±6.0 | 26.1±6.9 | 24.2±6.5 | <0.001 | <0.001 | <0.001 |
| CVLT, DFR | 7.39±3.21 | 6.81±3.07 | 7.82±3.25 | 8.55±3.27 | 6.61±2.93 | 7.91±3.23 | 6.90±3.12 | <0.001 | <0.001 | <0.001 |
| BVRT, error count | 6.12±4.93 | 5.49±4.84 | 6.58±4.95 | 5.72±4.65 | 6.38±5.10 | 5.32±4.58 | 6.88±5.13 | <0.001 | 0.019 | <0.001 |
| Digit span backwards | 5.68±2.15 | 5.72±2.26 | 5.65±2.06 | 6.31±2.27 | 5.25±1.95 | 5.77±2.19 | 5.59±2.10 | 0.52 | <0.001 | 0.14 |
| Digit span forward | 7.30±2.16 | 7.42±2.21 | 7.21±2.12 | 7.70±2.22 | 7.03±2.08 | 7.41±2.19 | 7.19±2.13 | 0.10 | <0.001 | 0.07 |
| Animal fluency | 18.9±5.5 | 19.6±5.5 | 18.4±5.4 | 20.2±6.0 | 18.0±4.9 | 19.6±5.7 | 18.2±5.2 | <0.001 | <0.001 | <0.001 |
| Brief test of Attention | 6.63±2.21 | 6.46±2.17 | 6.76±2.24 | 7.18±2.06 | 6.26±2.24 | 6.80±2.09 | 6.47±2.33 | 0.017 | <0.001 | 0.009 |
| Trailmaking test, A, sec. | 36.1±31.5 | 36.9±28.6 | 35.4±33.5 | 30.9±11.5 | 39.5±39.3 | 31.9±26.1 | 40.0±35.4 | 0.40 | <0.001 | <0.001 |
| Trailmaking test, B, sec. | 147±157 | 153±163 | 142±152 | 108±123 | 173±171 | 128±143 | 165±169 | 0.21 | <0.001 | <0.001 |
| Card rotation | 34.1±18.7 | 37.9±18.3 | 31.3±18.6 | 40.4±19.0 | 29.8±17.3 | 37.3±19.4 | 31.0±17.6 | <0.001 | <0.001 | <0.001 |
| Identical pictures | 23.6±6.6 | 22.9±6.6 | 24.1±6.6 | 25.7±6.8 | 22.1±6.1 | 26.0±6.6 | 21.3±5.8 | 0.001 | <0.001 | <0.001 |
| Clock drawing test | 8.84±1.20 | 8.92±1.18 | 8.77±1.22 | 9.05±1.17 | 8.70±1.20 | 8.84±1.20 | 8.84±1.20 | 0.026 | <0.001 | 0.99 |
Key BVRT=Benton Visual Retention Task; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Task; DFR=Delayed Free Recall; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; HS=High School; HUFA=highly unsaturated fatty acids; MMSE=Mini-Mental State Examination; PIR=Poverty Income Ratio; WRAT=Wide Range Achievement Test.
Values are mean±SD or percent.
P-value was based on 2-sided independent samples t-test when row variable is continuous and χ2 when row variable is categorical.
Women had higher dietary intakes than men in vitamins C and E per 1,000 kcal, whereas Whites had higher intakes of total carotenoids, vitamin E, folate than African-Americans but lower intakes of n-3 HUFA (%energy) and vitamin C. Older participants had higher intakes of vitamin B-6 and folate compared to their younger counterparts. Mean CES-D score was higher among women compared to men (11.9 vs. 10.3, p<0.001), though no significant differences were noted by race or age. Women outperformed men on the MMSE, CVLT-List A and CVLT-DFR, the BTA and IP. Men performed better on BVRT, AF, CR and CDT. No sex differences were detected for DS-F, DS-B, Trails A or B. Whites outperformed African-Americans on all cognitive tests, and older participants had a poorer performance than younger participants, on all tests except Digits span backwards and forward as well as the clock drawing test.
When examining the relationship between CES-D score and all other variables (Table S1, Supplemental Digital Content 2), certain patterns emerged. Importantly, a higher level of depressive symptoms was associated with poorer cognitive performance on all tests, with the exception of Trailmaking Test, A. Moreover, depressive symptoms were inversely but weakly related to age, they were significantly higher among women, and among individuals with lower educational attainment, income and literacy. Current smokers and unmarried individuals also had higher levels of depressive symptoms compared to non-smokers and married participants, respectively. Among dietary intake covariates, only total carotenoids were found to be significantly and inversely related to CES-D.
Table 2 presents findings from a series of OLS regression models independent associations between socio-demographic, lifestyle and dietary correlates with the main dietary antioxidant exposures. Among key findings, age and education were positively linked to vitamin E intake per 1,000 kcal/d, African-Americans had higher intakes of vitamin C compared to Whites, whereas men had lower intakes of both vitamins A and E compared to women. Current smokers had lower intake of vitamin C which was also inversely related to BMI and ever use of drugs but positively associated with total energy intake.
Table 2.
Socio-demographic, lifestyle and dietary correlates of each of the selected dietary antioxidants: multiple OLS regression models (N=1,274); HANDLS study
| Total carotenoids, µg per 1,000 kcal/d |
Vitamin A, RE per 1,000 kcal/d |
Vitamin C, mg per 1,000 kcal/d |
Vitamin E, mg per 1,000 kcal/d |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (SEE) | Pwald | β | (SEE) | P | β | (SEE) | Pwald | β | (SEE) | Pwald | |
| Age | −7.5 | (13.0) | 0.56 | +1.83 | (0.79) | 0.020 | −0.19 | (0.11) | 0.09 | +0.01 | (0.01) | 0.18 |
| Men vs. Women | +322.4 | (264.4) | 0.22 | −35.1 | (16.0) | 0.029 | +0.20 | (2.26) | 0.93 | −0.42 | (0.11) | <0.001 |
| African-American vs. White | −512.4 | (265.4) | 0.05 | +28.0 | (16.1) | 0.08 | +11.9 | (2.2) | <0.001 | −0.16 | (0.11) | 0.14 |
| Married vs. Unmarried | −44.6 | (46.8) | 0.34 | −2.2 | (2.8) | 0.44 | +2.1 | (2.2) | 0.33 | −0.03 | (0.02) | 0.16 |
| Education | ||||||||||||
| <HS | Ref | Ref | Ref | Ref | ||||||||
| HS | +298.0 | (502.1) | 0.55 | −1.2 | (30.5) | 0.97 | +0.35 | (4.29) | 0.94 | −0.03 | (0.20) | 0.87 |
| >HS | +132.5 | (545.2) | 0.81 | −8.1 | (33.2) | 0.81 | −1.34 | (4.66) | 0.77 | +0.45 | (0.22) | 0.041 |
| Missing | +1,010.1 | (896.2) | 0.26 | −12.8 | (54.5) | 0.82 | −6.97 | (7.66) | 0.36 | +0.43 | (0.37) | 0.24 |
| Literacy (WRAT score) | +27.6 | (17.6) | 0.12 | −0.65 | (1.07) | 0.55 | +0.08 | (0.15) | 0.60 | +0.01 | (0.01) | 0.06 |
| PIR <125% | −363.5 | (255.4) | 0.16 | +2.22 | (15.55) | 0.89 | +2.10 | (2.18) | 0.34 | +0.03 | (0.10) | 0.75 |
| Current smoking status | ||||||||||||
| Currently smoking | +10.9 | (278.3) | 0.97 | −19.1 | (16.9) | 0.26 | −5.6 | (2.4) | 0.019 | −0.20 | (0.11) | 0.08 |
| Missing | +564.7 | (939.3) | 0.55 | −40.9 | (57.1) | 0.47 | +2.0 | (8.7) | 0.81 | −0.36 | (0.38) | 0.34 |
| Ever use of illicit drugs | ||||||||||||
| Never used | Ref | Ref | Ref | Ref | ||||||||
| Used any type | −232.2 | (276.8) | 0.40 | −19.1 | (16.9) | 0.26 | −5.4 | (2.3) | 0.023 | +0.07 | (0.11) | 0.51 |
| Missing | −619.4 | (1,018.6) | 0.54 | −40.9 | (57.1) | 0.47 | +2.0 | (8.7) | 0.81 | +0.59 | (0.42) | 0.16 |
| Body mass index, kg.m−2 | +2.90 | (16.4) | 0.86 | −0.75 | (1.00) | 0.45 | −0.12 | (0.14) | 0.38 | −0.004 | (0.007) | 0.50 |
| Energy, kcal/d | −0.10 | (0.13) | 0.46 | −0.01 | (0.01) | 0.19 | −0.003 | (0.001) | 0.006 | +0.000 | (0.000) | 0.05 |
| Total carotenoids, µg per 1,000 kcal/d | __ | +0.02 | (0.00) | <0.001 | +0.001 | (0.000) | <0.001 | |||||
| Vitamin A, RE per 1,000kcal/d | +5.27 | (0.44) | <0.001 | __ | +0.007 | (0.004) | 0.06 | −0.000 | (0.000) | 0.95 | ||
| Vitamin C, mg per 1,000kcal/d | +19.07 | (3.27) | <0.001 | +0.37 | (0.20) | 0.06 | __ | +0.006 | (0.001) | <0.001 | ||
| Vitamin E, mg per 1,000kcal/d | +508.2 | (67.8) | <0.001 | −0.28 | (4.22) | 0.95 | +2.66 | (0.59) | <0.001 | __ | ||
| Vitamin B-6, mg per 1,000kcal/d | +227.0 | (347.8) | 0.51 | −75.8 | (21.0) | <0.001 | +15.7 | (2.9) | <0.001 | +0.92 | (0.14) | <0.001 |
| Vitamin B-12, mg per 1,000kcal/d | −490.4 | (42.1) | <0.001 | +82.2 | (1.4) | <0.001 | −1.12 | (0.38) | 0.003 | −0.02 | (0.02) | 0.34 |
| Folate, mg per 1,000kcal/d | +2.45 | (1.62) | 0.13 | +0.55 | (0.10) | <0.001 | +0.04 | (0.01) | 0.002 | +0.003 | (0.001) | <0.001 |
| n-3 HUFA, % energy/d | +2,343 | (476.6) | <0.001 | −309.8 | (27.9) | <0.001 | −0.12 | (4.11) | 0.98 | +0.62 | (0.20) | 0.002 |
Key HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; HS=High School; HUFA=Highly Unsaturated Fatty Acids; OLS=Ordinary Least Square; PIR=Poverty Income Ratio; RE=Retinol Equivalent; SEE=Standard error of the estimate; WRAT=Wide Range Achievement Test.
Among dietary correlates of four antioxidant exposures, total carotenoids were positively associated with intakes of vitamin A, C and E, n-3 HUFA (% energy) but inversely related to vitamin B-12 (per 1,000 kcal/d). Vitamin A was positively related to vitamin B-12 and folate intakes (and total carotenoids), but inversely related to vitamin B-6 and n-3 HUFA (%energy) intakes. We additionally detected a positive relationship between vitamin C intake and vitamins E, B-6 and folate intakes, and an inverse relationship with vitamin B-12 intake. Finally, vitamins B-6, folate and n-3 HUFA (in addition to vitamin C) were positively and independently related to vitamin E intake.
Table 3 displays associations between the four dietary antioxidant exposures and cognitive performance in separate models, based on multiple regression analyses. Among those exposures, vitamin C was not associated with cognitive performance in the total population. After Bonferroni correction, 1 SD (~2.02 mg) higher intake of vitamin E per 1,000 kcal was associated with a 0.64-point higher score on the CVLT-List A (reflecting the verbal memory domain, p=0.001), independently of other antioxidants, dietary and socio-demographic, lifestyle and health-related factors included in the model. Moreover, higher intake of vitamin E in the diet was linked to better performance on AF (reflecting language/verbal test performance, β=+0.53, p=0.001). Table 3 also presents sub-group analyses by sex. Although the associations between vitamin E and verbal memory and fluency were restricted to women, there was no statistically significant effect modification by sex. Furthermore, vitamin E was positively associated with performance on IP (reflecting psychomotor speed) among women after familywise Bonferroni correction (β=+0.68, p=0.001). Moreover, Table S2 (Supplemental Digital Content 3) shows findings from covariates entered into the model for the total population, presenting only those that were statistically significant at a type I error 0.05. Aside from the findings for age, sex and race which were consistent with the bivariate results in Table 1, other notable findings included the following: Cognitive performance was better with higher literacy (all tests), higher educational attainment (MMSE, CVLT-List A, BVRT, AF, Trails B and IP), above poverty income (MMSE, BVRT, AF, BTA, Trails A and B, IP), lower CES-D score (all tests), higher BMI (CVLT-List A and CVLT-DFR), lower vitamin B-12 intake (BVRT), drug users vs. not (AF, BTA, Trails B) and higher energy intake (CR and IP).
Table 3.
Cognitive function test scores by SD increase in dietary antioxidant intake: OLS and poisson regression models (all and stratified by sex): HANDLS study1
| All (N=1,274) |
Men (N=541) |
Women (N=733) |
Psex2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | ||
| MMSE, error count | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −0.02 | (0.02) | 0.38 | −0.03 | (0.03) | 0.33 | −0.02 | (0.03) | 0.64 | 0.38 |
| Vitamin A, per 541 RE/1,000 kcal/d | −0.05 | (0.04) | 0.26 | −0.09 | (0.06) | 0.15 | −0.00 | (0.06) | 0.97 | 0.65 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.03 | (0.02) | 0.17 | +0.03 | (0.03) | 0.32 | +0.03 | (0.03) | 0.35 | 0.74 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | −0.03 | (0.03) | 0.26 | −0.02 | (0.04) | 0.68 | −0.05 | (0.03) | 0.14 | 0.77 |
| CVLT, List A | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −0.32 | (0.19) | 0.08 | −0.47 | (0.27) | 0.09 | −0.20 | (0.26) | 0.44 | 0.42 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.18 | (0.34) | 0.59 | +0.31 | (0.48) | 0.52 | +0.12 | (0.51) | 0.82 | 0.65 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | −0.02 | (0.18) | 0.90 | −0.05 | (0.28) | 0.85 | −0.06 | (0.25) | 0.82 | 1.00 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.64 | (0.19) | 0.001 | +0.48 | (0.34) | 0.16 | +0.72 | (0.24) | 0.002 | 0.38 |
| CVLT, DFR | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −0.09 | (0.09) | 0.29 | −0.18 | (0.13) | 0.19 | −0.03 | (0.13) | 0.82 | 0.74 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.23 | (0.17) | 0.17 | +0.54 | (0.23) | 0.021 | −0.01 | (0.25) | 0.97 | 0.22 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.03 | (0.09) | 0.70 | −0.00 | (0.13) | 0.98 | +0.04 | (0.12) | 0.73 | 0.75 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.22 | (0.09) | 0.007 | +0.31 | (0.16) | 0.06 | +0.22 | (0.12) | 0.06 | 0.78 |
| BVRT, error count | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.11 | (0.15) | 0.44 | +0.11 | (0.22) | 0.61 | −0.00 | (0.20) | 1.00 | 0.47 |
| Vitamin A, per 541 RE/1,000 kcal/d | −0.67 | (0.27) | 0.013 | −0.52 | (0.39) | 0.18 | −0.61 | (0.40) | 0.13 | 0.41 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.00 | (0.14) | 1.00 | −0.05 | (0.22) | 0.81 | +0.11 | (0.19) | 0.57 | 0.75 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | −0.29 | (0.15) | 0.06 | +0.12 | (0.27) | 0.66 | −0.48 | (0.19) | 0.010 | 0.09 |
| Digit span backwards | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.07 | (0.06) | 0.24 | +0.10 | (0.10) | 0.30 | +0.11 | (0.08) | 0.16 | 0.35 |
| Vitamin A, per 541 RE/1,000 kcal/d | −0.08 | (0.11) | 0.51 | −0.20 | (0.17) | 0.23 | +0.01 | (0.15) | 0.95 | 0.33 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.03 | (0.06) | 0.65 | −0.05 | (0.10) | 0.64 | +0.06 | (0.07) | 0.44 | 0.33 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.05 | (0.06) | 0.41 | +0.13 | (0.12) | 0.26 | +0.02 | (0.07) | 0.78 | 0.55 |
| Digit span forward | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.03 | (0.06) | 0.61 | +0.10 | (0.10) | 0.30 | +0.00 | (0.09) | 0.97 | 0.30 |
| Vitamin A, per 541 RE/1,000 kcal/d | −0.19 | (0.12) | 0.11 | −0.28 | (0.17) | 0.11 | −0.15 | (0.17) | 0.39 | 0.17 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | −0.08 | (0.06) | 0.18 | −0.08 | (0.10) | 0.41 | −0.09 | (0.08) | 0.25 | 0.55 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.08 | (0.07) | 0.18 | +0.12 | (0.12) | 0.34 | +0.09 | (0.08) | 0.28 | 0.53 |
| Animal fluency | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.04 | (0.16) | 0.81 | +0.39 | (0.25) | 0.12 | −0.15 | (0.22) | 0.50 | 0.21 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.48 | (0.29) | 0.11 | +0.21 | (0.44) | 0.63 | +0.58 | (0.43) | 0.18 | 0.49 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | −0.02 | (0.16) | 0.92 | −0.27 | (0.25) | 0.29 | +0.10 | (0.21) | 0.63 | 0.66 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.53 | (0.16) | 0.001 | +0.48 | (0.30) | 0.11 | +0.52 | (0.20) | 0.009 | 0.93 |
| Brief test of Attention | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −0.00 | (0.07) | 0.99 | −0.13 | (0.10) | 0.21 | +0.08 | (0.09) | 0.34 | 0.09 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.18 | (0.12) | 0.14 | +0.09 | (0.17) | 0.59 | +0.27 | (0.18) | 0.14 | 0.42 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | −0.05 | (0.06) | 0.48 | +0.03 | (0.10) | 0.77 | −0.13 | (0.09) | 0.15 | 0.61 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | −0.04 | (0.07) | 0.59 | −0.01 | (0.12) | 0.94 | −0.05 | (0.08) | 0.57 | 0.81 |
| Trailmaking test, A, sec. | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.69 | (1.00) | 0.49 | +1.12 | (1.41) | 0.43 | +0.20 | (1.46) | 0.89 | 0.80 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.26 | (1.85) | 0.89 | −0.83 | (2.45) | 0.73 | +1.22 | (2.88) | 0.67 | 0.66 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | −1.25 | (0.98) | 0.20 | −1.33 | (1.41) | 0.34 | −1.09 | (1.38) | 0.43 | 0.76 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.88 | (1.03) | 0.39 | +0.29 | (1.71) | 0.87 | +0.98 | (1.34) | 0.46 | 0.69 |
| Trailmaking test, B, sec. | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +8.50 | (4.64) | 0.07 | +4.77 | (7.26) | 0.51 | +7.93 | (6.22) | 0.20 | 0.77 |
| Vitamin A, per 541 RE/1,000 kcal/d | −9.86 | (8.53) | 0.25 | −11.8 | (12.6) | 0.34 | −8.04 | (12.24) | 0.51 | 0.48 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +3.34 | (4.53) | 0.46 | +3.10 | (7.25) | 0.67 | +3.63 | (5.88) | 0.54 | 0.99 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | −2.60 | (4.75) | 0.58 | +8.44 | (8.81) | 0.34 | −8.92 | (5.70) | 0.12 | 0.60 |
| Card rotation | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −1.14 | (0.53) | 0.033 | −0.66 | (0.82) | 0.42 | −1.31 | (0.72) | 0.07 | 0.97 |
| Vitamin A, per 541 RE/1,000 kcal/d | +1.07 | (0.98) | 0.28 | −0.54 | (1.43) | 0.70 | +2.20 | (1.43) | 0.12 | 0.41 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.19 | (0.52) | 0.71 | −0.01 | (0.82) | 0.99 | +0.05 | (0.68) | 0.94 | 0.88 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +1.05 | (0.55) | 0.05 | +0.27 | (0.99) | 0.79 | +1.40 | (0.66) | 0.035 | 0.43 |
| Identical pictures | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | −0.13 | (0.17) | 0.44 | −0.33 | (0.26) | 0.20 | +0.02 | (0.23) | 0.94 | 0.20 |
| Vitamin A, per 541 RE/1,000 kcal/d | +0.25 | (0.31) | 0.42 | −0.08 | (0.45) | 0.86 | +0.61 | (0.45) | 0.18 | 0.19 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.15 | (0.17) | 0.40 | +0.34 | (0.26) | 0.19 | −0.09 | (0.22) | 0.69 | 0.29 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.38 | (0.17) | 0.030 | −0.09 | (0.32) | 0.78 | +0.68 | (0.21) | 0.001 | 0.13 |
| Clock drawing test | ||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | +0.03 | (0.04) | 0.48 | +0.08 | (0.06) | 0.16 | +0.01 | (0.05) | 0.90 | 0.45 |
| Vitamin A, per 541 RE/1,000 kcal/d | −0.11 | (0.07) | 0.13 | −0.18 | (0.10) | 0.07 | −0.06 | (0.10) | 0.53 | 0.26 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | +0.04 | (0.04) | 0.37 | +0.04 | (0.06) | 0.49 | +0.01 | (0.05) | 0.90 | 0.51 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | +0.05 | (0.04) | 0.17 | −0.04 | (0.06) | 0.49 | +0.09 | (0.05) | 0.06 | 0.33 |
Key BVRT=Benton Visual Retention Task; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Task; DFR=Delayed Free Recal; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; OLS=Ordinary Least Square; SD=Standard Deviation; SEE=Standard error of the estimate; WRAT=Wide Range Achievement Test.
Multivariate OLS or poisson (MMSE error count) models adjusted for age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, every use of illicit drugs, body mass index, and selected nutrients expressed per 1,000 kcal, namely vitamin B-6, B-12, folate, n-3 HUFA and CES-D total score.
P for interaction term exposure×sex in model with exposure main effect and main effect of sex and other covariates listed above.
Table 4 shows findings from sub-group analyses by race and age group (<48y vs. ≥48y), while separately evaluating two-way interactions between dietary antioxidant exposures and these potential effect modifiers in relation to cognitive performance. Among stratum-specific associations that survived multiple testing, higher intake of vitamin E in the younger group (<48y) was linked to better performance on CVLT-List A (β=+1.06, p<0.001), CVLT-DFR (β=+0.46, p=0.001; p for interaction by age group<0.05) and AF (β=+0.77, p=0.003). Tests of effect modification only showed a few other instances of heterogeneity between strata (e.g. vitamin E vs. MMSE by race and age group; total carotenoids vs. BVRT by race). A consistent, though mariginally significant result was found whereby vitamin E was positively related to IP among the younger group (β=+0.67, p=0.016). Similarly, higher vitamin E intake was marginally related to smaller number of errors among among Whites in both MMSE and BVRT (0.004<p<0.05, with a signficant racial difference in the case of MMSE (p=0.033).
Table 4.
Cognitive function test scores by SD increase in dietary antioxidant intake: OLS and poisson regression models (stratified by race and age): HANDLS study1,2
| Prace2 | Page3 | Whites (N= 513) |
African- Americans (N=761) |
Age<48y (N=621) |
Age≥48y (N=653) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | |||
| MMSE, error count | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.11 | 0.49 | +0.06 | (0.04) | 0.15 | −0.05 | (0.03) | 0.07 | −0.01 | (0.03) | 0.67 | −0.05 | (0.03) | 0.12 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.30 | 0.92 | −0.08 | (0.10) | 0.44 | −0.05 | (0.05) | 0.36 | +0.10 | (0.06) | 0.08 | +0.03 | (0.06) | 0.60 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.59 | 0.07 | +0.06 | (0.04) | 0.12 | +0.02 | (0.03) | 0.54 | +0.01 | (0.03) | 0.76 | +0.04 | (0.03) | 0.14 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.033 | 0.042 | −0.13 | (0.05) | 0.009 | −0.01 | (0.03) | 0.67 | −0.07 | (0.04) | 0.07 | −0.00 | (0.03) | 0.93 |
| CVLT, List A | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.43 | 0.67 | −0.17 | (0.31) | 0.58 | −0.32 | (0.23) | 0.18 | −0.35 | (0.26) | 0.18 | +0.26 | (0.27) | 0.35 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.45 | 0.22 | −0.88 | (0.77) | 0.25 | +0.16 | (0.46) | 0.73 | +0.69 | (0.48) | 0.15 | −0.45 | (0.50) | 0.37 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.80 | 0.71 | −0.28 | (0.36) | 0.43 | +0.19 | (0.21) | 0.37 | +0.04 | (0.26) | 0.87 | −0.09 | (0.25) | 0.82 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.21 | 0.06 | +0.63 | (0.32) | 0.05 | +0.58 | (0.24) | 0.014 | +1.06 | (0.29) | <0.001 | +0.27 | (0.25) | 0.29 |
| CVLT, DFR | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.61 | 0.63 | −0.04 | (0.15) | 0.78 | −0.07 | (0.12) | 0.56 | −0.14 | (0.12) | 0.26 | −0.02 | (0.14) | 0.89 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.42 | 0.32 | −0.12 | (0.36) | 0.63 | +0.03 | (0.23) | 0.88 | +0.49 | (0.22) | 0.031 | −0.15 | (0.25) | 0.56 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.99 | 0.51 | −0.08 | (0.17) | 0.63 | +0.09 | (0.10) | 0.37 | +0.06 | (0.12) | 0.59 | +0.03 | (0.13) | 0.84 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.51 | 0.016 | +0.21 | (0.15) | 0.16 | +0.22 | (0.12) | 0.06 | +0.46 | (0.14) | 0.001 | +0.08 | (0.13) | 0.52 |
| BVRT, error count | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.033 | 0.24 | −0.41 | (0.20) | 0.039 | +0.42 | (0.21) | 0.046 | −0.10 | (0.19) | 0.61 | +0.29 | (0.23) | 0.23 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.63 | 0.91 | +0.34 | (0.49) | 0.49 | −1.01 | (0.41) | 0.015 | −0.53 | (0.35) | 0.13 | −0.77 | (0.43) | 0.07 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.88 | 0.53 | +0.22 | (0.23) | 0.33 | −0.04 | (0.19) | 0.82 | −0.04 | (0.19) | 0.84 | −0.01 | (0.22) | 0.95 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.12 | 0.92 | −0.53 | (0.20) | 0.009 | −0.13 | (0.21) | 0.55 | −0.13 | (0.22) | 0.56 | −0.35 | (0.22) | 0.10 |
| Digit span backwards | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.92 | 0.23 | +0.07 | (0.10) | 0.49 | +0.07 | (0.08) | 0.33 | +0.03 | (0.08) | 0.72 | +0.17 | (0.09) | 0.05 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.79 | 0.46 | −0.25 | (0.25) | 0.31 | +0.01 | (0.15) | 0.97 | −0.06 | (0.16) | 0.70 | +0.12 | (0.16) | 0.48 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.69 | 0.58 | −0.05 | (0.11) | 0.69 | +0.07 | (0.07) | 0.27 | −0.08 | (0.09) | 0.37 | −0.03 | (0.08) | 0.72 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.31 | 0.35 | +0.08 | (0.10) | 0.45 | +0.04 | (0.08) | 0.57 | −0.02 | (0.10) | 0.87 | +0.12 | (0.08) | 0.15 |
| Digit span forward | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.94 | 0.93 | −0.03 | (0.10) | 0.80 | +0.07 | (0.08) | 0.40 | −0.00 | (0.09) | 0.97 | +0.08 | (0.10) | 0.38 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.69 | 0.23 | −0.35 | (0.25) | 0.16 | −0.23 | (0.17) | 0.17 | −0.30 | (0.16) | 0.06 | −0.13 | (0.18) | 0.46 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.64 | 0.09 | −0.06 | (0.11) | 0.59 | −0.06 | (0.08) | 0.42 | +0.00 | (0.09) | 0.06 | −0.16 | (0.09) | 0.08 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.11 | 0.23 | +0.18 | (0.10) | 0.09 | +0.04 | (0.09) | 0.66 | +0.14 | (0.10) | 0.17 | +0.05 | (0.09) | 0.54 |
| Animal fluency | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.30 | 0.48 | +0.14 | (0.27) | 0.61 | −0.04 | (0.20) | 0.85 | +0.02 | (0.22) | 0.91 | +0.02 | (0.24) | 0.94 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.30 | 0.75 | +0.99 | (0.65) | 0.13 | +0.33 | (0.40) | 0.42 | +0.81 | (0.41) | 0.05 | +0.07 | (0.43) | 0.88 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.93 | 0.87 | −0.13 | (0.30) | 0.68 | −0.03 | (0.18) | 0.86 | −0.03 | (0.23) | 0.89 | +0.04 | (0.22) | 0.87 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.29 | 0.15 | +0.50 | (0.27) | 0.07 | +0.45 | (0.20) | 0.027 | +0.77 | (0.26) | 0.003 | +0.34 | (0.22) | 0.12 |
| Brief test of Attention | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.59 | 0.27 | +0.03 | (0.10) | 0.75 | +0.00 | (0.09) | 0.96 | +0.10 | (0.09) | 0.26 | −0.08 | (0.11) | 0.45 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.66 | 0.11 | −0.04 | (0.24) | 0.86 | +0.35 | (0.18) | 0.05 | +0.17 | (0.16) | 0.28 | +0.19 | (0.19) | 0.32 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.15 | 0.96 | −0.22 | (0.11) | 0.06 | +0.03 | (0.08) | 0.67 | −0.07 | (0.09) | 0.40 | −0.01 | (0.10) | 0.95 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.38 | 0.96 | +0.06 | (0.10) | 0.58 | −0.09 | (0.09) | 0.31 | +0.06 | (0.10) | 0.57 | −0.04 | (0.10) | 0.65 |
| Trailmaking test, A, sec. | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.38 | 0.88 | −0.36 | (0.54) | 0.51 | +0.88 | (1.69) | 0.60 | +1.31 | (1.17) | 0.26 | −0.64 | (1.70) | 0.71 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.72 | 0.95 | +2.58 | (1.33) | 0.05 | +0.72 | (3.34) | 0.83 | −0.78 | (2.14) | 0.72 | +2.56 | (3.11) | 0.41 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.87 | 0.96 | +0.21 | (0.61) | 0.69 | −1.98 | (1.51) | 0.19 | −0.07 | (1.18) | 0.95 | −1.82 | (1.59) | 0.25 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.07 | 0.06 | −1.00 | (0.55) | 0.07 | +1.99 | (1.70) | 0.24 | −1.60 | (1.33) | 0.23 | +2.96 | (1.57) | 0.06 |
| Trailmaking test, B, sec. | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.41 | 0.60 | −0.17 | (5.75) | 0.98 | +12.1 | (6.9) | 0.08 | +4.92 | (5.93) | 0.41 | +10.4 | (7.4) | 0.16 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.92 | 0.46 | +6.48 | (14.14) | 0.65 | −22.1 | (13.6) | 0.11 | −18.4 | (10.9) | 0.09 | +0.33 | (13.5) | 0.98 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.73 | 0.64 | +2.75 | (6.51) | 0.67 | +4.25 | (6.16) | 0.49 | +5.68 | (5.98) | 0.34 | +0.14 | (6.89) | 0.98 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.88 | 0.38 | +0.44 | (5.88) | 0.94 | −5.97 | (6.97) | 0.39 | +0.72 | (6.76) | 0.91 | −1.20 | (6.89) | 0.86 |
| Card rotation | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.37 | 0.31 | −0.46 | (0.89) | 0.61 | −1.55 | (0.68) | 0.023 | −1.55 | (0.76) | 0.041 | −0.61 | (0.78) | 0.43 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.87 | 0.13 | −0.26 | (2.19) | 0.91 | +2.07 | (1.35) | 0.13 | +1.21 | (1.40) | 0.39 | +1.14 | (1.41) | 0.42 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.43 | 0.69 | −1.03 | (1.01) | 0.31 | +0.69 | (0.61) | 0.26 | +0.15 | (0.77) | 0.84 | +0.18 | (0.72) | 0.80 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.16 | 0.97 | +1.72 | (0.91) | 0.06 | +0.68 | (0.69) | 0.32 | +1.35 | (0.86) | 0.12 | +0.66 | (0.72) | 0.36 |
| Identical pictures | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.32 | 1.00 | −0.05 | (0.27) | 0.85 | −0.31 | (0.22) | 0.17 | −0.24 | (0.24) | 0.32 | +0.00 | (0.25) | 0.99 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.08 | 0.77 | +0.52 | (0.67) | 0.44 | +0.79 | (0.44) | 0.10 | +0.30 | (0.45) | 0.50 | +0.33 | (0.45) | 0.47 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.54 | 0.11 | −0.28 | (0.31) | 0.36 | +0.33 | (0.20) | 0.10 | +0.37 | (0.25) | 0.13 | −0.08 | (0.23) | 0.74 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.05 | 0.18 | +0.63 | (0.28) | 0.028 | +0.25 | (0.22) | 0.27 | +0.67 | (0.28) | 0.016 | +0.25 | (0.23) | 0.28 |
| Clock drawing test | ||||||||||||||
| Total carotenoids, per 4.83 mg/1,000 kcal/d | 0.54 | 0.83 | +0.07 | (0.06) | 0.22 | −0.00 | (0.05) | 0.95 | +0.06 | (0.05) | 0.26 | −0.00 | (0.06) | 0.95 |
| Vitamin A, per 541 RE/1,000 kcal/d | 0.42 | 0.05 | −0.07 | (0.14) | 0.63 | −0.10 | (0.10) | 0.32 | −0.13 | (0.09) | 0.17 | −0.09 | (0.11) | 0.41 |
| Vitamin C, per 40.4 mg/1,000 kcal/d | 0.74 | 0.10 | −0.01 | (0.07) | 0.90 | +0.06 | (0.05) | 0.17 | +0.02 | (0.05) | 0.72 | +0.10 | (0.05) | 0.07 |
| Vitamin E, per 2.02 mg/1,000 kcal/d | 0.79 | 0.84 | +0.01 | (0.06) | 0.88 | +0.08 | (0.05) | 0.13 | +0.07 | (0.06) | 0.21 | +0.01 | (0.05) | 0.89 |
Key BVRT=Benton Visual Retention Task; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Task; DFR=Delayed Free Recall; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; OLS=Ordinary Least Square; SD=Standard Deviation; SEE=Standard error of the estimate; WRAT=Wide Range Achievement Test.
Multivariate OLS or poisson (MMSE error count) models adjusted for age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, every use of illicit drugs, body mass index, and selected nutrients expressed per 1,000 kcal, namely vitamin B-6, B-12, folate, n-3 highly unsaturated fatty acids and CES-D total score.
P for interaction term exposure × race in model with exposure main effect and main effect of race and other covariates listed above.
P for interaction term exposure × age in model with exposure main effect and main effect of age group and other covariates listed above.
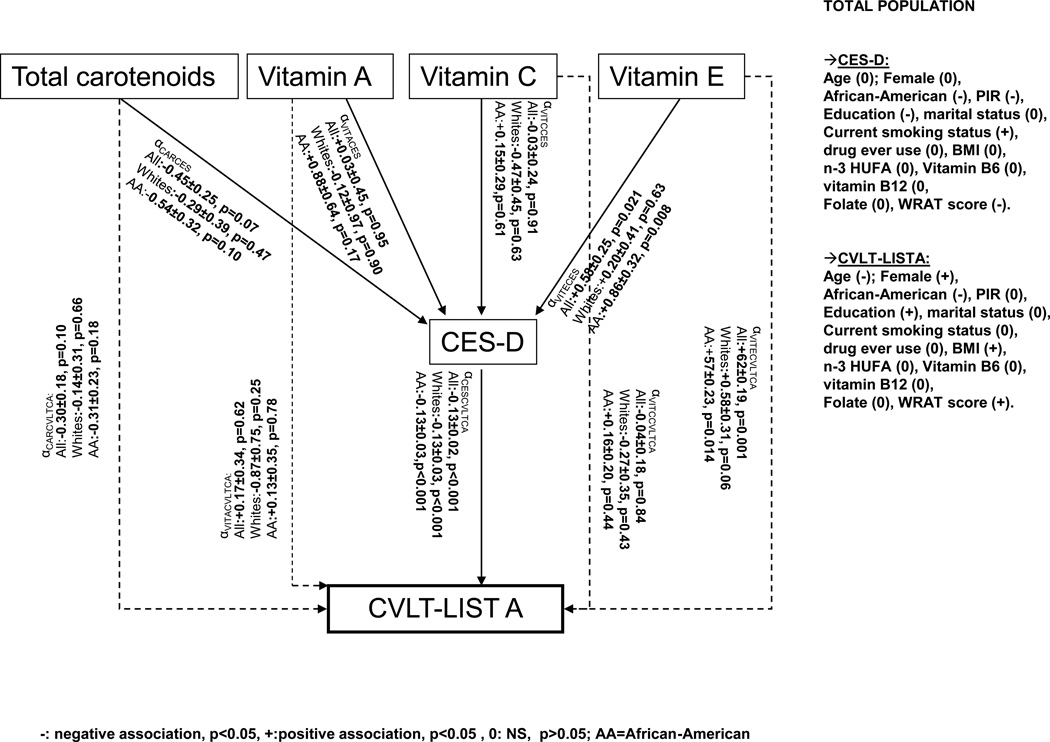
Multiple linear regression models were conducted to test mediation of the antioxidant-cognition association through CES-D scores (Table S3, Supplemental Digital Content 4). In the overall population, the total effects of vitamin E on CVLT-List A and CVLT-DFR indicated a putative protective effect, whereas there were positive association between vitamin E and CES-D scores and an inverse relationship between those two cognitive test scores and CES-D. When CES-D was entered into the model with antioxidants vs. CVLT-List A and CVLT-DFR, the net effect of vitamin E was markedly altered compared to the total effect, with a significant S-G test (p=0.032 and 0.035, respectively). In addition, the respective MPs were −13% and −16%. In all other total effects under study, CES-D did not show an appreciable mediating effect, especially when examining the S-G test.
Figure 1 displays the findings from a structural equations model where CVLT-List A is shown as an example for cognitive test score outcomes and predicted by the four antioxidants whose total effect is allowed to be partially mediated by CES-D score, by including a direct effect from each antioxidant into the cognitive test score. Our findings are in line with the S-G test and the MP estimate (~−13%). In addition, when stratifying the SEM by race, we found that associations in which vitamin E was positively associated with CES-D, CES-D inversely related to CVLT-List A while vitamin E had a positive and significant direct effect on CVLT-List A mainly among African-Americans. Among whites, the path coefficient (α) from vitamin E into CES-D was non-significant.
FIGURE 1.
Structural Equation Model (SEM) for associations between antioxidants and a test of verbal memory (CVLT-List A): mediating effects of depressive symptoms (CES-D): HANDLS study
Key: AA=African-American; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; HS=High School; HUFA=Highly Unsaturated Fatty Acids; OLS=Ordinary Least Square; PIR=Poverty Income Ratio; RE=Retinol Equivalent; SEE=Standard error of the estimate; WRAT=Wide Range Achievement Test.
Note: Path coefficients between antioxidant exoosure and CES-D or cognitive scores are denoted by α and labelled by to the predictor and otucome variables of each path.
When relaxing the assumption of additivity between the CES-D score (mediator) and each of the antioxidant exposures by allowing for interaction, we computed four distinctive estimates to assess mediation of antioxidant-CS relationship through CES-D (Table 5). Our findings were in line with the previous mediation analysis (Table S1), whereby vitamin E’s total effect on CVLT-List A and CVLT-DFR appeared to be partially mediated through CES-D with a significant NIE and a MP estimated at 13–16%.
Table 5.
Mediating effect of CES-D score on the total effect of dietary antioxidants (per 1 SD) on cognitive function test scores: mediation analysis relaxing the addititivity assumption and allowing for interaction between CES-D and the antioxidant exposure: HANDLS study1
| Controlled direct effect | Natural direct effect |
Natural indirect effect | Marginal total effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | |
| CVLT, List A | ||||||||||||
| Total carotenoids | −0.31 | (0.19) | 0.10 | −0.30 | (0.19) | 0.11 | +0.04 | (0.03) | 0.11 | −0.26 | (0.19) | 0.17 |
| Vitamin A | +0.23 | (0.36) | 0.51 | +0.22 | (0.34) | 0.52 | −0.00 | (0.07) | 0.95 | +0.22 | (0.35) | 0.53 |
| Vitamin C | −0.03 | (0.18) | 0.86 | −0.03 | (0.18) | 0.86 | +0.00 | (0.03) | 0.91 | −0.03 | (0.19) | 0.87 |
| Vitamin E | +0.63 | (0.19) | 0.001 | +0.62 | (0.19) | 0.001 | −0.07 | (0.04) | 0.040 | +0.55 | (0.19) | 0.005 |
| CVLT, DFR | ||||||||||||
| Total carotenoids | −0.09 | (0.09) | 0.30 | −0.09 | (0.09) | 0.31 | +0.02 | (0.01) | 0.12 | −0..07 | (0.09) | 0.42 |
| Vitamin A | +0.25 | (0.17) | 0.13 | +0.25 | (0.17) | 0.13 | −0.00 | (0.03) | 0.95 | +0.25 | (0.16) | 0.14 |
| Vitamin C | −0.04 | (0.09) | 0.69 | +0.04 | (0.09) | 0.69 | +0.00 | (0.01) | 0.91 | +0.04 | (0.09) | 0.68 |
| Vitamin E | +0.23 | (0.09) | 0.013 | +0.23 | (0.09) | 0.012 | −0.03 | (0.01) | 0.049 | +0.20 | (0.09) | 0.029 |
| BVRT, error count | ||||||||||||
| Total carotenoids | +0.10 | (0.15) | 0.48 | +0.10 | (0.15) | 0.51 | −0.01 | (0.01) | 0.33 | +0.08 | (0.15) | 0.56 |
| Vitamin A | −0.63 | (0.27) | 0.021 | −0.63 | (0.27) | 0.020 | +0.00 | (0.02) | 0.95 | −0.63 | (0.27) | 0.021 |
| Vitamin C | +0.02 | (0.15) | 0.84 | +0.03 | (0.15) | 0.86 | −0.00 | (0.01) | 0.91 | +0.03 | (0.15) | 0.86 |
| Vitamin E | −0.30 | (0.15) | 0.05 | −0.29 | (0.15) | 0.06 | +0.04 | (0.02) | 0.06 | −0.25 | (0.15) | 0.10 |
| Animal fluency | ||||||||||||
| Total carotenoids | +0.04 | (0.16) | 0.78 | +0.04 | (0.16) | 0.81 | +0.03 | (0.02) | 0.12 | +0.07 | (0.16) | 0.65 |
| Vitamin A | +0.44 | (0.30) | 0.13 | +0.45 | (0.30) | 0.13 | −0.00 | (0.02) | 0.95 | +0.45 | (0.30) | 0.13 |
| Vitamin C | −0.00 | (0.16) | 0.99 | −0.00 | (0.16) | 1.00 | −0.00 | (0.02) | 0.91 | +0.00 | (0.16) | 1.00 |
| Vitamin E | +0.57 | (0.16) | 0.001 | +0.57 | (0.16) | 0.001 | −0.05 | (0.03) | 0.05 | +0.52 | (0.17) | 0.002 |
| Card rotation | ||||||||||||
| Total carotenoids | −1.15 | (0.53) | 0.031 | −1.15 | (0.53) | 0.031 | +0.06 | (0.05) | 0.21 | −1.09 | (0.53) | 0.042 |
| Vitamin A | +0.94 | (0.99) | 0.34 | +0.95 | (0.99) | 0.33 | −0.00 | (0.04) | 0.95 | +0.95 | (0.99) | 0.34 |
| Vitamin C | +0.19 | (0.53) | 0.71 | +0.19 | (0.53) | 0.71 | +0.00 | (0.04) | 0.91 | +0.20 | (0.53) | 0.71 |
| Vitamin E | +1.08 | (0.55) | 0.05 | +1.07 | (0.55) | 0.05 | −0.11 | (0.07) | 0.10 | +0.96 | (0.55) | 0.08 |
Key BVRT=Benton Visual Retention Task; CDE=Controlled Direct Effect; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Task; DFR=Delayed Free Recall; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; MTE=Marginal Total Effect; NDE=Natural Direct Effect; NIE=Natural Indirect Effect; OLS=Ordinary Least Square; SD=Standard Deviation; SEE=Standard Error of the Estimate; WRAT=Wide Range Achievement Test.
Multivariate OLS models adjusted for age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, every use of illicit drugs, body mass index, and selected nutrients expressed per 1,000 kcal, namely vitamin B-6, B-12, folate, n-3 highly unsaturated fatty acids. CES-D score was entered as potential mediator alternatively for each antioxidant exposure, while others are kept in the model as covariates. Interaction between CES-D and each of the antioxidant exposure was allowed. Four parameters were estimated with SEE and p-values: CDE, NDE NIE, MTE. Those are described in more detail in statistical analysis section. For CDE, CES-D was set at a value of 11.0 (an approximation of the sample mean). Only results with significant total effects at type I error of 0.05 for at least one antioxidant are presented.
DISCUSSION
This is one of very few studies that examined the association between antioxidants and cognitive functioning in various domains among young and middle-aged US adults, and is the first to examine potential moderation by sex, race and age and mediation by depressive symptoms. Among key findings, dietary vitamin E intake was positively associated with performance in domains of verbal memory (total population and the younger group (<48y)), verbal fluency (total population and the younger group (Age<48y)) and psychomotor speed (women). Vitamin E was positively linked to CES-D among African-Americans, yet its positive association with verbal memory was only partially mediated by depressive symptoms (total population).
Vitamin E has not only antioxidant activity but also functions in other independent roles such as inhibiting brain protein kinase C activity. This ability is most likely attributed to the multiple isoforms of Vitamin E. (37, 81) It is recognized that the diet contains several Vitamin E isoforms while the results of this study reflect intake of only one form of Vitamin E, alpha tocopherol. Due to the increased use of oils in the US diet, there has been an increase in the gamma tocopherol, the form that has similar antioxidant capacity but greater anti-inflammatory properties compared alpha tocopherol, Morris reported that only α and γ-tocopherols found in foods were linked to slower rate of cognitive decline over a 6y period (82). This observation was corroborated by Commenges and colleagues (20).
It is worth noting, however, that in the case of vitamin E, our study population had on average a level of alpha tocopherol consumption that meets roughly 50% of Estimated Average Requirement (EAR) (Mean±SD: 6.8±5.0 mg/d vs. EAR=12 mg./d), with only 10.1% of the distribution being adequate. This is in contrast with vitamin A (38.8% meeting the EAR) and vitamin C (41.4% meeting the EAR). (83) Thus, our findings should be interpreted in light of this difference in distributions of intakes compared to other studies whose selected population consumed higher amounts of vitamin E or included supplemental intake in addition to dietary sources. (e.g. (32–35)) It is unclear from other publications when the Vitamin E intake included all isoforms due to a lack of description in the diet methodology. Morris and colleagues (34) reported that dietary intake of vitamin E, but not other antioxidants, was associated with a reduced risk of incident AD, although this association was restricted to individuals without the Apolipoprotein E ε4 genotype. Similar findings were reported with cognitive decline as an outcome. (35)
Vitamin C, which is essential for the reduction of vitamin E, was not associated with cognitive performance in the HANDLS study population. This finding differs from the results of a few recent prospective cohort studies which examined the association of other dietary antioxidants with various cognitive outcomes. One cross-sectional study found that participants in the lowest 10th percentile of vitamin C intakes had poorer performance on abstract thinking and problem-solving task. (33) Another study reported that high dietary intake of vitamins C and E may reduce the risk of AD (22). This relationship most pronounced among smokers. The inverse association between vitamin C intake and cognitive impairment as assessed by the MMSE was corroborated by Paleologos and colleagues, (84) while Sato and colleagues only found this association in men (36).
When evaluating the association between antioxidants and cognitive domains, one study found that past intakes of vitamins A and E were associated with better performance on visuospatial recall and/or abstraction performance. (32) These results were similar to ours, though some of our related findings did not survive multiple testing correction. Another study, suggested that dietary antioxidants were not able to reduce AD risk. (24) Similarly, Laurin and colleagues (23) found no association between midlife dietary intake of vitamins E and C and dementia incidence. At least four other cohort studies came to a similar conclusion, especially after adjustment for potentially confounding factors. (16, 85–87) In addition to examining associations of cognition with vitamins A, C and E, other studies found that carotenoids, particularly β-carotene intake, may have beneficial effects of various cognitive outcomes (16) though others were not able to detect such an association (35, 86, 87).
Epidemiologic studies examining relationships between supplemental antioxidants and various cognitive outcomes found mixed results. In fact, vitamin C supplement use was related to lower AD risk in one cohort study, (88) whereas vitamin E and vitamin C supplements in combination were associated with reduced prevalence and incidence of AD and cognitive decline in three other cohort studies (89–91). Yet Grodstein and colleagues, found this effect to be specific to Vitamin E supplements. (92) The putative protective effect of supplemental antioxidant use against adverse cognitive health outcomes was replicated in a large cohort study (93). In addition, a post-hoc analysis of a large trial found that a combination of supplements including but not limited to β-carotene, vitamin C and E can improve verbal memory in the long-term (six years after the trial). (31) However, there was little evidence of a cognitive benefit from use of antioxidant supplements, particularly vitamins C and E, according to at least five independent cohort studies. (24, 94–97) Finally, a recent randomized controlled trial examining transition from mild cognitive impairment to AD, found no significant association between treatment with supplemental vitamin E and the outcome of interest. (98)
Our study has notable strengths. First, it is one of the largest studies examining the primary question of interest, it made use of extensive cognitive function tests, a valid measure of depressive symptoms (the CES-D) and advanced multivariate techniques such as structural equations modeling, mediation analyses, and Heckman selection models among others. Second, it is one of few to use the average of two 24-hr recalls while estimating usual dietary intakes of antioxidants.
Our study also has limitations. First, its cross-sectional design precluded temporality ascertainment, highlighting the importance of conducting further longitudinal studies in a U.S. community. Moreover, due to lack of factorial invariance between race and sex groups as well as poverty income ratio groups, the use of cognitive domains --obtained from confirmatory factor analysis-- that were comparable between those groups was not feasible in this study. Data on supplemental intakes of antioxidants were not available for the baseline wave and thus were not accounted for in estimating total intakes. Generally speaking, it was estimated that between 2003–2006, more than half of adults used dietary supplements. (99) In addition, supplement users also had higher intakes of vitamins A, C and E from foods and in total than non-users. However, they also tended to exceed the tolerable upper intake level for vitamins A and C compared to non-users. (100) Whether antioxidants are obtained from diet or supplements, plasma concentration would be a more sensitive indicator of antioxidant and oxidative stress status, while reducing reporting bias. (45, 88–92) Although, the baseline HANDLS study did not incorporate these measures, future waves may support this analysis, thereby enhancing our understanding of these associations in an urban, low-income population.
In conclusion, our study indicated that dietary intakes of selected antioxidant nutrients and cognition are closely related, although these relationships may vary according to sex, race and depressive status and are specific to certain domains of cognition as well as the nutrient. In particular, and among key findings, we found that vitamin E was positively associated with performance in domains of verbal memory and fluency in the total population and psychomotor speed among women. The association between vitamin E and verbal memory was only partially mediated by depressive symptoms. Future cohort studies and dietary antioxidant interventions should focus on association of dietary vitamin E with age-related cognitive decline, particularly in the domains of verbal memory, verbal fluency, and psychomotor speed.
Supplementary Material
Acknowledgements
This study was entirely supported by the National Institute on Aging, Intramural Research Program (NIA/NIH/IRP).
Abbreviations
- AA
African-American
- AD
Alzheimer’s Disease
- AF
Animal Fluency
- AMPM
Automated Multiple Pass Method
- BTA
Brief Test of Attention
- BVRT
Benton Visual Retention Task
- CDE
Controlled Direct Effect
- CDT
Clock Drawing Test
- CES-D
Center for Epidemiologic Studies-Depression
- CR
Card Rotation
- CS
Cognitive Score
- CVLT-List A
California Verbal Learning Test, immediate free recall, List A
- CVLT-DFR
California Verbal Learning Test, delayed free recall, List A
- DS-B
Digit Span Backwards test
- DS-F
Digit Span Forward test
- EAR
Estimated Average Requirement
- HANDLS
Healthy Aging in Neighborhoods of Diversity across the Life Span
- HS
High School
- HUFA
Highly Unsaturated Fatty Acids
- IP
Identical Pictures
- M
Mediator
- MMSE
Mini-Mental State Examination
- MP
Mediation Proportion
- MRV
Medical Research Vehicles
- MTE
Marginal Total Effect
- NDE
Natural Direct Effect
- NIE
Natural Indirect Effect
- OLS
Ordinary Least Square
- PIR
Poverty Income Ratio
- RAA
Relaxing the Assumption for Additivity
- RE
Retinol Equivalent
- ROS
Reactive Oxygen Species
- SD
Standard Deviation
- SEE
Standard error of the estimate
- SEM
Structural Equations Modeling
- S-G
Sobel-Goodman Test
- Trails A
Trailmaking Test, Part A
- Trails B
Trailmaking Test Part B
- USDA
U.S. Department of Agriculture
- WRAT
Wide Range Achievement Test
- X
Antioxidant Exposure
Footnotes
MAB had full access to the data used in this manuscript and completed all the statistical analyses.
Disclosure statement: The authors declare no conflict of interest.
Author contributions:
MAB: Study concept, literature search and review, plan of analysis, data management, statistical analysis, write-up and revision of the manuscript.
MTFK: Data acquisition, write-up of parts of the manuscript, revision of the manuscript.
MKT: Data acquisition, write-up of parts of the manusript, revision of the manuscript.
HAB: Literature review, plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
JSK: Literature review, plan of analysis, statistical analysis, write-up of parts of the manuscript, revision of the manuscript.
Mason, M. A.: Plan of analysis, data management, revision of the manuscript.
Evans, M. K.: Data acquisition, revision of the manuscript.
Zonderman, A. B.: Data acquisition, plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
REFERENCES
- 1.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–S9. [PubMed] [Google Scholar]
- 2.Hendrie HC. Epidemiology of dementia and Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6:S3–S18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 3.2008 Alzheimer's disease facts and figures. Alzheimers Dement. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.2009 Alzheimer's disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 8.Mao G, Pan X, Zhu BB, Zhang Y, Yuan F, Huang J, Lovell MA, Lee MP, Markesbery WR, Li GM, Gu L. Identification and characterization of OGG1 mutations in patients with Alzheimer's disease. Nucleic acids research. 2007;35:2759–2766. doi: 10.1093/nar/gkm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob KD, Noren Hooten N, Tadokoro T, Lohani A, Barnes J, Evans MK. Alzheimer's disease-associated polymorphisms in human OGG1 alter catalytic activity and sensitize cells to DNA damage. Free radical biology & medicine. 2013;63:115–125. doi: 10.1016/j.freeradbiomed.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behl C. Amyloid beta-protein toxicity and oxidative stress in Alzheimer's disease. Cell and tissue research. 1997;290:471–480. doi: 10.1007/s004410050955. [DOI] [PubMed] [Google Scholar]
- 11.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 12.Grundman M. Vitamin E and Alzheimer disease: the basis for additional clinical trials. Am J Clin Nutr. 2000;71:630S–636S. doi: 10.1093/ajcn/71.2.630s. [DOI] [PubMed] [Google Scholar]
- 13.Ekinci FJ, Linsley MD, Shea TB. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res. 2000;76:389–395. doi: 10.1016/s0169-328x(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 14.Carty JL, Bevan R, Waller H, Mistry N, Cooke M, Lunec J, Griffiths HR. The effects of vitamin C supplementation on protein oxidation in healthy volunteers. Biochem Biophys Res Commun. 2000;273:729–735. doi: 10.1006/bbrc.2000.3014. [DOI] [PubMed] [Google Scholar]
- 15.Brennan LA, Morris GM, Wasson GR, Hannigan BM, Barnett YA. The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. The British journal of nutrition. 2000;84:195–202. doi: 10.1017/s0007114500001422. [DOI] [PubMed] [Google Scholar]
- 16.Jama JW, Launer LJ, Witteman JC, den Breeijen JH, Breteler MM, Grobbee DE, Hofman A. Dietary antioxidants and cognitive function in a population-based sample of older persons. The Rotterdam Study. American journal of epidemiology. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 17.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. American journal of epidemiology. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 18.Ortega RM, Requejo AM, Andres P, Lopez-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. The American journal of clinical nutrition. 1997;66:803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Kang SA, Lee HO, Lee BH, Park JS, Kim JH, Jung IK, Park YJ, Lee JE. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health. 2001;115:133–138. doi: 10.1038/sj/ph/1900729. [DOI] [PubMed] [Google Scholar]
- 20.Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JC, Welsh-Bohmer KA. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. The journal of nutrition, health & aging. 2007;11:230–237. [PubMed] [Google Scholar]
- 21.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 22.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA : the journal of the American Medical Association. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 23.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol. 2004;159:959–967. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 25.Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009;63:9–17. doi: 10.18926/AMO/31854. [DOI] [PubMed] [Google Scholar]
- 26.Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59:304–306. doi: 10.1038/sj.ejcn.1602072. [DOI] [PubMed] [Google Scholar]
- 27.Niti M, Yap KB, Kua EH, Ng TP. APOE-epsilon4, depressive symptoms, and cognitive decline in Chinese older adults: Singapore Longitudinal Aging Studies. J Gerontol A Biol Sci Med Sci. 2009;64:306–311. doi: 10.1093/gerona/gln013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy M, O'Leary E. Depression, cognitive reserve and memory performance in older adults. Int J Geriatr Psychiatry. 2009 doi: 10.1002/gps.2404. [DOI] [PubMed] [Google Scholar]
- 29.Panza F, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Caselli RJ, Todarello O, Pellicani V, Santamato A, Scapicchio P, Maggi S, Scafato E, Gandin C, Capurso A, Solfrizzi V. Depressive symptoms, vascular risk factors and mild cognitive impairment. The Italian longitudinal study on aging. Dement Geriatr Cogn Disord. 2008;25:336–346. doi: 10.1159/000119522. [DOI] [PubMed] [Google Scholar]
- 30.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesse-Guyot K-GE, Fezeu L, Jeandel C, Ferry M, Andreeva V, Amieva H, Hercberg S, Galan P. French adults' cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: a post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. The American journal of clinical nutrition. 2011;94:892–899. doi: 10.3945/ajcn.110.007815. [DOI] [PubMed] [Google Scholar]
- 32.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. The American journal of clinical nutrition. 1997;65:20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA : the journal of the American Medical Association. 1983;249:2917–2921. [PubMed] [Google Scholar]
- 34.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 35.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 36.Sato R, Helzlsouer KJ, Comstock GW, Hoffman SC, Norkus EP, Fried LP. A cross-sectional study of vitamin C and cognitive function in older adults: the differential effects of gender. The journal of nutrition, health & aging. 2006;10:37–44. [PubMed] [Google Scholar]
- 37.IOM. The essential guide to nutrient requirements. Washington, DC: National Academy of Science; 2006. Dietary reference intakes. [Google Scholar]
- 38.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Agriculture (USDA) Vol. 2007. Center for Nutrition Policy and Promotion; 2005. Healthy Eating Index 2005. http://www.cnpp.usda.gov/Publications/HEI/healthyeatingindex2005factsheet.pdf. [Google Scholar]
- 40.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72:1223–1231. doi: 10.1093/ajcn/72.5.1223. [DOI] [PubMed] [Google Scholar]
- 41.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & disease. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- 42.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 44.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, Staples RC, Cleveland LE. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. The American journal of clinical nutrition. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 45.United States Department of Agriculture (USDA), Agriculture Research Service. USDA; 2008. [Accessed: February, 2008]. Database for Analyzing Dietary Sources of Nutrients Using USDA Survey Food Codes version 3.0. http://www.ars.usda.gov/Services/docs.htm?docid=17031. [Google Scholar]
- 46.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 47.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res. 2004;126:177–187. doi: 10.1016/j.psychres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson GS. Wide Range Achievement Test–Revision 3. Wilmington, DE: Jastak Association; 1993. [Google Scholar]
- 49.Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 50.Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. 2008;14:277–285. doi: 10.1089/acm.2007.0663. [DOI] [PubMed] [Google Scholar]
- 51.Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, Liebson E, Price LL, Griffith J, Rosenberg I, Folstein MF. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2004;12:631–638. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- 52.D'Anci KE, Rosenberg IH. Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7:659–664. doi: 10.1097/00075197-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and the risk of depression in Finnish middle-aged men. A prospective follow-up study. Psychother Psychosom. 2004;73:334–339. doi: 10.1159/000080385. [DOI] [PubMed] [Google Scholar]
- 54.Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54:382–390. doi: 10.1111/j.1753-4887.1996.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 55.Kivela SL, Pahkala K, Eronen A. Depression in the aged: relation to folate and vitamins C and B12. Biol Psychiatry. 1989;26:210–213. doi: 10.1016/0006-3223(89)90027-9. [DOI] [PubMed] [Google Scholar]
- 56.Levitt AJ, Joffe RT. Folate, B12, and life course of depressive illness. Biol Psychiatry. 1989;25:867–872. doi: 10.1016/0006-3223(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 57.Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, Mishima N. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–147. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–945. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- 59.Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P, Bartali B, Corsi A, Senin U, Ferrucci L. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26:987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, Alessandri JM, Galan P, Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2008;78:171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 62.Strom M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. Am J Clin Nutr. 2009;90:149–155. doi: 10.3945/ajcn.2009.27552. [DOI] [PubMed] [Google Scholar]
- 63.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 64.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 65.Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 66.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 67.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and risk of cognitive decline among older adults: The Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr. 2007;85:1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 68.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 69.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–280. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 70.Willet WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 71.STATA. Statistics/Data Analysis: Release 13.0. Texas: Stata Corporation; 2013. [Google Scholar]
- 72.Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, Keiding N. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 2005;16:114–120. doi: 10.1097/01.ede.0000147107.76079.07. [DOI] [PubMed] [Google Scholar]
- 73.Beydoun MA, Wang Y. How do socio-economic status, perceived economic barriers and nutritional benefits affect quality of dietary intake among US adults? European journal of clinical nutrition. 2008;62:303–313. doi: 10.1038/sj.ejcn.1602700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]
- 75.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Shroff MR, Mason MA, Evans MK, Zonderman AB. The sex-specific role of plasma folate in mediating the association of dietary quality with depressive symptoms. J Nutr. 2010;140:338–347. doi: 10.3945/jn.109.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology. 2013;24:175–176. doi: 10.1097/EDE.0b013e3182781410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- 79.Beydoun MA, Kuczmarski MT, Mason MA, Ling SM, Evans MK, Zonderman AB. Role of depressive symptoms in explaining socioeconomic status disparities in dietary quality and central adiposity among US adults: a structural equation modeling approach. The American journal of clinical nutrition. 2009;90:1084–1095. doi: 10.3945/ajcn.2009.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hochberg Y, Tamhane AC. Multiple comparison procedures. New York: Wiley; 1987. [Google Scholar]
- 81.Cook-Mills JM. Isoforms of Vitamin E Differentially Regulate PKC and Inflammation: A Review. Journal of clinical & cellular immunology. 2013;4 doi: 10.4172/2155-9899.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 83.(USDA) USDoA. Dietary guidance: Dietary Reference Intakes. Food and Nutrition Information Center; 2014. [Google Scholar]
- 84.Paleologos M, Cumming RG, Lazarus R. Cohort study of vitamin C intake and cognitive impairment. American journal of epidemiology. 1998;148:45–50. doi: 10.1093/oxfordjournals.aje.a009559. [DOI] [PubMed] [Google Scholar]
- 85.Peacock JM, Folsom AR, Knopman DS, Mosley TH, Goff DC, Jr, Szklo M. Dietary antioxidant intake and cognitive performance in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study investigators. Public Health Nutr. 2000;3:337–343. doi: 10.1017/s1368980000000380. [DOI] [PubMed] [Google Scholar]
- 86.McNeill G, Jia X, Whalley LJ, Fox HC, Corley J, Gow AJ, Brett CE, Starr JM, Deary IJ. Antioxidant and B vitamin intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Eur J Clin Nutr. 2011;65:619–626. doi: 10.1038/ejcn.2011.2. [DOI] [PubMed] [Google Scholar]
- 87.Devore EE, Kang JH, Stampfer MJ, Grodstein F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am J Clin Nutr. 2010;92:1157–1164. doi: 10.3945/ajcn.2010.29634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA, Field TS, Evans DA. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 89.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 90.Maxwell CJ, Hicks MS, Hogan DB, Basran J, Ebly EM. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement Geriatr Cogn Disord. 2005;20:45–51. doi: 10.1159/000085074. [DOI] [PubMed] [Google Scholar]
- 91.Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, Munger RG, Norton MC, Tschanz JT, Lyketsos CG, Breitner JC, Welsh-Bohmer K. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement. 2008;4:223–227. doi: 10.1016/j.jalz.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. The American journal of clinical nutrition. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 93.Gray SL, Hanlon JT, Landerman LR, Artz M, Schmader KE, Fillenbaum GG. Is antioxidant use protective of cognitive function in the community-dwelling elderly? Am J Geriatr Pharmacother. 2003;1:3–10. doi: 10.1016/s1543-5946(03)80011-9. [DOI] [PubMed] [Google Scholar]
- 94.Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, Havlik R, White LR. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 95.Mendelsohn AB, Belle SH, Stoehr GP, Ganguli M. Use of antioxidant supplements and its association with cognitive function in a rural elderly cohort: the MoVIES Project. Monongahela Valley Independent Elders Survey. American journal of epidemiology. 1998;148:38–44. doi: 10.1093/oxfordjournals.aje.a009556. [DOI] [PubMed] [Google Scholar]
- 96.Fillenbaum GG, Kuchibhatla MN, Hanlon JT, Artz MB, Pieper CF, Schmader KE, Dysken MW, Gray SL. Dementia and Alzheimer's disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann Pharmacother. 2005;39:2009–2014. doi: 10.1345/aph.1G280. [DOI] [PubMed] [Google Scholar]
- 97.Gray SL, Anderson ML, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, Larson E. Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults. J Am Geriatr Soc. 2008;56:291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 98.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ Alzheimer's Disease Cooperative Study G. Vitamin E and donepezil for the treatment of mild cognitive impairment. The New England journal of medicine. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 99.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. The Journal of nutrition. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. Journal of the Academy of Nutrition and Dietetics. 2012;112:657–663. e4. doi: 10.1016/j.jand.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.