Abstract
Objectives
We sought to systematically review and meta-analyze the available data on the association between timing of antibiotic administration and mortality in severe sepsis and septic shock.
Data Sources and Study Selection
A comprehensive search was performed using a pre-defined protocol. Inclusion criteria: adult patients with severe sepsis or septic shock, reported time to antibiotic administration in relation to ED triage and/or shock recognition, and mortality. Exclusion criteria: immunosuppressed populations, review article, editorial, or non-human studies.
Data Extraction
Two reviewers screened abstracts with a third reviewer arbitrating. The effect of time to antibiotic administration on mortality was based on current guideline recommendations: 1) administration within 3 hours of ED triage; 2) administration within 1 hour of severe sepsis/septic shock recognition. Odds Ratios (OR) were calculated using a random effect model. The primary outcome was mortality.
Data Synthesis
1123 publications were identified and 11 were included in the analysis. Among the 11 included studies, 16,178 patients were evaluable for antibiotic administration from ED triage. Patients who received antibiotics more than 3 hours after ED triage (< 3 hours reference), had a pooled OR for mortality of 1.16 (0.92 to 1.46, p = 0.21). A total of 11,017 patients were evaluable for antibiotic administration from severe sepsis/septic shock recognition. Patients who received antibiotics more than 1 hour after severe sepsis/shock recognition (< 1 hour reference) had a pooled OR for mortality of 1.46 (0.89 to 2.40, p = 0.13). There was no increased mortality in the pooled ORs for each hourly delay from <1 to >5 hours in antibiotic administration from severe sepsis/shock recognition.
Conclusion
Using the available pooled data we found no significant mortality benefit of administering antibiotics within 3 hours of ED triage or within 1 hour of shock recognition in severe sepsis and septic shock. These results suggest that currently recommended timing metrics as measures of quality of care are not supported by the available evidence.
Keywords: Septic shock, severe sepsis, antibiotics, timing of antibiotics, shock recognition, anti-bacterial agents
Introduction
Severe sepsis and septic shock remain a major cause of emergency department (ED) visits and intensive care unit (ICU) admissions, and are associated with significant morbidity, mortality, and health care costs.(1–2) Previous studies have suggested improved outcomes with the implementation of a structured resuscitation, focusing largely on intravenous (IV) fluid resuscitation, timely broad-spectrum antibiotics, and vasopressor therapy. (3–7) While some authors have suggested the primacy of timely antibiotics administration for improved mortality in severe sepsis and septic shock,(8–9) previously published research evaluating the association of the time to antibiotic administration on outcomes has produced disparate results.
In 2006, Kumar and colleagues reported a 7.6% increase in mortality in sepsis patients for each hourly delay after the onset of shock.(10) Though subsequent studies have failed to demonstrate such substantial results, several have reported increased mortality associated with delays in antibiotic administration either from shock recognition or time from ED triage. (8–10) Other studies have not demonstrated any increase in mortality with delay of antibiotic administration based on triage time.(11–12)
The most recent Surviving Sepsis Campaign (SSC) guidelines include specific recommendations regarding the timing of antibiotics: “The administration of effective intravenous antimicrobials within the first hour of recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C) should be the goal of therapy”.(13) Additionally, the SSC recommend a ‘sepsis bundle’ which requires administration of broad spectrum antibiotics within three hours from ED triage. The authors of the SSC guidelines note that achieving these goals may not be operationally feasible in some cases and acknowledge that previous research has shown that compliance with guidelines regarding antibiotic administration frequently is not achieved.(12–14) Despite these limitations, time to antibiotics administration has gained increasing focus as a potential metric for the quality of care of patients with severe sepsis and septic shock.(15)
To our knowledge, no previous study has pooled the available data to evaluate the impact of time to antibiotics on sepsis outcomes. Our objective was to perform a systematic review of the published literature and to meta-analyze the available data on the association between the timing of antibiotics and mortality in severe sepsis and septic shock.
Methods
We developed and followed a comprehensive protocol and data collection instrument that followed PRISMA(16) recommendations prior to the start of the study. As this was not a study of human subjects but rather a synthesis of the previously published literature, it was exempt from institutional review board approval.
Search Strategy
A comprehensive literature search was performed using a pre-defined, written protocol of The Cochrane Database, CINAHL, Pubmed, and Scopus databases with no start date to January 2015. The search criteria, developed with the help of a medical librarian, used the following Medical Subject Headings terms:
Septic shock OR Severe sepsis OR Sepsis AND
Anti-bacterial agents OR Antibiotics
Inclusion and Exclusion Criteria and Outcomes
Manuscripts were eligible for inclusion if they evaluated human patients with severe sepsis or septic shock, reported timing of antibiotic administration from ED triage and/or septic shock/severe sepsis recognition, and reported mortality data. Studies involving non-humans, patients less 18 years old, and those focused solely on neutropenic or immunocompromised subjects were excluded. Review articles, editorials, case studies, and letters to the editor were excluded, though bibliographies were evaluated for relevant articles. Given an anticipated limited availability of high-quality clinical trials evaluating our stated objective, all study types, except those previously mentioned, were eligible for inclusion. If the time to antibiotics or mortality was not explicitly reported, the study was potentially eligible for inclusion pending author contact. The primary outcome was mortality.
Study Selection
Two authors (SAS and JP) independently reviewed abstracts of all relevant studies yielded from the initial search. In cases of disagreement, a review of the full article was conducted and inclusion determined by a third reviewer (AEJ). The full manuscript of each study passing the relevance screen was independently reviewed for eligibility by two authors (SAS and WRM). In cases of disagreement, a third reviewer (AEJ) determined inclusion. Data abstraction was performed using a standard data collection form for each study identified for final inclusion. For manuscripts that did not include complete data for inclusion in the meta-analysis portion, corresponding authors were contacted for additional information.
Quality Assessment
Though there is limited validity of scoring non-randomized control trials for quality,(17) we elected to utilize a scoring system to determine study quality given the anticipated inclusion of multiple study types. Therefore, we developed pre-determined a scoring system for all included studies based on commonly excepted measures of quality, with four categories were scored between 0–2 (Table 1).
Table 1.
Scoring Criteria for Included Manuscripts in Systematic Review. Maximum score of 8: 0–3 low quality, 4–6 moderate quality, >6 high quality.
| Score | Study Design | Identification of septic patients | Population Sampling | Data on Timing of antibiotics |
|---|---|---|---|---|
| 2 | Implementation | Standard, consensus definition | Consecutive or random | Prospectively entered |
| 1 | Prospective | Non-standard criteria | Convenience | Described record extraction |
| 0 | Retrospective | Not defined or unknown | Not specified or unknown | Not described or unknown |
Timing of Antibiotic Administration and Statistical Analysis
The effect of time to antibiotic administration on mortality was assessed in two ways based upon the SSC Guideline recommendations (13): 1) Antibiotic administration within three hours of hospital presentation/ED triage; 2) Antibiotic administration within one hour of severe sepsis/septic shock recognition.
To assess the association between mortality and the time to antibiotics from triage, the antibiotic timing was categorized as within 3 hours of triage or 3 hours and longer from triage with the former used as the reference group. To evaluate association between mortality and the time from septic shock/severe sepsis recognition to antibiotic administration, the antibiotic timing was categorized as within 1 hour or more than 1 hour from shock/severe sepsis recognition. We also performed a sensitivity analysis of the effect of time to antibiotics from severe sepsis/shock recognition in hourly increments (1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) using < 1 hour as the reference group. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using a random effect model.(18) Publication bias was assessed using funnel and L’Abbe plots. Heterogeneity was assessed using Cochran Q test.
Results
Study Inclusion
Our comprehensive literature search yielded 1123 publications for possible inclusion. Of these, 36 were deemed relevant and eligible for full review with good inter-rater agreement (98.5%) in those identified. After full review and adjudication, 18 manuscripts were deemed potentially eligible for inclusion. Of these, 9 contained data for meta-analysis and 9 required author contact for clarification of the data. After author contact 2 provided additional data, leaving 11 articles for the full meta-analysis (Figure 1). A summary of reasons for exclusion at each stage of the analysis is shown in Table 2. Of the 11 included articles, 3 contained only data for timing from triage, 5 contained only data for timing from severe sepsis/septic shock recognition, and 3 contained data for both time points. All of the studies included in the meta-analysis were considered moderate to high quality (>4 points) by our quality score (Table 3)
Figure 1.
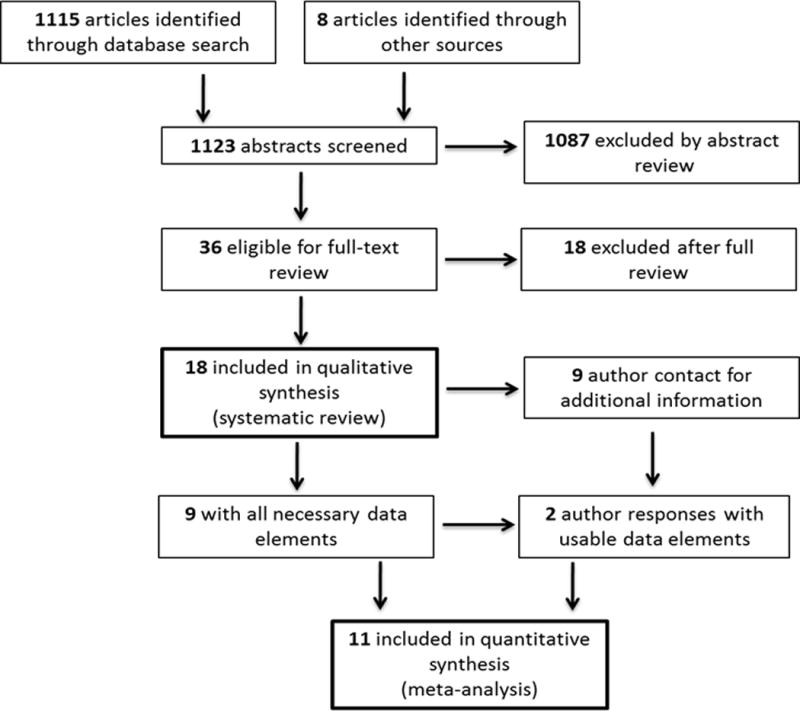
Flowchart for Inclusion
Table 2.
Summary of reasons for exclusion at each stage of search
| Reason for Exclusion | No of Reports |
|---|---|
| After relevance screen | |
| No antibiotic timing | 131 |
| Mortality data | 10 |
| Wrong focus/wrong group | 608 |
| Editorial, Letter, Conference paper | 104 |
| Neutropenic/Immuncompromised | 64 |
| Non-human study | 73 |
| Pediatrics | 10 |
| Non-English | 4 |
| Antibiotic prophylaxis | 83 |
| Total | 1087 |
| After manuscript review | |
| Wrong focus/wrong group | 9 |
| Review/Abstract | 3 |
| Mortality data | 5 |
| Non-English | 1 |
| Total | 18 |
| After author contact | |
| Failed author contact | 5 |
| Positive author response but unable to use data | 2 |
| Total | 7 |
Table 3.
Summary of included studies.
| Author/ Year Published |
Patient Location |
Primary Outcome |
Study Sites |
Years Conducted |
Number of Patients |
Timing of Antibiotics |
Quality Scoring* (Max=8) |
Reason for Exclusion |
Primary Finding |
|---|---|---|---|---|---|---|---|---|---|
| Bloos (2014) | ICU | 28-day mortality | Multi-center, German | ‘10–‘11 | 1011 | Sepsis recognition | 6 | Included | No significant association between TTA and survival |
| Bruce (2015) | ED | 3 hour SSC targets, in-hospital mortality | Single center, U.S. | ‘11–‘12 | 195 | Triage | 4 | Included | Protocol implementation reduced TTA but did not significantly improve mortality |
| Cullen (2013) | ICU | TTA | Single center, Australia | ‘05–‘08 | 89 | Sepsis recognition | 4 | Mortality data | Median TTA and time to appropriate antibiotics exceeded 1 hour |
| Ferrer (2009) | ICU | In-hospital mortality | Multi-center, Europe | ‘05–‘07 | 2796 | Sepsis recognition | 8 | Included | Early antibiotic treatment was associated with improved mortality |
| Ferrer (2014) | ED/ICU | In-hospital mortality | Multi-center U.S, Europe, South America | ‘05–‘10 | 17,990 | Sepsis recognition and triage | 5 | Included | Delay in antibiotic associated with no increased mortality in unadjusted analysis but increased mortality in adjusted analysis |
| Gaieski (2010) | ED | In-hospital mortality | Single center, U.S. | ‘05–‘06 | 261 | Sepsis recognition and triage | 5 | Included | No significant association from triage TTA and mortality. Significant association with mortality and appropriate antibiotics in < 1 hour |
| Gurnani (2010) | ICU | TTA and Appropriate fluid resuscitation | Single center, U.S. | ‘06–‘07 | 118 | Sepsis recognition | 8 | Mortality data | A sepsis protocol emphasizing early antibiotics and adequate fluids improved clinical outcomes |
| Hitti (2012) | ED | TTA from order | Single center, U.S. | 2/08–5/08 | 110 | Triage | 5 | Mortality data | Storing antibiotics in the ED reduces TTA |
| Hutchison (2011) | Not Specified | Discharge status, hospital/ICU LOS, TTA, cost | Single center, U.S. | ‘08–‘09 | 119 | Triage | 6 | Mortality data | Significant reduction in TTA, but no difference in mortality |
| Joo (2014) | ED | In-hospital mortality | Single center, Korea | ‘08–‘12 | 591 | Triage | 4 | Included | Antibiotics within 3 hours of ED arrival associated with improved mortality |
| Kumar (2006) | ED/Ward/ICU | In-hospital mortality | Multi-center, U.S., Canada | ‘89–‘04 | 2731 | Sepsis recognition | 5 | Included | Antibiotics in 1st hour of hypotension increased survival |
| Mok (2014) | Ward/ICU | TTA | Single center, Canadian | ‘09–‘10 | 100 | Sepsis recognition | 5 | Mortality data | Median TTA exceeded 1 hour time frame |
| Puskarich (2011) | ED | In-hospital mortality | Multi-center, U.S. | ‘07–‘09 | 291 | Sepsis recognition and triage | 7 | Included | No increase in mortality with hourly delays from triage or sepsis recognition |
| Ryoo (2015) | ED | 28 day mortality | Single center, U.S. | ‘10–‘12 | 426 | Sepsis recognition | 4 | Included | Mortality did not increase with hourly delays to antibiotics |
| Tipler (2013) | ED/ICU | TTA from physician order | Single center, U.S. | ‘08–‘10 | 209 | Sepsis recognition | 5 | Mortality data | A sepsis protocol improved TTA, but TTA still > 1 hour time frame |
| Venkatesh (2013) | ED | Time to septic shock recognition | Single center, U.S. | ‘06–‘08 | 267 | Sepsis recognition and triage | 5 | Mortality data | TTA from triage would misclassify performance on a large number of pts |
| Vilella (2014) | ED | TTA, appropriate antibiotics | Single center, U.S. | ‘10–‘11 | 184 | Triage | 5 | Included | Times to 1st and last antibiotics were not associated with survival |
| Yokota (2014) | ICU | Bundle compliance, appropriate antibiotics | Single center, Brazil | ‘05–‘12 | 1279 | Sepsis recognition | 5 | Included | Appropriate antibiotics improved mortality |
Abbreviations: ED – emergency department; ICU – intensive care unit; TTA – time to antibiotic; US – United States; LOS – length of stay.
Study Descriptions and Analyses
A list of 18 studies potentially eligible for inclusion were systematically reviewed and summarized in tabular format for the study characteristics, main findings, justification for inclusion/exclusion in meta-analysis, and quality assessment and listed in Table 3.
Six of the 11 included studies contained the necessary data on 16,178 patients for inclusion in the analysis of the effect of time to antibiotic administration from triage on mortality. A total of 10,208 patients receiving antibiotics within 3 hours of triage of whom 2574 died and 5970 patients receiving antibiotics in 3 or more hours after triage of whom 1793 died. As seen in Figure 2, the pooled OR for patients who received antibiotics 3 or more hours after triage was 1.16 (95% CI 0.92 to 1.46, p = 0.21) as compared to those that received antibiotics within 3 hours of triage. No statistical heterogeneity (p = 0.09) or publication bias was observed.
Figure 2.
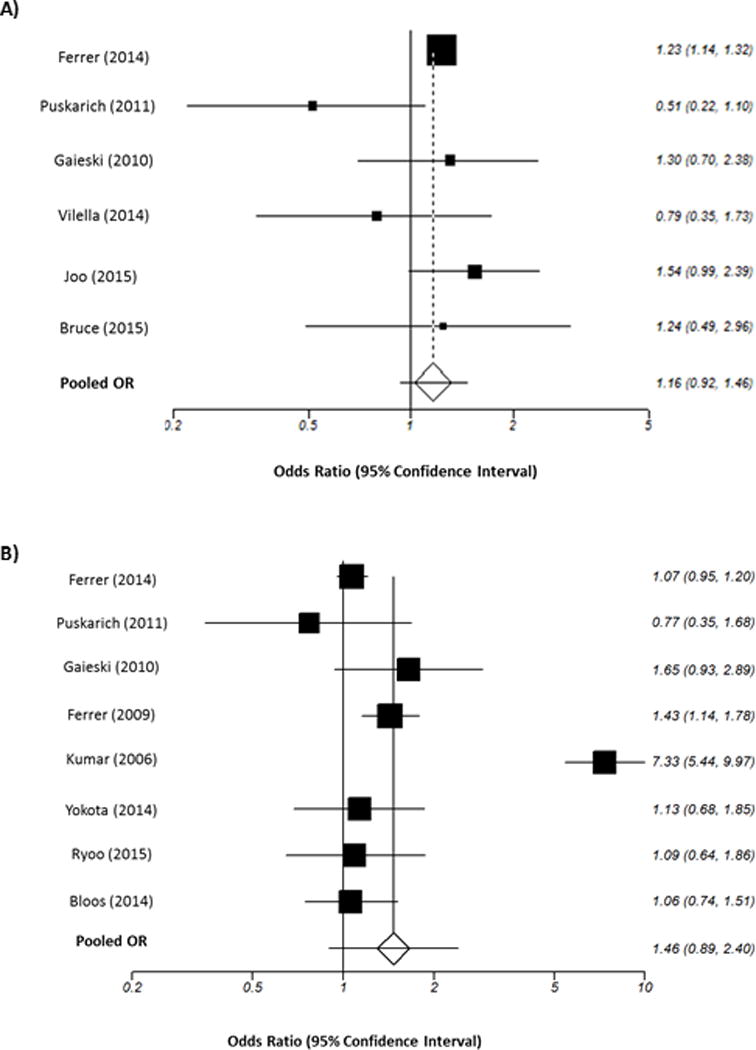
Summary Forrest plots
2A: Pooled odds ratios for mortality and time to antibiotics in less than or more than three hours from triage time; 2B: Pooled odds ratios for mortality and time to antibiotics in less than or more than one hour from severe sepsis/shock recognition
Eight of the 11 studies contained the necessary data on 11,017 patients for inclusion in analysis of the effect of time to antibiotics administration from severe sepsis/septic shock recognition. A total of 3335 patients were included in the within 1 hour of recognition group of whom 1174 died and 7682 patients received antibiotics in 1 or more hours after severe sepsis/shock recognition of whom 3581 deaths. The pooled OR for patients who received antibiotics in more than 1 hour of severe sepsis/shock recognition was 1.46 (95% CI 0.89 to 2.40, p = 0.13) compared to those who received antibiotics within 1 hour of severe sepsis/septic shock recognition (Figure 2). Although we did find statistical heterogeneity (p < 0.001) there was evidence of no publication bias. The total number of included patients from each study are listed in Table 4.
Table 4.
Total number of patients included in meta-analysis from each study
| Author (Date) | Number of patients |
|---|---|
|
Meta-analysis based on triage time
| |
| Ferrer (2014) | 14639 |
| Puskarich (2011) | 308 |
| Gaieski (2010) | 261 |
| Vilella (2014) | 184 |
| Joo (2014) | 591 |
| Bruce (2015) | 195 |
|
| |
|
Meta-analysis based on severe sepsis/shock recognition
| |
| Ferrer (2014) | 5062 |
| Puskarich (2011) | 172 |
| Gaieski (2010) | 261 |
| Ferrer (2009) | 1737 |
| Kumar (2006) | 2174 |
| Yokota (2014) | 358 |
| Ryoo (2015) | 426 |
| Bloos (2014) | 827 |
In the sensitivity analysis, 4 of the 11 studies contained complete data at every time point between less than 1 hour and more than 5 hours for further assessment of the effect of hourly delays to antibiotic administration from severe sepsis/shock recognition. The groups contained 848 deaths of 2318 patients in the < 1 hour group, 471 deaths of 1298 patients in the 1–2 hour group, 323 deaths of 853 patients in the 2–3 hour group, 245 deaths of 615 patients in the 3–4 hour group, 193 deaths of 453 patients in the 4–5 hour group, and 1537 deaths of 2386 patients in the > 5 hours group. We observed no statistical significant increased mortality in the pooled ORs for each hourly incremental delay in antibiotic administration from severe sepsis/shock recognition (Table 5).
Table 5.
Odds Ratios for mortality from with each hourly incremental delay in antibiotic administration from severe sepsis/septic shock recognition.
| Author | < 1 hour | 1–2 hours | 2–3 hours | 3–4 hours | 4–5 hours | > 5 hours |
|---|---|---|---|---|---|---|
|
Ferrer (2014) |
Ref | 0.94 (0.80, 1.12) |
0.89 (0.73, 1.08) |
0.92 (0.73, 1.15) |
0.97 (0.75, 1.25) |
1.38 (1.18, 1.61) |
|
Gaieski (2010) |
Ref | 1.65 (0.84, 3.20) |
1.38 (0.44, 3.96) |
1.72 (0.42, 6.36) |
4.13 (0.45, 50.6) |
0.92 (0.02, 11.82) |
|
Kumar (2006) |
Ref | 1.67 (1.10, 2.53) |
2.59 (1.67, 4.01) |
3.01 (1.94, 4.67) |
3.98 (2.45, 6.47) |
15.23 (11.1, 21.1) |
|
Ryoo (2015) |
Ref | 0.91 (0.47, 1.75) |
1.31 (0.62, 2.71) |
1.17 (0.39, 3.14) |
1.10 (0.30, 3.39) |
1.30 (0.34, 4.13) |
|
Pooled OR (95% CI) |
Ref |
1.21 (0.84, 1.72) |
1.42 (0.76, 2.67) |
1.53 (0.72, 3.28) |
1.90 (0.72, 5.01) |
2.47 (0.46, 13.36) |
Abbreviations: OR – Odds ratio; Ref – reference value; CI – confidence interval.
Discussion
The SSC international guidelines for the management of severe sepsis and septic shock recommend administering antibiotics within 1 hour of recognition and within 3 hours of ED triage. (13) Using the available published data, our results indicate that in patients with severe sepsis and septic shock antibiotic administration within three hours of ED triage and/or within one hour of shock recognition is not associated with significant improvement in mortality. Our findings do not support the SSC guideline recommendations on timing of antibiotic administration and raise concern about the use of time to antibiotic administration as currently recommended as a specific metric of treatment quality in sepsis care.(13)
The recognition and treatment of severe sepsis and septic shock remains a complex, and challenging burden for clinicians with a persistently high mortality rate.(1–2;12) In the past 15 years, research has suggested that an early structured approach to recognition and treatment of sepsis improves outcomes likely due to a combination of factors including heightened recognition or awareness, early reversal of microcirculatory or endothelial dysfunction, reversal of hypoperfusion, and/or eradication of infectious nidus.(3–5;7) However, the results of studies focusing on the impact of timing of antibiotic administration have been inconsistent. (8–9;11;14;19)
While it is recognized that failure to administer effective anti-microbial therapy will at some time point be detrimental to patient outcomes, the exact time frame when this shift begins to occur remains unknown. Furthermore, no randomized clinical trials examine the impact of the timing of antibiotics on outcomes directly,(20) and for obvious reasons it is unlikely any direct experimental investigation will be planned in the near future given current guideline recommendations and ethical concerns regarding patient safety of such a design.(13) Thus our results represent the most comprehensive and robust analysis of the differentiation and true impact of timing of antibiotic administration on outcome during the earliest phases of sepsis care.
There are multiple potential explanations for our findings of no mortality benefit when antibiotics are given within three hours of triage or one hour of severe sepsis/septic shock recognition. First given the complexity of the pathophysiologic insult of sepsis and resulting organ dysfunction, it is unlikely that a limited single point in time intervention, such as administration of a single dose of antibiotics, would have a profound and singular impact on survival. In fact, no other therapeutic agent has ever been shown to provide this effect despite many decades of research. As recently found in the ProCESS trial, many of the aggressive interventions targeted over the last several years, may not be as not as impactful as initially reported.(21) Second, it is plausible that in some patients the initiation of resuscitation prior to the administration of antibiotics provides the most ideal circumstance for the host to have a sustained and robust hemodynamic response to the propagation of the inflammatory cascade and resultant insult that can be instigated by release of components during bacterial lysis.(22–25)
Time to antibiotic administration is a logical and tempting metric to target when considering the quality of sepsis care. Venkatesh and colleagues(12) examined whether using the SSC recommendation metric of three hours from ED triage to antibiotic administration could adequately characterize what is realized in practice. In this study the triage-based metric performed poorly, misclassifying 23.4% of patients, likely due to the variable progression and clinical course in severe sepsis and septic shock. Furthermore, Villar and colleagues (26) found that 15% of patients with documented severe sepsis and septic shock don’t meet diagnostic criteria until more than three hours after hospital arrival. Both studies concluded that a triage-based metric was inadequate to evaluate ED performance in severe sepsis and septic shock and suggested that time to antibiotics from triage is not a reliable quality metric.(12;26) Our results provide quantitative data to support these conclusions in that we found no mortality benefit when antibiotics were administered within 3 hours of triage or 1 hour of severe sepsis/septic shock recognition.
We believe that an incorrect interpretation of this report would be that early administration of antibiotics is not of substantial importance. Antimicrobial administration is largely considered the cornerstone therapy for bacterial infections and a mandatory component of the management of severe sepsis and septic shock. Rather, our results should serve to highlight the importance of data driven and evidence based metrics for measuring quality in the care of acute critical conditions such as sepsis, rather than empiric, arbitrary or non-evidence based metrics that do not have patient oriented outcome benefit, are not operationally feasible and/or cannot be practically achieved in a comprehensive individual and systems change approach.
As a systematic review and meta-analysis of previously published literature, our results are limited by the inherent flaws and shortcomings of the included parent studies. Also, no randomized clinical trials have directly examined the effect of time to antibiotic administration on outcomes, our data was derived from cohort studies and different patient populations. While a randomized trial of immediate versus delay antibiotic administration would be difficult to design and implement, given the current variability of associative data, such a trial would be a substantial contribution to the current evidence base. Third, there was evidence of statistical heterogeneity among the included studies evaluating time to antibiotic administration from severe sepsis/shock recognition. While this is a limitation, given the large sample size in this study the findings appear to be robust and maintain validity.
Several publications appeared to have patient populations that had the potential to be included in our analysis but did not contain data that would allow for inclusion and analysis. We attempted author contact in these cases on three different occasions. We received responses in half of these requests and no response, either positive or negative, in half of requests. Although we followed recommended methodology for making valiant attempts to obtain all potential data for inclusion, it remains possible that their inclusion could have altered the results of this study and the lack of their inclusion heightens the possibility of information bias in our report.
Finally, we did not limit our study to appropriate or effective antibiotics (defined as an identified organism with in vitro sensitivity to an administered antibiotic). This was an a priori decision and viewed by the authors as the most clinically relevant and valid approach. Our rational for this decision were: 1) our primary aims were to evaluate the antibiotic recommendations of the SSC guidelines, which recommend that broad spectrum antibiotics that are likely effective based on patient history and local antibiogram resistant patterns, but do not specify that the antibiotics should be “appropriate” (i.e. sensitive to the subsequently cultured microbe); 2) including appropriateness of antibiotic choice into a meta-analysis would introduce irreconcilable clinical heterogeneity because undoubtedly the standard definition of appropriateness would vary greatly between papers including deciding on which cultures to include, what constitutes a positive culture, and how to handle conditions in which cultures are expected to be negative (such as cellulitis); 3) half or more of sepsis cases are culture negative and information on the offending organism and sensitivities are almost never available to treating clinicians at the time of antibiotic choice and administration, often taking between 12–120 hours for bacterial speciation and sensitivities using traditional blood culture techniques. (27–28). In a post-hoc review of the studies included in the meta-analysis, several studies mentioned appropriate antibiotics, but only one contained usable population level data on the effect of appropriate or effective antibiotics on mortality. Among the studies that mentioned appropriate antibiotic therapy, there was vast differences in definitions for appropriate or effective, highlighting the clinical heterogeneity with this definition. Some examples of the various definitions include: a) One study included culture-negative shock, but guideline-adherent broad-spectrum antibiotics and for culture-positive patients, in vitro activity against causative organism. This paper only evaluates those with appropriate antibiotics administered within 6 hours of first antibiotic treatment; b) One study reported only culture-positive patients, and the appropriateness of therapy is within 24 hours of diagnosis, not the initial dose of antibiotics; c) One study discussed but never defined appropriate antibiotics; d) One study defined local institutional antibiotic guideline adherence as appropriate regardless of culture results. While we recognize that the impact of appropriate or effective antibiotics in the early resuscitation of severe sepsis and septic shock remains an important question, there do not appear to be data available for meta-analysis of this subgroup and we suggest that future investigations should address this question with standard definitions and approaches.
Conclusion
In this comprehensive analysis of pooled data from the available literature in patients with severe sepsis and septic shock, administration of antibiotics within three hours of ED triage or within one hour of recognition of severe sepsis/septic shock did not confer mortality benefit. These results suggest that currently recommended specific timing metrics in international guidelines are not supported by the currently available evidence. Future stakeholders should consider these data when developing metrics to measure quality of care in severe sepsis and septic shock.
Acknowledgments
Copyright form disclosures: Dr. Jones’ institution received grant support from the National Institutes of Health (NIH). Dr. Sterling received support for article research from the NIH. Her institution received grant support from the NIH (T32 training grant). Dr. Puskarich’s institution received grant support from the Emergency Medicine Foundation (Career Development Award) and NIGMS (K23 GM113041-01).
Footnotes
The authors have no conflicts of interest to report
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001 Jul;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007 Aug;35(8):1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007 Aug;132(2):425–32. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, Kline JA. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008 Oct;36(10):2734–9. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007 Apr;35(4):1105–12. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 7.Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care. 2009;13(5):R167. doi: 10.1186/cc8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014 Aug;42(8):1749–55. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 9.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010 Apr;38(4):1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 11.Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, Kline JA, Jones AE. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011 Sep;39(9):2066–71. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesh AK, Avula U, Bartimus H, Reif J, Schmidt MJ, Powell ES. Time to antibiotics for septic shock: evaluating a proposed performance measure. Am J Emerg Med. 2013 Apr;31(4):680–3. doi: 10.1016/j.ajem.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 14.Vilella AL, Seifert CF. Timing and appropriateness of initial antibiotic therapy in newly presenting septic patients. Am J Emerg Med. 2014 Jan;32(1):7–13. doi: 10.1016/j.ajem.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Healthcare Improvement. Sepsis Resuscitation Bundle: Improve Time to Broad-Spectrum Antibiotics. 2015 [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Reeves B, Deeks J, Higgins J, Wells G. Including non-randomized studies. In: Higgines J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgines JPT, Rothstein HR. Fixed-Effect Versus Random-Effects Models Introduction to Meta-Analysis. John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 19.Hutchison RW, Govathoti DA, Fehlis K, Zheng Q, Cottrell JH, Franklin N, Montgomery GM. Improving severe sepsis outcomes: cost and time to first antibiotic dose. Dimens Crit Care Nurs. 2011 Sep;30(5):277–82. doi: 10.1097/DCC.0b013e318227756d. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui S, Razzak J. Early versus late pre-intensive care unit admission broad spectrum antibiotics for severe sepsis in adults. Cochrane Database Syst Rev. 2010;(10):CD007081. doi: 10.1002/14651858.CD007081.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014 May 1;370(18):1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenep JL, Mogan KA. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984 Sep;150(3):380–8. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- 23.Peng ZY, Wang HZ, Srisawat N, Wen X, Rimmele T, Bishop J, Singbartl K, Murugan R, Kellum JA. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012 Feb;40(2):538–43. doi: 10.1097/CCM.0b013e31822f0d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepper PM, Held TK, Schneider EM, Bolke E, Gerlach H, Trautmann M. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 2002 Jul;28(7):824–33. doi: 10.1007/s00134-002-1330-6. [DOI] [PubMed] [Google Scholar]
- 25.Mignon F, Piagnerelli M, Van N M, Vincent JL. Effect of empiric antibiotic treatment on plasma endotoxin activity in septic patients. Infection. 2014 Jun;42(3):521–8. doi: 10.1007/s15010-014-0586-4. [DOI] [PubMed] [Google Scholar]
- 26.Villar J, Clement JP, Stotts J, Linnen D, Rubin DJ, Thompson D, Gomez A, Fee C. Many emergency department patients with severe sepsis and septic shock do not meet diagnostic criteria within 3 hours of arrival. Ann Emerg Med. 2014 Jul;64(1):48–54. doi: 10.1016/j.annemergmed.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Kirn TJ, Weinstein MP. Update on blood cultures: how to obtain, process, report, and interpret. Clin Microbiol Infect. 2013 Jun;19(6):513–20. doi: 10.1111/1469-0691.12180. [DOI] [PubMed] [Google Scholar]
- 28.Kerremans JJ, Verboom P, Stijnen T, Hakkaart-van RL, Goessens W, Verbrugh HA, Vos MC. Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J Antimicrob Chemother. 2008 Feb;61(2):428–35. doi: 10.1093/jac/dkm497. [DOI] [PubMed] [Google Scholar]


