Abstract
Transcatheter management of valvular and structural heart disease is the most growing aspect of interventional cardiology. While the early experience was limited to patients who were not candidate for surgery, the continuous improvement in the efficacy and safety expanded its use to different degree depending on the procedure and the disease involved. The cost of these procedures is a major concern for health care in developed world. Cost-effectiveness of these transcatheter structural procedures varies depending on the procedure itself, the burden of the underlying disease, the feasibility and cost of both the Transcatheter and surgical procedures. In this review, we turn now to a specific discussion of the medical economics of percutaneous valvular and structural interventions.
Keywords: Cost- Effectiveness, Transcatheter Structural Intervention, Transcatheter Aortic Valve Replacement, Surgery
INTRODUCTION
Structural heart disease is the field of interventional cardiology that may well witness the greatest growth in the next 10 years.1 The field of structural interventional cardiology was first developed with transcatheter valvuloplasty of the mitral valve followed by the introduction of closure devices of atrial septal defects (ASD) and patent foramen ovale (PFO). Importantly, the recent introduction of transcatheter aortic valve replacement (TAVR) and the development of new transcatheter techniques to manage mitral regurgitation promises an expected growth of 30% in the volume of structural procedure over the next decade.1 This estimated growth is based on the expected increase in incidence of aortic and mitral valve disease in the future due to aging of the population in addition to greater penetration of these methods to reach broader populations. Analysis based on three large population-based epidemiological studies showed that the prevalence of aortic and mitral valve disease in the population was estimated to be 2.5% but ranged from less than 1% for those under 54 years old to 4% to 8% by age 65 to 74 and 12% to 14% over age 75.2
The approval of the Edwards Sapien valve (Edwards Lifesciences, Irvine, CA) in 2007 and the CoreValve (Medtronic, Minneapolis, MN) shortly thereafter in Europe lead to the performance of more than 60,000 TAVR procedures outside of the United States (US) in 2011.1 In the United States, the Edwards Sapien valve was approved in 2011 and the CoreValve was approved in 2014. It is expected that TAVR volume will grow from 2000 per year in 2012 to 25,000 per year after 2015, especially after TAVR was shown to be a viable option in not only inoperable patients but also in operable but high risk patients.1 Similarly, many advances in the transcatheter management of mitral regurgitation are evolving.3 Currently the MitraClip is the only transcatheter-approved therapy for mitral regurgitation in the US, but there are many other devices available around the world.
All these advances in the transcatheter management of previously surgically managed conditions led to a different degree of change in practice around the world. Most of these transcatheter procedures are effective and are associated with comparable outcomes to surgery while being less invasive. However, they are associated in many cases with higher costs. In light of this, we turn now to a specific discussion of the medical economics of percutaneous valvular and structural interventions.
COST EFFECTIVENESS ANALYSIS AND DECISION MAKING
The primary goal of cost-effectiveness analysis is to evaluate different health care intervention options in common terms so that policy and other decision makers can be informed of the most efficient method of producing extra health benefits from among the alternative ways that health care dollars can be distributed. The metric used to assess incremental cost effectiveness is the incremental cost-effectiveness ratio (ICER). An ICER is defined as the ratio of incremental costs to incremental health benefits of treatment 1 compared to treatment 2, or ICER = (C1 – C2)/(HB1 – HB2); where C1 and C2 are cost for treatment 1 and 2, respectively and HB is the health benefit of treatment 1 and 2, respectively.4
The ICER defines the cost that should be assumed for gaining one unit of output. In other words, if one of the alternatives is the usual practice, then it will tell us how much it will cost to gain a unit of outcome when moving from the usual practice to a new alternative. The health benefit may be measured in any sensible unit, such number of myocardial infarctions averted, but most studies use the conventional option of measuring clinical benefits as either the number of added life-years (LYs) or quality adjusted life years (QALYs).4,5 Both of these approaches require estimation of life expectancy with and without the intervention being considered.
When assessing whether a treatment is cost effective, a requirement for threshold can arises when policy makers seek a benchmark to compare different treatments and judge different studies. In general, wealthier countries may be willing to pay more (i.e. accept higher threshold) for a given treatment than poorer countries.4,6 In the United States, a cost-effectiveness ratio < $50,000 per LY or QALY is frequently regarded as economically attractive, in part because it approximates the cost of providing chronic hemodialysis to patients with renal failure, at a cost that meets willingness-to-pay through Medicare.4 Conversely, an ICER of > $100,000 per added LY or QALY is frequently regarded as economically unattractive. The range between these two benchmarks is the gray zone in which there is no consensus on whether a treatment is economically acceptable.4 However, assigning the same ICER threshold for different treatment in wide range of diseases and different burden of this disease may not be reasonable. Some countries may assign a general threshold for most cases, but allow for a higher threshold for treatment that relive considerable burden of illness, i.e. “the rule of rescue”. For example in Netherland, the average acceptable ICER is around €20,000, but an ICER of €80,000 per QALY will be acceptable for illnesses associated with a considerable disease burden.7 Similarly in Great Britain the limit of acceptable ICER varies between £20,000 and £30,000 depending on the burden of the disease.8
TRANSCATHETER VERSUS SURGICAL CLOSURE OF SECUNDUM ASD IN ADULTS
Transcatheter ASD closure was shown to be a feasible option for the management of secundum ASD in adults.9 Outcome analysis of a cohort of 718 ASD closures performed between 1988 and 2005 that included 383 surgical ASD closures and 335 transcatheter ASD closures showed no difference in mortality at 5 years between the two strategies (5.3% transcather vs. 6.3% surgical; P = 1.00), but transcatheter intervention was associated with a higher re-intervention rate at 5 years (7.9% vs. 0.3%, p = 0.0038).9 Following ASD closure, there was no difference in new-onset CHF (5.0% vs. 3.0%, p= 0.30) or stroke/TIA (1.6% vs. 1.8%, p0.99) between patients undergoing surgical versus transcatheter ASD closure. Furthermore, there was a higher outpatient physician visits per patient (7.5 vs. 6.4, p 0.001) and critical care days per patient (0.24 vs. 0.14, p = 0.001) in the surgical cohort. There was no difference in the emergency department visits between the 2 groups (0.92 vs. 0.98, p = 0.61).9
Cost effectiveness on this cohort showed that the 5-year cost of surgical closure was $15,304 versus $11,060 for the transcatheter alternative. At 5 years, transcatheter closure was marginally more effective than surgery (4.683 ± 0.379 LY versus 4.618 ± 0.638 LY).10 So it seems that whenever feasible, transcatheter ASD closure may offer a very promising cost effective alternative surgical closure.
MEDICAL ECONOMICS OF TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR)
It is estimated that 3.4% of the population older than 75 years has severe aortic stenosis with 350,000 patients in the US alone.11 Forty percent of these patients do not get surgical intervention secondary to being considered either inoperable or at very high surgical risk. TAVR was developed as an alternative for surgical valve replacement. TAVR treats aortic stenosis by displacing and functionally replacing the native valve with a bioprosthetic valve delivered on a catheter (Edward Sapien) or trileaflet porcine pericardial valve on a self-expanding nitinol frame (CoreValve) through the femoral artery (transfemoral approach, TF- TAVR) or the left ventricular apex (transapical placement, TA- TAVR). It is estimated that there are approximately 102,558 TAVR candidates in North America and 189,836 TAVR candidates in Europe.11
Cost Effectiveness of Transcatheter Aortic Valve Replacement vs. Medical Therapy in Inoperable patients
Symptomatic severe aortic stenosis in the absence of definitive treatment leads to progressive symptoms, functional decline, and death.12 In patients who are considered not candidates for surgical aortic valve replacement (SAVR), medical therapy was not effective in slowing the expected outcomes. The introduction of TAVR offered an alternative for this patients’ population.
The evidence supporting the use of TAVR in patients with severe aortic stenosis who are not surgical candidates comes from Cohort B of the placement of aortic transcatheter valves (PARTNER) trial that randomized 358 patients with aortic stenosis who were not considered to be suitable surgical candidates to either medical management (inclusive of balloon valvuloplasty) or TAVR. The trial found an impressive absolute 20% reduction in 1-year mortality for TAVR compared with standard therapy in this population (30.7% TAVR vs. 50.7% standard therapy; HR= 0.55; 95% CI; [0.40 – 0.74]; P<0.001). However, TAVR was associated with a higher incidence of major strokes (5.0% vs. 1.1%, P=0.06) and major vascular complications (16.2% vs. 1.1%, P<0.001).12
An economic evaluation of the PARTNER B cohort showed that TAVR was associated with a mean cost of $42,806 for the initial procedure and $78,542 for the hospitalization. Follow-up costs through 12 months were lower with TAVR compared to standard therapy ($29,289 vs. $53,621; P<0.001). The cumulative 1-year costs remained higher with TAVR ($106,076 vs. $53,621; P<0.001). TAVR would increase discounted life expectancy by 1.6 years (1.3 quality-adjusted life-years) at an incremental cost of $79,837. TAVR use was associated with ICER of $50,200 per LY gained and $61,889 per QALY gained.13 Figure-1 summarizes the cost assessment of medical management vs. TAVR in Partner trial Cohort B.
Figure-1.
The Cost Assessment Of Medical Management vs. TAVR in Partner trial Cohort B.
Another cost stimulation model based on the same cohort for an estimated 10-year time horizon showed that TAVR was cost effective at 24 months with ICER of £18,500 ($31,450) per QALY gained.14 So even though TAVR in this group was associated with a considerable increase in cost, it was still associated with what might be considered an acceptable ICER per LY and a potentially acceptable ICER per QALY over time.
The recent US trial evaluating the Medtronic self-expanding CoreValve compared to standard therapy in extreme risk patients has yielded similar improvement in outcomes with significant reduction in the incidence of death and stroke (26.0% CoreValve vs. 43.0% standard therapy; P < 0.0001).15 An economic evaluation of CoreValve use in this population is expected in the near future.
Cost Effectiveness of Transcatheter Aortic Valve Replacement vs. Surgical Aortic Valve Replacement in High-Risk Patients
As TAVR offered a less invasive approach for aortic valve replacement, the question of its applicability and effectiveness in high-risk surgical patients came to the forefront. Cohort A of the PARTNER trial tried to answered this by randomizing 699 high-risk patients with severe aortic stenosis to undergo either TAVR (by either a transfemoral TAVR [TF-TAVR] or a transapical TAVR [TA-TAVR] approach) or SAVR. The results showed that TAVR was non-inferior to SAVR in the primary endpoint of death at 1 year (24.2% TAVR vs. 26.8% SAVR; P=0.44). The rates of major stroke were non statistically different at 1 year (5.1% TAVR vs. 2.4%, SAVR; P=0.07). At 30 days, major vascular complications were significantly more frequent with TAVR (11.0% TAVR vs. 3.2% SAVR; P<0.001), while adverse events including major bleeding were more frequent after SAVR (9.3% TAVR vs. 19.5% SAVR, P<0.001).16
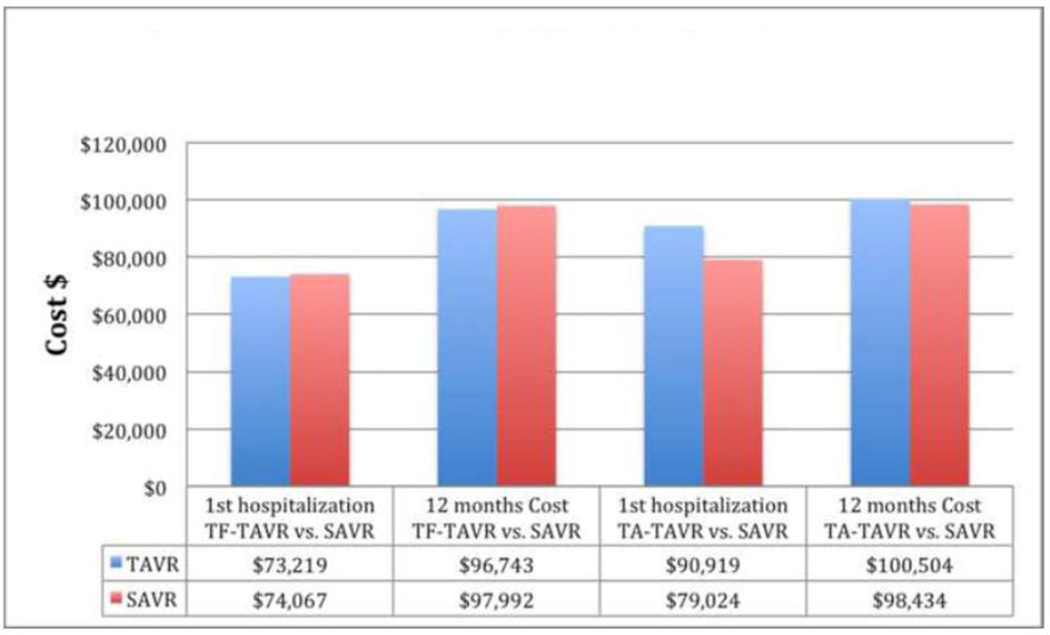
Cost effectiveness analysis of Cohort A of the PARTNER trial compared SAVR with both TF-TAVR and TA-TAVR. In the TF-TAVR cohort, the overall admission costs were not significantly different between groups ($73,219 TAVR vs. $74,067 SAVR; P=0.84) and the 12 months costs were non-significantly lower for TF-TAVR ($96,743 TF-TAVR vs. $97,992 SAVR; P=0.88) with a mean difference of −$1,250 (95% CI [−$18,132 to $13,867]; P=0.88). Both life expectancy and quality-adjusted life expectancy were slightly higher with TF-TAVR, making TF-TAVR economically dominant compared with SAVR in 55.7% of replicates with an ICER of $50,000/QALY gained in 70.9%.17
On the other hand, in the TA-TAVR cohort, the total admission costs remained non-significantly higher with TA-TAVR ($90,919 TA-TAVR vs. $79,024 SAVR; P=0.06). The 12-month cost was slightly non-significantly higher with TA-TAVR ($100,504 TA-TAVR vs. $98,434 SAVR; P=0.22) with a mean difference of $2,070 (95% CI [−$9,960 to $13,499]; P=0.22). Life expectancy was similar for the TA-TAVR and SAVR groups, whereas quality adjusted life expectancy tended to be less with TA-TAVR. Secondary to these, TA-TAVR was economically dominated by SAVR in the base case and economically attractive in only 7.1% of replicates. So in summary, while TF-TAVR seems to be an economically attractive alternative to SAVR in high surgical risk patient, it is unclear whether it is the case for TA-TAVR. Figure-2 shows the cost assessment of TAVR vs. SAVR in partner trial cohort A.
Figure-2.
The cost assessment of TAVR vs. SAVR in partner trial cohort A.
Recently data from the US trial evaluating the Medtronic self-expanding CoreValve comparing CoreValve TAVR to SAVR in high-risk patients also showed CoreValve use to be associated with significant reduction in mortality (14.2% CoreValve TAVR vs. 19.1% SAVR; P<0.001 for noninferiority; P = 0.04 for superiority).18
An economic analysis of CoreValve in this population showed that while CoreValve TAVR improved 1-month quality of life and 12-month survival relative to SAVR, TAVR was associated with $11,260 per patient higher index admission costs [$69,592 vs. $58, 332; P<0.01]. The difference was less pronounced with TF-TAVR [$67,477 vs. $58,690; P<0.01], while it was significantly higher with non TF-TAVR approach [$79,790 vs. $56,446; P<0.01]. At 12 months, TF-TAVR was still associated with a slightly higher cost [$98,358 vs. $89,151]. The difference was still significantly higher with non TF-TAVR [$108,556 vs. $87,256]. Overall TAVR use was associated with ICER of $57,000 per LY gained and $67,059 per QALY gained.19 For TF-TAVR, the ICER was $48,330 per LY gained and $55,534 per QALY gained. For non TF-TAVR, the ICER was $98,141 per LY gained and $118,247 per QALY gained. Figure-3 shows the cost assessment of TAVR vs. SAVR in U.S. CoreValve Trial.
Figure-3.
The cost assessment of TAVR vs. SAVR in U.S. CoreValve Trial.
Transcatheter Aortic Valve Replacement vs. Surgical Aortic Valve Replacement in Intermediate-Risk Patients
SAVR is the standard of care in patients with severe aortic stenosis and intermediate or average surgical risk. Randomized trials comparing TAVR and SAVR in this population are currently ongoing (e.g., SURTAVI and PARTNER II); therefore, the comparative effectiveness and cost-effectiveness of TAVR and SAVR in intermediate-risk patients is not yet known.20
Data comparing the cost of both procedures comes from a single center study performed at the Erasmus Medical Center in the Netherlands comparing CoreValve TAVR with SAVR. Despite the fact that the hospital length of stay was shorter with TAVR, the cost of the TAVR procedure and follow up for the TAVR patient was higher resulting in higher in-hospital (€40,802 TAVR vs. €33,354 SAVR; p=0.01) and total costs (€46,217 TAVR vs. €35,511 SAVR; p=0.009).21
COST EFFECTIVENESS OF PERCUTANEOUS MITRAL VALVE REPAIR
Data comparing percutaneous mitral valve repair with surgical mitral valve surgery comes from the EndoVascular Edge-to-Edge Repair Study (EVEREST) II trial that randomly assigned 279 patients with moderately severe or severe (grade 3+ or 4+) mitral regurgitation in a 2:1 ratio to undergo either percutaneous repair with the MitraClip (company, location) or conventional surgery for repair or replacement of the mitral valve. Results showed that surgical repair was associated with higher freedom from death, from surgery for mitral-valve dysfunction, and from grade 3+ or 4+ mitral regurgitation at 12 months (55% MitraClip vs. 73% mitral surgery; P=0.007). This was mainly due to increased need for definitive surgery following use of the MitraClip (20% MitraClip vs. 2% surgery). There was no difference in death (6% in each group) or freedom from grade 3+ or 4+ mitral regurgitation (21% MitraClip vs. 20% surgery). Patients' quality of life improved from baseline to 12-months in the two study groups, yet surgery was associated with a transient decrease in the quality of life at 30 days.3
Cost effectiveness analysis of EVEREST II showed that the potential cost-effectiveness of MitraClip compared with mitral valve surgery varied depending primarily on MitraClip price and acute procedural success. Economic assessment using the study price of $18,000 showed that the clip strategy reduced costs by $2,200/patient, making MitraClip economically dominant. But using a clip price of $26,200 (approximate European sales price), the overall costs were higher with the clip strategy by $6,192 and the ICER ratio was unfavorable (>$400,000 per QALY gained). In a sensitivity analysis limited to patients with acute procedural success, the QALY gain was larger, the cost-offsets with the clip was greater, and cost-effectiveness was more favorable (dominant at a MitraClip price of $18,000 and around $54,000 per QALY gained at a price of $26,200).22 In summary, although MitraClip may seems an economically reasonable option in some economic models; the clinical outcomes of MitraClip are not comparable to surgery.
DISCUSSION
The field of structural heart disease is a growing field. The field started with the development of transcatheter closure of ASD/PFO. While the transcatheter approach was shown to be an effective and probably dominant strategy when compared to surgery, the original demand of this procedure decreased overtime secondary to conflicting data in regards of the benefit of this closure in preventing recurrent stroke.23,24
The introduction of TAVR in inoperable patients first and then in high-risk patients introduced a whole new horizon for interventional cardiologists and cardiothoracic surgeons. This offered the chance to perform the procedure in a large population of patients who were either not candidate for aortic replacement or at a very high risk to undergo the procedure. The cost of the procedure and the potential economic effects of its widespread use especially in an aging US population leading to a higher prevalence of aortic stenosis constitute a major concern and a potential burden on the health care system. Cost effectiveness studies show that TAVR have a reasonable ICER for LY and QALY in inoperable and high-risk patients. The transfemoral approach seems to be more cost effectives than non-femoral approaches in most available models. Transcatheter management of mitral regurgitation even a broader techniques to the field. MitraClip is currently the only available techniques in the U.S. Additional studies evaluating MitraClip and other transcatheter interventions concerning mitral valve will be essential in the future.
Many considerations should be kept in mind while assessing the medical economics of structural heart disease. First, many of the interventional techniques are under development and while the original cost of these devices is high, continuous development and involvement of many manufactures will allow overtime for more reasonable costs. Second, there is a learning curve for the whole institution where the procedure is being done, that involves the whole cardiac team the including interventional cardiologist and cardiothoracic surgeon performing the procedures, operating room/ Catheterization laboratory staff, as well as imaging techniques and post procedure care. Third, performing the procedure in the catherization laboratory and with sedation instead of performing it in the operating room under general anesthesia will decrease the cost as well. Finally, the use of smaller access sheet will result in lower incidence of vascular complications that will result in turn in lower cost.
In Summary, many interventional structural procedures offered an alternative to surgery in the management of structural and valvular heart disease. Although most of these procedures are associated with higher risk, they still offer a cost effective alternative. Expected future improvement in the design and cost of these devices coupled with higher level of experience of those who perform the procedures will offer a higher success rate, less rate of complications, lower hospital stay and overall a lower cost.
Acknowledgments
Funding Source: Funded in part by U54-GM104941 from the National Institute of General Medical Sciences of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faxon DP, Williams DO. The changing face of interventional cardiology. Circ Cardiovasc Interv. 2012;5:325–327. doi: 10.1161/CIRCINTERVENTIONS.112.971671. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 4.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part I. Circulation. 2002;106:516–520. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]
- 5.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions-- standardizing data on outcomes. N Engl J Med. 1998;339:380–386. doi: 10.1056/NEJM199808063390606. [DOI] [PubMed] [Google Scholar]
- 6.Gilder SS. London letter. Can Med Assoc J. 1971;104:473–481. [PMC free article] [PubMed] [Google Scholar]
- 7.Simoens S. How to assess the value of medicines? Frontiers in Pharmacology. 2010;1 doi: 10.3389/fphar.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–452. doi: 10.1002/hec.864. [DOI] [PubMed] [Google Scholar]
- 9.Kotowycz MA, Therrien J, Ionescu-Ittu R, et al. Long-term outcomes after surgical versus transcatheter closure of atrial septal defects in adults. JACC Cardiovasc Interv. 2013;6:497–503. doi: 10.1016/j.jcin.2012.12.126. [DOI] [PubMed] [Google Scholar]
- 10.Mylotte D, Quenneville SP, Kotowycz MA, et al. Long-term cost-effectiveness of transcatheter versus surgical closure of secundum atrial septal defect in adults. Int J Cardiol. 2014;172:109–114. doi: 10.1016/j.ijcard.2013.12.144. [DOI] [PubMed] [Google Scholar]
- 11.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B) Circulation. 2012;125:1102–1109. doi: 10.1161/CIRCULATIONAHA.111.054072. [DOI] [PubMed] [Google Scholar]
- 14.Watt M, Mealing S, Eaton J, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart. 2012;98:370–376. doi: 10.1136/heartjnl-2011-300444. [DOI] [PubMed] [Google Scholar]
- 15.Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 16.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds MR, Magnuson EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A) J Am Coll Cardiol. 2012;60:2683–2692. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds MR. Cost-Effectiveness of Transcatheter Aortic Valve Replacement with a Self-Expanding Prosthesis Compared with Surgical Aortic Valve Replacement in High Risk Patients. J Am Coll Cardiol. 2014;64 doi: 10.1016/j.jacc.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadey G, Reynolds MR. Cost-Effectiveness Considerations in Transcatheter Management of Valvular Heart Disease. Can J Cardiol. 2014 doi: 10.1016/j.cjca.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg. 2012;94:1954–1960. doi: 10.1016/j.athoracsur.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds MR, Galper B, Apruzzese P ea. Cost effectiveness of the MitraClip compared with mitral valve surgery: 12-month results from the EVEREST II randomized controlled trial. J Am Coll Cardiol. 2012;60:B229. (abstract). [Google Scholar]
- 23.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 24.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422–431. doi: 10.1161/STROKEAHA.111.631648. [DOI] [PMC free article] [PubMed] [Google Scholar]