Abstract
Diabetic retinopathy is a major cause of visual impairment, which continues to increase in prevalence as more and more people develop diabetes. Despite the importance of vision, the retina is one of the smallest tissues in the body, and specialized techniques to study the retinopathy have been developed. This chapter will summarize several methods used to (i) induce diabetes, (ii) maintain the diabetic animals throughout the months required for the development of typical vascular histopathology, (iii) evaluate vascular histopathology of diabetic retinopathy, and (iv) quantitate abnormalities implicated in the development of the retinopathy.
Keywords: Diabetes, streptozotocin, retinopathy, retina, mouse
INTRODUCTION
Diabetic retinopathy is a common complication of diabetes, and one of the leading causes of visual loss in working age populations in developed countries. Major risk factors of diabetic retinopathy are the degree and duration of hyperglycemia. The retinal lesions that develop in type 1 diabetes are not different from those that develop in type 2 diabetes, although the severity and/or incidence of the lesions may differ. Clinically detectable characteristics of DR have focused on damage to the retinal vasculature. Based on vascular changes, DR is subdivided into an early nonproliferative stage including progressive capillary occlusion and degeneration (NPDR), and a more advanced, proliferative or neovascular stage (PDR).
Animal models are being used by numerous investigators to study the pathogenesis of diabetic retinopathy. To date, animal models of diabetic retinopathy have not been found to reproducibly develop the advanced, neovascular phase of the disease, but they have proved useful in investigating the earlier stage of the retinopathy. This chapter will summarize methods used to (i) induce diabetes, (ii) maintain the diabetic animals throughout the months required for the development of typical vascular histopathology, (iii) evaluate vascular histopathology of diabetic retinopathy, and (iv) quantitate abnormalities implicated in the development of the retinopathy. Additional important alterations in neural retina and in vascular permeability are beyond the scope of this review, and have been addressed by others.
Basic Protocol 1 describes the induction of diabetes using streptozotocin, while basic protocol 2 describes the care and maintenance of diabetic animals. Basic Protocol 3 describes the enucleation of the eye and subsequent removal of the retina. Basic protocol 4 describes the isolation of the retinal vasculature from fixed eyes and the quantification of capillary loss due to diabetes. Basic protocol 5 describes the measurement of superoxide from the retina using lucigenin. Basic protocols 6 and 7 describe techniques for enumerating white blood cells that are attached to the retinal vasculature (leukostasis) in fresh and fixed tissue respectively.
BASIC PROTOCOLS
NOTE: All protocols using live animals have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University or Louis Stokes VA Medical Center, and conform to governmental regulations regarding the care and use of laboratory animals.
BASIC PROTOCOL 1
INDUCTION OF DIABETES USING STREPTOZOTOCIN
Types of diabetes in rodent models
Mice: Studies of diabetic retinopathy using mouse models of type 1 diabetes have utilized chemically induced diabetes (Berkowitz et al., 2009; Du et al., 2013; Feit-Leichman et al., 2005; Geraldes et al., 2009; Gubitosi-Klug et al., 2008; Talahalli et al., 2013; Tang et al., 2013; Xu et al., 2014) or the spontaneous diabetes of the Ins2Akita mouse (Barber et al., 2005; Han et al., 2013; Huang et al., 2011; McLenachan et al., 2013; Muir et al., 2012; Smith et al., 2008; Vagaja et al., 2013). Mouse models of Type 2 diabetes also are available. db/db mice develop a type 2 diabetes due to loss of function mutation in the leptin receptor gene. The KK mouse strain exhibits glucose intolerance and insulin resistance, and becomes obese with aging. Both strains have been used for studies of early stages of diabetic retinopathy (Barile et al., 2005; Bogdanov et al., 2014; Cheung et al., 2005; Krady et al., 2005; Li et al., 2009; Ning et al., 2004; Tang et al., 2011).
Rats: Streptozotocin-diabetic or alloxan-diabetic rats have been a valuable model for research into the pathogenesis of diabetic retinopathy (Asnaghi et al., 2003; Dagher et al., 2004; Hammes et al., 1993; Kern and Engerman, 1994; Kowluru et al., 2004; Kowluru et al., 2001; Stitt et al., 2002). Because these chemicals damage insulin production, they are used as models of type 1 diabetes. Other strains that spontaneously develop an insulin-deficient diabetes, including the diabetic BB (BioBreeding) or BBW (BioBreeding Wistar) rat (Marliss et al., 1982), the Torii (SDT) rat (Shinohara et al., 2000), and the WBN/Kob rat (Nakama et al., 1985) also have been studied with respect to development of retinopathy. Some models of type 2 diabetes also have been studied with respect to their susceptibility to develop retinal lesions in diabetes (Zucker diabetic fatty rat, ZDF/Gmi-fa, Goto-Kakizaki rat, Otsuka Long-Evans Tokushima fatty (OLETF) rat, BBZDP/Wor, Obese Koletsky (SHROB) rat, spontaneously hypertensive/NIH-corpulent rat strain (SHR/N-cp). Most of these strains show some degree of hyperglycemia, impaired glucose tolerance, and defective insulin response to glucose, with or without obesity and hypertension, but few have been used in long-term studies of diabetic retinopathy.
Studies of chemically induced diabetes enjoy certain advantages, in that genetic changes found in many type 2 animal models are not an issue. The generation and maintenance of diabetic rodents is a critical part of diabetes research pertaining to retinopathy, because the retinopathy takes so long (many months in rodents, and decades in patients) to become manifest.
NOTE: Streptozotocin has been identified as a potential carcinogen, and appropriate care must be taken when handling this chemical.
Materials
Unless otherwise specified all materials were purchased form Sigma chemical or Fisher Scientific.
Citrate buffer (47mM Sodium Citrate, 53mM Citric Acid in distilled water; pH 4.5). May be stored frozen for 6 months.
-
Streptozotocin (STZ) solution (60mg/Kg body weight for mice or 55mg/Kg for rats in a volume of 200µL citrate buffer), Streptozotocin (MP Biomedicals, Santa Anna, CA)
Solution is extremely photosensitive and has about a 15 minute half-life in citrate buffer. Dissolve just before use, and filter sterilize.
-
Insulin
For mice: Dilute NPH insulin 1:10 with diluent (provided free by Eli Lilly and Co upon request) and refrigerate.
For rats: Use NPH insulin (Humulin (NPH); Eli Lilly and Co) undiluted.
Inject animals with streptozotocin
-
1.
Obtain baseline weight.
-
2.
Fast animals for 6 and 12 hours for mice and rats, respectively, prior to STZ injection.
-
3.
Inject the STZ solution IP.
A useful schedule for mice is to remove food from animals to be injected each morning at 8am, then inject with STZ at 2pm. Repeat for a total of 5 consecutive days.
Rats only require a single injection of STZ.
Allow each animal access to food immediately following the injection.
Verify the diabetic status of animals
-
4.
Animals are weighed every other day after the last STZ injection for about 10 days. After animals have been assigned to the “diabetic” group, they are weighed once per week. Additional details are provided in basic protocol 2 (Clinical measures of diabetes severity).
-
5.
Between 7 and 15 days after the last STZ injection, measure fasting blood glucose (FBG; fasted for 6 hours) concentration on 3 different days spaced at least two days apart.
The onset of diabetes is defined as the date at which the FBG readings are >275 mg/dL.
If FBG is not elevated beyond normal levels two weeks after the last STZ injection and body weight continues to increase, the animal likely is not diabetic. Induction of diabetes will require another round of injections. The dose of STZ for next round of injections should be increased to 70mg/Kg body weight for mice or 60mg/Kg for rats.
Insulin therapy for insulin-deficient diabetic rats and mice
-
6.
If a diabetic animal is progressively losing weight over a 7–14 day period, subcutaneous injections of exogenous insulin are needed.
A slight fluctuation in day to day body weight of ±2g per 25g mouse or ±20g per 250g rat is not uncommon. Look for a trend in weight loss over time to determine if exogenous insulin is required.
Typical insulin dosing schedule for diabetic rodents is 0.2 units of insulin (20 µl of diluted insulin) for mice and 2 units (20 µl) of full-strength insulin for rats. Initially we administer insulin twice per week for animals showing loss of body weight. Use trial and error to select the best insulin dose and frequency of injection for each animal, with the goal of inhibiting the weight loss while not inhibiting hyperglycemia.
BASIC PROTOCOL 2
CLINICAL MEASURES OF DIABETES SEVERITY
In this protocol we cover the basic care and required glucose testing of diabetic animals.
Materials
Blood glucose test strips and glucose meter. Most glucose meters will suffice.
Scale to measure body weight.
Method to measure HbA1c (or glycated hemoglobin). There are several methods available. We currently use BioRad VARIANT Classic system (BioRad Clinical Diagnostics, Hercules, CA, USA)
-
Blood glucose can be measured in rodents fasted for 5 to 6 hours to assess the severity of hyperglycemia or diabetic status. Blood for this assay is collected from tail by tail-snip or tail-vein nick. We do not recommend retro-orbital collection as it may induce complications in the eye. The numerical value obtained depends on the method used to assay glucose, and so the normal value should be established for each laboratory.
Nondiabetic C57BL/6J mice assayed in our laboratory typically have FBG values of 140 ± 40 mg/dL, and non-diabetic Lewis rats typically have FBG values of 100 ± 20 mg/dL.
Diabetic rodents have FBG values > 275 mg/dL.
HbA1c or glycated hemoglobin are measures of the severity of hyperglycemia over time (due to the formation of adducts of glucose accumulating on hemoglobin). These parameters can be measured using a variety of methods, including UPLC, boronate affinity columns, and electrophoretic methods. In our laboratory HbA1c values of non-diabetic rodents is typically 3.0%, and diabetic rodents between 8% to 13%.
Body weight of diabetic rodents should stabilize within 2–3 weeks after injection of STZ. This weight is typically within 10% of their pre-STZ baseline weight. While some diabetic rodents may never require insulin therapy and will still either maintain or show slight and steady increases in body weight, it is not uncommon for most STZ-diabetic animals to require insulin therapy in order to prevent weight loss.
Polyphagia and polydipsia will both become apparent in diabetic rodents within a week of successful STZ injection/induction. Animal care personnel should be alerted to the increased husbandry needs of diabetic rodents. Diabetic animals will require additional drinking water and food changes/replenishment, as well as increased frequency of changing cage bedding. This additional care is important for maintaining the health of these animals.
BASIC PROTOCOL 3
ISOLATION OF FRESH RETINA
Both molecular (Western blot, PCR) and histologic (wholemounts, cryosections) benefit from use of isolated retina. The method described below is suitable for isolation of retinas for these analyses.
Materials
Small dissecting scissors (curved blunt end 22mm ROBOZ RS 5983)
Lint free tissue (such as Kimwipes)
Razor blades (GEM single edge stainless steel, Teflon coated, Electron Microscopy Sciences (EMS), 71970)
Pink Dental wax (EMS, 72670)
Forceps (Dumont #5, Biological grade tip, EMS, 72700-D) (or Dumont, electronic grade tip, EMS, 0103-2-PO)
Micro dissecting spring scissors (Vannas, 3mm straight ROBOZ, RS-5620
Micro spatula (Fine Science Tools, FST 10091-12)
Isolation of the eye
-
1.
Anesthetize or euthanize the animal.
-
2.
Proptose the eye. Turn mouse on side and place thumb on head just below eye but above jaw and index finger just above eye (but not on top of head). Gently push down with fingers and spread them apart. The eye should bulge out of the socket exposing the attachment of the muscle to the eye.
-
3.
Enucleate the eye. While eye is proptosed, use curved scissors to scoop under the eye and cut the muscular attachment. Be careful not to cut off the back of the eye during the isolation.
Isolation of the retina
-
4.
Remove the anterior section of the eye.
Place the eye on small piece of wet lint-free tissue on top of dental wax to prevent the eye from moving while opening the globe. Under a dissecting microscope, hold the remnants of the muscle that was attached to the outside of the posterior eye with micro-forceps, and orient eye so that cornea faces to a side.
Use a Teflon-coated razor blade to make an incision 1–2 mm behind and parallel to the limbus (cornea-sclera junction). While holding the muscle with the forceps, draw the blade across limbus with very little downward force. Continue cutting with razor until anterior eye is totally separated from posterior eye. Do not saw back and forth.
Remove and discard anterior segment of eye, including cornea, lens (and vitreous if possible). The posterior of the eye (the eye cup) should be moved to the dental wax (if the retina comes in contact with tissue paper, it will stick to the paper).
-
5.
Gently detach the retina from the sclera/RPE. The retina is firmly attached to the sclera only at the limbus and at the optic nerve. If you cut 1–2 mm behind the limbus (described in 4b above), the retinal attachment at the limbus already has been cut off. Note avoid pulling on fresh retina.
Grab the muscle on outside of sclera with tweezers or if possible the sclera at the cut edge but not the retina. Place the spatula between sclera and retina working all the way around the perimeter of the eye cup. Where the retina is attached to the sclera/optic nerve, gently use spatula or micro-scissors to break the connections at the limbus.
Cut junction of retina with optic nerve- Use the micro spatula to scoop under the retina and sever the optic nerve or use the micro scissors to cut the sclera and then the optic nerve.
Remove vitreous and ciliary muscle that remain adherent to the periphery of the retina. If the cut to remove the anterior eye is too close to the limbus, muscle can remain attached to the retina. Carefully grasp the ribbon of muscle with the tweezers, and gently pull away to remove both the muscle and vitreous.
Immediately transfer the isolated retina into buffer or flash freeze on dry ice.
BASIC PROTOCOL 4
ISOLATION OF RETINAL VESSELS FOR HISTOLOGY
In this protocol, we describe the isolation, staining and quantitative assessment of the blood vessels in the retina. In diabetic rodents, vascular abnormalities are in general limited to capillary loss, leukostasis and vascular permeability. It is important to note that the vascular abnormalities can be observed in non-diabetic animals and non-diabetic patients, but increase in the frequency and severity with diabetes.
Comparison of elastase method to original trypsin digest method. Previously, investigators isolated retinal vessels for histologic analysis using the “trypsin digest method” (Engerman and Kern, 1987; Engerman and Kern, 1993; Kern and Engerman, 1994; Kern and Engerman, 1996; Kuwabara and Cogan, 1960), so named because a crude trypsin solution was used as the protease to isolate the vasculature from the neural retina. The crude trypsin that worked best for this procedure is no longer available (pure trypsin does not work properly), so elastase has been substituted (Laver et al., 1993). Results are very comparable.
Materials
Formalin (10% buffered)
Elastase solution (40 units elastase/ml in 100 mM sodium phosphate buffer, 150 mM sodium chloride, and 5 mM EDTA, at pH6.5). Aliquot and store at −20°C.
Elastase (Calbiochen Company, #324682)
Activating solution (100 mM Tris-base pH 8.5 in water)
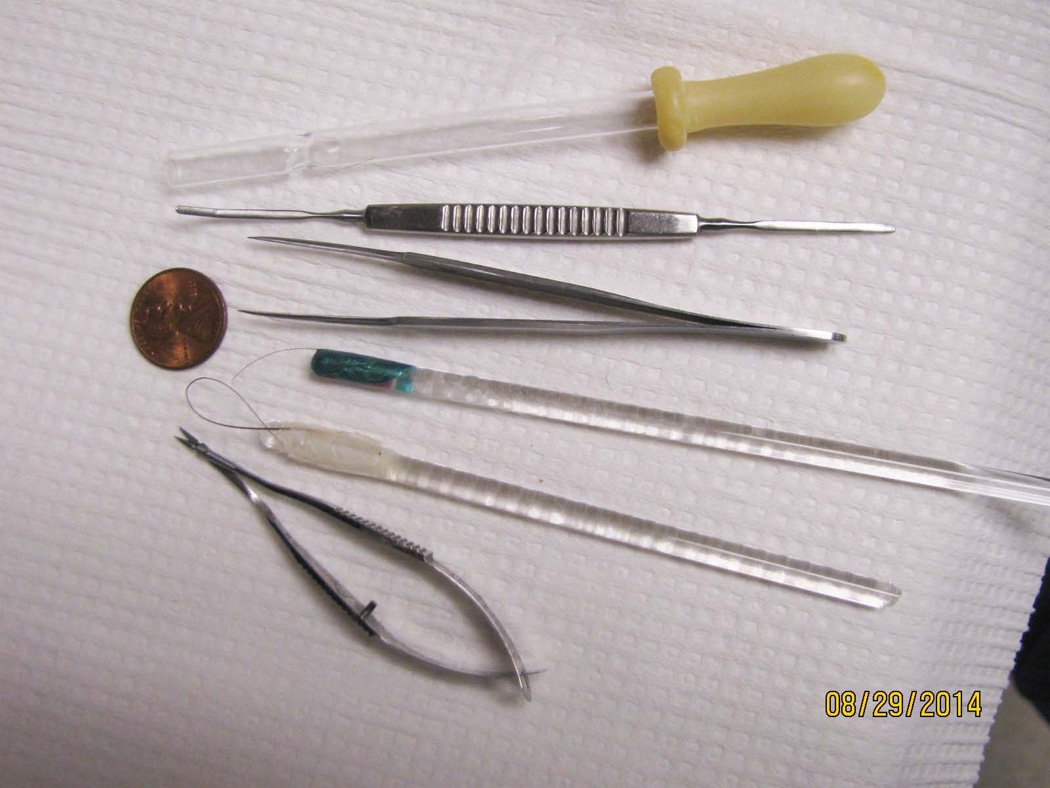
2 “brushes” of a single Sable hair or cat whisker glued to the end of glass a rod with fingernail polish. An additional brush glued at both ends of the hair to the rod so that it forms a loop is helpful (Figure 1).
-
Glass transfer pipette (modified Pasteur pipette)
-
Snap off thin end of Pasteur pipette and cover with rubber bulb.
Pasteur pipette (borosilicate glass, Fisher, 1367820B)
Rubber pipette bulb (Fisher, 0344821)
-
Mounting dish (specifications available upon request).
-
Superfrost Plus treated slides (Fisher, 12-550-15)
Slides should be from unopened box or slides can be cleaned using sulfuric acid, water, acetone and finally ethanol.
Periodic acid solution (35mM periodic acid, 12mM Sodium acetate in water)
Schiff reagent base (Sigma, 3952016)
Harris modified Hematoxylin Filter before use to remove precipitates
Permount mounting media
Glass coverslips (22x40)
7.4 mM Ammonium Hydroxide in water
Fig 1.
Tools used to isolate retinal vasculature in the elastase or trypsin digestion methods. Shown are an inverted pasteur pipette (used to pick up the fragile retina or vasculature), micro-spatula, toothed forceps, 2 single hair brushes, and Vannas micro-scissors. A penny is added for size comparison.
Removal and fixation of the eye
-
1.
Euthanize mouse and enucleate eyes as previously described in basic protocol 3.
-
2.
Fix eye in 10% buffered formalin for at least 1 week (there is no known upper limit as to how long the tissue can stay in formalin and still work with regard to vessel isolation).
-
3.
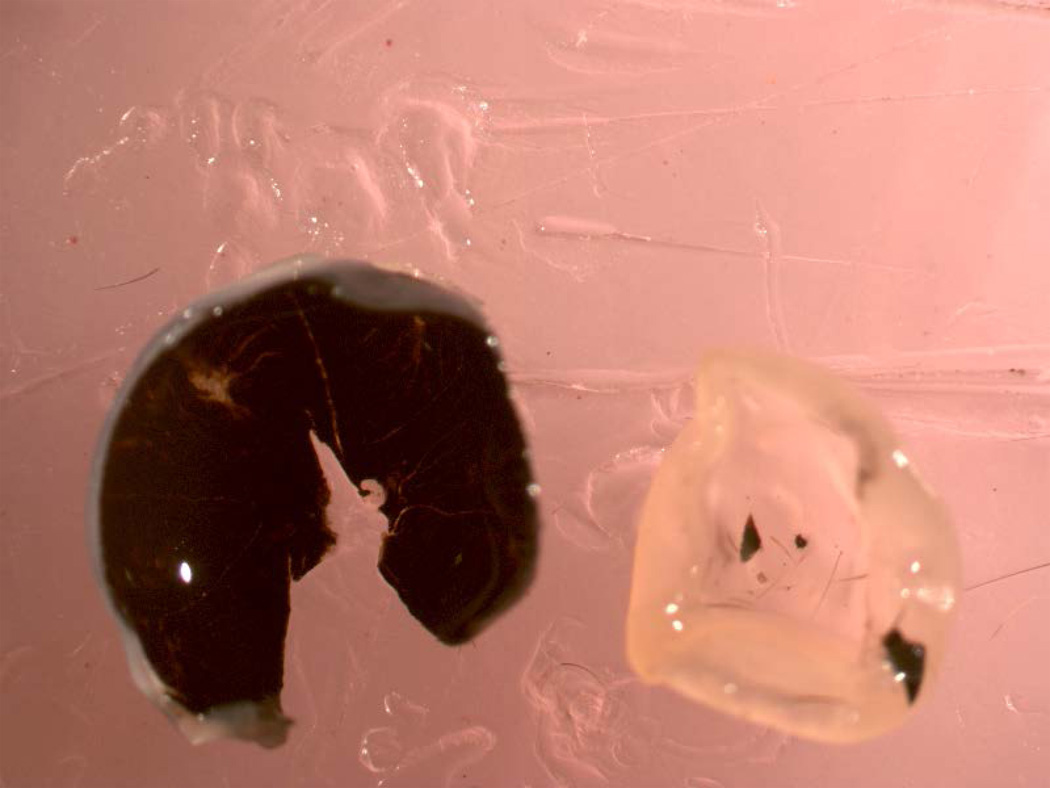
Cut off and discard the anterior half of the eye, and isolate retina as described in basic protocol 3, except that micro-scissors are recommended to keep the optic nerve head attached to the retina following cutting the optic nerve between the retina and the sclera. Keeping the nerve head attached to the retina during the vessel isolation procedure is desirable, because it keeps the vasculature together (Figures 2–4). Using micro scissors, make a single radial cut in the retina radially part way to the optic nerve to help relieve some of the curvature in the retina.
Fig 2.
The eye has been bissected into anterior and posterior halves. The scissors are beginning to separate the retina from the underlying RPE/choroid.
Fig 4.
Retina has been totally isolated from the sclera.
Digestion and removal of non-vascular tissue
-
4.
Remove excess fixative by rinsing the retina overnight in water. When doing many samples at the same time, it is helpful to place each retina in a small disposable plastic biopsy cassette with the sample ID written on the cassette. Put all samples in a large beaker containing water, and have a small trickle of water continually flowing into and out of the beaker overnight.
-
5.
Incubate each retina in a micro-centrifuge tube or 48-well plate containing 800uL of elastase solution at 37°C for at least two and a half hours. The enzyme is minimally active at these conditions, but it will soak into the tissue.
-
6.
Transfer each retina and some of the elastase solution (a drop or two) into 1mL or more of the activating solution (Tris buffer pH 8.5) and incubate at room temperature for at least 12 hours. Elastase is optimally active at pH 8.5.
-
7.
Transfer the retina to a shallow dish such as the top of a petri dish containing lint free, deionized water.
-
8.
Under dissecting microscope with side illumination, use the single hair brushes to remove neural and glial tissue from the vascular tree.
Use one of the whisker brushes to hold down the retina on the bottom of the dish.
Use the middle of the second brush to push into the outer nuclear layer or backside of the retina repeatedly, thus disrupting the neural cells by leaving ridges and creases. If the tissue does not easily deform with a thin hair brush then incubate the retina in the activating solution for an additional 12 hours or more (Figure 5).
Gently tap the single hair across the surface of the retina to dislodge neural tissue. Note that the outer nuclear layer and photoreceptors can come off in big sheets as they have no vasculature holding them to the rest of the retina.
Move the brush strokes progressively from the optic nerve toward the periphery so that the non-vascular tissue is swept out and away from the vasculature.
Remnants of the vitreous or inner limiting membrane commonly are still attached to the retina at this point. They look like a sheet of transparent tissue attached to the optic nerve or retina. Grasp the vitreous with a pair of tweezers and gently brush the vasculature backwards and away from the vitreous. Use micro-scissors to cut the vitreous away from the optic nerve or retina (discard the vitreous) (Figure 6).
Continue to gently tap on the retina with the hair (do not use the tip of the hair) to break up any patches of tissue remaining attached to the vasculature. If the tissue does not break up easily then transfer the retina back to the activating solution with a small amount of the elastase solution and incubate overnight at room temperature.
Remove any hairs or lint from the vascular bed, then transfer the vasculature to a dish of fresh water and remove small chunks of neural tissue from the vasculature by gently brushing the single hair over the vasculature from the optic nerve head towards the periphery.
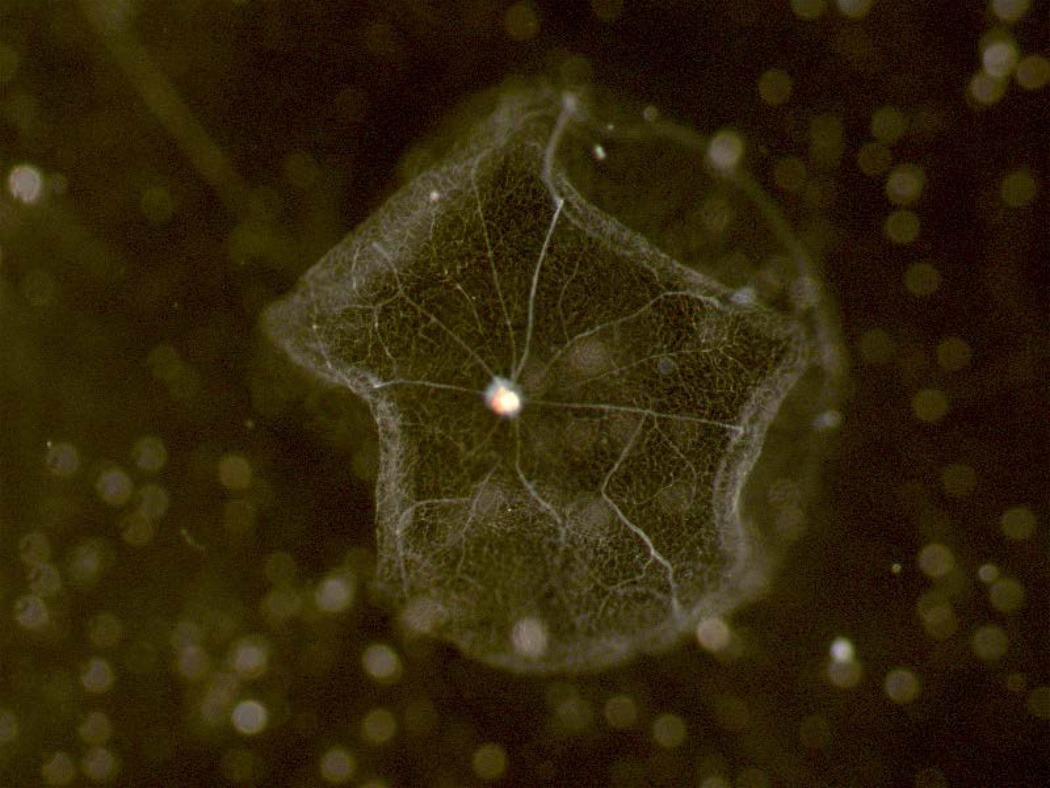
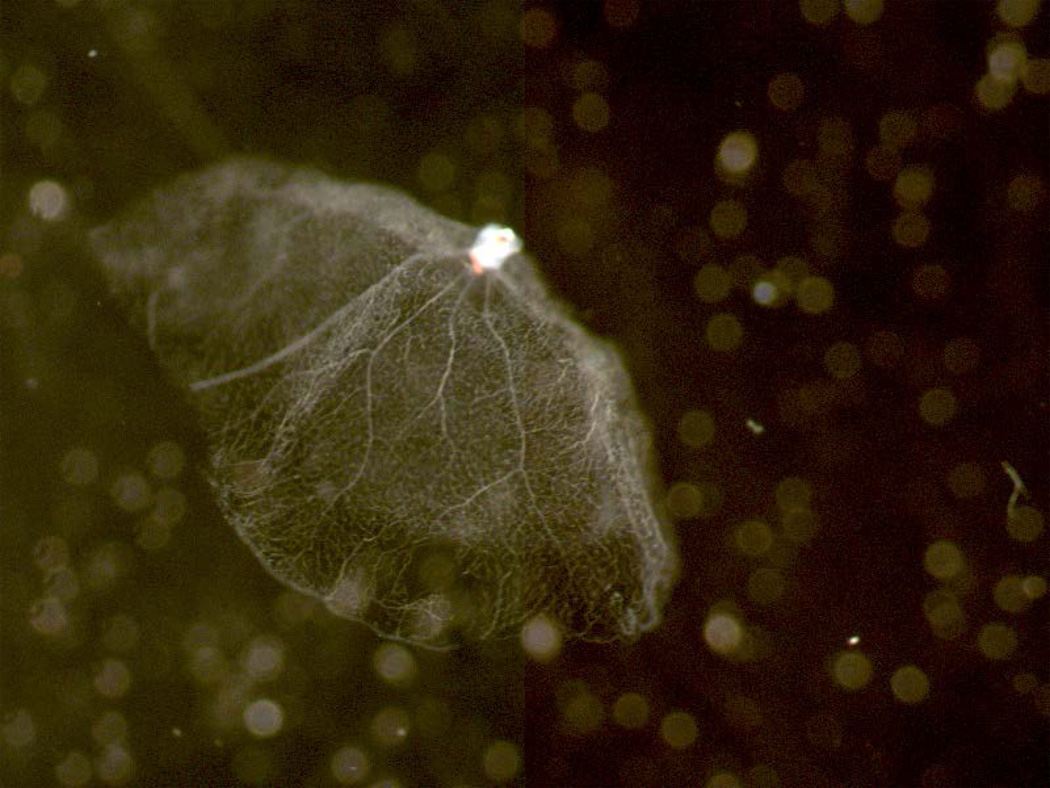
When the vascular network has been well cleaned, transfer it to a fresh dish of clean water (Figures 7–8).
Fig. 5.
Using hair “brushes” to immobilize the retina, and remove neuronal cells using a gentle stroking motion across the surface of the retina. The partially digested retina in this high magnification photomicrograph is seen as a whitish mass in the center of the photo, and the two brown “lines” are hairs being used to immobilize (hair loop at bottom left of the photo) and gently stroke (thinner hair in upper right) the retina to brush off weakly adherent neuronal cells.
Fig 6.
Use of microscissors to remove adherent vitreous from partially cleaned retina. Vitreous (bottom) is very clear and colorless compared to the ivory/grey color of the partially cleaned retina (top).
Fig 7.
Clean preparation of retinal vasculature looking down and into the bowl-shape of the isolated vasculature while it floats in water.
Fig 8.
Clean preparation of retinal vasculature viewed from the side while it floats in water. The bowl shape of the retina is preserved.
Mounting the isolated retinal vasculature on a microscope slide
-
9.
Place clean mounting dish under dissecting scope. A photo of the mounting dish is shown in (Figure 9).
-
10.
Use a black background under the mounting dish to help generated contrast to see the illuminated vasculature. Fill dish with double distilled water. Illuminate the mounting dish from the sides.
-
11.
Place a clean, labeled microscope slide into the mounting dish (underwater) using forceps.
Note: Oil from your skin will impair the attachment of the vasculature to the microscope slide. Avoid touching the microscope slide or water in the mounting dish with your fingers.
-
12.
Suck the cleaned retinal vasculature into the modified Pasteur pipette, and gently dispense the vasculature into the water of the mounting dish (just above the microscope slide).
The cleaned microvasculature is essentially invisible in normal light. Maintain visual contact with the vasculature as you are transferring it, so that you don’t lose it.
-
13.
The isolated retinal vasculature in water does not rapidly sink or rise to the surface. While the vasculature is floating in water above the microscope slide, use the whisker brush to “open up” the vasculature
-
14.
Use the brushes to gently push the vasculature down onto the glass slide, and then use the hair to tack down the opened vasculature down onto the center of the slide. The vasculature should stick to the slide as it touches. If the vessels do not stick to the microscope slide, the microscope slide is not clean enough. Clean slides in ethanol, then xylene, then H2SO4, and finally clean distilled water or use a fresh box of slides.
-
15.
Slowly drain water from under microscope section using mounting dish to minimize currents (water currents might dislodge the vasculature from the slide, causing the retina to fold or become lost).
-
16.
Allow vasculature to air dry overnight before staining.
Fig 9.
Mounting dish/dissecting microscope set-up. The dissecting dish contains an elevated platform (black plexiglass) upon which sits a clean microscope slide. The whole preparation is submerged under clean water, and then the cleaned retina is gently floated above the microscope slide (highlighted in red), and tacked down to the glass slide using the single hair brushes. Water then drains slowly out of the chamber to the top of the photo.
Periodic acid-Schiff staining of isolated retinal vasculature
-
17.
Re-hydrate the vasculature (previously dried onto microscope slide) in water for 15 min.
-
18.
Immerse the slide in freshly prepared periodic acid solution for 8 minutes.
-
19.
Rinse briefly in de-ionized water.
-
20.
Immerse in Schiff reagent for 15 minutes.
-
21.
Wash in running tap water (lukewarm) for 5 minutes, change the water and repeat at least twice more until the water does not turn pink.
-
22.
Check slides to insure vessels are dark pink. If not, repeat periodic acid and Schiff staining.
-
23.
Immerse in Harris hematoxylin for 15 minutes.
-
24.
Wash in running tap water for 5 minutes, dump out the water and repeat at least twice more.
-
25.
Dip up to 20 times in ammonium hydroxide solution. This step will darken the nuclear stain.
-
26.
Dehydrate through different concentrations of (70, 80, 95, and 100%) ethanol and then xylene for about 2 minutes each.
-
27.
Affix cover glass with Permount mounting media and allow to dry overnight.
Quantitating diabetes-induced retinal histopathology
-
28.
Observe the stained vasculature under a microscope and count the degenerate (acellular) capillaries and pericyte ghosts.
-
Degenerate (acellular) capillaries
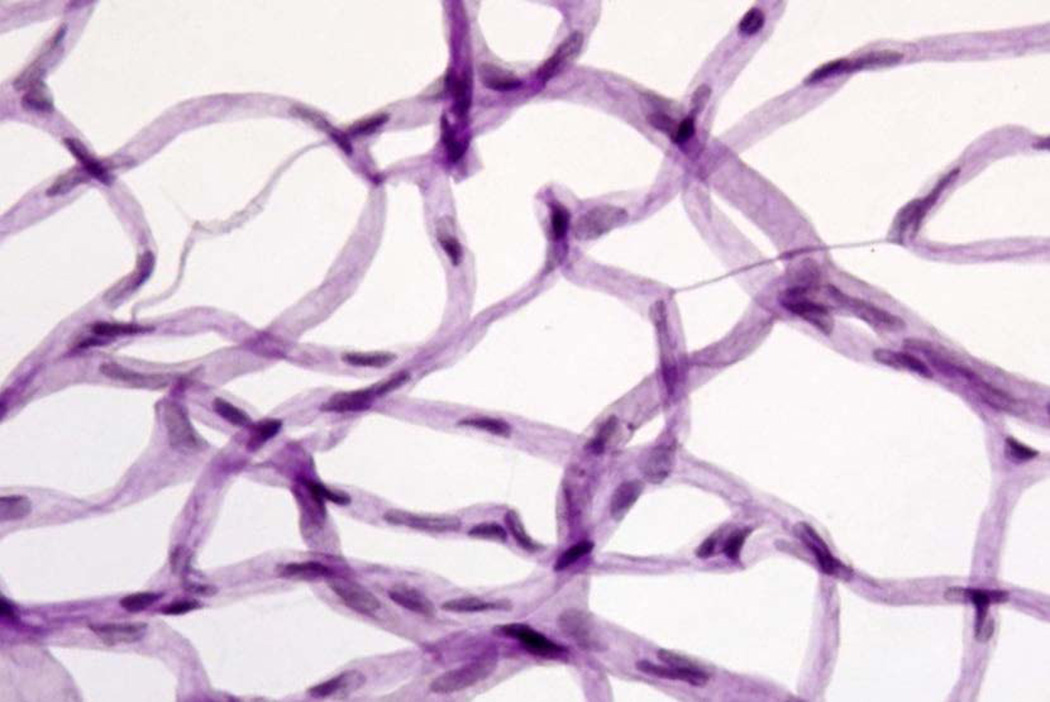
Acellular capillaries are quantitated per square mm in six to seven fields centered midway between the optic nerve and the periphery (200x magnification) in a masked manner (Figure 10).
Acellular capillaries are defined as capillary-sized vessel tubes having no nuclei anywhere along their length. Exclude capillaries which are not at least as wide as 1/5 the width of a normal capillary at some point along their length. (Vessel remnants that are very thin have been called “strands”, and are believed to be a result of remodeling of the vasculature soon after birth). Exclude “bridging” capillaries which are not at least as long as 4 times the width of a normal capillary.
-
Pericyte “ghosts”
Pericyte “ghosts” are estimated from the prevalence of protruding “bumps” in the capillary basement membranes from which pericyte nuclei have disappeared
At least 1,000 capillary cells (endothelial cells and pericytes) six field areas in the mid-retina were examined (at 400x magnification) in a masked manner
Exclude ghosts on any acellular vessels.
-
Fig 10.
PAS-H stain of isolated retinal vasculature that has been dried onto glass microscope slide. Pericyte nuclei in capillaries tend to be more circular and densely stained, whereas endothelial nuclei in capillaries tend to be more oblong and less densely stained. Degenerate (acellular) capillaries are shown.
Basic Protocol 5
MEASUREMENT OF RETINAL SUPEROXIDE
Generation of superoxide by the retina is increased in diabetic animals, and therapies that inhibit the diabetes-induced production of superoxide by the retina have been found also to inhibit the later degeneration of retinal capillaries at longer durations of diabetes (Berkowitz et al., 2009; Kanwar et al., 2007; Kowluru et al., 2001; Li et al., 2012; Veenstra et al., 2013). Lucigenin, which emits light upon reaction with the superoxide anion, is used to measure superoxide anion production.
Materials
Kreb-HEPES buffer (20mM HEPES, 5mM or 25mM glucose in 0.12mM CaCl2 0.08mM MgSO4 HBSS) Ca and Mg are 1/10 of normal HBSS. Use 25mM glucose solution for tissues from diabetic animals.
D-(+)-Glucose solution (45% in water, tissue culture grade, Sigma, G8769)
Hank’s Balanced Salt Solution ((HBSS) without phenol red, Mg or Ca, Cell Grow, 21-022-CV)
Hank’s Balanced Salt Solution ((HBSS) without phenol red, 1.2mM CaCl2, 0.81mM MgSO4, Cell Grow, 21-023-CV)
HEPES Buffer Solution (1M, Gibco, 15630-106) (store in dark)
-
5mM Lucigenin in 5mM glucose Kreb-HEPES buffer
-
Lucigenin- N,N′-Dimethyl-9,9′-biacridinium dinitrate (Sigma, M8010)
Sterile filter and store at 4°C in the dark for up to 1 week.
Centrifuge at 12k x g for 5 min.
Luminometer (such as GlowMax 20/20 luminometer from Promega). Set measurement interval to 3 seconds.
-
Bradford protein assay (as per manufacturer’s instructions; Biorad 500-0006)
10x cell lysis buffer (Cell Signaling, 9803)
Prepare retina for measurement
-
1.
Isolate fresh retina (see basic protocol 3).
-
2.
Immediately add retina to 200µL of pre-warmed Kreb-HEPES buffer in a glass tube, and incubate for 10 minutes at 37°C and 5% CO2.
-
3.
Add 25uL of pre-warmed lucigenin buffer per 200uL of Kreb-HEPES buffer to tube, and incubate for 5 minutes at 37°C and 5% CO2.
Measure luminescence
-
4.
Gently (but quickly) mix contents of tube, measure the luminescence in luminometer three times consecutively for 3 seconds each reading and then replace the tube in the incubator.
Gently swirl the tissue with several quick flicks of the wrist while holding tube between forefinger and thumb, but do not vortex. Shaking too vigorously will increase the background luminescence and often activate the tissue, potentially resulting in a sudden spike of luminescence. Too little agitation will give a poor reading.
-
5.
Measure samples three times at 4 minutes intervals, and average the 9 values for each animal.
-
6.
Transfer the entire contents of the tube to a 1.5mL micro-centrifuge tube, add 1/10 volume 10x cell lysis buffer, sonicate, and measure the protein concentration.
-
7.
Express results as RLU/mg protein of retina.
Basic protocol 6
Quantification of diabetes-induced adherent leukocytes in retinal vasculature (Leukostasis)
Leukostasis is defined as the attachment of leukocytes to the lumen of the vasculature. It is characteristic of inflammation, and is increased in the retina of diabetic rodents, and has been postulated to contribute to retinal capillary degeneration. In the method described below, the animal is perfused with buffer to remove free blood, followed by perfusion with Concanavalin A. Concanavalin A binds to all cells, but is especially bright staining where leukocytes are bound to the vasculature.
Materials
Concanavalin A solution (1mg /mL in PBS of Concanavalin A FITC (Vector FL-1001)
Pressure infuser (Infusurge, 4010, Ethox Bufflo NY)
IV Catheter set with regulating clamp (70 inches, Baxter, 2C5417s)
Gavage Needle (20g (1.25mm OD barrel tip) x 30mm, Fine Science, 18060-20)
Saline (0.9%, veterinary grade, 1000 mL Baxter 04925-04-10)
2 4-way stopcock Luer lock IV line valve (Baxter, 2C6204)
1 IV extension set (2.3ml, 21”, Luer lock, Baxter 2C2662)
2 IV extension sets (3.9ml, 36”, Luer lock, Baxter 2C2667)
0.22 micron syringe filter.
-
10 mL syringe.
Connect in series the bag of saline in the pressure infuser, the catheter set, a 4-way valve, the two 3 foot extensions of IV line, the second 4 way valve, a 0.22um filter, the 1 foot IV line extension and finally the gavage needle.
Purge lines and ports of all air bubbles and set flow rate to 18 mL/min at 150mmHg (Janssen et al., 2002).
Attach a 10mL syringe filled with Concanavalin A solution to 4-way valve closest to the gavage needle. After perfusing with saline, turn the valves to shut off flow to the gavage needle and fill the tubing extensions displacing saline out the open port of the other valve. Then return the valves to their original position and perfuse with the Concanavalin solution at the proper flow rate and pressure.
Perfuse the mouse
-
1.
Anesthetize mouse, and open the chest cavity to expose the heart.
-
2.
Cut the left ventricle, and insert the gavage needle up through the heart into the aorta until just the very end is sticking into the aorta.
-
3.
Clamp with hemostats lightly across the ventricles and cut open the right atria.
-
4.
Perfuse for 2 minutes with pre-warmed saline, followed by 10 mL of pre-warmed Concanavalin A-FITC solution for 2 minutes, and then an additional 2 minutes of saline.
Isolate retina and count leukocytes
-
5.
Enucleate the eye and isolate the retina.
-
6.
Make 4–5 radial cuts into retina to allow it to lay flat (cloverleaf pattern).
-
7.
Lay out the unfixed retina on slide with a small amount of PBS, and count FITC labeled leukocytes in blood vessels using fluorescence microscopy. We recommend viewing at 100x magnification (10x objective). Express count as leukocytes per retina.
BASIC PROTOCOL 7
IMMUNOHISTOCHEMISTRY OF VASCULATURE AND LEUKOCYTES IN RETINAL WHOLE MOUNT
This protocol describes a technique in which non-perfused vasculature and leukocytes can be visualized and enumerated in the whole fixed retina. This technique can be modified to identify specific subtypes of leukocytes with the addition of appropriate antibodies.
Materials
Perfusion system from Basic Protocol 6.
Concanavalin A solution 200uL of stock solution in 10mL PBS per mouse.
Concanavalin A stock solution 5mg/mL in 10mM Hepes, 0.15M NaCl, 0.1 mM CaCl2, 0.01mM MnCl2, 0.08% NaN3 (Vector labs, L1000).
Fixation buffer 2% paraformaldehyde in 1x PBS pH 7.2.
Triton X −100.
Demasking buffer (10mM Sodium Citrate, 0.05% Tween 20, 0.01% Sodium Azide, pH 6.0)
TBST (20mM Tris base, 150mM sodium chloride, 0.05% Tween-20 at pH 7.6)
Blocking buffer- TBST with 2% BSA, 2% normal goat serum, 2% normal donkey serum, 2% normal rabbit serum, 0.05% sodium azide at pH 6.8 (use pH 7.3–7.4 for primary antibodies).
Normal Goat Serum (Invitrogen, 16210064)
Normal Donkey serum (Jackson IR 017-000-121)
Normal Rabbit serum (Jackson IR 011-000-120)
Bovine serum albumin (BSA) heat shock isolation (Amgen, 0332)
Hoechst dye (1:1000 Thermo-Fisher, 62249)
Rabbit anti-collagen IV (1:1000, Abcam, Ab6586)
Donkey anti-Rabbit AF488 conjugated (1:1000 Jackson Immuno Research, 711-545-152)
Rat IgG2b anti-CD45 clone 30F-11 PE conjugated (1:500 BD 553081)
Goat Anti-PE (1:1000 Rockland 600-101-387) pre-labeled with Dylight 594 (Thermo-Fisher 46412)
Goat anti-Concanavalin A (1:500, Vector labs, AS2004) pre-labeled with Dylight 650 (Thermo-Fisher, 62274)
Vascular labeling and initial fixation
-
1.
Anesthetize or euthanize the mouse, open the chest cavity and perfuse mouse with 18mL/min of saline for 2 minutes to remove non-adherent blood cells.
-
2.
Label the vasculature by perfusion with 10mL of the Concanavalin A solution (about 2 minutes) followed by perfusion with a saline wash for 2 minutes.
-
3.
Perfuse with the fixation buffer until the front legs of the mouse move together (about 30 seconds), then immediately stop the perfusion. This step limits leukocyte migration out of the vasculature.
Isolation of the eye and additional fixation
-
4.
Proptose and enucleate the eyes as described in basic protocol 3.
-
5.
Make a small incision (1/8 of eye) with a Teflon razor blade just below the limbus (cornea-sclera junction) using a razor blade to enhance fixative access to the retina. Don’t cut off the anterior segment or remove the lens yet
-
6.
Immerse eyes in cold fixation buffer on ice for 30 minutes. To reduce background auto-fluorescence, transfer eyes after 10 minutes of fixation to cold fixation solution with 0.3% Triton-X 100 (v/v) on ice for the remaining 20 minutes.
-
7.
After 30 minutes of fixation on ice, continue fixing the eyes for an additional 3.5 hours at room temp. Total fixation time is 4 hours. Longer fixation times will increase the stability of the retina over time but will also increase the background fluorescence.
-
8.
Rinse each eye in PBS for a few minutes at room temperature. Remove the anterior segment, and isolate the retina from the posterior segment as described in basic protocol 3.
Antigen retrieval
-
9.
Incubate the retina overnight in PBS overnight at 4°C to remove residual fixative.
-
10.
Incubate retina in de-masking buffer at 56–65°C for 24 to 12 hours respectively in a covered container (Jiao et al., 1999). Boiling causes opacification of the retina. This procedure works even on retina that has been fixed for months, however the incubation time must be increased to 36 hours.
-
11.
Rinse retina in PBS at room temperature for several hours to remove de-masking buffer.
Immunohistochemistry
-
12.
Block retina in 1mL of blocking buffer (pH 6.8) overnight at 4°C.
-
13.
Incubate retina with dilutions indicated above (about 0.2 ug/mL) primary antibodies in 1mL of fresh blocking buffer (pH 7.3–7.4) for 12–24 hours at 4°C.
-
14.
De-stain for 1 day (or 5 half-lives) in at least 1 mL of TBST, during which the buffer should be changed every 4–6 hours (at least 3 times).
-
15.
Incubate with secondary antibody and Hoechst nuclear stain at 1:1000 in blocking buffer (pH 7.3–7.4) overnight at 4°C.
-
16.
De-stain as previously described then rinse for a few hours in TBS or PBS to reduce Tween 20 concentration.
-
17.
Mount retina on a slide in a well that is at least one coverslip thickness deep to prevent the retina from being crushed/deformed under the coverslip.
To protect the retina from damage by coverslip, cut strips of coverslip affixed to the slide with fingernail polish can be used to form the well but the nail polish must be allowed to thoroughly dry and then the slide must be soaked in PBS for at least a day to remove any remaining solvent from the nail polish.
-
18.
Cover with anti-fade medium and a glass coverslip for at least an hour prior to viewing under fluorescent microscope at appropriate wavelengths.
-
19.
Count adherent leukocytes in the vasculature and non-perfused vasculature.
COMMENTARY
Background Information
Diabetic retinopathy is diagnosed and subdivided into proliferative and non-proliferative stages by observations of abnormalities in the vasculature of the retina in diabetic patients. Loss of the retinal vasculature in the non-proliferative stage is thought to initiate a hypoxic environment in the retina which leads to an outgrowth of blood vessels from the retina into the vitreous, which is believed to contribute to visual impairment and loss. Good regulation of glucose levels in both experimental animals (Engerman et al., 1977) and patients (1993; Ratner, 2001; United Kingdom Prospective Diabetes Study, 1998) has been shown to inhibit the diabetes-induced increase in vascular lesions of the retina, and in humans, the progression of diabetic retinopathy into the proliferative stage. Therefore animal models and techniques which recapitulate the vascular abnormalities in the pre-proliferative stage are important tools in understanding the biological mechanism of the disease. The increase in diabetes induced retinal acellular capillaries, which are devoid of nuclei of supporting endothelial and pericyte cells, is one of the few clinically meaningful endpoints which can be measured in both humans and experimental animals. Inhibition of the diabetes-induced increase in superoxide generation by the retina at two months of diabetes correlates with therapies which inhibit diabetes induced retinal vascular loss at longer durations of diabetes in rodent models.
Critical Parameters and trouble shooting
Induction and measurement of experimental diabetes
Immediate injection of the streptozotocin after reconstitution into fasted animals followed by access to food is critical to the uptake of the active form of the drug and induction of diabetes. Different strains of rodents can differ in their sensitivity to the STZ. The protocol was developed for C57BL/6J male mice at 25g and for Lewis male rats at approximately 250 grams body-weight (8 to 10 weeks of age). Multiple injections of a small dose of streptozotocin in mice are more effective in inducing diabetes and decreasing the incidence of toxicity and development of cancer in both the liver and kidneys. Animals which fail to become diabetic after the first round of injections can have the dose increased slightly to induce diabetes. After three rounds of injections rodents which fail to become diabetic should be euthanized. The induction of diabetes in female mice is more problematic than in male mice, because diabetes is less severe in female rodents.
Maintenance of body weight in diabetic rodents with small injections of insulin is critical for long term experiments. While fasted blood glucose is a good indicator of the presence of diabetes, glucose levels will change throughout the day depending on the activity and feeding habits of the animal. Therefore, a low glucose value in a mouse with previously high blood glucose should be repeated after allowing some additional time for the rodent to feed and rest. Many animals which have only slightly elevated glucose levels soon after injection of STZ will develop more severe diabetes within a few weeks. Occasionally there are some animals that revert to a non-diabetic status after several months, and therefore the diabetic status needs to be monitored over the course of the experiment by both fasted blood glucose levels and by measurement of HbA1C.
Isolation of fresh retina
The critical step in the isolation the retina is the enuculation of the eye without cutting off the back of the eye. Proptose the eye so that it bulges out before cutting it away with scissors. Pulling on the eye while cutting will cause the optic nerve to be stretched (and possibly ripped away from the retina). Razor blades should be changed frequently, because dull blades cut poorly. The lens can deflect the razor blade as it is pushed through the eye causing a large portion of the retina to be cut off if one is not careful. Rotate the eye while cutting and or remove the lens until enough skill is obtained to cut and open the eye cleanly. The initial removal of the anterior part of the eye is much easier when done on wet lint free paper, however the paper must be removed prior to scooping out the retina onto the dental wax.
Isolation of fixed vasculature
The retina must be digested enough that the tissue easily flakes off from the vasculature. If the non-vascular tissue does not deform and flake away easily after digestion incubate the retina in the activating solution with a small amount of the elastase solution overnight at room temperature. If the digested retina sticks to the single hair brush, dip the hair in 10% formalin or the elastase solution for a few seconds and then rinse it in water. This “coats” sticky areas on the hair. If the hair continues to stick to the vasculature look for a small piece of remaining vitreous and remove it with the micro scissors. Occasionally the vasculature will ball up or become very sticky. Often this due to a small remnant of vitreous. Add some protein such as a few drops of BSA to the water and use the modified pipette to suck up and expel the vasculature several times. Allowing the vasculature to incubate in the protein solution for several hours is also often helpful. If the vasculature is wound around the tools use the pipette to move the protein solution over the vasculature and when possible either cut off the edge of the vasculature where attached or use a second hair to carefully slide it off the tool. Identifying the acellular capillaries is tedious time consuming work and will require the observer to move the focus up and down to determine the insertion and end point of the capillaries. Do not count short bridging capillaries. The diabetes-induced acellular capillaries are not uniformly distributed through the retina, and are most frequent in the superior temporal quadrant (Kern and Engerman, 1995; Tang et al., 2003). Aging will increase the number of acellular capillaries near the periphery (limbus) of the retina. We have not found it necessary to profuse the animal prior enucleating the eye to allow easy visualization of nuclei within the isolated retinal vessel preparations.
Measurement of superoxide
Crushing or pulling on the isolated retina can cause an elevation in superoxide production which slowly decreases over the course of about 30 minutes. A failure to gently swirl the retina in the tube just prior to measurement will lead decreased detection of superoxide, where as excessive shaking, or the failure to filter the lucigenin may lead to abnormally high measurements. Use an empty glass tube as an initial blank for the luminometer. The value of the measurement should not change if the tube is measured when the luminometer is draped with a light blocking heavy blanket to eliminate the possibility of light leaks. Measurement luminescence of sample buffer with the lucigenin (25 µl) should give a similar value to the glass blank; if it is higher than that, centrifuge or remake the solutions. Vortexing or hard shaking of the tube containing the lucigenin solution should give a brief elevated count. If it does not then the lucigenin is likely oxidized. This technique can also be used to determine the upper limit of shaking the tube too hard. The light emitted from the sample will decrease with both reagent consumption (time) and temperature loss. Therefore, a slight decrease in the superoxide detected will occur between readings. If the retina is folded over or curled up in the tube, the amount of superoxide detected will be decreased. To fix this, carefully remove the retina from the tube, remove any vitreous, cut the retina in a cloverleaf pattern, rinse it in Kreb buffer and allow the retina to incubate for an additional 10 minutes before restarting the measurement. If the retina is cut excessively from the edge to the optic nerve as opposed to about ¼ of that distance in a clover leaf pattern, then the retina may bend back and forth like a butterfly during the mixing process resulting is a very high superoxide measurement and damage to the retina.
Leukostasis
Poor perfusion of the mouse will prevent labeling of the vasculature and subsequent analysis of leukocyte adherent to the lumen, whereas excessive pressure from a rapid squeeze of a syringe can cause blood vessels to burst. Poor vascular perfusion is often due to use of cold fluids (causing vasoconstriction), excessive blood loss (greater than 500 µL), or kinking of the vasculature due to the weight and placement of the perfusion needle. Check the placement of the end of the needle which should just protrude from the heart. If the needle is inserted into the pulmonary vein, then the lungs will fill when the flow is started. During the perfusion, move the heart and needle slightly from side to side and up and down. This will often result in an increase of blood exiting from the heart if the vasculature is kinked. The tongue, kidneys and liver should blanch during the perfusion, however these organs are downstream of the blood vessels which supply the eyes and therefore do not guarantee a good profusion of the retina. Caution must be used when viewing the retina under high magnification as the bends in the capillaries can appear to be labeled leukocytes adherent to the vasculature; focusing the microscope up and down while observing the area will usually allow differentiation of leukostasis from a vessel diving into the retina.
Immunohistochemistry of whole retina
Exposing the interior of the eye to the fixation solution quickly helps stablize the retina and inhibit leukocyte migration out of the vasculature into the tissue. Antigen recovery after formalin fixation must be done in low heat. Sufficient time to block and de-stain are important to reducing the background fluorescence, especially in whole-mount preparations. As a control for immunohistochemistry, use a non-specific antibody of the same host species, concentration and fluorochrome conjugate as the primary antibody. Determine the half-life of the antibody into and out of the tissue after measuring the intensity of the fluorescence after 30 minutes and 3 hours of staining and de-staining respectively. The use of “pre-screened” secondary antibodies are not sufficient controls to account for interactions between the primary antibody and off-target binding such as binding to the Fc receptor.
Anticipated results
Induction and measurement of experimental diabetes
Greater than 80–90% of the male rodents become diabetic after injection of streptozotocin, but there is variation in the severity of the diabetes. Most of the experimentally diabetic animals survive the first two months of diabetes, while mortality is higher for long-term experiments. It is possible that up to half of the experimental diabetic animals will die by 8 months of diabetes.
Isolation of fixed retinal vasculature
The number of acellular capillaries per retina will vary with the user, the animals, and the duration of diabetes, but in general, the number of degenerate capillaries in animals having diabetes of more than 9 months duration is about 2–3 times greater than in nondiabetic animals.
Measurement of superoxide
Superoxide generated by retina from diabetic animals should be about twice that generated by samples from non-diabetic animals.
Leukostasis in fresh retina
There are 3–10 leukocytes adherent to the vasculature per retina from a diabetic animal, while only 1–2 are seen in non-diabetic animals.
Immunohistochemistry of whole retina
Light staining of the plexiform layers with antibody as opposed to punctate staining of discrete cells is indicative of non-specific staining which does increase in the retina from diabetic animals. The outer segment of the photoreceptors is often autofluorescent in the red and green channels (488 and 595 nm).
Time Considerations
Induction and measurement of experimental diabetes
The first round of streptozotocin injections requires one week and at least two weeks to confirm that the animals are diabetic. Experienced personnel familiar with IP injections can expect to inject about 20 mice in 30 minutes. Measurement of blood glucose and weight requires about 3 minutes per mouse.
Molecular and biochemical changes in the retina due to diabetes are commonly measured about 10 weeks after the onset of diabetes, whereas retinal vasculature loss is best measured after 30–40 weeks of diabetes.
Isolation of fresh retina
The removal of the eye and the isolation of fresh retina requires less than 5 minutes per mouse with practice. The novice user should practice for several days with whole fixed eyes before attempting to isolate the retina from unfixed eyes. The unfixed retina will be severely damaged by attempts to grab it directly.
Isolation of fixed retinal vasculature
The digestion step can vary between two to four days. Experienced users can expect to clean and mount up to 10 retina per day after enzymatic digestion. The novice user will be limited to about 3 retina per day which will have patches of remaining tissue and often sections of damaged vasculature. Staining of the mounted vasculature requires several hours. An experienced user can expect to count the acellular capillaries at the rate of one to two hours per eye. Novice users will require several days of counting and re-counting a diabetic and non-diabetic sample until they can reliably identify the diabetes-induced acellular capillaries in the mouse retinal vasculature.
Measurement of superoxide
Isolation and incubation of the retina prior to adding the substrate will require 10 to 20 minutes. Once the substrate has been added and incubated for 5 minutes, the luminescence of one retina can be measured in a minute.
Measurement of leukostasis in fresh retina
The procedure requires about 30 minutes from start to finish. The majority of the time will be used perfusing the animal.
Immunohistochemistry of whole retina
The procedure generally takes a week to complete. The majority of the time will be the various 12 hour incubation and wash steps. Documentation of items of interest in the retina can range from an hour to about 12 hours to photograph the entire retina.
Fig 3.
Isolation of retina by sliding a microspatula between RPE/choroid and retina.
Acknowledgements
This work was supported by grants from the National Eye Institute (R01EY00300, R01EY022938 and R24EY021126) and the Medical Research Service of the Department of Veteran Affairs.
Footnotes
Conflicts of interest
We have no conflicts of interest to report.
Literature cited
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009;50:2351–2358. doi: 10.1167/iovs.08-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov P, Corraliza L, Villena JA, Carvalho AR, Garcia-Arumi J, Ramos D, Ruberte J, Simo R, Hernandez C. The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One. 2014;9:e97302. doi: 10.1371/journal.pone.0097302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110:16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Bloodworth JMB, Jr, Nelson S. Relationship of microvascular disease in diabetes to metabolic control. Diabetes. 1977;26:760–769. doi: 10.2337/diab.26.8.760. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Aldose reductase inhibition fails to prevent retinopathy in diabetic and galactosemic dogs. Diabetes. 1993;42:820–825. doi: 10.2337/diab.42.6.820. [DOI] [PubMed] [Google Scholar]
- Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, Chen DF. Vascular Damage in a Mouse Model of Diabetic Retinopathy: Relation to Neuronal and Glial Changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-Lipoxygenase, Contributes to Degeneration of Retinal Capillaries in a Mouse Model of Diabetic Retinopathy. Diabetes. 2008;57:1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes H-P, Klinzing I, Wiegand S, Bretzel RG, Cohen AM, Federlin K. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen diabetic rat. Invest Ophthalmol Vis Sci. 1993;34:2092–2096. [PubMed] [Google Scholar]
- Han Z, Guo J, Conley SM, Naash MI. Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:574–584. doi: 10.1167/iovs.12-10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B, Debets J, Leenders P, Smits J. Chronic measurement of cardiac output in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R928–R935. doi: 10.1152/ajpregu.00406.2001. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods. 1999;93:149–162. doi: 10.1016/s0165-0270(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13:863–867. doi: 10.3109/02713689409015087. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp. Eye Res. 1995;60:545–549. doi: 10.1016/s0014-4835(05)80069-7. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch. Ophthalmol. 1996;114:986–990. doi: 10.1001/archopht.1996.01100140194013. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru A, Chakrabarti S, Khan Z. Potential contributory role of H-Ras, a small G-protein, in the development of retinopathy in diabetic rats. Diabetes. 2004;53:775–783. doi: 10.2337/diabetes.53.3.775. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Studies of retinal vascular patterns: I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Laver NM, Robison WG, Jr, Pfeffer BA. Novel procedures for isolating intact retinal vascular beds from diabetic humans and animal models. Invest Ophthalmol Vis Sci. 1993;34:2097–2104. [PubMed] [Google Scholar]
- Li G, Veenstra AA, Talahalli RR, Wang X, Gubitosi-Klug RA, Sheibani N, Kern TS. Marrow-Derived Cells Regulate the Development of Early Diabetic Retinopathy and Tactile Allodynia in Mice. Diabetes. 2012;61:3294–3303. doi: 10.2337/db11-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliss EB, Nakhooda AF, Poussier P, Sima AA. The diabetic syndrome of the ‘BB’ Wistar rat: possible relevance to type 1 (insulin-dependent) diabetes in man. Diabetologia. 1982;22:225–232. doi: 10.1007/BF00281296. [DOI] [PubMed] [Google Scholar]
- McLenachan S, Chen X, McMenamin PG, Rakoczy EP. Absence of clinical correlates of diabetic retinopathy in the Ins2Akita retina. Clin Experiment Ophthalmol. 2013;41:582–592. doi: 10.1111/ceo.12084. [DOI] [PubMed] [Google Scholar]
- Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53:6488–6494. doi: 10.1167/iovs.12-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama K, Shichinohe K, Kobayashi K, Naito K, Uchida O, Yasuhara K, Tobe M. Spontaneous diabetes-like syndrome in WBN/KOB rats. Acta Diabetol Lat. 1985;22:335–342. doi: 10.1007/BF02624752. [DOI] [PubMed] [Google Scholar]
- Ning X, Baoyu Q, Yuzhen L, Shuli S, Reed E, Li QQ. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int J Mol Med. 2004;13:87–92. [PubMed] [Google Scholar]
- Ratner RE. Glycemic control in the prevention of diabetic complications. Clin Cornerstone. 2001;4:24–37. doi: 10.1016/s1098-3597(01)90027-4. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Masuyama T, Shoda T, Takahashi T, Katsuda Y, Komeda K, Kuroki M, Kakehashi A, Kanazawa Y. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. International journal of experimental diabetes research. 2000;1:89–100. doi: 10.1155/EDR.2000.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR, Anderson NL. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- Talahalli R, Zarini S, Tang J, Li G, Murphy R, Kern TS, Gubitosi-Klug RA. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. J Leukoc Biol. 2013;93:135–143. doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Du Y, Petrash JM, Sheibani N, Kern TS. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS One. 2013;8:e62081. doi: 10.1371/journal.pone.0062081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Mohr S, Du Y, Kern TS. Non-uniform distribution of lesions and biochemical abnormalities within the retina of diabetic humans. Curr. Eye Res. 2003;27:7–13. doi: 10.1076/ceyr.27.2.7.15455. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Experimental biology and medicine. 2011;236:1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Vagaja NN, Binz N, McLenachan S, Rakoczy EP, McMenamin PG. Influence of endotoxin-mediated retinal inflammation on phenotype of diabetic retinopathy in Ins2 Akita mice. Br J Ophthalmol. 2013;97:1343–1350. doi: 10.1136/bjophthalmol-2013-303201. [DOI] [PubMed] [Google Scholar]
- Veenstra AA, Tang J, Kern TS. Antagonism of CD11b with Neutrophil Inhibitory Factor (NIF) Inhibits Vascular Lesions in Diabetic Retinopathy. PLoS One. 2013;8:e78405. doi: 10.1371/journal.pone.0078405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, Du Y, Kern TS, Thimmulappa R, Barber AJ, Biswal S, Duh EJ. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57:204–213. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]