Abstract
Plastic, as a form of marine litter, is found in varying quantities and sizes around the globe from surface waters to deep-sea sediments. Identifying patterns of microplastic distribution will benefit an understanding of the scale of their potential effect on the environment and organisms. As sea ice extent is reducing in the Arctic, heightened shipping and fishing activity may increase marine pollution in the area. Microplastics may enter the region following ocean transport and local input, although baseline contamination measurements are still required. Here we present the first study of microplastics in Arctic waters, south and southwest of Svalbard, Norway. Microplastics were found in surface (top 16 cm) and sub-surface (6 m depth) samples using two independent techniques. Origins and pathways bringing microplastic to the Arctic remain unclear. Particle composition (95% fibres) suggests they may either result from the breakdown of larger items (transported over large distances by prevailing currents, or derived from local vessel activity), or input in sewage and wastewater from coastal areas. Concurrent observations of high zooplankton abundance suggest a high probability for marine biota to encounter microplastics and a potential for trophic interactions. Further research is required to understand the effects of microplastic-biota interaction within this productive environment.
Contamination of the world’s open oceans, enclosed seas and coastal waters by synthetic non-biodegradable material has become a high profile environmental concern1. Of this debris, plastic (both macro- and micro-) make up the largest quantity and can be related to increased production of anthropogenic materials and growing dependence on plastic products. World-wide production rates for 2013 were estimated at 299 million tonnes2. The longevity of plastics in the environment means that they can be distributed huge distances from their origin, and accumulate on remote beaches and on the seafloor3. Once in the ocean, mechanical and biological processes cause plastics to break down into microplastics (<5 mm)4,5. Microplastics may also enter the environment directly as granules, pellets, fibres and powders used in the production of larger plastic products, abrasive scrubs in personal care products, medicines and as a consequence of washing synthetic clothing6.
Microplastic monitoring tends to focus in coastal areas or in the open ocean in convergent zones and gyres7. Microplastics have been found worldwide, from surface waters8 to deep sea sediments9, and in freshwater systems10. Microplastics are difficult to mechanically remove from the ocean and their distribution and fate has still to be studied in depth. Recently, microplastic presence was reported in ice cores from remote areas of the Arctic Ocean, at levels greater than those reported for Pacific Gyre surface waters11. Whilst models propose the transport and a potential accumulation zone of microplastics at higher northern latitudes, including the Barents Sea12,13; field studies have not yet validated microplastic distribution within polar waters.
Polar waters support distinct and highly productive marine food webs and ecosystems14 which may be vulnerable to marine pollution. Prevailing ocean currents, wind currents, and migratory species can transport containments to the Arctic. In particular, the accumulation of persistent organic pollutants (POPs) has been well documented15. Recent quantitative analysis found the levels of plastic ingested by 87.5% of northern fulmars (Fulmarus glacialis) to exceed the OSPAR ecological quality objective16. Whilst the particular challenges posed by microplastics to polar organisms remain uncertain, interactions with microplastics have been observed for zooplankton17, invertebrates18, fish19, seabirds16, and mammals20. Effects of ingestion could include reduced feeding, energy depletion, injury, death or a toxicological response to contaminants associated with the plastics. As such, microplastics and marine litter have been incorporated into national and international policies, and legislation, to assess the combined risks on the environment and biota (e.g. EU Marine Strategy Framework Directive (2008/56/EC), NOAA Marine Debris Programme). Determination of the relationships between sources and sinks of microplastics will help to identify areas possibly vulnerable to microplastic accumulation.
The marine environment of the west Spitsbergen shelf has Atlantic water, Arctic water, brine and freshwater inputs21,22,23. Several inflowing and significant current systems transporting water, nutrients and associated biota to Arctic regions from the North Atlantic may also transport microplastics. Furthermore, as commercial activity increases in response to sea ice decline and a growing economic demand, the threat of marine pollution will also rise. This could suggest heightened levels of microplastics in the surface waters, thus increasing the likelihood of marine biota interacting with the particles. The Arctic region supports an important and diverse ecosystem from planktonic communities to marine mammals24. There are also several developing and important fisheries in the area25, which could be affected if organisms interact with marine contaminants. Considering the potential implications of microplastics7, there is an urgent need to assess the levels in the Arctic. This will allow for future microplastic monitoring which could lead to a risk assessment of the potential impacts of decreasing sea ice, increasing shipping and commercial activity.
This study aims to describe the distribution and abundance of polar microplastics. Two independent techniques were used to sample microplastics from surface and sub-surface waters. Water samples were collected using a manta net in the top 16 cm of surface water and sub-surface samples from the vessel’s on-board seawater pump, situated 6 m below the surface. Environmental variables including temperature, salinity, wind speed, wind direction and boat speed were collected from the vessel’s underway systems to assess their influence on the number of microplastics sampled. The present study represents the first assessment of microplastic distribution and abundance in surface and sub-surface Arctic waters south and southwest of Svalbard, Norway.
Results
Surface samples
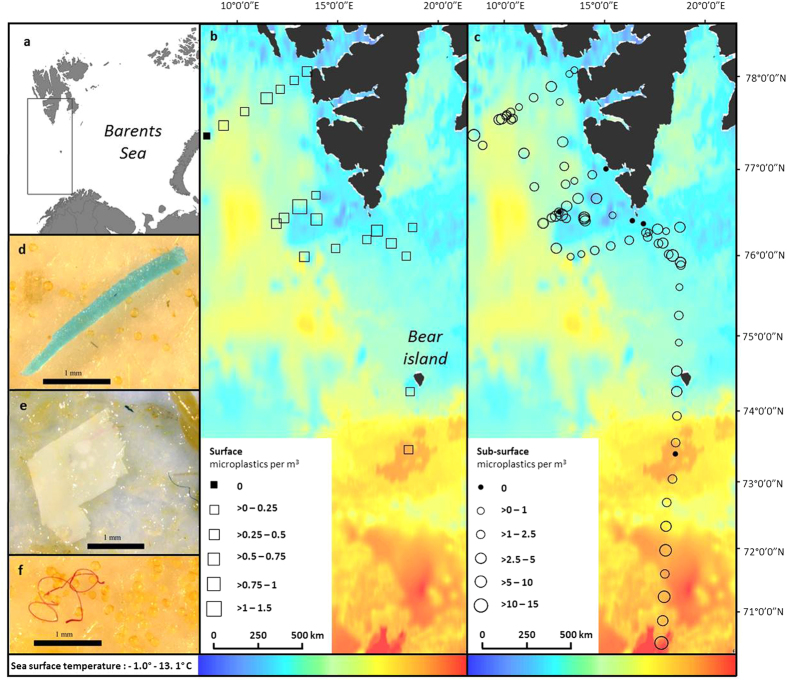
Surface samples were collected in the top 16 cm of seawater using a manta net. Microplastics were found in 20 out of 21 (95%) samples (Fig. 1b). Microplastic abundance ranged between 0 and 1.31 particles per m3, and averaged 0.34 (±0.31 SD) particles per m3. The single sample which was free from microplastics was found furthest offshore. Distances covered by the manta net tows ranged from 0.55 to 1.85 km. Mean zooplankton abundance in surface samples was 623.65 individuals per m3 (±838.11 SD). There was no significant correlation between zooplankton abundance and microplastic abundance (Pearson’s, p = 0.20).
Figure 1. Map of sample locations during research cruise (created using ArcGIS) and example microplastic pictures.
(a) Location of survey area, (b) surface sampling positions and microplastic abundance per m3, (c) sub-surface sampling positions and microplastic abundance per m3, (d) plastic fragment, (e) plastic film, (f) plastic fibre. SST source satellite data from: JPL OurOcean Project. 2010. GHRSST Level 4 G1SST Global Foundation Sea Surface Temperature Analysis. Ver. 1. PO.DAAC, CA, USA. Dataset accessed [2015-08-03] at http://dx.doi.org/10.5067/GHG1S-4FP01.
Sub-surface samples
Sub-surface samples were collected at a depth of 6 m using the vessel’s on-board seawater pump. Each sample consisted of 2,000 litres of sub-surface seawater filtered over a 2 hour period and the number of microplastics were standardised to m3. Total sub-surface sampling effort filtered 150,000 litres of seawater. Out of the 75 sub-surface samples, 93 % contained microplastics. Microplastic abundance ranged between 0 and 11.5 particles per m3, and averaged 2.68 (±2.95 SD) particles per m3 (Fig. 1c).
Microplastic classification
A total of 665 particles were identified using visual identification methods (261 from the surface samples and 404 from the sub-surface samples). Three different types of particle were identified (fibres, fragments and films; Fig. 1d–f) with fibres being the most abundant (95%), followed by fragments (4.9%) and films (<0.1%). Black (45%) and blue (29%) were the most abundant colours. Particle size ranged between 0.25 mm and 7.71 mm with an average length of 1.93 mm (±1.22 SD). Of those particles subjected to FT-IR analysis, polymers identified included polyester (15%), polyamide (15%), polyethylene (5%), acrylic (10%), polyvinyl chloride (5%), cellulose (possibly Rayon, see discussion) (30%) and 20% of particles were of unknown origin.
Data analysis
Average microplastic values from the surface waters 0.34 (±0.31 D) particles per m3 were less than those reported in sub-surface waters using the underway system 2.68 (±2.95 SD) particles per m3.
Whilst microplastics appear to be ubiquitous across the survey area the standardised values (microplastic count per m3) sampled by the manta trawl and sub-surface system were very different (GLM: p < 0.001, Table 1). The best fit GLM model (equation 1) of the number of microplastics per sample showed that sea surface temperature was a significant predictor of microplastic abundance (at the 0.1 significance level, Table 1).
Table 1. Generalised linear model (GLM) results for significant variables only based on the best fit model: (count = SST + SSS + method + SST:SSS).
| Response variable | Explanatory variables | p | AIC |
|---|---|---|---|
| Count | SST | 0.08 | 611.3 |
| SSS | 0.69 | ||
| method | <0.001 | ||
| SST * SSS | 0.13 |
The response variable was the number of microplastics per sample. Gaussian distribution was assumed for the response variable. Results displayed are explanatory variables included in the final best fit model and their significance based on Analysis of Variance. Also provided is the AIC value for the model. References to the explanatory variables can be found in Table 3.
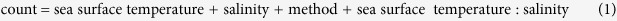 |
Contamination control
Sources of contamination were mitigated through the application of established methods and controls26. Procedural blanks used during sampling did not indicate any sources of potential contamination.
Discussion
Microplastics were found across the survey area in more than 90% of samples, both in the surface waters using a manta net and at 6 m depth using the vessels underway seawater pump. Microplastic abundance values in surface waters were of the same order of magnitude as those found in the North Pacific and North Atlantic, greater than the Californian current system, south and equatorial Atlantic, but less than those reported for the closed water system of the Mediterranean (Table 2). However, the variances around these values are not reported in all studies, hence precluding a determination of the potential statistical difference between them. Although the use of the sub-surface pump to collect marine debris is a relatively new method, microplastic abundance in this study (average: 2.68 ± 2.95) is not significantly higher (T-test, t = −0.84, p = 0.4) than reported using the same method in the North Atlantic (average: 2.46 microplastics per m3 26). No evidence of a microplastic accumulation zone was observed. However, studies have reported increasing numbers of micro and macroplastics towards centres of ocean gyres8,27,28,29. This accumulation has been attributed to the convergence of surface currents and local wind conditions29.
Table 2. Abundance of microplastics observed in manta net and neuston net studies from around the world.
| Location | n/m2 | n/m3 | Particle abundance |
|---|---|---|---|
| Arctic waters (This study) | 0.028 | 0.34 | 0–1.31/m3 |
| Bering Sea48 | . | 0.004–0.19 | . |
| North Pacific subtropical gyre49 | . | 0.116 | . |
| North Pacific subtropical gyre28 | 0.02–0.45 | . | . |
| South Californian current system50 | . | 0.011–0.033 | 0.00–3.14/m3 |
| South Pacific29 | 0.027 | . | 0–0.40/m2 |
| North Atlantic51 | . | 0.01–0.04 | . |
| North Atlantic subtropical gyre27 | 0.0015 | . | 0–0.2/m2 |
| Portuguese coast52 | . | 0.02–0.036 | . |
| Equatorial Atlantic53 | . | 0.01 | . |
| South Atlantic54 | . | 0.03 | . |
| Mediterranean37 | 0.12 | . | 0–0.89/m2 |
| Mediterranean55 | 0.25 | . | . |
| Mediterranean56 | . | 0.15 | 0.01–0.35/m3 |
Surface currents, wind and boat movement can cause turbulence that would be expected to redistribute particles within the water column, however, less microplastics were observed on the surface than in the sub-surface samples from this study. At present, we cannot exclude that this result could be affected by differences in the sampling methods. The two methods used cannot be directly compared as surface (manta net) and sub-surface (underway pump) sampling have some different features: the manta net filters a large volume of surface water over a short period of time and small distance (in this study up to 45 times the volume of water was sampled by the sub-surface underway pump in total) and slowly towed by the vessel; whereas the sub-surface system filters small volumes of water over larger distances, using the intake from the underway pump of the vessel. Furthermore, additional differences may arise when considering the geographical distance samples were collected over. The manta net covers a shorter distance and there would be less chance of the sampling masking the presence of possible patchiness in a smaller area compared to the sub-surface pump. Future studies with simultaneous depth sampling such as multi-nets30 could explain the vertical distribution of microplastics.
The number of microplastics in the study area might also be affected by the influence of water masses from adjacent areas. During the survey, marked differences in the sea-surface temperature were apparent. In general there is a cooling trend northwards and the cold Arctic Front was apparent north of Bear Island where negative anomalies in temperature and salinity marked the presence of a cyclonic gyre, or eddy, from the Barents Sea31. As sampling moved closer to shore, reduced salinity indicated the influence of Arctic waters and freshwater inputs from the fjords. The sub-surface samples that did not contain microplastics were reported close to the shore suggesting that unpolluted freshwater might dilute microplastic levels whereas more saline waters offshore, containing more Atlantic water, were possibly transporting microplastics from densely populated areas along the North Atlantic to the Arctic. Despite clear changes in polar fronts found throughout this survey31, microplastic abundances did not change significantly with salinity, however there was a marked, but not statistically significant, difference with fewer particles in colder, less saline waters. These findings could be explained by the connection between the Barents Sea and the North Atlantic. Ocean drifters released within six potential accumulation zones found that tracers from the North Atlantic gyres were advected north-eastward towards the Barents Sea, while tracers from the Barents Sea were advected south-westward towards the North Atlantic12. Lower numbers of microplastics were found near the cold front of the Barents Sea. Less dense Arctic water is the dominant water body in the Barents Sea and will float above the Atlantic water, suggesting that microplastic values collected here were influenced by unpolluted or diluted Arctic waters. Microplastics in Arctic water could be diluted by unpolluted freshwater rather than increased. These conclusions contrast strongly with the results of Obbard and colleagues11 that suggest accumulation of microplastics in the less dense, seasonally formed sea ice. The melt of ephemeral sea ice should contribute most to the less dense Arctic water. Possibly, at the time of year we sampled, the influence of unpolluted freshwater flows from the nearby Svalbard archipelago outweighed the potential microplastic input from summer sea ice melt.
Although our values are similar to those from other locations (Table 2), it is possible that the sea ice concentrates and retains microplastics, acting as a sink. As sea ice forms, it concentrates particles which are less dense than seawater and levels of microplastics in sea ice have been estimated in several orders of magnitude higher than surface waters11. If sea ice could act as a sink, it will be important to understand how long particles will be retained in the ice, and if/when they are released, where they will end up. Consequently, the current and forecast reduction in sea ice extent and volume32 could result in the release of trapped particles11. Inter- and intra-annual sampling will be necessary to compare to data collected in summer months, and to ascertain the role of sea ice formation and thawing processes in retaining and/or releasing entrained microplastics.
Microplastics found during our sampling were mainly fibres. This result suggests that microplastics in this area are probably from the break down products of larger plastic items such as fibres from shipping activity or fishing equipment, recreation and offshore industries (e.g. oil and gas)6. They have likely arrived in the Arctic after long periods at sea, and may have been transport over large distances. The input of microplastic fibres arising from the washing of textiles may also be a source6. However, the Arctic is not surrounded by dense urban populations, as such the input of debris from urban areas would be less likely than the transport of plastics on ocean currents, or input from shipping and commercial activity. Little is known about marine litter pollution and microplastics in Norway, although initial assessments found plastics on many Norwegian beaches including Svalbard33 and it is estimated that annual Norwegian emissions of microplastic are approximately 8,000 tonnes34. Furthermore, Arctic waters are very productive with intense fishing and ship traffic, and a large proportion of marine debris may be a result of fishing activities or loss at sea. These larger items will break down overtime to form microplastics. The direct input of microplastics to the area is at present unknown, and as such studies focusing on local and regional input, sewage and waste treatment works are required to find potential local or short-range sources. The most abundant polymers identified in this study (Rayon, polyester and polyamide, 30%, 15% and 15% respectively) were also the most abundant polymers identified in sea ice (Rayon: 54%, polyester: 21%, polyamide: 16%)11. Polymers identified have a range of uses, including packaging, textiles and fishing gear; as such we are unable to suggest specific sources. FT-IR reported 30% of fibres to be cellulose, however cellulose and the semi-synthetic polymer Rayon, have almost identical FT-IR spectra26. Without further analytical techniques, the classification must be used with caution. A review of previous studies found that the most commonly reported polymers included polyethylene and polypropylene which have low densities35, and are likely to float in sea water. However, as well as finding low density microplastics, we also found particles with higher densities (polyester, polyamide, acrylic, polyvinyl chloride) which are commonly used in textiles and packaging. As such particles would be more likely to sink and their presence means that some local generation of microplastics cannot be dismissed. Our observation of polymers of different densities in surface waters could also suggest that turbulence30, wind36,37 and storm events38 may redistribute particles in the water column.
The ubiquitous presence of microplastic in the surface waters of the Arctic Ocean heightens the chance that marine organisms inhabiting the same area will encounter microplastic particles. The Arctic waters support a large quantity of filter feeding organisms, from copepods, to fish and baleen whales, which could actively target or passively ingest microplastics floating in the surrounding water7. Previous studies have not focused on hyponeuston in the Arctic. A high abundance of zooplankton in the surface waters (our sampling collected animals present in the uppermost 16 cm of water) suggests a high probability of encounter between microplastics and fauna just beneath the surface. Within marine ecosystems zooplankton play a vital ecological role, as both primary and secondary consumers within the food web39. Copepods are one of the most abundant components of the zooplankton and use chemo- and mechano-receptors to detect their prey40. Some have the ability to distinguish between their prey (such as micro-algae or detritus) and plastic beads41. Effects of plastic ingestion by zooplankton observed in laboratory studies include altered feeding capacity, decreased fecundity and mortality17,42,43. Zooplankton collected in surface samples consisted primarily of calanoid copepods, which may actively feed on organic and synthetic particles in the surface waters. However the microplastics observed were mainly fibres, on average 1.93 mm long, and thus are unlikely to be ingested by zooplankton. Even so, further fragmentation of microplastics into nanoplastics might increase potential interaction with zooplankton that mistake plastics for prey. Secondary trophic impacts may also occur at higher trophic levels if ingested nanoplastics are transferred within the prey items of fish, birds and mammals44. In particular, organisms such as baleen whales that usually feed on aggregations of planktivorous fish, crustacea45, and cephalopods46 both in the water column and at the surface have a high likelihood of primary and secondary ingestion of microplastics whilst feeding. If sea ice is acting as a sink11 (and subsequent source of microplastics), such ecological interactions will be exacerbated by reduced sea ice extent and volume.
Conclusion
This is the first study to show, by two independent methods, the presence and distribution of microplastics in the Arctic waters south and southwest of Svalbard, Norway, up to 78° of latitude. Microplastic found were mainly fibres (95%). This implies transport of microplastics to the Arctic possibly over long distances and periods, although the input from local sources (fishing, commercial activities and sewage) should not be overlooked. At present, we are unable to determine the source of microplastics found in this study, although it is possible that prevailing winds and surface water transport from Norway and the northeast Atlantic influence the input of microplastics to Arctic waters. We did not observe any accumulation areas but pole-ward transport pathways need to be understood to confirm whether the Arctic is acting as a sink for microplastic particles. Furthermore, the influence of local input needs to be addressed. Whilst the ecological effects of microplastic distribution in the Arctic remain uncertain, high likelihood of encounter, interaction and ingestion by marine organisms suggests there may be ecological impacts. Understanding such impacts is particularly important in biologically rich waters such as the Arctic, which support ecologically and economically valuable species and systems.
Methods
Sample collection
Samples were collected during an oceanographic and geophysical cruise on-board the R.V. G. O. Sars between June 5th and 15th 2014. Sampling started on departure from Tromsø, Norway and was carried out southwest of the Svalbard archipelago, Norway, up to the latitude of 78.07° (Fig. 1).
Surface sample collection
Surface samples were collected using a manta trawl (0.61 m wide ×0.16 m vertical opening, 0.333 mm mesh, 3 metres long). The net was deployed from the stern of the vessel and sampled the top 10–16 cm of the water column. The net was towed in a straight line for, on average, 20 minutes at an average speed of 1.2 knots. Vessel speed was reduced to minimise the effect of vessel movement. Tow duration was maintained, however the length of the tow varied with vessel speed and surface currents, as such the volume of water filtered for each tow was not kept as a standard. A calibrated flow meter (Hydrobios) was attached to the mouth of the net to allow for calculation of the amount of water filtered. After the allocated collection time, the net was returned to the stern of the vessel where it was rinsed from the outside with a deck hose. The cod-end was removed and taken to the laboratory where it was rinsed and volume reduced, using pre-filtered water, into a sieve (200 μm). The volume reduced sample was transferred to a large collecting jar and stored in formalin (4% final concentration) for analysis. Analysis of plankton rich samples, followed an adaption of previously published procedures. Firstly, samples were gently shaken and placed in graduated cylinders to separate microplastics from zooplankton by gravity37. After standing for 24 hours, the organic material sank forming a concentrated deposit in the bottom of the cylinders whereas plastics floated in the overlying supernatant. The volume of the settled zooplankton was recorded before the supernatant was removed. The supernatant was filtered under vacuum onto GF/C paper (47 mm), and analysed under a Leica M205 C stereo microscope for the presence of microplastics (see microplastic identification). From the remaining material, the solution was homogenised and the entire samples or replicate aliquots of 5 ml were analysed by counting at least 1000 individual zooplankton. The number of individuals found in the aliquots was multiplied for the total zooplankton volume (during this analysis the possible presence of sunk microplastic particles was investigated). A potential source of underestimation in this method is the sinking of bio-fouled or denser particles, thus excluding them from the supernatant. Any plastic found within the zooplankton replicate aliquots were added to the initial count. It is noted that this problem would only be relevant in samples which contained a large quantity of zooplankton and methods previously developed47 could be used to reduce underestimation of microplastic particles. To calculate the zooplankton abundance (individuals per m3), the zooplankton in the total volume collected was divided by the volume filtered using the estimation of the flow meter (excluding stations 20 and 22 where we used the volume calculated by distance travelled, due to error with the flow meter).
Sub-surface sample collection
Seawater was collected from a continuous intake located on the drop keel in the centre of the vessel at a depth of 6 m. Seawater was pumped aboard using an IWAKI Magnetic Drive Pump (MDM25 160 ECFF 0221-E2) (Japan), 2.2 kW power (2 Bar vacuum) and particles were collected using previously published methodology26. Seawater was passed under pressure through stainless steel pipes to the deck of the vessel. Samples were collected using a hose connected directly to the seawater system. A marine grade stainless steel sieve (250 μm), in a simple covered sampling stage, filtered suspended particulate matter from a known volume of water. Calculation of the flow rate made it possible to determine the period of time required to filter a 2,000 litre volume of water (2hr). After the required elapsed time to filter 2,000 litres, any items retained on the sieve were re-suspended using filtered water and subsequently filtered under vacuum onto GF/C (47 mm diameter) filter paper. Filter papers were folded, placed in Eppendorf tubes, labelled and stored at – 20 °C. On return to the laboratory, filter papers were analysed under a stereo microscope and particles were counted and categorised following (26, see below microplastic identification). Environmental data (temperature, salinity, wind speed, wind direction, boat speed) were collected from the vessels underway systems.
Contamination control
To mitigate sample contamination all glass vessels were acid washed and rinsed with pre-filtered sea water before and after use. All consumables were taken directly from packaging, and considered sterile. Samples and equipment were covered where possible to minimise periods of exposure, and rinsed with filtered water. Filters were analysed microscopically for fibres and evidence of microplastic contamination before use. Procedural blanks (absent of biological material or microplastics) were run in parallel with samples containing desiccated material, solutions or trawled material. Procedural blanks were analysed in the same way as other samples for microplastics using a stereomicroscope after filtering under pressure onto GF/C paper. Personal protective equipment, lab coats and gloves were worn at all times.
Microplastic identification
Filter papers were visually examined under a dissecting microscope fitted with a polariser (Olympus SZX10 with a mounted Q-imaging Retiga 2000R camera and Leica M205 C), and photographs of all potential microplastics were recorded. Particles were assigned to product type categories: fibres, fragments and films (Fig. 1d,e,f). Potential microplastics were confirmed and accepted based on features such as colour: homogenously coloured, shininess or unnatural colours, if they had unnatural forms (no cellular or organic structures visible), fibres were equally thick throughout their length and had three dimensional bending. Many fibres were not uniform in thickness or colour and subsequently rejected. A subsample of microplastics (n = 30) was selected to represent the diversity of particle types found, and subjected to Fourier Transform Infrared spectroscopy (FT-IR) analysis (Perkin Elmer Spectrum Two). This confirmed the presence of cellulosic fibres (matt, non-uniform fibres) and synthetic fibres (shiny, uniform fibres). Any fibres suspected of being of a cellulosic or semi-synthetic origin were rejected from the analysis.
Statistical Analysis
All statistical analysis was carried out in R. Data were tested for normality and homogeneity of variance. Environmental variables were averaged for the entire sample duration of each sub-surface sample (2 hr duration). Sea state could not be reliably integrated over the two hour sample period therefore wind speed which was directly recorded from the ships instruments was used. Pairwise scatter plots were used to highlight potential explanatory variables and interactions between variables. To understand the influence of environmental factors on the number of particles per sample, an integrated Generalised Linear Model (GLM) was developed for both the surface samples and the sub-surface samples. Using a stepwise process and AIC scores to include only the main effects, the GLM was analysed using an Analysis of Variance to determine which explanatory variables (Table 3) were significant predictors of the number of microplastics per m3. Non-significant variables were eliminated until the final model obtained (Table 1). Zooplankton data were unavailable for the sub-surface samples. Pearson’s correlation was carried out to look for a relationship between the microplastic and zooplankton abundance in manta net samples. Sea surface temperature source satellite data downloaded from: JPL OurOcean Project. 2010. GHRSST Level 4 G1SST Global Foundation Sea Surface Temperature Analysis. Ver. 1. PO.DAAC, CA, USA. Dataset accessed [2015-08-03] at http://dx.doi.org/10.5067/GHG1S-4FP01.
Table 3. List of variables assessed in the derivation of the best fitting Generalised Linear Model (GLM).
| Variables | Abbreviation | Descriptor | |
|---|---|---|---|
| Dependant variable | count | count | number of particles per sample |
| Environmental data | sea surface temperature | SST | in °C |
| sea surface salinity | SSS | in % | |
| wind speed | WS | in metres per second | |
| wind direction | WD | in degrees | |
| distance to shore | DS | in km | |
| Vessel information | boat speed | BS | in knots |
| Sample information | volume filtered | VOL | in litres |
| sample length | SL | in km | |
| Sample method | method | method | 1. underway system |
| 2. manta net |
Additional Information
How to cite this article: Lusher, A. L. et al. Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci. Rep. 5, 14947; doi: 10.1038/srep14947 (2015).
Acknowledgments
Amy Lusher was supported by an Irish Research Council Postgraduate Scholarship [Project ID: GOIPG/2013/284] and a GMIT 40th Anniversary Studentship. Data collection was supported by EUROFLEETS2 polar call (POLAR PLASTICS and PREPARED projects). The authors thank the crew of the R.V. G. O. Sars for their assistance and Heidi Acampora for helping with the sample collection during G. O. Sars Cruise No. 191.
Footnotes
Author Contributions A.L., I.O’C. and R.O. conceived and designed the sampling protocol. A.L. and V.T. conducted the experimental work and analysed the data. A.L. and V.T. wrote the paper. I.O’C. and R.O. reviewed the manuscript.
References
- Rochman C. M. et al. Policy: Classify plastic waste as hazardous. Nature 494(7436), 169–171 (2013). [DOI] [PubMed] [Google Scholar]
- PlasticsEurope. Plastics – the Facts 2014/2015. Available at: http://www.plasticseurope.org/ (Accessed 1st May 2015).
- Barnes D. K., Galgani F., Thompson R. C., & Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos. T. Roy. Soc. B. 364(1526), 1985–1998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrady A. L. Plastics and the environment (ed. Andrady A). (John Wiley & Sons, 2003). [Google Scholar]
- Arthur C., Baker J. & Bamford H. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, NOAA Technical Memorandum NOS-OR & R-30.NOAA, Silver Spring, September 9–11, 2008, 530 (2009).
- Browne M. A. in Marine Anthropogenic Litter (eds Bergmann M., Gutow L. & Klages M.). Ch. 9, 229–244 (Springer, 2015). [Google Scholar]
- Lusher A. L. in Marine Anthropogenic Litter (eds Bergmann M., Gutow L. & Klages M.). Ch. 10, 245–308 (Springer, 2015). [Google Scholar]
- Cózar A. et al. Plastic debris in the open ocean. P. Natl. Acad. Sci. USA. 111(28), 10239–10244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall L. C. et al. (2014). The deep sea is a major sink for microplastic debris. Roy. Soc. Open. Sci. 1(4), 140317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. et al. Microplastics in freshwater ecosystems: what we know and what we need to know. Enviro. Sci. Europe. 26(12), 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbard R. W. et al. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future, 2(6), 315–320 (2014). [Google Scholar]
- Van Sebille E., England M. H. & Froyland G. Origin, dynamics and evolution of ocean garbage patches from observed surface drifters. Environ. Res. Lett. 7(4), 044040 (2012). [Google Scholar]
- Zarfl C. & Matthies M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 60(10), 1810–1814 (2010). [DOI] [PubMed] [Google Scholar]
- Gradinger R. et al. in: Life in the world’s oceans. (ed McIntyre A.). Ch. 10, 183–202 (Blackwell Publishing, 2010). [Google Scholar]
- Byrne S. C. Persistent Organic Pollutants in the Arctic: A Report for the Delegates of the 4th Conference of the Parties Stockholm Convention on Persistent Organic Pollutants (2009) Available at: http://www.akaction.org/Publications/Stockholm_Convention_PDFs/POPs_in_the_Arctic_ACAT_May_2009.pdf (Accessed: 1st July 2015).
- Trevail A. M., Gabrielsen G. W., Kühn S. & Van Franeker J. A. Elevated levels of ingested plastic in a high Arctic seabird, the northern fulmar (Fulmarus glacialis). Polar Biol. 1–7 (2015). [Google Scholar]
- Cole M. et al. Microplastic ingestion by zooplankton. Envir. Sci. Technol. 47(12), 6646–6655 (2013). [DOI] [PubMed] [Google Scholar]
- Murray F. & Cowie P. R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 62(6), 1207–1217 (2011). [DOI] [PubMed] [Google Scholar]
- Lusher A. L., McHugh M. & Thompson R. C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 67(1), 94–99 (2013). [DOI] [PubMed] [Google Scholar]
- Lusher A. L. et al. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 199, 185–191 (2015). [DOI] [PubMed] [Google Scholar]
- Coachman L. K.& Aagaard K. in Marine Geology and Oceanography of the Arctic Seas (ed. Herman Y). Ch. 1, 1–72 (Springer Berlin Heidelberg, 1974). [Google Scholar]
- Cottier F. R. & Venables E. J. On the double-diffusive and cabbeling environment of the Arctic Front, West Spitsbergen. Polar Res. 26(2), 152–159 (2007). [Google Scholar]
- Nilsen F., Cottier F., Skogseth R. & Mattsson S. Fjord–shelf exchanges controlled by ice and brine production: the interannual variation of Atlantic Water in Isfjorden, Svalbard. Cont. Shelf Res. 28(14), 1838–1853 (2008). [Google Scholar]
- McBride M. M. et al. Krill, climate, and contrasting future scenarios for Arctic and Antarctic fisheries. ICES J. Mar. Sci. fsu002, 1–22 (2014). [Google Scholar]
- Christiansen J. S., Mecklenburg C. W. & Karamushko O. V. Arctic marine fishes and their fisheries in light of global change. Glob. Change Bio. 20(2), 352–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusher A. L., Burke A., O’Connor I. & Officer R. Microplastic pollution in the Northeast Atlantic Ocean: validated and opportunistic sampling. Mar. Pollut. Bull. 88(1–2), 325–333 (2014). [DOI] [PubMed] [Google Scholar]
- Law K. L. et al. Plastic accumulation in the North Atlantic subtropical gyre. Science, 329(5996), 1185–1188 (2010). [DOI] [PubMed] [Google Scholar]
- Goldstein M. C., Titmus A. J. & Ford M. Scales of spatial heterogeneity of plastic marine debris in the northeast Pacific Ocean. Plos One 8(11), e80020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M. et al. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 68, 71–76 (2013). [DOI] [PubMed] [Google Scholar]
- Reisser J et al. The vertical distribution of buoyant plastics at sea. Biogeosciences Discuss. 11(11), 16207–16226 (2014). [Google Scholar]
- Lucchi R. G. et al. PREPARED present and past flow regime on contourite drifts west of Spitsbergen. EUROFLEETS-2 Cruise Summary Report, R/V G. O. Sars, Cruise No. 191. Available at: 10.13140/2.1.1975.376. (Accessed: 1st May 2015). [DOI]
- Sea Ice Prediction Network. Arctic Sea Ice Outlook 2014: Post season report. Available at: http://www.arcus.org/sipn/sea-ice-outlook/2014/post-season. (Accessed 1st May 2015).
- Strand J. et al. Marine litter in Nordic waters. Technical report. (2015) Available at: http://norden.diva-portal.org/smash/get/diva2:824655/FULLTEXT01.pdf (Accessed: 1st July 2015).
- Norwegian Environmental Agency. Sources of Microplastic-Pollution to the Marine Environment. (2014) Available at: http://www.miljodirektoratet.no/Documents/publikasjoner/M321/M321.pdf (Accessed: 1st July 2015).
- Hidalgo-Ruz V., Gutow L., Thompson R. C. & Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Envir. Sci. Tech. 46(6), 3060–3075 (2012). [DOI] [PubMed] [Google Scholar]
- Kukulka T., Proskurowski G., Morét-Ferguson S., Meyer D. W. & Law K. L. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 39, L07601 (2012). [Google Scholar]
- Collignon A. et al. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 64, 861–864 (2012). [DOI] [PubMed] [Google Scholar]
- Lattin G. L., Moore C. J., Zellers A. F., Moore S. L. & Weisberg S. B. A comparison of neustonic plastic and zooplankton at different depths near the southern Californian shore. Mar. Pollut. Bull. 49, 291–294 (2004). [DOI] [PubMed] [Google Scholar]
- Mitra A. et al. Bridging the gap between marine biogeochemical and fisheries sciences; configuring the zooplankton link. Prog. Oceanogr. 129, 176–199 (2014). [Google Scholar]
- Mauchline J. The biology of calanoid copepods in Advances in Marine Biology, Vol. 33 (eds Blaxter J. H. S., Southward A. J. & Tyler P. A.) (Academic Press, S. Diego, 1998). [Google Scholar]
- Paffenhöfer G. A. & Van Sant K. B. The feeding response of a marine planktonic copepod to quantity and quality of particles. Mar. Ecol. Prog. Ser. 27, 55–65 (1985). [Google Scholar]
- Lee K.-W., Shim W. J., Kwon O. Y. & Kang J.-H. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ. Sci. Technol. 47(19), 11278–11283 (2013). [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Filemn E., Halsband C. & Galloway T. S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 49, 1130–1137 (2015). [DOI] [PubMed] [Google Scholar]
- Eriksson C. & Burton H. Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. Ambio 32, 380–384 (2003). [DOI] [PubMed] [Google Scholar]
- Ryan C. et al. Prey preferences of sympatric fin (Balaenoptera physalus) and humpback (Megaptera novaeangliae) whales revealed by stable isotope mixing models. Mar. Mammal Sci. 30(1), 242–258 (2014). [Google Scholar]
- Gardiner K. & Dick T. A. Arctic cephalopod distributions and their associated predators. Polar Res. 29(2), 209–227 (2010). [Google Scholar]
- Cole M., Webb H., Lindeque P. K., Fileman E. S., Halsband C. & Galloway T. S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 4, 4528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. J., Watson W., Bowlin N. M. & Sheavly S. B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 71(1), 41–52 (2011). [DOI] [PubMed] [Google Scholar]
- Goldstein M. C., Rosenberg M. & Cheng L. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biology Lett. 8(5), 817–820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan L. R., Ohman M. D., Doyle M. J. & Watson W. Occurrence of plastic micro-debris in the southern California Current system. Cal. Coop. Ocean. Fish. 50, 123–133 (2009). [Google Scholar]
- Thompson R. C. et al. Lost at sea: where is all plastic? Science, 304, 838 (2004). [DOI] [PubMed] [Google Scholar]
- Frias J. P. G. L., Otero V. & Sobral P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 95, 89–95 (2014). [DOI] [PubMed] [Google Scholar]
- Ivar do Sul J. A., Costa M. F., Barletta M. & Cysneiros F. J. A. Pelagic microplastics around an archipelago of the Equatorial Atlantic. Mar. Pollut. Bull. 75(1), 305–309 (2013). [DOI] [PubMed] [Google Scholar]
- Ivar do Sul J. A., Costa M. F. & Fillmann G. Microplastics in the pelagic environment around oceanic islands of the Western Tropical Atlantic Ocean, Water. Air. Soil. Poll. 225(7), 1–13 (2014). [Google Scholar]
- Cózar A. et al. Plastic accumulation in the Mediterranean Sea. Plos One 10(4), e0121762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia G. A. et al. Amount and distribution of neustonic micro-plastic off the western Sardinian coast (Central-Western Mediterranean Sea). Mar. Environ. Res. 100, 10–16 (2014). [DOI] [PubMed] [Google Scholar]