Abstract
The blood-brain barrier (BBB) maintains the optimal microenvironment in the central nervous system (CNS) for proper brain function. The BBB is comprised of specialized CNS endothelial cells with fundamental molecular properties essential for the function and integrity of the BBB. The restrictive nature of the BBB hinders delivery of therapeutics for many neurological disorders. In addition, recent evidence shows that BBB dysfunction can precede or hasten the progression of several neurological diseases. Despite the physiological significance of the BBB in health and disease, major discoveries of the molecular regulators of BBB formation and function have only occurred recently. This review will highlight recent findings describing the molecular determinants and core cellular pathways that confer BBB properties upon CNS endothelial cells.
Keywords: Blood-brain barrier
History of the blood-brain barrier
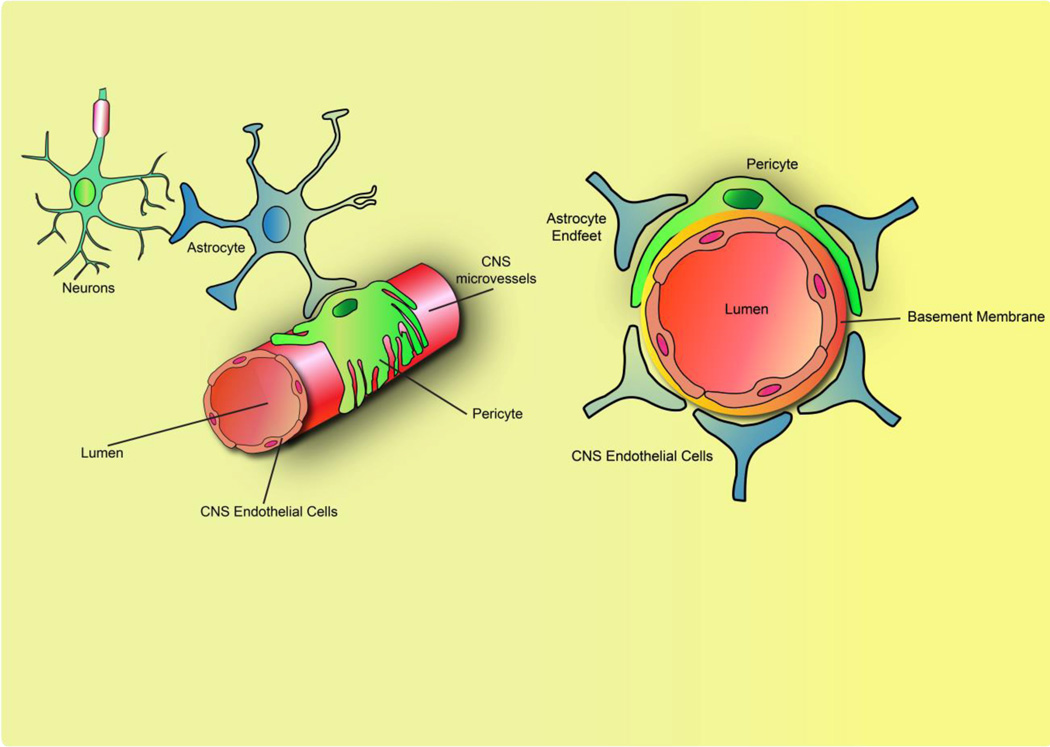
The BBB partitions the brain from circulating blood and functions to a) shield the brain from potential blood-borne toxins, b) meet the metabolic demands of the brain, and c) regulate the homeostatic environment in the CNS for proper neuronal function[1]. The functional BBB is comprised of CNS endothelial cells, pericytes, astrocytes and neurons that collectively form a functional “neurovascular unit” (NVU)(Figure 1) [2].
Figure 1. The functional BBB is dependent on the neurovascular unit.
The blood-brain barrier (BBB) is localized at central nervous system (CNS) microvessels, comprising of a single layer of continuous, nonfenestrated endothelial cells. Surrounding the aluminal surface of the CNS endothelial cells are the basement membrane, pericytes, and astrocyte endfeet, collectively known as the neurovascular unit (NVU). The BBB properties are not intrinsic to CNS endothelial cells but require the continuous functional interactions with the NVU.
The BBB was first observed over a century ago. Pioneering physiologists studying the cerebrospinal fluid (CSF) noticed that water-soluble dyes injected in the peripheral circulation stained several tissues except the brain[3]. Ehrlich argued that this phenomenon occurred because the CNS had low affinity for the dye[4]. However, Goldmann questioned this argument, as injection of the same dyes in the subarachnoid space colored the brain but not peripheral tissues[5]. Continuing from these studies, Lina Stern and colleagues performed experiments in which they injected several vehicles into the brain parenchyma and blood. The results from these dye studies prompted Stern to introduce the term, “blood-brain barrier” and suggest its physiological function in maintaining brain homeostasis[6]. Over the years, the concept of the BBB fascinated physiologists but the anatomical site of the BBB was highly disputed; specific possibilities included the endothelium, astrocytic end-feet, or the basement membrane. A seminal study by Reese and Karnovsky using electron microscopy (EM) and injection of electron-dense horseradish peroxidase (HRP) resolved this dispute [7]. In this work, ultrastructural analysis by EM was used to delineate astrocytic end-feet, and the luminal, abluminal, and basement membrane. Results revealed that the HRP was confined to the lumen of the CNS endothelium. Furthermore, EM revealed that the CNS endothelial cells are joined continuously by tight junction complexes and have limited intracellular vesicles[7]. Similar to Goldman’s experiments, HRP injection into the brain parachyma diffused past astrocytic end-feet and halted at the abluminal membrane of the endothelium, demonstrating that astrocytic end-feet do not significantly contribute to the physical barrier[8]. Thus, the site of the BBB is CNS capillaries comprised of a single, non-fenestrated, continuous endothelial cell layer.
Molecular properties of the BBB
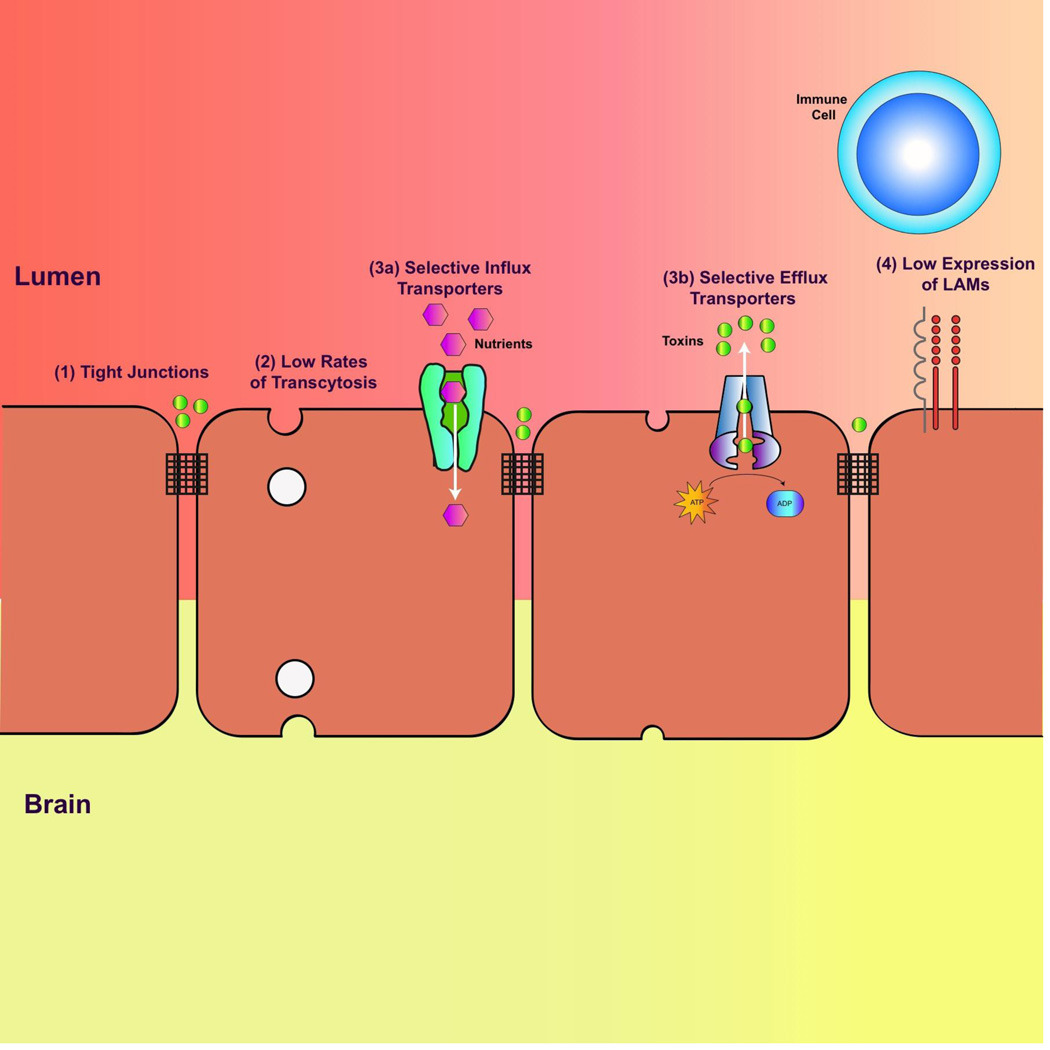
CNS endothelial cells are highly polarized with distinct luminal (apical) and abluminal (basolateral) compartments[9]. The polarized nature of CNS endothelial cells is reflected in their four fundamental barrier properties that contribute to BBB function and integrity (Figure 2)[10]. First, circumferential tight junction complexes at the lateral, apical membrane between CNS endothelial cells establish a high-resistance paracellular barrier to small hydrophilic molecules and ions[8,11]. Tight junction complexes are comprised of a) tight junction proteins such as claudins and occludin, b) adhesion molecules such as VE-cadherin and E-cadherin and c) junctional adhesion molecules[12,13]. These transmembrane proteins are further linked and stabilized to the cytoskeleton via multiple cytoplasmic adaptor proteins such as zonula occludens proteins [14]. Emerging studies have demonstrated that there is significant crosstalk among these tight junction complex proteins to regulate the restrictive barrier junction[15]. Second, in contrast to the peripheral endothelium, CNS endothelial cells display minimal vesicular trafficking, limiting the vesicle-mediated transcellular movement of cargo known as transcytosis[16]. Although CNS endothelial cells display limited transcytosis, it is still the preferred pathway for the selective transport of plasma macromolecules such as albumin and low-density lipoprotein[17]. Third, the establishment of the restrictive paracellular and transcellular barriers allows CNS endothelial cells to use highly polarized cellular transporters to dynamically regulate the influx of nutrients and efflux of metabolic waste and toxins between the blood and the brain parenchyma. The major class of known efflux transporters is the ATP-binding cassette (ABC) transporters- including Pgp, BCRP and MRP- mostly localized at the luminal membrane[18–20]. These efflux transporters hydrolyze ATP to transport a wide array of substrates against their concentration gradients into the blood[19,20]. CNS endothelial cells also express specialized nutrient transporters that facilitate the transport of ions, macromolecules and proteins from the blood to the brain. Many of these transporters belong to the superfamily solute carrier proteins (SLC) of facilitative transporters that includes sugar transporters such as SLC2A1 (GLUT1), and cationic amino acid transporters such as SLC7A1 [21,22]. It is surprising to note that although SLCs play a vital role in metabolism and nutrition, they are particularly understudied[23]. Fourth, CNS endothelial cells lack the expression of leukocyte adhesion molecules (LAMs) such as E-selectin and Icam1[24]. The lack of these luminal surface molecules prevents the entry of immune cells from the blood into the parenchyma, resulting in a paucity of immune cells in the brain microenvironment[25]. As a result, the healthy brain is “immune privileged”, where introduced antigens do not elicit the development of adaptive immune responses[26]. These fundamental molecular characteristics confer BBB properties on CNS endothelial cells to regulate brain homeostasis.
Figure 2. The four fundamental molecular properties of CNS endothelial cells that contribute to BBB integrity and function.
(1) Specialized tight junction complexes between endothelial cells prevent paracellular flux. (2) CNS endothelial cells have low rates of transcytosis, limiting transcellular flux. (3a) CNS endothelial cells mediate the selective uptake of nutrients and molecules from the blood using selective influx transporters and (3b) efflux of toxins against their concentration gradient with ATP-dependent selective efflux transporters. (4) The low expression of leukocyte adhesion molecules (LAMs) contributes to the low level of immune surveillance in the CNS.
Identifying molecular regulators of BBB function and integrity in CNS endothelial cells
The four fundamental BBB properties listed above are not intrinsic to CNS endothelial cells but are induced and regulated by the neural environment[27]. Transplantation studies using chick/quail chimeras have demonstrated that nonvascularized brain fragments transplanted into the coelomic cavity were soon vascularized by abdominal vessels that developed BBB characteristics, such as exclusion of circulating dye and low number of vesicles[28]. In contrast, nonvascularized embryonic mesoderm tissues grafted in the CNS were soon vascularized by neural vessels that failed to displayed BBB properties[28]. This seminal experiment demonstrated that: 1) BBB properties are not inherent to CNS endothelial cells and 2) the neural environment provides inductive cues to CNS endothelial cells to activate genetic programs in order to acquire BBB properties. Although the identities of these signals and genetic programs have been elusive, recent advances in purification and gene expression profiling of CNS endothelial cells have elucidated novel molecular mediators that confer barrier properties upon CNS endothelial cells.
The most well-characterized genetic program inducing BBB properties in CNS endothelial cells (Table 1a) is β-catenin signaling[29–31]. Daneman et al. purified CNS and peripheral endothelial cells using fluorescence-activated cell sorting (FACS) from Tie2GFP mice, a transgenic GFP reporter for endothelial cells[31]. Microarray analysis indicated that many downstream effectors of Wnt/ β-catenin signaling are enriched in CNS endothelial cells, suggesting that β-catenin signaling may mediate CNS vasculature functions. Indeed, various studies have demonstrated that canonical Wnt signaling is essential for both CNS-specific angiogenesis and barriergenesis. For example, endothelial cell specific deletion of β-catenin disrupts CNS-angiogenesis, resulting in gross vascular malformations and hemorrhages whereas peripheral angiogenesis remains largely undisrupted[31]. In addition, endothelial cell-specific deletion of β-catenin disrupts barriergenesis, downregulating Glut1 expression, a marker commonly used for BBB formation, in CNS vasculature[29–31]. Zhou et al. [28] demonstrated that postnatal, endothelial cell-specific deletion of β-catenin results in BBB breakdown, exemplified by extravasation of dyes and downregulation of tight junction protein expression, showing that barrier dysfunction is not a consequence of disrupted angiogenesis. Recently, many of the receptors upstream of B-catenin - including Frizzled receptors, co-receptors LRP5 /LRP6 and auxiliary receptor GPR124 - as well as the Wnt ligands (please see next section) necessary for β-catenin activation have been identified[32–40]. Loss-of-functions of these genes results in CNS vasculature dysfunction and largely resembles β-catenin mutants[33,34].
Table 1.
| a | ||||
|---|---|---|---|---|
| Tight Junctions |
Transcytosis | (25–34) | ||
| Yes | Yes | Yes** | (25–27) | |
| Yes | Yes | Yes** | (28–30), (70) | |
| Yes | Yes | Yes** | (28–35) | |
| Yes | Yes | Yes** | (25–27) (36) | |
| Yes | Yes | ND | (37) | |
| No | No | Yes | (38–44) | |
| No* | Most likely*** | ND | (45) | |
| b | ||||
|---|---|---|---|---|
| Tight Junctions | Transcytosis | |||
| Neural Progenitors; Bergmann Glia |
Yes | Yes | Yes** | (25–27) (29) (32) |
| Astrocytes | ND | Yes | ND | (47) |
| Gut Microbiota | No | Yes | ND | (49) |
No vascular defects were observed during embroynic analysis of LSR knockout mice
Loss-of-function of Wnt signaling results in elevated expression of PLVAP, a marker for fenestarted endothelial cells and transcytosis
Although ultrastructural analysis by EM revealed no defects in tight junction complexes, loss of LSR affects tight junctions
ND: Not Determined
A recent study identified novel downstream targets of β-catenin signaling that mediate both CNS angiogenesis and barriergenesis. Tam et al. used antibody-based FACS to isolate endothelial cells from CNS and non-CNS tissues at three developmental ages.[41] Microarray analysis indicated dr6, troy and spock2 are highly enriched in CNS endothelial cells. Indeed, these genes are essential for CNS vasculature function as dr6, troy and spock2 knockdown in zebrafish resulted in vascular malformation and barrier dysfunction. In contrast, loss-of-function of other genes enriched in CNS endothelial cells, abcyap1r1 and tspn5, resulted in vascular morphogenesis defects but exhibited no barrier dysfunction. The authors demonstrated that in vitro activation of β-catenin with recombinant Wnt ligands upregulates the expression of dr6 and troy, suggesting that these genes are downstream effectors of β-catenin. However, it is difficult to determine if these genes specifically regulate barriergenesis, or if barrier dysfunction is a consequence of vascular malformations. Temporal deletion of these genes after CNS angiogenesis can clarify this point.
Genes that specifically regulate BBB function and integrity independent of CNS angiogenesis have been identified via gene profiling of purified CNS endothelial cells. Ben-Zvi et al. mapped the development of mouse cortical barriergenesis at E15.5 and performed microarray analysis from FACS purified Tie2GFP+ CNS and lung endothelial cells at E13.5, a time when barrier properties are actively forming[42]. The microarray analysis indicated that major facilitator domain containing protein 2A (Mfsd2a) is enriched in CNS endothelial cells. Mfsd2a is expressed specifically in CNS vasculature and not in the choroid plexus, a structure that lacks BBB. Mfsd2a knockout mice display BBB dysfunction due to unregulated bulk flow of transcytosis. However, vascular development and patterning remain unaffected, suggesting Mfsd2a specifically regulates BBB integrity independent of angiogenesis. Surprisingly, Mfsd2a has putative dual physiological functions[43,44]. Not only is Mfsd2a essential for the CNS endothelium to maintain low rates of transcytosis, but also Nguyen et al. reported that Mfsd2a is a transporter for omega-3 fatty acids across the CNS endothelium[45]. Lipidomics revealed that brains of Mfsd2a knockout mice exhibit decreased docosahexaenoic acid (DHA), an omega-3 fatty acid essential for neuronal function, and elevated arachidonic acid, an omega-6 fatty acid. Furthermore, Mfsd2a knockout mice display fewer neurons in the hippocampus and cerebellum, microcephaly and other neurological deficits. These altered brain fatty acids and behavioral abnormalities are reminiscent of omega-3 fatty acid deficiency[46]. In fact, recent human genetics studies identified loss-of-function missense mutations in MFSD2A as a recessive cause of microcephaly [47,48]. One study identified two MFSD2A missense mutations that result in severe intellectual disability, seizures and early lethality, whereas a second study identified a milder MFSD2A missense mutation that results in patients with intellectual disability alone. Similar to GLUT1, Mfsd2a has dual physiological functions at the BBB – maintaining barrier integrity and transporting nutrients across the barrier[49,50]. It will be essential for future studies to determine if Mfsd2a’s dual physiological functions of (1) maintaining BBB integrity and (2) transporting essential nutrients act simultaneously or if one function is required for the other.
Another recently discovered gene that mediates barriergenesis independent of angiogenesis is lipolysis-stimulated lipoprotein receptor (LSR). Sohet and Daneman et al. also purified TIE2GFP+ CNS and peripheral endothelial cells and performed microarray analysis to identify lsr, another gene enriched in CNS endothelial cells[51,52]. Although lsr is expressed in many cell-types in peripheral tissues, it is expressed specifically in endothelial cells in the brain. LSR was initially reported to mediate clearance of triglyceride-rich lipoproteins and low-density lipoproteins but the physiological function of LSR in BBB was just recently explored[53]. Lsr knockout mice were reported not to display any vascular malformations or hemorrhage but still display BBB dysfunction. Indeed, LSR is essential for BBB integrity, as lsr knockout embryos exhibit extravasation of small molecular weight tracers but not larger molecular weight tracers, a phenotype that is reminiscent of the claudin5 knockout mouse[54]. This size selective permeability dysfunction may be a common phenotype observed when disrupting tight junction molecules. The extravasation of small tracers is most likely mediated via paracellular entry. In contrast, larger molecules most likely leak out through transcytosis, such as observed in Mfsd2a knockout mice, where HRP (44 kDa) and 70 kDa dextran tracers leak out of the CNS vasculature. However, the molecular mechanism underlying LSR regulation of BBB integrity is still unknown. Although LSR is localized at the tricellular tight junctions (where two bicellular tight junctions meet) in the functional BBB, lsr knockout mice display no obvious disruption in TJ complexes by EM. Because lsr has known physiological functions in peripheral tissues, conditional deletion of LSR will determine if a cell autonomous function of LSR regulates BBB integrity.
Identifying inductive signals that confer BBB properties
Recent studies have identified key inductive signals in the CNS microenvironment that confer CNS endothelial cells with BBB properties (Table 1b). It is evident that these inductive signals originate from the NVU. As mentioned above, the most well-characterized signal that mediates BBB function is canonical Wnt signaling[29–31,33]. Neural progenitors in the neuroepithelium secrete Wnt7a/Wnt7b, whereas in the cerebellum, Bergmann glia secrete Norrin. These secreted ligands bind to classical components of canonical Wnt signaling such as the Frizzled receptors and co-receptors LRP5 /LRP6 that are expressed on CNS endothelial cells to drive β-catenin signaling. Disruption of these Wnt ligands phenocopies β-catenin mutants, impairing CNS angiogenesis and displaying loss of vessel numbers, vascular malformations, hemorrhages, and BBB dysfunction[31–33].
Another inductive signal essential for barriergenesis is Sonic hedgehog (Shh) signaling. Although Hedgehog signaling has been well-characterized for neuronal development and angiogenesis, Alvarez et al. demonstrated that astrocyte-secreted Shh is essential for BBB integrity and CNS immune quiescence[55,56]. Astrocytes express shh whereas CNS endothelial cells robustly express Hedgehog signaling components-Patched-1, Smoothened and Gil. Astrocyte-conditioned medium or recombinant Shh were sufficient to (a) elevate tight junction protein expression and transendothelial electrical resistance (TEER), a technique used to measure the integrity of tight junctions dynamics in cell culture, and (b) suppress permeability of various tracers in vitro. Furthermore, endothelial cell-specific disruption of Hedgehog signaling in vivo results in normal vascular formation but BBB dysfunction through suppressed expression of tight junction proteins and extravasation of plasma proteins. Hedgehog signaling is also essential to establish immune quiescence in the CNS. For example, Hedgehog signaling is sufficient to suppress chemokines and LAM expression in ECs in vitro. Furthermore, Hedgehog signaling in leukocytes suppresses expression of proinflammatory cytokines, such as Tumor Necrosis Factor (TNF), reducing neuroinflammatory processes. Hedgehog signaling has a protective role in neuroinflammatory diseases such as Multiple Sclerosis (MS). MS patients display elevated Hedgehog signaling components in the CNS and pharmacological blockade of Hedgehog signaling in EAE models results in greater severity of the disease, with increases in proinflammatory cytokines, leukocyte accumulation in the CNS and demyelination. It should be noted that the above studies focus on Hedgehog signaling as essential for BBB maintenance, not necessarily induction, as astrocytes are born and manifest in the NVU around birth. Although Shh is robustly expressed in the CNS during embryonic development and Shh knockout mice display reduced tight junction expression at E13.5, shortly before BBB maturation, the early roles of Hedgehog signaling during barriergenesis is still not well-characterized. Therefore, it will be interesting to explore the early inductive roles of Shh during BBB development before the onset of astrocyte-mediated Shh signaling to maintain BBB integrity.
Although it is well established that the neural microenvironment contains factors that induce CNS endothelial cells to manifest BBB properties, recent studies demonstrate that environmental cues and factors extrinsic to the CNS can impact BBB development and integrity as well. Braniste et al. demonstrated that gut microbiota influences the regulation of the BBB through epigenetic control of tight junction expression in CNS endothelial cells[57]. Emerging studies have demonstrated that an organism’s microbiota influences many physiological functions, including behavior[58]. Furthermore, gut microbiota has been reported to influence tissue barrier systems[59]. Comparing pathogen-free (control) and germ-free mice (altered microbiota), these authors discovered that germ-free mice display BBB dysfunction in both embryonic development and postnatal life, due to downregulation of tight junction protein expression. Indeed, unlike pathogen-free mice, germ-free mice display extravasation of Evans blue dye. Consistent with low tight junction expression, ultrastructure analysis by EM revealed disruption of tight junction complexes. Remarkably, transplanting fecal matter from pathogen-free mice to recolonize the intestinal microbiota of germ-free mice can restore the dysfunctional BBB observed in germ-free adult mice. Indeed, germ-free mice with recolonized microbiota have restored tight junction protein expression in the CNS with accompanying restriction of dye tracers to CNS endothelium. The molecular determinants from microbiota impacting BBB integrity in these experiments were short-chain fatty acids such as butyrate, which has been reported to strengthen the integrity of the intestinal epithelial barrier. Indeed, treatment with butyrate was sufficient to elevate tight junction protein expression and restore the BBB integrity in the germ-free mice. The authors suggest that butyrate epigenetically regulates tight junction expression in the CNS by increasing histone acetylation. This crosstalk between microbiota and the BBB is intriguing and provocative. It will be interesting to explore if short chain fatty acids directly increase BBB integrity or cause secondary effects on other signaling pathways throughout the body. Furthermore, it will be of clinical interest to explore how the use of strong antibiotics that eliminate gut microbiota influences the BBB.
Another recent study highlighted how foreign microbes impact the BBB. Acute bacterial meningitis is an infection in the CNS that causes neural damage and can result in mental impairment, seizures, paralysis and death if untreated[60]. To induce meningitis, bacteria must first breach the BBB[61]. However, it is unclear how bacteria penetrate through the BBB. Kim et al. demonstrated that blood-borne bacteria such as group B Streptococcus (GBS) can weaken BBB integrity by upregulating the expression of Snail1, a zinc finger transcription factor, in host CNS endothelial cells that subsequently suppresses tight junction protein expression[62]. Exposure of GBS to CNS endothelial cells in vitro and in vivo upregulates Snail1 expression and downregulates tight junction protein expression, with accompanying increases in GBS counts in the brain. Furthermore, transgenic dominant-negative Snai1 zebrafish are more resistent to GBS-mediated lethality. It is interesting that bacteria can manipulate the gene expression in host CNS endothelial cells to weaken the integrity of the BBB. It will be of clinical interest for future studies to explore what bacterial molecules interact with host CNS endothelial cells to alter gene expression and to determine if bacteria breach the BBB via weakened tight junctions.
Future directions of BBB research
Although the BBB research community has recently made significant strides in identifying novel molecular regulators and inductive signals that mediate BBB function and integrity, the field is still in its infancy, with many fundamental questions waiting to be answered (Box 2). Further refinements in cell type purification techniques and next-generation sequencing technologies will unravel key molecular regulators and core pathways essential for BBB formation and function. Currently, only a single CNS endothelial cell RNAseq dataset from mouse exists but nevertheless has been fruitful to compare gene expression among multiple purified cell types in the CNS[63]. However, this dataset is limited because it is only from normal developing mice at age P7. The RNAseq approach to unravel the transcriptome of BBB and other purified cell types in the NVU at different developmental, physiological, aging and disease contexts will address many questions about BBB regulation. Next-generation sequencing will be invaluable to address the underappreciated heterogeneity of the BBB. For example, different brain regions use different Wnt/ β-catenin molecular components for BBB function. Norrin is the Wnt signal in the retina and cerebellum whereas Wnt 7a/7b are the signals in the forebrain. [33]. Furthermore, it would interesting to explore how the molecular signatures of the CNS endothelial cells in circumventricular organs (the regions in the CNS that do not display BBB such as median eminence) differ from CNS endothelial cells displaying BBB[64–66].
Box 2. Outstanding questions.
What are the repertoires of molecules and genetic programs that mediate BBB formation and function? Cell type–specific purification methods and gene profiling have facilitated recent strides in elucidating molecular mediators and cellular pathways that confer CNS endothelial cells with fundamental BBB properties. Therefore, improvement in these experimental techniques such as using highly sensitive, unbiased, and high-throughput next generation sequencing technologies will unravel key molecular regulators and core pathways essential for the BBB.
What are the minimal core components necessary for endothelial cells in vitro to recapitulate the BBB properties displayed in vivo? Recent studies have demonstrated that activation of Wnt or Shh signaling is sufficient to elevate certain BBB properties in endothelial cells in vitro. A robust BBB in vitro system would be essential for high-throughput screening of drugs that can modulate the permeability of the BBB.
What are the molecular and cellular mechanisms of how these key molecules mediate BBB properties?
How tightly coupled is the relationship between CNS angiogenesis and barriergenesis? These two developmental processes may be more independent than once appreciated, especially with emerging studies demonstrating a) that CNS angiogenesis continues well after barriergenesis and throughout postnatal life and b) the identity of genes that specifically regulate barriergenesis independent of angiogenesis such as Mfsd2a.
How can we target the molecular regulators of BBB function to manipulate BBB properties for delivery of therapeutics? A major focus in BBB research has targeted the transferrin receptor to hijack clathrin mediated transcytosis for the delivery of therapeutics.
Transcriptomics has proven invaluable in identifying genes that regulate BBB function and integrity[67]. But the genes from these datasets are still merely candidates until validated that they indeed mediate BBB regulation. Therefore, the field needs high-throughput screening to not only validate candidate genes but also to discover drugs that can modulate BBB permeability. The advent of genome editing methods such as CRISPR-Cas9 has facilitated the generation of in vivo loss-of-function transgenesis but this process is still arduous and too low throughput to validate a list of candidates from transcriptomics datasets[68]. Thus, the use of more tractable model organisms with simpler BBB and robust loss-of-function genetic manipulations could accelerate validation of candidates.
The ideal high-throughput screening method would be an in vitro BBB system that reproduces the properties of the BBB in vivo, such as CNS endothelial cell polarity and restrictive paracellular and transcellular permeability. Because the functional BBB requires the interaction among the multiple cell types of the NVU, CNS endothelial cells readily lose their BBB properties ex vivo[69]. Several studies demonstrated that co-culture with astrocytes and pericytes to mimic the NVU can enhance BBB properties in endothelial cells [70]. Furthermore, stimulation of cultured endothelial cells with Wnt and Shh is sufficient to induce BBB properties without the need to co-culture other cell types, suggesting that activating core BBB pathways is sufficient to elicit BBB properties, and further emphasizing the need to determine the molecular regulators of BBB function activated by these signaling pathways[55,71]. Intriguingly, studies reported that human pluripotent stem cells treated with retinoic acid can differentiate to endothelial cells displaying BBB properties[72,73]. The development of these new technologies will accelerate the discoveries of key molecules and essential signaling pathways in BBB and neuroscience research
Although the BBB field has made significant progress in identifying key molecules that mediate BBB function, the molecular and cellular mechanisms of how these molecules are mediating BBB function and integrity are still poorly understood. This gap in knowledge is partly due to the limitations in the current technologies for BBB research. Currently, EM analyses in conjunction with dye tracers are the main techniques to monitor BBB properties[27]. But these techniques only provide a static snapshot of the BBB and do not provide essential information such as kinetics of vesicular trafficking. There is a pressing need to develop an in vivo high-resolution technique to monitor BBB properties such as tight junction complexes and transcytosis in real time.
A comprehensive understanding of the molecular constituents and mechanisms of BBB function and integrity would offer novel strategies for CNS therapeutics. Although the functional BBB is essential for proper neuronal function, the restrictive BBB is an impediment to deliver therapeutics, including recombinant proteins, antibodies and even small molecules, to the brain parenchyma[74]. Thus, a major focus of BBB research is identifying strategies to enhance delivery of therapeutics across the BBB. Here, we will highlight three promising methods to manipulate BBB properties to deliver drugs. First, several groups have demonstrated that hijacking receptor-mediated transcytosis pathways could deliver large, genetically engineered proteins across barrier endothelium[74]. The transferrin receptor (TfR), which binds to its ligand transferrin-bound iron and undergoes clathrin-mediated transcytosis to facilitate iron delivery to the brain, has been the main target of this work[75,76]. For example, chimeric monoclonal antibodies with α-Tfn fused to α-Aβ antibodies has been successful in hijacking the TfR pathway to reduce Aβ in an Alzheimer’s disease mouse model [77]. Second, scanning ultrasound (SUS), in which systemic injected circulating microbubbles causes transient opening of tight junctions when activated with ultrasound, has been reported to safely and transiently permeabilize the BBB[78,79]. Third, as we identify mediators of BBB function and better understand the molecular and cellular pathway underlying BBB regulation, we could target and manipulate these genes to enhance therapeutic delivery. For example, functional blocking of Frizzled4 antibodies has been shown to permeabilize the blood-retina barrier, offering a temporal opportunity for enhanced drug delivery[80]. As we further understand the cellular pathways and molecular mechanisms that regulate BBB function and integrity, we can develop creative strategies to manipulate these molecules to enhance drug delivery. Answering fundamental questions in BBB research and identifying molecular constituents of barrier regulation will enhance the development of therapeutics to modulate the BBB for drug delivery and neurologic disorders.
Concluding remarks
The BBB is comprised of specialized CNS endothelial cells that regulate CNS homeostasis to ensure proper neuronal function. In this review, we have highlighted that improvements in experimental tools have facilitated the recent findings of molecular constituents that mediate BBB function and integrity. These discoveries have greatly expanded our molecular and cellular understanding of this specialized vasculature that has fascinated physiologists for more than a century. Nevertheless, these discoveries open many more fundamental questions waiting to be resolved. We have emphasized the pressing demand for refinement in experimental technologies that will certainly accelerate our discoveries for novel molecules and our understanding of their cellular mechanisms that mediate BBB function. We believe that these findings will directly benefit therapeutics for neurological disorders in both drug delivery and repairing the dysfunctional barrier in certain neurological diseases.
Box 1. Trends Box.
The blood-brain barrier (BBB) is comprised of CNS endothelial cells that display specialized molecular properties essential for BBB function and integrity.
These molecular BBB properties are not intrinsic to CNS endothelial cells but have to be induced by the environment.
The formation, function and maintenance of the BBB require the functional interaction between CNS endothelial cells and the neurovascular units (NVU).
Advances in gene profiling and cell-type purification methods have progressed the identification of molecular mediators and core cellular pathways involved in BBB function and integrity.
A comprehensive understanding of key molecules and cellular pathways involved in BBB function would offer novel strategies for CNS therapeutics.
Acknowledgments
The authors are grateful to the colleagues, friends and lab members who contributed to reading and editing this review. This review received funding from NIH Pioneer Award (1DP1NS092473-01).
Glossary
- Angiogenesis
the development of new vessels from proliferation of pre-existing endothelial cells.
- Blood-brain barrier
a physiological barrier comprised of a thin layer of continuous, non-fenestrated CNS endothelial cells that regulates the brain microenvironment for proper neuronal function.
- Endothelial cells
mesoderm derived cells that line vasculatures of the circulatory system.
- Immune Privilege
introduction of antigens without eliciting an inflammatory adaptive immune response.
- Neurovascular Unit
the functional interactions among neurons, glia, pericytes and endothelial cells.
- Tight Junctions
a junctional complex between two cells that is essential for cell polarity, barrier functions, and cell adhesions.
- Transcytosis
Vesicular trafficking from the luminal to the abluminal plasma membrane and vice-versa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreone BJ, et al. Neuronal and Vascular Interactions. Annu. Rev. Neurosci. 2015 doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermeier B, et al. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich P. Das Sauerstoff-Bedürfniss des Organismus. eine farbenanalytische Studie. Berlin. 1885 [Google Scholar]
- 4.Ehrlich P. Ueber die beziehungen von chemischer constitution, verteilung und pharmakologischer wirkung. in Gesammelte Arbeiten zur Immunitaetsforschung. 1904 [Google Scholar]
- 5.Goldmann EE. Die Aussere und innere skeretion des gesunden Organismus im Lichte der "Vitalen Farbung". 1909 [Google Scholar]
- 6.Vein AA. Science and Fate: Lina Stern (1878–1968), A Neurophysiologist and Biochemist. Journal of the History of the Neurosciences. 2008;17:195–206. doi: 10.1080/09647040601138478. [DOI] [PubMed] [Google Scholar]
- 7.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz AL, Goldstein GW. Polarity of the Blood-Brain Barrier: Neutral Amino Acid Transport into Isolated Brain Capillaries. Science. 1978;202:225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- 10.Daneman R, Prat A. The Blood-Brain Barrier. Cold Spring Harbor Perspectives in Biology. 2015;7:a020412–a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappenheimer JR. Filtration, Diffusion and Molecular Sieving Through Peripheral Capillary Membranes. A Contribution to the Pore Theory of Capillary Permeability’. American Journal of Physiology. 1951;167:1–34. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Siegenthaler JA, et al. “Sealing off the CNS”: cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol. 2013;23:1057–1064. doi: 10.1016/j.conb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W-Y, et al. Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther. 2012;18:609–615. doi: 10.1111/j.1755-5949.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol. Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 17.Xiao G, Gan L-S. Receptor-Mediated Endocytosis and Brain Delivery of Therapeutic Biologics. International Journal of Cell Biology. 2013;2013:1–14. doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schinkel AH, et al. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schinkel AH, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 20.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Simpson IA, et al. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders NR, et al. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol. Aspects Med. 2013;34:742–752. doi: 10.1016/j.mam.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 23.César-Razquin A, et al. A Call for Systematic Research on Solute Carriers. Cell. 2015;162:478–487. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 26.Muldoon LL, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan N, Ben-Zvi A. The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin. Cell Dev. Biol. 2014 doi: 10.1016/j.semcdb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev. Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 29.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 30.Liebner S, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman R, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y, et al. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Developmental Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhnert F, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posokhova E, et al. GPR124 Functions as a WNT7-Specific Coactivator of Canonical β-Catenin Signaling. Cell Reports. 2015;10:123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson KD, et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cullen M, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanhollebeke B, et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. Elife. 2015;4 doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, et al. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE. 2012;7:e30203. doi: 10.1371/journal.pone.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam SJ, et al. Death receptors DR6 and TROY regulate brain vascular development. Developmental Cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betsholtz C. Physiology: Double function at the blood-brain barrier. Nature. 2014;509:432–433. doi: 10.1038/nature13339. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 46.Lafourcade M, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 47.Guemez-Gamboa A, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 2015 doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alakbarzade V, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet. 2015 doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 49.Winkler EA, et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng P-P, et al. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010;68:835–844. doi: 10.1002/ana.22318. [DOI] [PubMed] [Google Scholar]
- 51.Sohet F, et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J. Cell Biol. 2015;208:703–711. doi: 10.1083/jcb.201410131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daneman R, et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yen FT, et al. Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 2008;283:25650–25659. doi: 10.1074/jbc.M801027200. [DOI] [PubMed] [Google Scholar]
- 54.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez JI, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 56.Martí E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends in Neurosciences. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- 57.Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158–263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsiao EY, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013 doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Asmakh M, Hedin L. Microbiota and the control of blood-tissue barriers. 2015 doi: 10.1080/21688370.2015.1039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brouwer MC, et al. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin. Microbiol. Rev. 2010;23:467–492. doi: 10.1128/CMR.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Sorge NM, Doran KS. Defense at the border: the blood–brain barrier versus bacterial foreigners. Future Microbiology. 2012;7:383–394. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BJ, et al. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J. Clin. Invest. 2015;125:2473–2483. doi: 10.1172/JCI74159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gross PM, et al. The microcirculation of rat circumventricular organs and pituitary gland. Brain Research Bulletin. 1987;18:73–85. doi: 10.1016/0361-9230(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 66.Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood - Broadwell - 2004 - Journal of Comparative Neurology - Wiley Online Library. Journal of Comparative. 1976 doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- 67.Huntley MA, et al. Dissecting gene expression at the blood-brain barrier. Front Neurosci. 2014;8:355. doi: 10.3389/fnins.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilhelm I, et al. In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars) 2011 doi: 10.55782/ane-2011-1828. [DOI] [PubMed] [Google Scholar]
- 70.Hatherell K, et al. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods. 2011;199:223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Paolinelli R, et al. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS ONE. 2013;8:e70233. doi: 10.1371/journal.pone.0070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lippmann ES, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lippmann ES, et al. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pardridge WM. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones AR, Shusta EV. Blood–Brain Barrier Transport of Therapeutics via Receptor-Mediation - Springer. Pharm Res. 2007 doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10:459–472. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niewoehner J, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 78.McDannold N, et al. Temporary Disruption of the Blood–Brain Barrier by Use of Ultrasound and Microbubbles: Safety and Efficacy Evaluation in Rhesus Macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samiotaki G, et al. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J. Cereb. Blood Flow Metab. 2015;35:611–622. doi: 10.1038/jcbfm.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paes KT, et al. Frizzled 4 is required for retinal angiogenesis and maintenance of the blood-retina barrier. Investigative Ophthalmology & Visual Science. 2011;52:6452–6461. doi: 10.1167/iovs.10-7146. [DOI] [PubMed] [Google Scholar]