Abstract
Tumor neo-angiogenesis is regulated, in part, by the hypoxia-inducible gene HIF1. Evidence suggests HIF1 associates with polymerized microtubules and traffics to the nucleus. This study investigated the role of HIF1 in mediating the antitumor activity of two steroid-based sulfamate ester microtubule disruptors, STX140 and STX243, in vitro and in vivo. The effects of STX140, STX243 and the parental compound 2-methoxyestradiol (STX66) on HIF1α and HIF2α protein expression were assessed in vitro in MCF-7 and MDA-MB-231 cells cultured under hypoxia. More pertinently, their effects were examined on HIF1-regulated genes in vivo in mice bearing MCF-7 or MDA-MB-231 tumors. The level of mRNA expression of Vascular Endothelial Growth Factor (VEGF), Glucose Transporter 1 (GLUTI), Phosphoglycerate Kinase (PGK), ATP-binding cassette sub-family B member 1 (ABCB1) and Carbonic Anhydrase IX (CAIX) was quantified by Real-time Polymerase Chain Reaction (RT-PCR). Despite inhibiting nuclear HIF1α protein accumulation under hypoxia in vitro, STX140 and STX243 did not significantly regulate the expression of four out of five HIF1α-regulated genes in vitro and in vivo. Only CAIX mRNA expression was down-regulated both in vitro and in vivo. Immunoblot analysis showed that STX140 and STX243 reduced CAIX protein expression in vitro. These compounds had no effect on HIF2α translocation. The potential for inhibition of CAIX by STX140 and STX243 was examined by docking the ligands to the active site in comparison with a known sulfamate-based inhibitor. Microtubule disruption and antitumor activity of STX140 and STX243 is most likely HIF1-independent and may, at least in part, be mediated by inhibition of CAIX expression and activity.
Keywords: HIF1A, CAIX, VEGF, MCF-7 cells, MDA-MB-231 cells, xenografts, microtubule disruptor, anti-angiogenic
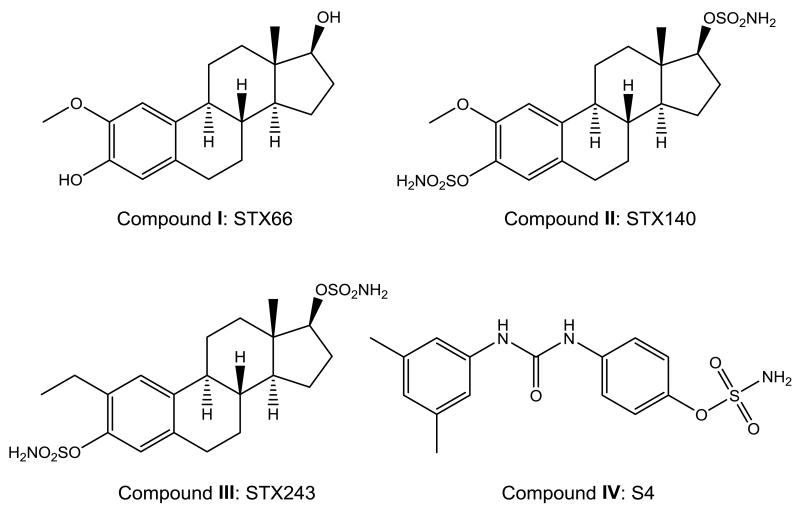
Angiogenesis, the creation of new blood vessels, is induced in tumors by secretion of growth factors, such as vascular endothelial growth factor (VEGF). Much research into targeting angiogenesis has been undertaken to discover new therapeutic agents for cancer therapy. 2-Methoxyestradiol (STX66; Figure 1, compound I) has shown promise in vitro by being an anti-angiogenic and antiproliferative agent (1, 2) and has progressed to numerous clinical trials (3). However, 2-methoxyestradiol has poor in vivo efficacy because of poor bioavailability (3, 4).
Figure 1.
Structures of study compounds. I: 2 Methoxyestradiol, STX66; II: 2-thoxyestradiol-3,17-O,O-bis-sulfamate, STX140; III: 2-Methylestradiol-3,17-O,O-bis-sulfamate, STX243; IV: S4.
2-Methoxyestradiol-3,17-O,O-bis-sulfamate (STX140; Figure 1, compound II) and 2-ethylestradiol-3,17-O,O-bis-sulfamate (STX243; Figure 1, compound III) are a new class of steroid A-ring-modified anticancer compound (5). They compete with colchicine for tubulin binding and disrupt interphase microtubules, leading to cell-cycle arrest and apoptosis in vitro and in vivo (6-9). They have excellent oral bioavailability in vivo (10) and inhibit angiogenesis in vitro and in vivo (10, 11). STX140 and STX243 can cause endothelial cell apoptosis in vitro (7, 8, 11, 12), but this may not fully explain their anti-angiogenic activity. The hypothesis that the anti-angiogenic activities of microtubule disruptors are mediated by inhibition of hypoxia inducible factor 1 (HIF1) was proposed by Mabjeesh et al. (13).
HIF1 is a heterodimer consisting of a constitutively expressed β subunit and an α subunit, the expression of which is tightly regulated by intracellular oxygen concentration. Under normoxia, HIF1α is rapidly and continuously degraded by ubiquitination and proteosomal degradation (14, 15). Under hypoxic conditions, HIF1α accumulates and is translocated to the nucleus, where it forms an active complex with HIF1β and activates transcription of about 60 target genes (16). Hypoxia stimulates the transcription of many genes such as those for glucose transporters (e.g. GLUT1), growth factors (e.g. VEGF), survival proteins and enzymes [e.g. carbonic anhydrase IX (CAIX), phosphoglycerate kinase (PGK)] and ATP binding cassette, subfamily B, member 1 (ABCB1; multidrug-resistant protein) (17-20). HIF1α activity is also controlled in an oxygen-independent manner through the regulation of two main signaling pathways: the phosphatidylinositol 3-kinase (PI-3K) and mitogen-activated protein kinase (MAPK) pathways (16), which can also regulate the activity of the CAIX enzyme (21). HIF1 is increased in human cancer as a result of the physiological prevention of degradation of the HIF1α subunit in response to intratumoral hypoxia and because of genetic alterations that activate oncogenes and inactivate tumor suppressor genes, making HIF1α an attractive target for cancer therapies (22).
2-Methoxyestradiol, like other microtubule-disrupting agents, interferes with the crosstalk between cancer cells and endothelial cells, which contributes to the antitumor and anti-angiogenic activity of this compound (13). It has also been shown that 2-methoxyestradiol inhibits nuclear accumulation of HIF1α, which leads to the inhibition of HIF1 transcriptional activity and to the subsequent down-regulation of VEGF expression. This suggests that microtubule disruption may be required for HIF1α inhibition (13). However, Mabjeesh et al. failed to demonstrate this in an in vivo tumor model because of the technical challenge of staining for HIF1α in sections from xenografts. Escuin et al. hypothesized that all microtubule disruptors may inhibit angiogenesis via HIF1, by inhibiting the synthesis and the nuclear accumulation of HIF1α (23). Intriguingly, microtubule disruption can significantly down-regulate nuclear translocation of HIF1α protein and this therefore can directly affect HIF1 transcriptional activity (24, 25)
To date, however, none of the results obtained from in vitro studies has been confirmed in in vivo xenograft tumor models and, furthermore, the effects of microtubule disruptors on HIF1-regulated genes have not been studied. It is also not clear as to whether HIF1 inhibition can occur at therapeutically relevant concentrations of these agents (26).
More recently, HIF2α, the other oxygen-labile α subunit that can form a HIF1 complex with the stable β subunit, has been demonstrated to promote cell-cycle progression and increase target gene transcription in hypoxic renal clear carcinoma cells (27, 28). The actions of HIF1α and HIF2α are distinct and differ according to cell type. Although HIF2α involvement in the survival of hypoxic breast cancer cell lines remains controversial (29, 30), the expression of both α subunits seems essential for the growth of most tumors. Consequently, it is important to investigate whether microtubule disruptors inhibit HIF1 activity by reducing nuclear accumulation of HIF2α.
In this study, we investigated the effects of two new microtubule disruptors, STX140 and STX243, on HIF1α and HIF2α expression and translocation into the nucleus and, more pertinently, on HIF1-regulated genes in MCF-7 and MDA-MB-231 cells. In addition, in vivo tumor models were used to explore the mechanistic role of HIF1 in mediating the anti-angiogenic activities of these compounds. Computational modeling was used to explore docking of ligands to CAIX in comparison with those of known activity.
Materials and Methods
Drug synthesis
STX66 was synthesized as previously described (31). STX140 and STX243 were synthesized by reaction of the parent 2-methoxyestradiol and 2-ethylestradiol respectively with sulfamoyl chloride in dimethyl acetamide solution (5, 32). The compounds were tested alongside the known commercially available tubulin agent, paclitaxel (Sigma, Poole, UK).
Molecular modeling
Models of STX140, STX243, the known CAIX inhibitor S4 [Figure 1, compound IV, (33)] and acetazolamide were built and minimized in Discovery Studio (BIOVIA, San Diego, CA, USA). These compounds were docked into the ‘A’ chain of the 3IAI crystal structure of the CAIX catalytic domain (34). This structure has an acetazolamide molecule, a glycerol molecule, and several water molecules in the substrate-binding site. For the dockings, acetazolamide and glycerol were removed, with the substrate-binding site being defined as a sphere of 5Å radius centered on the centroid of the acetazolamide molecule: the centroid of the docked compounds was required to lie within this sphere. Each compound was docked 25 times using GOLD (35). The position of the docked compounds were assessed by visual inspection and the docking scores recorded.
Cell culture and cell treatment
MCF-7 estrogen receptor-positive (ER+ve) human breast cancer cells, MDA-MB-231 ER-negative (ER−ve) human breast cancer cells were obtained from the American Type Culture Collection (LGC Promochem, Teddington, UK). Cells were routinely cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal calf serum, 1% L-glutamine (200 mM), 1% non-essential amino acids (100 ×) and 1% bicarbonate (7.5%) from Sigma and maintained in a humidified incubator under 5% CO2 atmosphere at 37°C. For experiments using hypoxic conditions, cells were incubated under 1% O2 and 5% CO2 atmosphere at 37°C.
Xenograft models
Athymic, female MF1 nude mice (nu−/−) were purchased from Harlan (Bicester, Oxfordshire, UK) at 5 weeks of age (~20-25 g in weight). A total of 5×106 MCF-7 or 2×106 MDA-MB-231 cells were inoculated subcutaneously into the flanks of the animals. When tumors reached 100 to 150 mm3, mice were randomly divided into four treatment groups of five mice: vehicle (10% tetrahydrofuran: 90% propylene glycol), STX66 (40 or 75 mg/kg), STX140 (20 mg/kg) or STX243 (40 mg/kg). All compounds were given orally daily for 28 days for mice bearing MCF-7 xenografts and daily over 21 days for mice bearing MDA-MB-231 xenografts. A separate group of mice with MCF-7 xenografts was also treated intravenously twice weekly with paclitaxel at 15 mg/kg. Throughout the study, mice were weighed and tumor measurements were taken weekly. Tumor volumes were calculated using the formula (length × width2/2). At the conclusion of dosing (day 28 for mice with MCF-7 xenografts or day 21 for mice with MDA-MB-231 xenografts), the animals were euthanized and tumors removed for mRNA isolation. All experiments were carried out under conditions that complied with UK Home Office Animals (Scientific Procedures) Act 1986 and local institutional ethical guidelines. For ethical reasons, animals were removed from the study if the mean tumor diameter exceeded 1.5 cm.
Immunoblotting
MCF-7 and MDA-MB-231 were seeded at ~2.5×105 per T-25 flask in 5 ml medium and incubated at 37°C, with 5% CO2 in a humidified incubator. After 24 h either STX140 (0.5 μM), STX243 (0.5 μM) or STX66 (0.5 μM) were added to the cells which were incubated for a further 18 h under normoxia then for 6 h under hypoxia (atmosphere of 1% O2 and 5% CO2). Protein was prepared from treated cells using radioimmunoprecipitation assay lysis buffer (Sigma) and a Bradford assay was undertaken to determine protein concentrations.
To prepare nuclear extracts cells were seeded at ~ 1×106 per T-75 flask in 18 ml of medium and incubated at 37°C with 5% CO2 in a humidified incubator. After 24 h, the compounds were added and the cells were incubated for a further 18 h under normoxia then for 6 h under hypoxia (1% O2 and 5% CO2 atmosphere). At the end of treatment, the medium was removed and the cells were washed with 10 ml ice-cold PBS, scraped off and centrifuged for 5 min at 600×g at 4°C. The pellets were resuspended in 500 μl ice-cold lysis buffer (20 mM Tris pH 8, 20 mM NaCl, 0.5% NP40, 1 mM Dithiothreitol and 1 μl/ml protease inhibitor cocktail from Sigma) and left for 5 min on ice to lyse the cells. The supernatant, referred to as the cytoplasmic fraction, and the nuclear pellets were obtained by centrifugation at 600×g 1 min at 4°C. The nuclear pellets were then lysed with 100-200 μl of ice-cold buffer C (20 mM Hepes pH 8, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 2 mM Dithiothreitol and 1 μl/ml protease inhibitor cocktail from Sigma). The proteins were isolated from the nuclei by centrifugation at 16000×g for 10 min at 4°C. Supernatants containing the cytoplasmic fraction or nuclear proteins were frozen at −80°C until used and the protein concentrations were determined by a Bradford assay with the Bradford reagent (Sigma).
Following determination of protein concentrations, equal amounts of either nuclear or whole cell protein extracts (50 μg) were loaded into all wells and separated by electrophoresis through a 412% NuPAGE Bis-Tris gel (Invitrogen Ltd., Paisley, UK) and transferred to Hybond-P membrane (GE Healthcare, Bucks, UK). To further ensure equal protein loading and successful transfer, the membranes were stained and visualized with Ponceau S (Sigma) before proceeding with immunodetection. Membranes with uneven transfer of proteins were rejected.
Detection was carried out using primary antibodies to human HIF1α (ref. 610959; BD Biosciences, Erembodegen, Belgium), PI-3K (ref. sc1637; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or HIF2α (ref. NB100122SS; Novus Biological, Littleton, CO, USA). Alkaline phosphatase-conjugated anti-rabbit IgG (ref. 7054; Cell Signaling Technology, Boston, MA, USA) or anti-mouse (ref 7056; Cell Signaling) secondary antibodies were used for detection. Digital images and band intensities were quantified using Kodak 1D software, version 3.5 (Eastman Kodak Company, Rochester, New York, USA). The results shown are representative of three separate experiments.
Real-time Polymerase Chain Reaction (RT-PCR)
For tumor samples, 20 to 40 mg of tissue were excised from tumor xenografts and transferred to 2 ml of RNAlater solution (Ambion, Inc., Austin, TX, USA). The tissue sample was transferred to 600 μl of RNeasy lysis buffer (Qiagen, Ulm, Germany) plus 1% β-mercaptoethanol (Promega Ltd., Southampton, UK) and homogenized. The homogenate was centrifuged (3 min at 10 000×g) and the RNA was isolated from the supernatant using the RNeasy kit (Qiagen) and stored at −80°C. For the in vitro samples, cells were lysed with 350 μl of RNeasy lysis buffer (Qiagen) plus 1% β-mercaptoethanol (Promega) and homogenized with the QIAshredder columns (Qiagen). The RNA was isolated from the homogenate using the RNeasy kit (Qiagen) and stored at −80°C.
A 5 μg aliquot of each RNA sample was reverse transcribed in a final volume of 33 μl to generate a cDNA using the First-Strand cDNA Synthesis kit (GE Healthcare) and stored at −20°C. Reverse-transcription PCRs were performed in a Rotor Gene 2000 Real-time Cycler (Corbett Research, Cambridge, UK) with 2 μl cDNA in a final volume of 25 μl using Taqman Universal PCR Master Mix (Applied Biosystems, CA, USA). Primers and hydrolysis probes for VEGF165 (isoform A, Ref. Hs00900057_m1), GLUT1 (Ref. Hs00197884_m1), phospho-glycerate kinase (PGK; Ref. Hs99999906_m1), CAIX (Ref. Hs00154208_m1) and ABCB1 (Ref. Hs00184491_m1) genes and for the internal control human large ribosomal protein (RPLO; Ref. 4310879E) were synthesized by Applied Biosystems. Human-specific primers were used because human cancer cell lines were inoculated into the flanks of the mice to generate the xenografts. The conditions used were: 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 60°C for 45 s and 72°C for 45 s. The relative Ct values were calculated using the software rotorgene 6 (Corbett Research, Cambridge, UK) and the comparative Cts were calculated as:
Statistics
In vitro experiments were carried out in triplicate and data presented are representative of three or more different experiments. All data shown are the mean ± SEM. Student’s t-test was used to assess significance.
Results
HIF1α immunoblotting
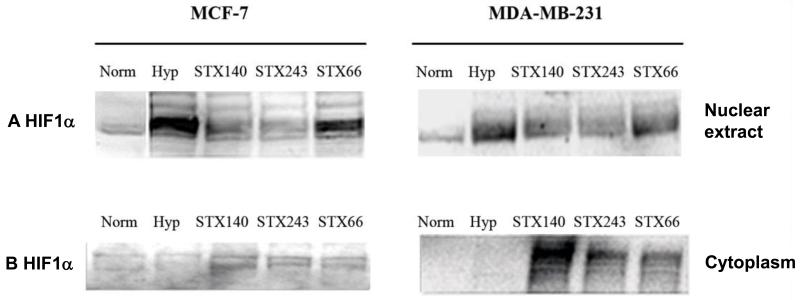
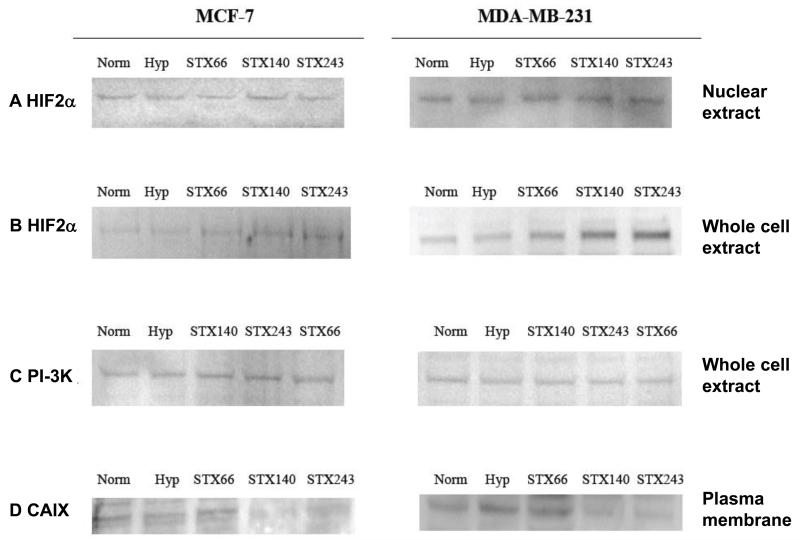
To investigate the role of HIF1α in mediating the anti-angiogenic activities of STX140 and STX243, HIF1A expression was initially examined in vitro. ER+ve MCF-7 and ER−ve MDA-MB-231 breast cancer cells were treated with STX140, STX243 or STX66 under normoxia for 18 h and then under hypoxia for 6 h, according to the protocol described by Escuin et al. (23). All the compounds were initially used at 0.5 μM to compare their efficacy. Nuclear extracts and cytoplasmic fractions were prepared and analyzed by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and immunoblotting with antibody to HIF1α. An increase of HIF1A expression was observed in the nuclei of the cells cultured under hypoxia (2.4-fold increase in MCF-7 cells and 1.37-fold increase in MDA-MB-231 cells, p<0.05, Figure 2A) compared to the HIF1α expression in nuclei of cells cultured under normoxia (Figure 2A). This indicates that 6 h hypoxia is enough to induce HIF1α expression. When both cell lines were treated with STX140 or STX243 (Figure 2A), nuclear HIF1α expression was lower than that in untreated cells cultured under hypoxia (p<0.05) and the steady-state level of HIF1α protein was similar to that in cells cultured under normoxia. In addition, no down-regulation of HIF1α protein expression was seen for both cell lines when treated with 0.5 μM STX66 (Figure 2A). These results show that STX140 and STX243 block nuclear accumulation of HIF1α in ER+ve and ER−ve breast cancer cells. Immunoblotting assays of the cytoplasmic fractions showed that HIF1α protein was not expressed or only very weakly expressed in the cytoplasm of both cell types under hypoxia and normoxia (Figure 2B). HIF1α was detected at 120 kDa in the cytoplasm of cells treated with STX140, STX243 or STX66 (Figure 2B).
Figure 2.
Hypoxia-inducible Factor 1α (HIF1α) protein expression in MCF-7 and MDA-MB-231 cells. MCF-7 and MDA-MB-231 cells were treated with 0.5 μM STX140, STX243 or STX66 under normoxia for 18 h and then under hypoxia (1% O2, 5% CO2) for 6 h. Equal amount of each nuclear extract (A) or cytoplasmic fraction (B) was resolved by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and blotted for HIF1α. The immunoblot shown is representative of three separate experiments and was quantified using Kodak 1D software. A: MCF-7 nuclear extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (241%); 3: STX140 (162%*); 4: STX243 (105%*); 5: STX66 (216%). MDA-MB-231 nuclear extracts, lane 1: control normoxia (100% intensity); 2: control, hypoxia (147%); 3: STX140 (128%*); 4: STX243 (123%*); 5: STX66 (144%). B: MCF-7 cytoplasmic extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (75%); 3: STX140 (146%*); 4: STX243 (134%*); 5: STX66 (122%*). MDA-MB-231 cytoplasmic extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (67%); 3: STX140 (259%*); 4: STX243 (173%*); 5: STX66 (162%*). *p<0.05, compared to control.
In vitro gene expression
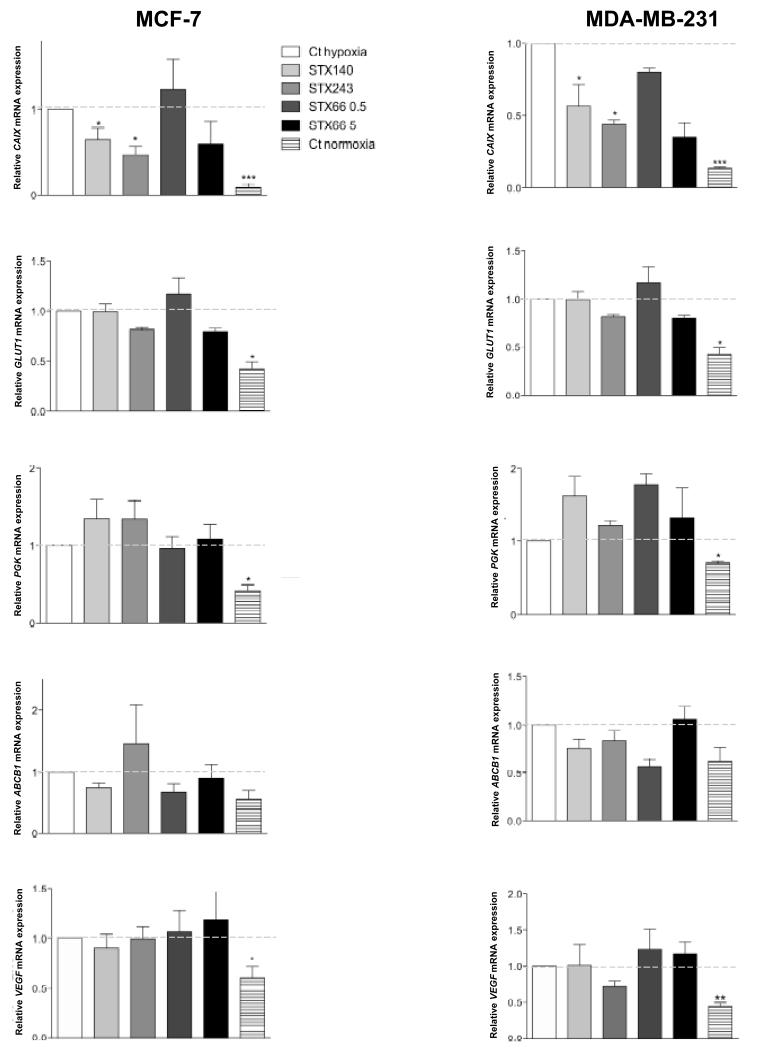
To examine the effects of STX140, STX243 and STX66 on HIF1 activity, the transcription of five HIF1-regulated genes was studied in MCF-7 and MDA-MB-231 cells treated with STX140, STX243 at 0.5 μM or STX66 at 0.5 μM and 5 μM under normoxia for 18 h and then under hypoxia for 6 h. Total RNA was extracted and reverse transcribed. The expression of CAIX, ABCB1, VEGF165, PGK and GLUT1 mRNA was quantified by RT-PCR (Figure 3). Although CAIX, GLUT1, PGK and VEGF165 were overexpressed under hypoxia compared to their expression level in cells cultured under normoxia, showing that 6 h hypoxia is enough to enhance their expression, only CAIX mRNA expression was significantly down-regulated by treatment with STX140 or STX243 in both cell lines (Figure 3). The mRNA expression of the other genes studied was neither significantly down-regulated nor up-regulated by treatment with the compounds tested and ABCB1 expression was not significantly induced by 6 h hypoxia in either cell line.
Figure 3.
Vascular Endothelial Growth Factor (VEGF)165, ATP-binding cassette sub-family B member 1 (ABCB1), Glucose Transporter 1 (GLUT1), Phosphoglycerate Kinase (PGK1) and Carbonic Anhydrase IX (CAIX) mRNA expression in MCF-7 and MDA-MB-231 cells. MCF-7 cells and MDA-MB-231 cells were seeded in T25 flasks, and treated with 0.5 μM STX140, STX243 or STX66, or with 5 μM STX66 under normoxia for 18 h and under hypoxia (1% O2, 5% CO2) for 6 h. Total mRNA was then prepared, reverse-transcribed and the relative quantity of VEGF165, ABCB1, GLUT1, PGK1 and CAIX were determined by RT-PCR. mRNA expression of untreated cells after 6 h hypoxia of each experiment was given the value 1. *p<0.05, **p<0.01, ***p<0.001, compared to control (Ct).
In vivo gene expressions
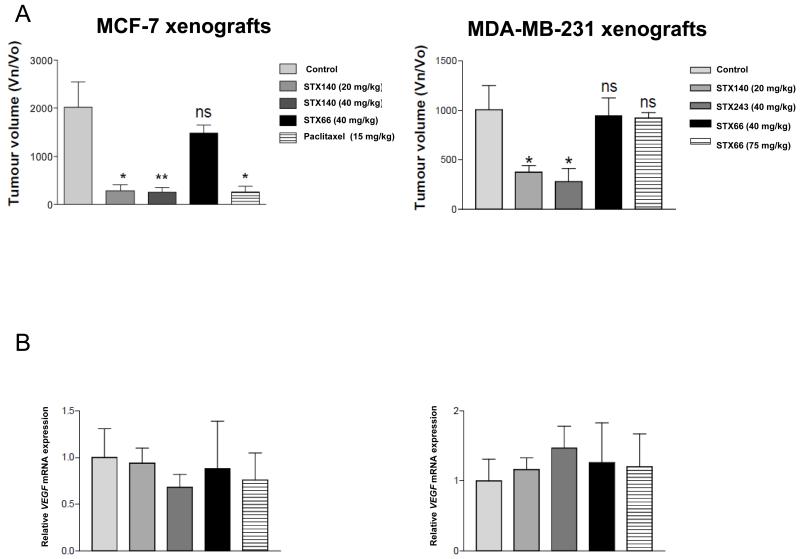
To analyze whether STX140, STX243 and STX66 had an effect on HIF1-regulated genes in vivo, the expression of CAIX, ABCB1, GLUT1, PGK and VEGF was analyzed from tissue taken from the MCF-7 and MDA-MB-231 xenograft models. STX140 at 20 mg/kg (p.o. daily) and STX243 at 40 mg/kg (p.o. daily) significantly inhibited both MCF-7 and MDA-MB-231 xenograft growth (p<0.05; Figure 4A). In contrast, STX66 at 40 mg/kg against MDA-MB-231 xenografts or 75 mg/kg (p.o. daily) had no effect on tumor growth in both models. Paclitaxel was efficacious at 15 mg/kg (i.v. twice weekly) when tested in the MCF-7 model (Figure 4A). The mRNA level for VEGF165 was not affected by any treatment in either xenograft model and the transcription level was similar to that in xenografts from untreated mice (Figure 4B). The transcription levels of the HIF1-regulated genes CAIX, ABCB1, GLUT1 and PGK were also quantified for each sample. Only CAIX mRNA expression was significantly down-regulated by STX140 in both xenograft models (p<0.05) and was also significantly down-regulated by STX243 in the MDA-MB-231 xenograft model (p<0.05. Table I). PGK mRNA expression was significantly increased in MCF-7 xenografts treated with STX140 (p<0.05, Table I). Treatment with paclitaxel did not affect any of the HIF1-regulated genes studied (Table I). Although STX66 did not affect the growth of tumors in either model, it down-regulated CAIX in the MDA-MB-231 xenograft model (p<0.05, Table I).
Figure 4.
A: MCF-7 and MDA-MB-231 xenograft models. Mice were treated orally for 7 days per week with STX140, STX243, STX66 at the doses indicated. Mice bearing MCF-7 xenografts were also treated intravenously once weekly with paclitaxel at 15 mg/kg. Results show average tumor volumes at the end of dosing (28 days for MCF-7 and 21 days for MDA-MB-231 xenografts, n = 4-9 tumors); data are means ± SEM. *p<0.05, **p<0.01, compared to control. Volume at day measured/Volume at day 0 (Vn/Vo). B: VEGF165 mRNA expression in MCF-7 and MDA-MB-231 xenografts treated with STX140, STX243, STX66 or paclitaxel. Total mRNA was prepared from 20 to 40 mg of tissues excised after 21 days treatment for mice with MDA-MB-231 xenografts and after 28 days treatment for mice with MCF-7 xenografts. mRNA was reverse-transcribed and quantified by RT-PCR. Three to four tumor samples (n = 3 or 4) were submitted to a minimum of three independent amplifications, and each amplification was performed in duplicate. The results presented are the average of the relative Ct values from all of the experiments relative to vehicle-dosed animals (control).
Table I.
ATP-binding cassette sub-family B member 1 (ABCB1), Phosphoglycerate Kinase (PGK), Glucose Transporter 1 (GLUT1) and Carbonic Anhydrase IX (CAIX) mRNA expression in MCF-7 and MDA-MB-231 xenografts from mice dosed with STX140, STX243, STX66 or paclitaxel. Mice bearing MCF-7 xenografts were dosed daily with STX140 at 20 mg/kg, STX243 at 40 mg/kg, or STX66 at 40 mg/kg p.o. or weeklywith paclitaxel at 15 mg/kg i.v.. Mice bearing MDA-MB-231 xenografts were dosed daily with STX140 at 20 mg/kg, STX243 at 40 mg/kg, STX66 at 40 mg/kg or STX66 at 75 mg/kg p.o. Total mRNA was prepared from 20 to 40 mg of tissues excised after 21 days dosing for mice with MDA-MB-231 xenografts and after 28 days dosing for those with MCF-7 xenografts. mRNA was reverse transcribed and quantified by RT-PCR. Three to four tumor samples (n = 3 or 4) were submitted to a minimum of three independent amplifications, and each amplification was performed in duplicate. The results presented are the average of the Ct values from all of the experiments relative to vehicle-dosed animals (control).
| Gene Expression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCB1 | PGK | GLUT1 | CAIX | |||||||||
|
| ||||||||||||
| MCF-7 | Average | SDTEV | Ttest | Average | SDTEV | Ttest | Average | SDTEV | Ttest | Average | SDTEV | Ttest |
|
| ||||||||||||
| Control | 1.00 | 1.90 | 1.00 | 0.32 | 1.00 | 0.45 | 1.00 | 0.23 | ||||
| STX140, 20 mg/kg | 0.61 | 0.71 | 0.70 | 1.52 | 0.18 | 0.04 | 0.64 | 0.28 | 0.24 | 0.42 | 0.08 | 0.05 |
| STX243, 40 mg/kg | 0.14 | 0.07 | 0.40 | 0.97 | 0.21 | 0.91 | 0.66 | 0.26 | 0.25 | 0.46 | 0.22 | 0.10 |
| STX66, 75 mg/kg | 0.33 | 0.50 | 0.50 | 1.08 | 0.20 | 0.65 | 0.76 | 0.24 | 0.39 | 0.58 | 0.21 | 0.18 |
| Paclitaxel, 15 mg/kg | 0.22 | 0.26 | 0.40 | 1.05 | 0.18 | 0.78 | 0.89 | 0.13 | 0.66 | 0.71 | 0.50 | 0.39 |
| MDA-MB-231 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Control | 1.00 | 1.20 | 1.00 | 0.33 | 1.00 | 0.36 | 1.00 | 0.75 | ||||
| STX140, 20 mg/kg | 0.59 | 0.09 | 0.71 | 1.64 | 0.50 | 0.78 | 0.77 | 0.21 | 0.27 | 0.25 | 0.06 | 0.01 |
| STX243, 40 mg/kg | 0.80 | 0.17 | 0.84 | 1.48 | 0.53 | 0.13 | 0.77 | 0.20 | 0.53 | 0.47 | 0.30 | 0.02 |
| STX66, 40 mg/kg | 0.87 | 1.11 | 0.53 | 1.26 | 0.69 | 0.53 | 1.41 | 0.29 | 0.11 | 0.55 | 0.16 | 0.03 |
| STX66, 75 mg/kg | 0.26 | 0.20 | 0.54 | 1.45 | 0.55 | 0.21 | 1.23 | 0.07 | 0.23 | 0.72 | 0.34 | 0.71 |
In vitro study of HIF2α expression
The effects of STX140 and STX243 on HIF2 expression and HIF2α translocation into the nucleus were investigated. MCF-7 and MDA-MB-231 cells were treated with STX140, STX243 or STX66 at 0.5 μM under normoxia for 18 h and then under hypoxia for 6 h. Whole cell protein extracts and nuclear extracts were prepared, then 50 μg proteins were loaded and HIF2α expression was detected by immunoblotting with a human antibody to HIF2α. HIF2α protein was detected at 118 kDa. Immunoblot analysis showed that HIF2α expression in nuclear extracts of both cell lines was not affected by any treatment and was similar to that in untreated cells (Figure 5A). HIF2α protein expression in whole cell extracts from MCF-7 and MDA-MB-231 cells was not increased under hypoxia (Figure 5B). The steady-state level of HIF2α protein was higher in MDA-MB-231 cells treated under hypoxia with STX140 and STX243 (p<0.05) than that in untreated cells cultured under both normoxia and hypoxia (Figure 5B).
Figure 5.
Hypoxia-inducible Factor 2α (HIF2α), Phosphatidylinositol-3 Kinase (PI-3K) and Carbonic Anhydrase IX (CAIX) protein expression in MCF-7 and MDA-MB-231 cells. MCF-7 and MDA-MB-231 cells were treated with 0.5 μM STX140, STX243 or STX66 under normoxia for 18 h and then under hypoxia (1% O2, 5% CO2) for 6 h. Nuclear extracts (A) and total protein extracts (B) were prepared and analyzed by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and immunoblotting with the antibody to HIF2α or PI-3K (C). Cells were given an additional 48 h incubation without compound and plasma membrane fractions were prepared and analyzed by SDS-PAGE and immunoblotting with the antibody to CAIX (D). A: MCF-7 nuclear extracts, lane 1: control normoxia (100% intensity), 2: control hypoxia (99%); 3: STX66 (101%); 4: STX140 (101%); 5: STX243 (102%). MDA-MB-231 nuclear extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (101%); 3: STX66 (102%); 4: STX140 (104%); 5: STX243 (103%). B: MCF-7 total protein extracts; lane 1: control normoxia (100% intensity); 2: control hypoxia (102%); 3: STX66 (108%); 4: STX140 (119%); 5: STX243 (123%). MDA-MB-231 total protein extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (99%); 3: STX66 (108%); 4: STX140 (121%*); 5: STX243 (134%*). C: MCF-7 total protein extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (100.2%); 3: STX140 (100.3%); 4: STX243 (99.6%); 5: STX66 (100.3%). MDA-MB-231 total protein extracts, lane 1: control normoxia (100% intensity); 2: control hypoxia (99.8%); 3: STX140 (99.2%); 4: STX243 (98.8%); 5: STX66 (99.3%). D: MCF-7 plasma membrane fraction, lane 1: control normoxia (100% intensity); 2: control hypoxia (101.3%); 3:STX66 (103%); 4: STX140 (61.2%*); 5: STX243 (74.2%*). MDA-MB-231 plasma membrane fraction; 1: control (100% intensity); 2: control hypoxia (104%); 3: STX66 (99.23%); 4: STX140 (67.3%*); 5: STX243 (67.4%*). *p<0.05, compared to control.
In vitro study of PI-3K expression
As previous results showed that STX140 and STX243 down-regulate CAIX mRNA expression without affecting the other HIF1-regulated genes studied, the role of PI-3K protein in mediating the down-regulation of CAIX mRNA expression was investigated. MCF-7 cells and MDA-MB-231 cells were treated with STX140, STX243 or STX66 under normoxia for 18 h and then under hypoxia for 6 h. Cells were lysed and the protein extracts were analyzed by SDS-PAGE and immunoblotting with a human antibody to PI-3K. A band was detected at 85 kDa, the correct size for the α subunit of PI-3K, in MCF-7 and MDA-MB-231 cells regardless of treatment (Figure 5C). No changes were observed in PI-3K expression.
In vitro study of CAIX expression
MCF-7 and MDA-MB-231 cells were treated with 0.5 μM STX140, STX243 or STX66 under normoxia for 18 h followed by 6 h under hypoxia. Plasma membrane fractions were prepared and 50 μg of protein was analyzed by immunoblotting. No changes in CAIX expression were observed (data not shown). This may be due to the long half-life of CAIX protein (38 h) masking the changes observed in CAIX mRNA expression after compound treatment for just 24 h. Therefore, the initial experiment was repeated but the cells were given an additional compound-free, 48 h incubation, for any changes in CAIX mRNA expression to be reflected at the protein level. The results showed that CAIX protein expression is inhibited in cells treated with STX140 and STX243 (Figure 5D, p<0.05).
Modeling results
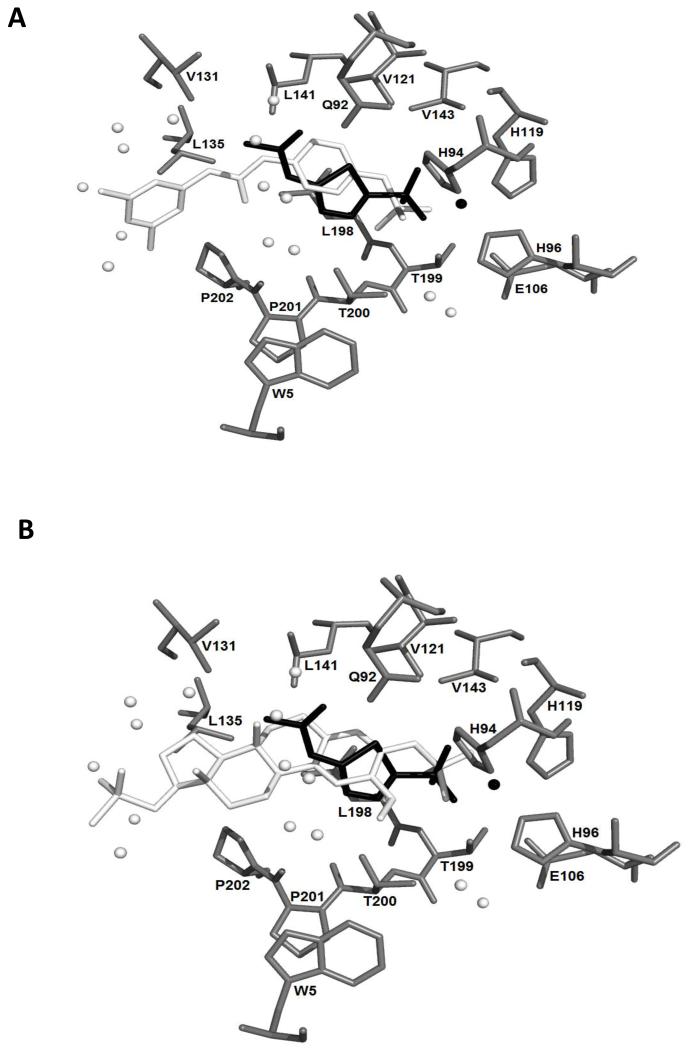
Compound S4 is a small molecule sulfamate ester derivative that inhibits CAIX with a Ki of 7nM (33). STX140, STX243, S4 and acetazolamide were docked into the 3IAI crystal structure of the CAIX catalytic domain (34). This structure has an acetazolamide molecule in the substrate-binding site, with the ligand sulfonamide group coordinated to the zinc ion in the binding site. With the acetazolamide removed, our docking protocols were able to recreate the observed crystallographic docking pose for this ligand (data not shown), giving confidence for the later studies. It was expected that the structurally related sulfamate group of the docked compounds would bind in a similar manner (35-37) and this indeed proved to be the case (Figure 6). All three compounds docked in the same region of the binding site: the docked poses of the two STX compounds were almost identical and the two sulfamates overlaid with the sulfonamido group of the acetazolomide. For each of the three compounds, all 25 docked poses were approximately the same. The docking scores of the best-ranked poses were 45.45 (S4), 14.35 (STX140) and 16.24 (STX243), with the higher score suggesting better binding.
Figure 6.
S4 (A) and STX140 (B) docked into the 3IAI ‘A’ chain crystal structure of CAIX. Amino acid residues are shown in grey and are labelled. The crystal structure acetazolamide is shown in black. The black sphere is a zinc ion. The docked ligands are shown in white. The white spheres are the oxygen atoms of water molecules.
Discussion
Angiogenesis, the creation of a new network of blood vessels supplying nutrients and oxygen, plays an essential role in tumor growth, tumor progression and metastasis. Therefore, angiogenesis inhibitors offer a potential therapy for cancer. Many anti-angiogenic agents identified have led to the development of a number of novel cancer therapies, such as Avastin (bevacizumab). Optimized sulfamoylated compounds based on 2-methoxyestradiol, such as STX140 and STX243, were derived from our earlier evidence that the addition of a sulfamate group can have profound effects on activity (39). They are potent inhibitors of in vitro and in vivo angiogenesis (10, 11, 40). Previous studies also showed that they are microtubule disruptors and bind to the colchicine site on tubulin, as does the parent steroid 2-methoxyestradiol. Nevertheless, the mechanisms linking microtubule disruption, anti-angiogenic activity and cell death, remain largely unknown. Escuin et al. demonstrated that the anti-angiogenic activity of microtubule disruptors occurs after the disruption of microtubules and is mediated by the post-transcriptional down-regulation of the pro-angiogenic transcription factor HIF1α protein (23). This was confirmed only with a non-cancer in vivo model for 2-methoxyestradiol when mice were dosed with 100 mg/kg 2-methoxyestradiol (41). So far, no studies have been carried out with in vivo cancer models.
Although the protocol described by Escuin et al. (23) was followed, the results obtained differ from their findings, since no diminution in nuclear HIF1α accumulation was seen in either of the cell lines treated with 2-methoxyestradiol (STX66). This may be due to the lower concentration used. The in vitro experiments were undertaken with 0.5 and 5 μM 2-methoxyestradiol, but using higher concentrations is clinically irrelevant as its poor bioavailability prevents it reaching plasma concentrations higher than 0.1 μM (3, 4, 10, 42). However, Chua et al. have shown that doses of 50 μM and above are required to affect HIF1α expression (43). STX140 and STX243 did inhibit nuclear HIF1α accumulation in MCF-7 and MDA-MB-231 cells, supporting the theory that microtubule disruptors inhibit nuclear HIF1α accumulation. STX140 and STX243 increased cytoplasmic HIF1α protein expression, suggesting they may increase HIF1α stability or inhibit its nuclear translocation. Cytoplasmic HIF2α expression was increased by STX140 or STX243 treatment, whereas nuclear HIF2α accumulation was unchanged, indicating that HIF2α translocation is not inhibited by STX140 or STX243.
To test whether the inhibition of nuclear accumulation of HIF1α, observed in vitro, mediated anti-angiogenic activity of microtubule disruptors by down-regulating HIF1 transcriptional factor activity, mRNA expression was measured for a cohort of HIF1-regulated genes. Uniquely, and in addition to samples from in vitro experiments, cancer xenograft tissue was also examined to test the relevance of this mechanism in vivo. No HIF1 immunochemistry was carried out on in vivo samples because the cellular composition of tumors is heterogeneous and cells may be necrotic. It is also known that tumor oxygen levels oscillate over both hours and days, causing periodic fluctuations of HIF expression (44). The study of mRNA expression within the tumor also overcomes potential user bias in assessing immunochemistry. For this reason, only non-necrotic tissues excised from the tumors were studied.
The mRNA was quantified for VEGF, GLUT1, ABCB1, PGK1 and CAIX, all of which are reported to be regulated by HIF1 (45). STX140 and STX243 did not inhibit the expression of the HIF1-regulated genes examined in vitro and in vivo, except CAIX, suggesting that they do not down-regulate HIF1 transcriptional factor activity. The fact that they do not down-regulate VEGF, one of the most important biomarkers for tumor angiogenesis, corroborates these results. Although no strictly HIF2α-regulated genes were studied, HIF1α/HIF2α-common targets, such as GLUT1, were not affected by any treatment, suggesting that they do not alter HIF2α activity. STX140 and STX243 might affect HIF1α and HIF2α stability or translocation without altering their activities. To assess the in vivo antitumor efficacy of STX140 and STX243, paclitaxel was included in the xenograft studies as a clinically proven competitor compound. Therefore, analysis of the effects of paclitaxel on HIF1-regulated genes in vivo was undertaken, despite the fact that paclitaxel did not form part of the in vitro study. It showed that expression of HIF1-regulated genes are not significantly altered by paclitaxel. HIF1 down-regulation would appear to have little mechanistic relevance to explain the link between microtubule disruption and anti-angiogenic effects that can be induced by these compounds. Moreover, it has also been shown that STX140 and STX243 are potent tumor growth inhibitors in non-VEGF-stimulated tumor models (46).
STX140 and STX243 down-regulated CAIX mRNA and protein expression in vitro and in vivo. No correlation was seen between tumor size and CAIX mRNA expression, suggesting that the down-regulation observed is not tumor size related, but is due to the effects of STX140 and STX243. The CAIX enzyme is overexpressed in many tumors, where it is involved in crucial processes related to cancer progression, such as pH regulation and cell adhesion, and it is associated with a poor prognosis (47). CAIX transcription can be strongly activated by hypoxia via HIF1 transcription (20). However, as HIF1 activity is not down-regulated by STX140 and STX243, the decrease in CAIX transcription is most likely to be HIF1-independent, and may be activated via the PI-3K pathway (48). However, in vitro PI-3K protein expression was not altered by treatment with STX140 or STX243. Another potential pathway implicated in regulating CAIX expression is through the anti-apoptotic oncogene B-cell lymphoma 2 (BCL2). Decreased expression of BCL2 directly leads to CAIX down-regulation (49), and it is known that STX140 can down-regulate BCL2 expression in breast cancer cells (50). Despite this, how STX140 and STX243 down-regulate CAIX mRNA expression remains to be fully resolved. Ho et al. showed that STX140 and STX243 directly inhibit CAII (12), another enzyme of the carbonic anhydrase family, whereas the parent 2-methoxyestradiol does not. This is due to the presence of a sulfamate group in STX140 and STX243 that directly coordinates with the zinc atom of CAII. Indeed, both STX140 and STX243 were previously co-crystallized with CAII and their binding modes elucidated (37). It is therefore very likely that STX140 and STX243 will also directly inhibit the CAIX enzyme, which is structurally similar to CAII. This direct enzyme inhibition could explain, at least in part, the ability of these compounds to inhibit angiogenesis and tumor growth.
We proposed earlier that such sulfamate esters might inhibit not only CAII, but also CAIX and CAXII (36, 38). Since this work, the X-ray crystal structure of CAIX has been reported (34). We therefore used computational docking to explore possible binding modes for STX140, STX243 and the recently reported potent sulfamate-based CAIX inhibitor S4 (IV, (33)). All successfully docked into the CAIX active site. The sulfamate and its interaction with the zinc ion is a major determinant of the binding pose, as the crystal structure of CAII with 17-O-sulfamoylated 2-methoxy-3-hydroxyestratriene, i.e. STX140 with the 3-O-sulfamoyl moiety replaced with a hydroxyl group, binds with the 17-O-sulfamoyl moiety interacting with the zinc, but if the 17-O-sulfamoyl moiety of STX140 is removed then the 3-O-sulfamoyl moiety interacts with the zinc (37). In the crystal structure of CAII with STX243, with sulfamates in both the 3- and17-positions, the 17-O-sulfamate interacts with the zinc ion in the binding site, at least in those crystals examined. This is presumed to be because of local steric hindrance exerted by the neighboring 2-methoxy group on the phenolic sulfamate, but this may of course simply represent the only binding mode that could be crystallized. However, the docking of STX243 (and STX140) into CAIX has the 3-O-sulfamate making this interaction. Although there are some amino acid and, therefore, structural differences between the binding sites of CAII and CAIX, we can see no obvious structural explanation for the difference in binding pose and possibly both are operative in vivo.
S4 Has a Ki of 7nM and a docking score of 45.45. If docking score is correlated with Ki then the docking scores of STX140 (14.35) and STX243 (16.24) suggest that the STX compounds may be weaker inhibitors of CAIX than S4. This may be related to the greater rigidity of the STX compounds compared to S4 and the consequent reduced ability to adapt to the shape of the binding site. Carbonic anhydrase inhibitors are known to be anticancer agents in vitro and in vivo. In binding to the related CAII, STX140 and STX243 had IC50 values of 379 and 290 nM respectively in direct comparison to acetazolamide, that had a value of 25 nM. S4 has a marked anti-metastatic effect (33). If this effect is a consequence of CAIX inhibition, then this may explain the antiproliferative effect of the STX compounds. Moreover, in a recent in vivo study (9), in addition to observed marked growth inhibition, tumors treated with STX140 were shown to exhibit no metastasis, in stark contrast to those treated with paclitaxel. This additional positive effect of STX140 may be due to a combination of its direct inhibition and down-regulation of CAIX.
In conclusion, we have shown that STX140 and STX243, two potent anti-proliferative, antitumor growth and anti-angiogenic microtubule disruptors down-regulate HIF1α accumulation in nuclei in vitro but do not inhibit HIF1 transcriptional activity in vitro and in vivo. We also showed that these compounds have no effect on nuclear HIF2α accumulation. Furthermore, we demonstrated that STX140 and STX243 down-regulate CAIX mRNA transcription and expression without affecting PI-3K protein expression. Therefore, the anti-angiogenic activity observed for these compounds and other microtubule disruptors, such as paclitaxel and 2-methoxyestradiol, is unlikely to be mediated by the inhibition of HIF1α-mediated gene transcription. The excellent in vivo efficacy of STX140 and STX243 may at least in part be due to a combination of the inhibition of transcription of CAIX, a marker of poor prognosis in breast cancer (51), and to direct inhibition of CAIX in hypoxic environments.
Acknowledgements
This work was supported by Sterix Ltd., a member of the IPSEN Group. B.V. L. Potter is a Wellcome Trust Senior Investigator (grant 101010). We would also like to thank Helena Tutill for her expert technical assistance. Finally, we would like to acknowledge the work of Professor Michael J. Reed who sadly passed away in 2009. Along with his many publications in the steroid field, Professor Reed also drove much of the work that is presented and referenced in this article. He is a great loss to science and sorely missed.
Footnotes
Competing Interests
This research was supported by Sterix Ltd., a member of the IPSEN Group (C. Stengel, S.P. Newman, M.P. Leese, and P.A. Foster,). B.V.L. Potter and A. Purohit were consultants to IPSEN.
References
- 1.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 2.Shang W, Konidari I, Schomberg DW. 2-Methoxyestradiol, an endogenous estradiol metabolite, differentially inhibits granulosa and endothelial cell mitosis: a potential follicular antiangiogenic regulator. Biol Rep. 2001;65:622–627. doi: 10.1095/biolreprod65.2.622. [DOI] [PubMed] [Google Scholar]
- 3.Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 4.Newman SP, Ireson CR, Tutill HJ, Day JM, Parsons MF, Leese MP, Potter BVL, Reed MJ, Purohit A. The role of 17β-hydroxysteroid dehydrogenases in modulating the activity of 2-methoxyestradiol in breast cancer cells. Cancer Res. 2006;66:324–330. doi: 10.1158/0008-5472.CAN-05-2391. [DOI] [PubMed] [Google Scholar]
- 5.Leese MP, Leblond B, Smith A, Newman SP, Di Fiore A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BVL. 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem. 2006;49:7683–7696. doi: 10.1021/jm060705x. [DOI] [PubMed] [Google Scholar]
- 6.Day JM, Newman SP, Comninos A, Solomon C, Purohit A, Leese MP, Potter BVL, Reed MJ. The effects of 2-substituted oestrogen sulphamates on the growth of prostate and ovarian cancer cells. J Steroid Biochem Mol Biol. 2003;84:317–325. doi: 10.1016/s0960-0760(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 7.Foster PA, Ho YT, Newman SP, Kasprzyk PG, Leese MP, Potter BVL, Reed MJ, Purohit A. 2-MeOE2bisMATE and 2-EtE2bisMATE induce cell-cycle arrest and apoptosis in breast cancer xenografts as shown by a novel ex vivo technique. Breast Cancer Res Treat. 2007;111:251–260. doi: 10.1007/s10549-007-9791-5. [DOI] [PubMed] [Google Scholar]
- 8.Raobaikady B, Reed MJ, Leese MP, Potter BVL, Purohit A. Inhibition of MDA-MB-231 cell cycle progression and cell proliferation by C-2-substituted oestradiol mono- and bis-3-O-sulphamates. Int J Cancer. 2005;117:150–9. doi: 10.1002/ijc.21066. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Losic F, Newman SP, Day JM, Reed MJ, Kasprzyk PG, Purohit A, Foster PA. STX140, but not paclitaxel, inhibits mammary tumour initiation and progression in C3(1)/SV40 T/t-antigen transgenic mice. PLoS One. 2013;8:e80305. doi: 10.1371/journal.pone.0080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Leese MP, Smith AC, Potter BVL, Reed MJ. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. British J Cancer. 2004;90:932–937. doi: 10.1038/sj.bjc.6601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman SP, Leese MP, Purohit A, James DR, Rennie CE, Potter BVL, Reed MJ. Inhibition of in vitro angiogenesis by 2-methoxy- and 2-ethyl-estrogen sulfamates. Int J Cancer. 2004;109:533–540. doi: 10.1002/ijc.20045. [DOI] [PubMed] [Google Scholar]
- 12.Ho YT, Newman SP, Purohit A, Leese MP, Potter BVL, Reed MJ. The effects of 2-methoy oestrogens and their sulphamoylated derivatives in conjuction with TNF-α on endothelial and fibroblast cell growth, morphology and apoptosis. J Steroid Biochem Mol Biol. 2003;86:189–96. doi: 10.1016/s0960-0760(03)00269-3. [DOI] [PubMed] [Google Scholar]
- 13.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 14.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. Embo J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 16.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 17.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 18.Laderoute KR, Grant TD, Murphy BJ, Sutherland RM. Enhanced epidermal growth factor receptor synthesis in human squamous carcinoma cells exposed to low levels of oxygen. Int J Cancer. 1992;52:428–432. doi: 10.1002/ijc.2910520317. [DOI] [PubMed] [Google Scholar]
- 19.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 20.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 21.Kopacek J, Barathova M, Dequiedt F, Sepelakova J, Kettmann R, Pastorek J, Pastorekova S. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta. 2005;1729:41–49. doi: 10.1016/j.bbaexp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Rohwer N, Zasada C, Kempa S, Cramer T. The growing complexity of HIF-1α’s role in tumorigenesis: DNA repair and beyond. Oncogene. 2013;32:3569–3576. doi: 10.1038/onc.2012.510. [DOI] [PubMed] [Google Scholar]
- 23.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor1α accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65:9021–9028. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbonaro M, O’Brate A, Giannakakou P. Microtubule disruption targets HIF1α mRNA to cytoplasmic P-bodies for translational repression. J Cell Biol. 2011;192:83–99. doi: 10.1083/jcb.201004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbonaro M, Escuin D, O’Brate A, Thadani-Mulero M, Giannakakou P. Microtubules regulate hypoxia-inducible factor 1a protein trafficking and activity: implications for taxane therapy. J Biol Chem. 2012;287:11859–11869. doi: 10.1074/jbc.M112.345587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powis G, Kirkpatrick L. Hypoxia-inducible factor1α as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 27.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-MYC transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. 2006. [DOI] [PubMed] [Google Scholar]
- 29.Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)1α and HIF-2α expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–7113. [PubMed] [Google Scholar]
- 30.Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris A. Predominant role of hypoxia-inducible transcription factor (HIF)1α versus HIF2α in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- 31.Leese MP, Hejaz HA, Mahon MF, Newman SP, Purohit A, Reed MJ, Potter BVL. A-ring-substituted estrogen-3-O-sulfamates: potent multitargeted anticancer agents. J Med Chem. 2005;48:5243–5526. doi: 10.1021/jm050066a. [DOI] [PubMed] [Google Scholar]
- 32.Leese MP, Leblond B, Newman SP, Purohit A, Reed MJ, Potter BVL. Anticancer activities of novel D-ring modified 2-substituted estrogen-3-O-sulfamates. J Steroid Biochem Mol Biol. 2005;94:239–251. doi: 10.1016/j.jsbmb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Gieling RG, Babur M, Mamnani L, Burrows N, Telfer BA, Carta F, Winum JY, Scozzafava A, Supuran CT, Williams KJ. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast cancer xenografts. J. Med Chem. 2012;55:5591–5600. doi: 10.1021/jm300529u. [DOI] [PubMed] [Google Scholar]
- 34.Alterio V, Hilva M, Di Foire A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pasterekova S, Pedone C, Scozzafava A, Monti SM, De Simone G. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA. 2009;106:16233–16238. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones G, Willet P, Glen RC. Molecular recognition of a receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995;245:43–53. doi: 10.1016/s0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 36.Ho YT, Purohit A, Vicker N, Newman SP, Robinson JJ, Leese MP, Ganeshapillai D, Woo LW, Potter BVL, Reed MJ. Inhibition of carbonic anhydrase II by steroidal and non-steroidal sulphamates. Biochem Biophys Res Commun. 2003;305:909–914. doi: 10.1016/s0006-291x(03)00865-9. [DOI] [PubMed] [Google Scholar]
- 37.Cozier GE, Leese MP, Lloyd MD, Acharya KR, Potter BVL. Structures of four human carbonic anhydrase II/inhibitor complexes reveal a second binding site for steroidal and non-steroidal inhibitors. Biochemistry. 2010;49:3464–3476. doi: 10.1021/bi902178w. [DOI] [PubMed] [Google Scholar]
- 38.Vicker N, Ho Y, Robinson JJ, Woo LWL, Purohit A, Reed MJ, Potter BVL. Docking studies of sulphamate inhibitors of estrone sulphatase in human carbonic anhydrase II. Med. Chem. Lett. 2003;13:863–865. doi: 10.1016/s0960-894x(03)00009-x. [DOI] [PubMed] [Google Scholar]
- 39.MacCarthy-Morrogh A, Townsend PA, Purohit A, Hejaz HAM, Potter BVL, Reed MJ, Packham G. Differential effects of estrone- and estrone-3-O-sulfamate derivatives on mitotic arrest, apoptosis and microtubule assembly in human breast cancer cells. Cancer Res. 2000;60:5441–5450. [PubMed] [Google Scholar]
- 40.Leese MP, Jourdan FL, Gaukroger K, Mahon MF, Newman SP, Foster PA, Stengel C, Regis-Lydi S, Ferrandis E, Di Foire A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BVL. Structure–activity relationships of C17 cyano-substituted estratrienes as anticancer agents. J Med Chem. 2008;51:1295–308. doi: 10.1021/jm701319c. [DOI] [PubMed] [Google Scholar]
- 41.Becker CM, Rohwer N, Funakoshi T, Cramer T, Bernhardt W, Birsner A, Folkman J, D’Amato RJ. 2-Methoxyestradiol inhibits hypoxia-inducible factor1α and suppresses growth of lesions in a mouse model of endometriosis. Am Journal Pathol. 2008;172:534–544. doi: 10.2353/ajpath.2008.061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor C, Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41–48. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- 43.Chua YS, Chua YL, Hagen T. Structure activity analysis of 2-methoxyestradiol analogues reveals targeting of microtubules as the major mechanism of antiproliferative and proapoptotic activity. Mol Cancer Ther. 2010;9:224–235. doi: 10.1158/1535-7163.MCT-09-1003. [DOI] [PubMed] [Google Scholar]
- 44.Dewhirst MW. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor1 targeting. Cancer Res. 2007;67:854–855. doi: 10.1158/0008-5472.CAN-06-4744. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. Targeting HIF1 for cancer therapy. Nature Reviews. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 46.Chander SK, Foster PA, Leese MP, Newman SP, Potter BVL, Purohit A, Reed MJ. In vivo inhibition of angiogenesis by sulphamoylated derivatives of 2-methoxyoestradiol. Br J Cancer. 2007;96:1368–1376. doi: 10.1038/sj.bjc.6603727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, Lerman MI, Stanbridge EJ. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 α stabilization: a role for phosphatidylinositol 3′-kinase. Cancer Res. 2002;62:4469–4477. [PubMed] [Google Scholar]
- 49.Anai S, Shiverick K, Medrano T, Nakamura K, Goodison S, Brown BD, Rosser CJ. Downregulation of BCL2 induces down-regulation of carbonic anhydrase IX, vascular endothelial growth factor, and pAKT and induses radiation sensitization. Urology. 2007;70:832–837. doi: 10.1016/j.urology.2007.06.1118. [DOI] [PubMed] [Google Scholar]
- 50.Newman SP, Foster PA, Stengel C, Day JM, Judde JG, Lassalle M, Prevost G, Leese MP, Potter BVL, Reed MJ, Purohit A. STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells. Clin Cancer Res. 2008;14:597–606. doi: 10.1158/1078-0432.CCR-07-1717. [DOI] [PubMed] [Google Scholar]
- 51.Generali D, Fox SB, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield SM, Bruzzi P, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Bottini A, Harris AL. Role of carbonic anhydrase IX expression in prediction of the efficacy and outcome of primary epirubicin/tamoxifen therapy for breast cancer. Endocr Relat Cancer. 2006;13:921–930. doi: 10.1677/erc.1.01216. [DOI] [PubMed] [Google Scholar]