Abstract
Background
Disease control in anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) with immunosuppression is effective but burdened by adverse events, especially infections. The study goal was to evaluate risks and types of infections in patients with AAV.
Methods
Biopsy-proven AAV patients (diagnosed 1/1991–6/2011) followed in an inception cohort were evaluated for adverse events. Severe infections (requiring intravenous antibiotics, intensive care unit, or causing death) were recorded. Infection number was grouped as none, 1–2 or ≥3. Cox regression was used to estimate hazard ratios with 95% confidence intervals.
Results
A total of 489 patients (median age 59; 47% female, 55% myeloperoxidase-ANCA) were followed for 2.8 years (median). At 1, 2 and 5 years cumulative incidence of infection was 51, 58 and 65% and severe infection was 22, 23 and 26%. Pulmonary and upper respiratory infections were most common (42 and 30% ever experienced each, respectively), highest in the first 3 months. Staphylococcus aureus was most frequently seen among positive cultures (41%, 78 S. aureus/192 total positive cultures), and only one Pneumocystis jiroveci pneumonia (6 weeks into treatment). All-cause death in 12 months was associated with infections (% deaths: 0 infections 3%; 1–2 infections 10%, ≥3 infections 13%, P = 0.002). Controlling for age, sex and kidney function, patients with severe infections were 4.2 times more likely to die within 12 months (95% CI 2.0–8.7; P = 0.001).
Conclusions
More infections increase the risk of a severe infection which increases risk of all-cause mortality. Respiratory and S. aureus infections are dominant. Targeted prophylactic therapy could decrease morbidity.
Keywords: ANCA, glomerulonephritis, immunosuppression, outcomes, risk factors
INTRODUCTION
Treatment of antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) has focused on suppression of the immune response and control of the pathogenesis of disease [1–5]. Currently, 80–85% of patients with AAV reach remission with effective immunosuppressive strategies [6–9]. This disease control has an associated burden of infection-related mortality during the 12 months following diagnosis of AAV [10]. AAV has transformed from a process likely to cause acute death [4] to one of achieving and preserving remission. However, the use of immunosuppressive agents places patients at risk for infections. Although there was hope that rituximab would reduce adverse events when compared with cyclophosphamide, this has not been the case [11–14]. The current challenge is to reduce long-term morbidity and mortality by tailoring immunosuppression to limit infections and other adverse events without increasing relapse.
The goal of this study was to use a prospective inception cohort of AAV followed since 1991 to clearly define patient-specific factors that influence infections as well as the types of organisms causing and temporal nature of these infections. Insights gained could elucidate mechanisms to improve outcomes in AAV and provide more appropriate prophylactic therapy.
MATERIALS AND METHODS
Study population
Patients from the Glomerular Disease Collaborative Network (GDCN) inception cohort were included in this study if they had AAV diagnosed between 01 January 1991 to 06 January 2011. The GDCN is a longitudinal, glomerular disease patient registry and bio bank repository that has been in existence for over 30 years and primarily enrolls patients from southeastern USA, previously described [9, 15]. Patients required diagnostic biopsy with evidence of pauci-immune glomerulonephritis or small vessel vasculitis with or without granulomatous inflammation. All patients had positive ANCA determination by immunofluorescence (IF) microscopy or antigen-specific, enzyme-linked immunosorbent assay (ELISA). ANCA positivity was classified as cytoplasmic ANCA (cANCA) and/or proteinase 3-ANCA (PR3-ANCA), or perinuclear ANCA (pANCA) and/or myeloperoxidase-ANCA (MPO-ANCA). A positive pANCA alone required a concurrent negative antinuclear antibody test result.
Age was noted at the time of initial diagnosis. Diagnostic disease categories were defined according to the modified Chapel Hill Consensus Conference nomenclature, and designated as granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), microscopic polyangiitis (MPA), or pauci-immune necrotizing and/or crescentic glomerulonephritis (GN) without overt signs of systemic vasculitis [16, 17].
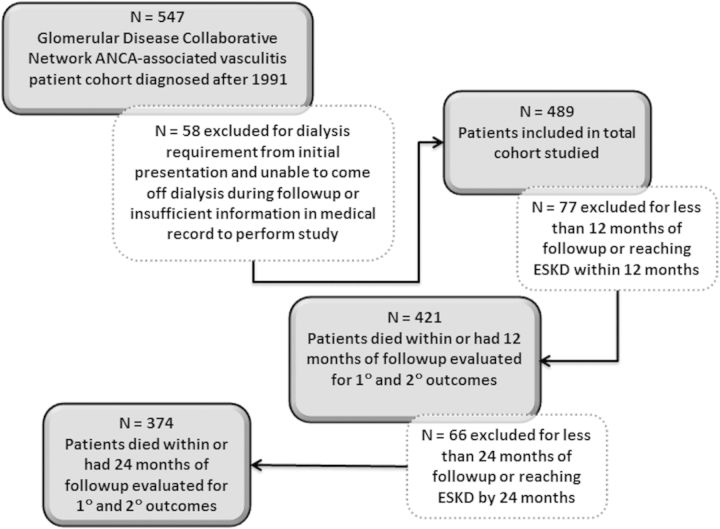
Infections and adverse events were obtained from medical records (see list in Table 1). Severe infections were defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death. Venothromboembolic events (VTE) were assumed to be related to pathogenicity of disease [18–21]. Relapse was defined as the appearance of dysmorphic red blood cells in the urine or signs of active vasculitic lesions in any other organ deemed severe enough to warrant a change in therapy. Proteinuria alone was not considered indicative of active glomerulonephritis. We also evaluated end-stage kidney disease (ESKD) and death from any cause. Patients were excluded for ESKD from the time of initial diagnosis (Figure 1).
Table 1.
Demographics and clinical characteristics for the entire cohort and for the 1- and 2-year cohorts
| Total cohort (N = 489) | 1-year cohort (patients followed for a minimum of 1 year or died within 1 year) (N = 421) | 2-year cohort (patients followed for a minimum of 2 years or died within 2 years) (N = 374) | |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 55 ± 19 | 55 ± 19 | 56 ± 19 |
| Median (IQR) | 59 (45, 70) | 59 (46, 70) | 59 (46, 71) |
| Sex | |||
| Female | 232 (47%) | 200 (48%) | 182 (49%) |
| ANCA specificity | |||
| MPO/P-ANCA | 269 (55%) | 231 (55%) | 198 (53%) |
| PR3/C-ANCA | 216 (44%) | 187 (44%) | 173 (46%) |
| Both PR3- and MPO-ANCA | 2 (0.4%) | 1 (0.2%) | 1 (0.3%) |
| ANCA Positive, no specificity | 2 (0.4%) | 2 (0.5%) | 2 (0.5%) |
| Clinical diagnosis | |||
| GPA | 122 (25%) | 113 (27%) | 103 (28%) |
| MPA | 257 (53%) | 219 (52%) | 203 (54%) |
| PICGN | 105 (22%) | 84 (20%) | 64 (17%) |
| EGPA | 5 (1%) | 5 (1%) | 4 (1%) |
| Organ involvement | |||
| Kidney | 462 (95%) | 396 (94%) | 350 (94%) |
| Lungs | 252 (52%) | 215 (51%) | 196 (52%) |
| ENT | 188 (39%) | 170 (40%) | 155 (41%) |
| Skin | 108 (22%) | 97 (23%) | 92 (25%) |
| GI | 46 (9%) | 38 (9%) | 33 (9%) |
| eGFR at onset (mL/min/1.73 m2) | |||
| Median (IQR) | 19 (12, 41) | 21 (12, 42) | 22 (12, 43) |
| Follow-up (years) | |||
| Median (IQR) | 2.8 (1.2, 6.2) | 3.4 (1.9, 7.1) | 3.8 (2.4, 7.5) |
| Death (any cause) over the entire follow-up, n (%) | 128 (26%) | 119 (28%) | 116 (31%) |
| Death from infection over the entire follow-up, n (%) | 15 (3%) | 15 (4%) | 15 (4%) |
| N (%) with at least 1 infection ever | 376 (77%) | 335 (80%) | 307 (82%) |
| N (%) with at least 1 severe infection evera | 114 (23%) | 91 (22%) | 96 (26%) |
| N (%) with at least 1 relapse ever | 194 (40%) | 194 (46%) | 194 (52%) |
| N (%) ESKD over entire follow-up | 94 (19%) | 69 (16%) | 60 (16%) |
| Infusions of methylprednisolone | |||
| N | 305 | 260 | 234 |
| Median (IQR) | 3 (3, 4) | 3 (3, 5) | 3 (3, 6) |
| Months of prednisone | |||
| N | 398 | 343 | 305 |
| Median (IQR) | 11 (5, 21) | 12 (5, 23) | 12 (6, 25) |
| Months of IV cyclophosphamide | |||
| N | 294 | 255 | 225 |
| Median (IQR) | 6 (3, 6) | 6 (3, 7) | 6 (3, 7) |
| Months of daily oral cyclophosphamide | |||
| N | 201 | 182 | 161 |
| Median (IQR) | 7 (3, 14) | 8 (4, 14) | 8 (4, 15) |
| Courses of rituximab | |||
| N | 90 | 88 | 85 |
| Median (IQR) | 2 (2, 4) | 2 (2, 4) | 2 (2, 4) |
| Months of azathioprine | |||
| N | 130 | 121 | 114 |
| Median (IQR) | 9 (2, 21) | 9 (2, 21) | 11 (2, 24) |
| Months of mycophenolate mofetil | |||
| N | 154 | 146 | 139 |
| Median (IQR) | 14 (4, 34) | 16 (5, 38) | 18 (5, 38) |
| Months of methotrexate | |||
| N | 39 | 38 | 37 |
| Median (IQR) | 19 (7, 30) | 20 (7, 30) | 20 (7, 30) |
| Venothromboembolic event, n (%) | 53 (11%) | 43 (10%) | 40 (11%) |
| Cancerb, n (%) | 65 (13%) | 61 (15%) | 56 (15%) |
| Avascular necrosis, n (%) | 8 (2%) | 8 (2%) | 8 (2%) |
| Osteopenia/osteoporosis, n (%) | 66 (14%) | 63 (15%) | 60 (16%) |
| Weight gain, yes n (%) from prednisone start to finish | 174 (36%) | 158 (38%) | 140 (37%) |
| Neuropsychiatric events, n (%) | 77 (16%) | 68 (16%) | 59 (16%) |
| Cataracts, n (%) | 37 (8%) | 37 (9%) | 35 (9%) |
| Myocardial Infarction, n (%) | 19 (4%) | 18 (4%) | 17 (5%) |
| Gastrointestinal bleed, n (%) | 53 (11%) | 45 (11%) | 41 (11%) |
| History of diabetes mellitus, n (%) | 38 (8%) | 30 (7%) | 25 (7%) |
| Steroid-induced diabetes mellitus, n (%) | 148 (30%) | 106 (25%) | 103 (29%) |
| Stroke, n (%) | 16 (3%) | 15 (4%) | 15 (4%) |
| Myopathy, n (%) | 44 (9%) | 42 (10%) | 37 (10%) |
| Acne, n (%) | 32 (7%) | 28 (7%) | 23 (6%) |
SD, standard deviation; IQR, Interquartile range; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; P, perinuclear; PR3, proteinase 3; C, cytoplasmic; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; PICGN, pauci-immune crescentic glomerulonephritis without systemic vasculitis (renal limited disease); EGPA, eosinophilic granulomatosis with polyangiitis; ENT, ear nose and throat; GI, gastrointestinal; ESKD, end-stage kidney disease; IV, intravenous.
aSevere infection defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death.
bAll cancers including non-melanomatous skin cancers that occurred after immunosuppression administration.
FIGURE 1:
Depiction of studied patient population.
We screened 547 patients and excluded 58 (ESKD at initial presentation or insufficient information) (Figure 1). This left 489 patients who were evaluated for overall occurrence of adverse events. To learn more about the first 2 years after initial diagnosis, we then evaluated the subset of patients who had a full 12 months of follow-up and evaluated the outcomes within this timeframe (‘1-year cohort’, n = 421, Figure 1). We then performed this same evaluation including the subset of patients with a full 24 months of follow-up (‘2-year cohort’, n = 374, Figure 1). Of note, patients who died from any cause were included in both the 1-year and 2-year cohort. Evaluation of patients within these set intervals of follow-up allowed us to study only those patients with equal amounts of time to have the events of interest. Those who reached ESKD within these timeframes were censored at the time of ESKD due to potential differences in reasons for adverse events and death following initiation of renal replacement therapy. Estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) at diagnosis was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation [22].
Patient participation was approved by the University of North Carolina Institutional Review Board, with informed consent provided by all patients for long-term follow-up of medical information.
Therapy
Months and number of infusions of intravenous (IV) immunosuppressive treatment were recorded. Rituximab therapy was recorded by course. For total cyclophosphamide exposure 1 IV infusion was considered 1 month of therapy and added to number of months of oral cyclophosphamide therapy. Patients in this cohort were most commonly treated with a regimen that included methylprednisolone 500 mg IV daily for 3 days, prednisone 60 mg daily for 4 weeks followed by a 12 week taper, cyclophosphamide 0.5–1 g/m2 monthly or daily oral 2 mg/kg for 3–6 months, azathioprine 2 mg/kg for 12 months, mycophenolate mofetil 1 g twice a day or rituximab therapy given as 1 g IV on day 1 and 15 (1 course) or 375 mg/m2 IV weekly for 4 weeks (1 course). The combination of immunosuppressive medications was decided by the attending physician and not per specific protocol for this study. Leukopenia was defined as white blood cell count of ≤3.0 × 109 per liter. Prophylactic medications included any trimethoprim sulfamethoxazole, dapsone, antiviral or antifungal or any other antimicrobial agent prescribed for the prevention of infection in the setting of a known immunocompromized state. The use of these medications in the setting of an active infection was not counted as prophylactic therapy.
Statistical analyses
Descriptive statistics included the number (n), percentage (%), mean and standard deviation (SD), or median with interquartile range (IQR). Comparisons between groups used Fisher's exact tests for categorical measures and Wilcoxon rank sum tests or Kruskal–Wallis tests for continuous measures. Incidence rates were reported for relapse and infection as per patient-year or per person month (for the 3 months pre- and post-relapse) of follow-up using all events, with a Poisson distribution used to calculate 95% confidence intervals (95% CI) and standard deviations (SD). Proportional hazards models were used for multivariable modeling of time to adverse event, relapse, or death, with hazards ratio (HR), 95% CI and P-values presented. Proportional odds models were used to estimate multivariable associations with number of infections (none versus 1–2 versus ≥3 infections), both with and without weighting severe infections (×2 for each). Logistic regression models were used to evaluate multivariable risk factors for severe infections in the 1-year and 2-year cohorts separately. For proportional odds and logistic models, odds ratio (OR), 95% CI and P-values are presented. Analyses were conducted using SAS software (version 9.3 SAS Institute, Cary, NC, USA). Exact P-values were reported with a two-sided P-value of <0.05 considered statistically significant.
RESULTS
Four hundred and eighty-nine patients with adequate follow-up available, diagnosed after 1991, and not designated as having ESKD at time of diagnosis were eligible for this study (Table 1, Figure 1). Forty-seven percent of the population was female and median age was 59 years (IQR 45, 70). Fifty-three percent of patients were diagnosed with MPA and 55% were MPO-ANCA positive (Table 1). Median follow-up of the cohort was 2.8 years (IQR 1.2, 6.2). Cumulative immunosuppression exposure is listed in Table 1.
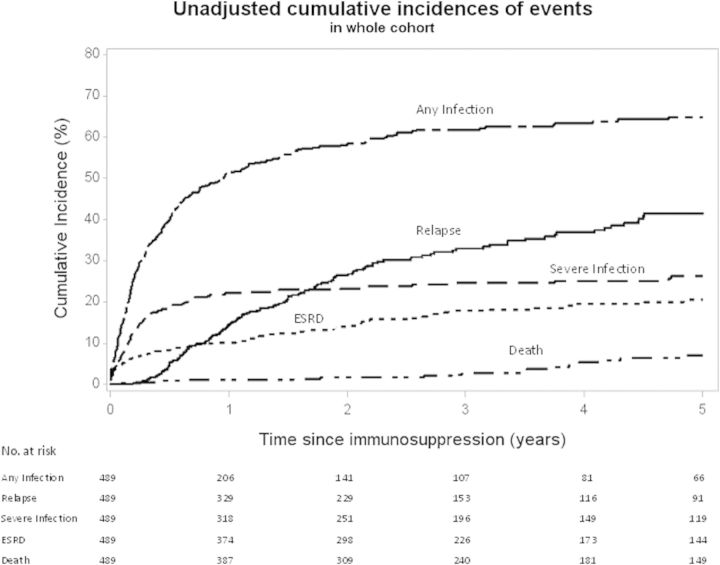
Cumulative incidence at 1, 2 and 5 years of any infection was 51, 58 and 65%, severe infection was 22, 23 and 26%, and relapse was 14, 27 and 41%, respectively, which are unadjusted estimates (Figure 2). One hundred and sixteen severe infections occurred in 52 patients after 12 months from start of therapy. Of these, 32 (28%) occurred during treatment for relapse and 84 (72%) occurred during maintenance therapy. Incidence of ESKD and death are also shown in Figure 2, with 17% of the patients progressing to ESKD after diagnosis and 7% dying in 5 years. Other adverse events and complications of therapy are listed in Table 1 and are reported as ever present over the duration of follow-up.
FIGURE 2:
Unadjusted cumulative incidences of events in the entire cohort.
Infections in the 1-year and 2-year cohorts
Of the 421 patients included in the detailed 1-year cohort, 237 (56%) experienced at least one infection during that year; 192 (46%) had 1–2 infections and 45 (11%) had ≥3 infections (Table 2). An increased number of infections per patient was associated with increasing age and steroid-induced diabetes mellitus (DM) (Table 2). Females and patients with more leukopenic episodes had a trend for more infections (Table 2). In multivariable proportional hazards models of increasing numbers of infections age (OR 1.01, 95% CI 1.00, 1.02, P = 0.021) and female sex (OR 1.75, 95% CI 1.18, 2.58, P = 0.005) were significant predictors, but not steroid-induced diabetes. Results were the same if severe infections were weighted more heavily than other infections and also if controlling for leukopenic episodes and eGFR, neither of which was associated with number of infections. Infections were associated with death from any cause and relapse within 12 months (Table 2). Greater number of infections was associated with a greater likelihood of severe infection. Infection number was not different between those who were and were not given prophylactic therapy. Of note, 90% of those given prophylactic therapy were treated with trimethoprim sulfamethoxazole.
Table 2.
(N = 421) Demographics and outcomes of patients in the 1-year cohort by infection number
| 0 infections in first 12 months (N = 184) | 1–2 infections in first 12 months (N = 192) | ≥3 infections in first 12 months (N = 45) | Pa | |
|---|---|---|---|---|
| Mean age years | 0.02 | |||
| Mean ± SD | 53 ± 19 | 57 ± 19 | 59 ± 17 | |
| Median (IQR) | 57 (45, 66) | 60 (47, 72) | 66 (48, 73) | |
| Female sex | 77 (42%) | 97 (51%) | 26 (58%) | 0.08 |
| Mean number of episodes of Leukopenia within 12 months | 0.09 | |||
| N | 64 | 80 | 25 | |
| Mean ± SD | 0.9 ± 0.9 | 1.1 ± 1.1 | 1.2 ± 0.9 | |
| Median (IQR) | 1 (0, 1) | 1 (0, 1) | 1 (1, 1) | |
| eGFR at onset (mL/min/1.73 m2) Median (IQR) |
22 (13, 45) | 20 (11, 41) | 18 (13, 33) | 0.24 |
| Diabetes mellitus induced by steroids within 12 months | 34 (19%) | 52 (27%) | 20 (44%) | 0.001 |
| Mean number of relapses within 12 months | 0.01 | |||
| N | 92 | 85 | 17 | |
| Mean ± SD | 0.2 ± 0.5 | 0.5 ± 0.6 | 0.4 ± 0.5 | |
| Median (IQR) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | |
| Prophylaxis given within 12 months | 47 (26) | 46 (24%) | 14 (31%) | 0.59 |
| Severe infectionb within 12 months | 0 (0%) | 69 (36%) | 29 (64%) | <0.0001 |
| Death from any cause within 12 months | 5 (3%) | 20 (10%) | 6 (13%) | 0.002 |
SD, standard deviation; IQR, interquartile range.
aP-values were calculated by Fisher exact test for categorical variables and Wilcoxon two samples for continuous variables.
bSevere infection defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death.
In the 2-year cohort (n = 374), 251 (67%) experienced at least one infection during that timeframe; 169 (45%) had 1–2 infections and 82 (22%) had ≥3 infections (Table 3). Infection results of the 2-year cohort were similar to patients followed for at least 12 months, except that female sex was more strongly associated with increasing number of infections (0 infections: 39% female, 1–2 infections: 52% female, ≥3 infections: 56% female, P = 0.03 versus males) (Table 3). Infections remained associated with death and relapse in the 2-year cohort (Table 3). A trend for an association between more leukopenic episodes and more infections remained (P = 0.06). Multivariable associations were virtually the same as in the 1-year cohort with age and female sex remaining predictors, but not other measures, and results were not changed when weighting severe infections (data not shown).
Table 3.
Demographics and outcomes of patients in the 2-year cohort by infection number (N = 374)
| 0 infections in first 24 months (N = 123) | 1–2 infections in first 24 months (N = 169) | ≥3 infections in first 24 months (N = 82) | Pa | |
|---|---|---|---|---|
| Mean age years | 0.01 | |||
| Mean ± SD | 52 ± 19 | 58 ± 18 | 57 ± 18 | |
| Median (IQR) | 56 (44, 65) | 61 (48, 72) | 63 (47, 71) | |
| Female Sex | 48 (39%) | 88 (52%) | 46 (56%) | 0.03 |
| Mean number of episodes of Leukopenia within 24 months | 0.06 | |||
| Mean ± SD | 1 ± 0.9 | 1.3 ± 1 | 1.5 ± 1 | |
| Median (IQR) | 1 (0, 1) | 1 (1, 2) | 1 (1, 2) | |
| eGFR at onset (mL/min/1.73 m2) Median (IQR2) |
24 (14, 52) | 22 (11, 41) | 20 (14, 39) | 0.14 |
| Diabetes mellitus induced by steroids within 24 months | 24 (20%) | 45 (27%) | 34 (42%) | 0.003 |
| Mean number of relapses within 24 months | 0.005 | |||
| N | 71 | 79 | 44 | |
| Mean ± SD | 0.6 ± 0.7 | 0.9 ± 0.8 | 0.9 ± 0.6 | |
| Median (IQR) | 0 (0, 1) | 1 (0, 1) | 1 (1, 1) | |
| Prophylaxis given within 24 months | 32 (26%) | 47 (28%) | 29 (35%) | 0.32 |
| Severe infectionb within 24 months | 0 (0%) | 52 (31%) | 44 (54%) | <0.0001 |
| Death from any cause | 5 (3%) | 20 (10%) | 6 (13%) | 0.13 |
SD, standard deviation; IQR, interquartile range.
aP-values were calculated by Fisher exact test for categorical variables and Wilcoxon two samples for continuous variables.
bSevere infection defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death.
To assess if infections predominantly occurred post-relapse due to additional immunosuppressive therapy, the incidence of infections 3 months prior to relapse and 3 months post-relapse was determined. First relapse was used to avoid repeated immunosuppressive treatments with multiple relapses. Among 88 patients who relapsed within 2 years, there were 15 infections in the 3 months prior and 25 in the 3 months post-relapse, with incidence rates of infection: 0.056 (0.031, 0.083) per person per month in the 3 months prior to relapse and 0.095 (0.063, 0.126) per person per month in the 3 months after relapse, P-value = 0.078. The trend for fewer infections prior to relapse does not rule out the potential that infections may contribute to initiating a relapse.
In the 2-year cohort, 96 experienced at least one severe infection within those 2 years (Table 4). Advancing age, female sex, steroid-induced diabetes and lower eGFR at diagnosis were associated with severe infections compared with no infections, while more leukopenic episodes showed a trend of an association with any infection (severe or not) compared with those with no infection. In a multivariable model of predictors of severe infections, age was no longer statistically significant, but the following measures remained important: female gender (OR 1.83 (95% CI 1.20, 2.81, P = 0.006), steroid-induced diabetes (OR 1.91 (95% CI 1.23, 2.98, P = 0.004) and lower eGFR (OR 0.990 (95% CI 0.981, 0.999, P = 0.028). Patients who had at least one severe infection were more likely to die from any cause compared with those with no infections or with those having non-severe infections (Table 4). By multivariable analysis controlling for age, sex, and eGFR, severe infection remained independently associated with death from any cause (HR = 3.75, 95% CI 1.99–7.03, P < 0.0001).
Table 4.
Comparison of demographics and outcomes of patients with no infections, non-severe infections and severe infections among those in the 2-year cohort (patients followed for a minimum of 2 years or who died within that timeframe) (N = 374)
| 0 infections (N = 123) | Patients with only non-severe infection (N = 155) | Severe infectiona (N = 96) | Pb values | |
|---|---|---|---|---|
| Age years | 0.0043 | |||
| Mean ± SD | 52 ± 19 | 56 ± 18 | 59 ± 18 | |
| Median (IQR) | 56 (44, 65) | 60 (45, 71) | 65 (50, 72) | |
| Female sex | 48 (39%) | 77 (50%) | 57 (59%) | 0.011 |
| ANCA specificity | 0.79 | |||
| MPO/P-ANCA | 65 (53%) | 82 (53%) | 51 (53%) | |
| PR3/C-ANCA | 58 (47%) | 72 (47%) | 43 (45%) | |
| Both PR3- and MPO-ANCA | 0 (0%) | 0 (0%) | 1 (1%) | |
| ANCA Positive, no specificity | 0 (0%) | 1 (1%) | 1 (1%) | |
| Diabetes mellitus, steroid induced, within 24 months | 24 (20%) | 36 (23%) | 43 (45%) | <0.0001 |
| Mean number of episodes of Leukopenia within 24 months | 0.099 | |||
| N | 44 | 64 | 45 | |
| Mean ± SD | 1 ± 0.9 | 1.4 ± 1.2 | 1.2 ± 0.8 | |
| Median (IQR) | 1 (0, 1) | 1 (1, 2) | 1 (1, 1) | |
| eGFR at onset (ml/min/1.73 m2) Median (IQR2) |
24 (14, 52) | 25 (12,46) | 17 (11, 30) | 0.005 |
| Incidence of relapse within 24 months (per-person-year) | 0.042 | 0.038 | 0.034 | |
| Death from any cause within 24 months | 8 (7%) | 9 (6%) | 24 (25%) | <0.0001 |
SD, standard deviation; IQR, interquartile range; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; P, perinuclear; PR3, proteinase 3; C, cytoplasmic.
aSevere infection defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death.
bP-values were calculated by Fisher exact test for categorical variables and Wilcoxon two samples for continuous variables.
Steroid-induced diabetes, corticosteroid treatment and severe infections
The association between steroid-induced diabetes, corticosteroid treatment and severe infections was further explored. Patients developed steroid-induced diabetes in a median of 1 month (28.5 days; IQR: 3 days to 4.0 months). In the 2-year cohort, patients who developed steroid-induced diabetes were more likely to receive pulse methylprednisolone for their initial treatment for vasculitis (58%) compared with those who did not develop steroid-induced diabetes (44%, P = 0.006). There was no difference in months of oral prednisone between those who did and did not develop steroid-induced diabetes (P = 0.92). Among those who did not receive methylprednisolone, 31% (14/48) with diabetes had a least one severe infection versus 20% (30/147) among those without diabetes (P = 0.17). Among those who did receive methylprednisolone, 45% (29/65) with diabetes had at least one severe infection versus 19% (22/114) among those without diabetes (P = 0.0005).
Timing of infections following immunosuppression
Pulmonary and upper respiratory infections were the most common, followed by systemic/organ threatening infections and urinary tract infections/pyelonephritis (Figure 3a). The greatest numbers of infections occurred in the first 3 months (Figure 3a). The time line of when infections occurred within the first 24 months is presented by infection type in Figure 3a. Systemic/organ threatening infections included sepsis or any infection that caused an organ-based infection such as meningitis, endocarditis and orbital infection. Viral infections encompassed, but were not limited to, herpes simplex virus, varicella-zoster virus and cytomegalovirus. Adenovirus and upper respiratory virus infections were encompassed within the upper respiratory infection (URI) categorization.
FIGURE 3:
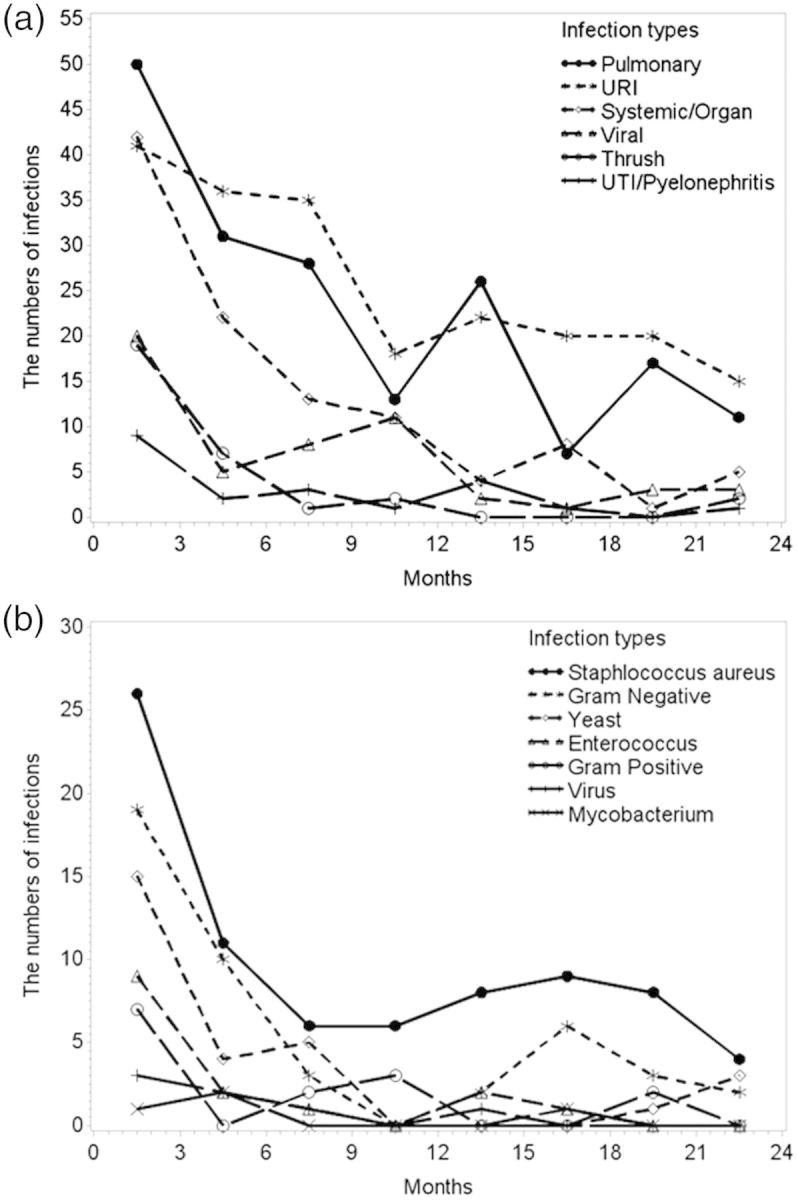
Number of infections experienced within 3 month time intervals from initiation of first therapy out to 24 months by (a) infection type and by (b) microbiologic culture type (among 128 patients cultured with 112 having 192 positive cultures). Multiple infections and cultures per person are shown.
Causative organisms for infections
To understand infectious etiology, we reviewed 128 patients who had an available culture within 24 months following treatment initiation. Of these, 95 patients had 249 positive cultures from urine (38%), the upper respiratory tract (15%), blood (13%), lung (10%), skin (4%), other sites (5%) or from an unspecified site (15%). Twenty-nine of the 249 (12%) cultures were from severe infections. Results are broken down as a temporal outline in 3-month intervals across 24 months (Figure 3b). Staphylococcus aureus was the most frequently grown infectious agent in culture (34%, 85 cultures positive for S. aureus/249 total positive cultures) and was found most commonly in urine (54% of those positive for S. aureus) and the upper respiratory tract (16%), but also in blood, lungs, skin and stool (<6% each), with 10% from unknown sites. Eighteen of the 85 (21%) S. aureus cultures were among patients who had received trimethoprim sulfamethoxazole as prophylaxis. Twelve (14%) of the 85 S. aureus cultures were from infections classified as severe. Gram negative organisms (25%, 64/249) and yeast (16%, 40/249) were the next most common categories (Figure 3b). There was one positive culture for Pneumocystis jiroveci pneumonia 6 weeks following treatment initiation, and this patient had not received prophylactic therapy.
Infection differences by gender
Types of infections were explored by sex to understand the trends for more total infections and severe infections in females compared with males. Among the entire cohort, women were far more likely than men to have urinary tract infections/pyelonephritis (73/232 = 32 versus 32/257 = 13%, respectively; P < 0.0001). Upper respiratory infections were seen in 9% more of the women than men (35 versus 26%, respectively; P = 0.02), but all other types of infections were similar by sex.
Influence of immunosuppression on infections
Within 2 years of follow-up, patients with severe infection had less exposure to cyclophosphamide [median 5 months (IQR: 2, 9)] compared with those who never experienced severe infection [median 6 months (IQR: 4,11); P = 0.008]. Duration of all other immunosuppressive therapies was similar between those with and without severe infection. Months of exposure to cyclophosphamide, prednisone, MMF, azathioprine, methotrexate, or infusions of methylprednisolone or rituximab was not different by number of infections (0 versus 1–2 versus ≥3). However, months of combined exposure to MMF, azathioprine or methotrexate in the 24-month cohort was higher among those who had no infections [median 14 months (IQR: 11, 18)] and those who had ≥3 infections [median 13 months (IQR: 7, 17)] compared with those who had 1 or 2 infections [median 8 months (IQR: 3,14); P = 0.0001]. These trends were similar in the 12-month cohort. Due to the dynamic interplay of treatment choices made in response to patients experiencing an infection, which usually involved decreasing, stopping or changing treatment, modeling of duration of immunosuppression therapy across infectious outcomes was not pursued.
Death
Cumulative incidence of death in the total cohort at 1, 2 and 5 years was =1, 2 and 7%, respectively, which are unadjusted estimates that are censored for loss of follow-up (Figure 2). Within the 1-year cohort, 31 of 421 (7%) died within 12 months; 29% with active disease, 13% from infectious complications, 29% from various causes after reaching ESKD, 10% from cardiovascular events and 19% from unknown causes prior to reaching ESKD. Advanced age was associated with death within 12 months (alive median age 57 years [IQR 45, 68]), versus those who died median age 72 [IQR 66, 74], P = < 0.0001), as was lower eGFR (alive median eGFR 22 mL/min/1.73 m2 [IQR 12, 43], versus those who died (median eGFR 12 [IQR 8, 20], P = 0.0002). Severe infection within 12 months was associated with death: 19% (19/98) with severe infections died compared with 4% (12/323) with no severe infection; P ≤ 0.0001. In multivariable analysis controlling for age, sex, eGFR and severe infection, age and severe infection remained associated with death within 12 months: HR for age/1-year increase = 1.06 (95% CI 1.02, 1.09, P = 0.0011); HR for severe infection = 4.20 (95% CI 2.0, 8.7; P = 0.001). There was a trend for lower eGFR to predict death (HR = 0.97, 95% CI 0.94, 1.0, P = 0.066). ANCA serotype, sex and involvement of specific organs were not associated with death at 12 months. Ten additional patients died between 12 and 24 months for a total of 41 deaths in 24 months. Results were virtually the same for the impact of age and severe infection on death at 24 months (Table 5).
Table 5.
Number of patients who ever experienced each category of infection over the entire follow-up by cohort (the 12-month cohort, the 24-month cohort and the total cohort)
| Infections | 1-Year follow-up cohorta (N = 421) | 2-Year follow-up cohortb (N = 374) | Total cohortc (n = 489) | Females from total cohort (N = 232) | Males from total cohort (N = 257) | P-valuesd |
|---|---|---|---|---|---|---|
| Pulmonary | 185 (44%) | 175 (47%) | 203 (42%) | 103 (44%) | 100 (39%) | 0.23 |
| Upper respiratory | 140 (33%) | 130 (35%) | 148 (30%) | 82 (35%) | 66 (26%) | 0.02 |
| Systemic/organ | 106 (25%) | 99 (27%) | 124 (25%) | 59 (25%) | 65 (25%) | 1.00 |
| Urinary tract/pyelonephritis | 93 (22%) | 87 (23%) | 105 (22%) | 73 (32%) | 32 (13%) | <0.0001 |
| Viral (N = 80)% | 78 (19%) | 66 (18%) | 80 (16%) | 45 (19%) | 35 (14%) | 0.088 |
| Cellulitis (N = 56)% | 53 (13%) | 49 (13%) | 56 (12%) | 25 (11%) | 31 (12%) | 0.67 |
| Thrush (N = 37)% | 32 (8%) | 28 (8%) | 37 (8%) | 19 (8%) | 18 (7%) | 0.73 |
| Otitis media (N = 28)% | 26 (6%) | 26 (7%) | 28 (6%) | 13 (6%) | 15 (6%) | 1.00 |
| Musculoskeletal (N = 11)% | 11 (3%) | 11 (3%) | 11 (2%) | 4 (2%) | 7 (3%) | 0.55 |
| Other (N = 7)% | 7 (2%) | 7 (2%) | 7 (1%) | 2 (1%) | 5 (2%) | 0.45 |
aPatients in this cohort died within or were followed for a complete 12 months.
bPatients in this cohort died within or were followed for a complete 24 months.
cAll patients with all available follow-up included in this cohort.
dP-values compare females and males and were calculated using Fisher Exact tests.
DISCUSSION
Efforts made to control vasculitis through immunosuppression are associated with the encumbrance of adverse events, most notably infections that increase patient morbidity and mortality. This work supports and expands upon mounting evidence that further reduction of mortality in vasculitis patients requires a reduction of infections [10, 14, 23–25]. We have found that increasing numbers of infections increase the chance of having a severe infection which in turn increases risk of all-cause mortality. The risk of mortality from severe infections, the high risk of infections in the first year and the predominance of pulmonary infections are similar to a recent report out of China [25]. However, our study considers the impact of severe and non-severe infections and uniquely shows temporal breakdown of organisms causative of infection.
The quandary of patient management hangs in the balance of controlling or preventing recurrence of active vasculitis in the face of increasing risk of infections from therapy. Death rates in vasculitis prior to the use of immunosuppression were 85% at 5 years [1–4] and it was not until effective immunosuppressive strategies were defined [5, 26] that vasculitis morphed from an acute cause of death to a disease of chronic morbidity [10, 23, 27]. Understanding the intertwined immunosuppression-associated outcomes of maintaining quiescent disease and also limiting infections suggests the need for patient-specific treatment strategies.
It is accepted that infections are related to immunosuppression. Within the first 12 months following diagnosis, 56% of our patient population had some form of infection and 23% had a severe infection. Finding strategies for prevention or reduction of respiratory infections and urinary tract infections/pyelonephritis, the most common causes of infection in our cohort, would have a substantial impact on reducing the impact of infection, including severe infections. Focus on prevention of severe infection by early detection and treatment with antimicrobials and reduction of immunosuppression may also reduce all-cause mortality.
Prophylactic therapy was not associated with number of infections in 1 or 2 years in this study, but uniform use of prophylaxis was not employed. When prescribed, the intention was to treat with prophylaxis therapy throughout induction therapy. However, without systematic implementation and information on compliance, we recognize the true impact of prophylactic therapy cannot be evaluated. This study provides a time line of when infections occur and which infectious agents are causative. Although we did not have comprehensive culture assessment for all infections, we believe our assessment of the microbiological data can guide infection prevention and prophylaxis more specifically in AAV. Importantly, there was only one case of Pneumocystis jiroveci pneumonia in this cohort. The highest proportion of positive cultures was Staphylococcus aureus. Previous studies note an association between presence of S. aureus and active vasculitis [28]. There could be a benefit in the first 3–6 months following treatment initiation to routinely provide patients with prophylaxis against Staphylococcus aureus, Gram negative and yeast infections. When looking at our treatment pattern for corticosteroid administration we note a correlation that we have previously noted between the time we prescribe corticosteroids and when infection burden is at its highest [29]. Thrush and other infections caused by yeast are of greatest burden in the time frame when steroids are prescribed in the highest doses. Could a steroid-sparing strategy in AAV control infections in general and/or those specifically associated with yeast? Also important to consider is that patients with steroid-induced diabetes mellitus (DM) are at increased risk of infection, especially severe infections. Steroid-induced DM occurs in 30% of our population, and keen attention to reduction in steroids is vital as they are associated with hyperglycemia as well as infection [29]. In our cohort the combined impact of methylprednisolone use and subsequent steroid-induced diabetes conferred the strongest risk for severe infections, but an association of steroid-induced diabetes with severe infection in the absence of use of methylprednisolone cannot be ruled out. Although the majority of AAV patients do not end up with steroid-induced DM, the morbidity this diagnosis carries is significant and speaks to the importance of steroid-sparing strategies in AAV to reduce morbidity, as are being piloted in other autoimmune diseases [30].
While attempts at universal reduction of overall infection burden are a critical step, we must also be conscious of treating each patient considering individual risks of adverse events and disease relapse with an attempt at patient specific/directed therapy. A difficulty with studying outcomes in vasculitis is that the outcomes of infection and relapse are intertwined. Infections may require a reduction in immunosuppression that could allow disease activity to emerge. Having an infection can also directly trigger a relapse [31–33]. Furthermore, increased immunosuppression used to treat relapse also puts patients at increased risk for infections.
Additionally, self-monitoring by use of routine urinary dip sticks for the presence of nitrites and leukocyte esterase to catch urinary tract infections early and trigger early directed antibiotic courses would likely be beneficial. Urinary dip stick use is inexpensive and easy for patients to incorporate into a self-care routine. Furthermore, routine urinary dip stick home evaluation could also be extremely valuable in the AAV population for early detection of relapse. We commonly recommend that patients with a history of glomerulonephritis on or off immunosuppression self-monitor with urinary dip-sticks. This enables the early detection of hematuria or increasing proteinuria and/or increasing nitrites and leukocyte esterase to prompt urgent clinic evaluation for relapse or infection. Catching either event early could prevent significant organ threatening impacts of the disease and development of severe infections.
This study is not a randomized controlled trial geared at providing clear-cut guidelines for treatment of AAV, but this real world experience of an inception cohort allows use of what we know from our patients in the past to improve how we treat patients in the future. Level of disease activity including treatment resistance and specific complications of vasculitis such as lung cavities or damage were not included in our analysis. Non-uniform treatment and lack of total doses of each immunosuppressive treatment in the cohort limit our ability to distinguish differences in infection risk by therapy type, dose or induction versus maintenance therapy. Furthermore, immunosuppressive treatments are typically reduced or changed in response to an infection, which is supported by our data showing that patients with more infections or severe infections have less exposure to immunosuppression than those with the lowest infectious burden. Of note, the patients who had 1–2 infections (rather than 0 or ≥3 infections) had the least exposure to remission maintenance therapy: MMF, azathioprine and/or methotrexate. This may indicate that patients who do not have infections are on remission maintenance longer and patients with higher numbers of infections or more severe infections are switched from cyclophosphamide to remission maintenance therapy sooner. Our analysis does not take into account patient frailty which also potentially impacts immunosuppression choices and adverse events.
In conclusion, this study provides information on the types of organisms causing infection and a time line of when these infections occur. This information allows for more directive and well-timed prophylactic therapy. This study provides fodder for constructing future randomized control trials which can control for variables not possible to control for in an inception cohort study. The focus of clinical care and future studies regarding therapy for vasculitis requires a shift from the sole emphasis on attaining and retaining remission towards a balance of immunosuppression for disease control and infection reduction. Part of finding this balance may involve down-titrating immunosuppressive therapy alongside early relapse monitoring with urine dip sticks and close follow-up. It is time to take a personalized approach rather than treating all patients with the same immunosuppression strategy. We must attempt to avoid the harm from our therapies with overuse above the requirements of disease control.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Zeek PM. Periarteritis nodosa and other forms of necrotizing angiitis. N Engl J Med 1953; 248: 764–772 [DOI] [PubMed] [Google Scholar]
- 2.Rose GA, Spencer H. Polyarteritis nodosa. Q J Med 1957; 26: 43–81 [PubMed] [Google Scholar]
- 3.Frohnert PP, Sheps SG. Long-term follow-up study of periarteritis nodosa. Am J Med 1967; 43: 8–14 [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS, Haynes BF, Katz P, et al. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983; 98: 76–85 [DOI] [PubMed] [Google Scholar]
- 5.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009; 68: 310–317 [DOI] [PubMed] [Google Scholar]
- 6.Guillevin L, Cordier JF, Lhote F, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis [see comments]. Arthritis Rheum 1997; 40: 2187–2198 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman GS, Leavitt RY, Fleisher TA, et al. Treatment of Wegener's granulomatosis with intermittent high-dose intravenous cyclophosphamide [see comments]. Am J Med 1990; 89: 403–410 [DOI] [PubMed] [Google Scholar]
- 8.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150: 670–680 [DOI] [PubMed] [Google Scholar]
- 9.Nachman PH, Hogan SL, Jennette JC, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7: 33–39 [DOI] [PubMed] [Google Scholar]
- 10.Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010; 69: 1036–1043 [DOI] [PubMed] [Google Scholar]
- 11.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369: 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 [DOI] [PubMed] [Google Scholar]
- 14.Hogan J, Avasare R, Radhakrishnan J. Is newer safer? Adverse events associated with first-line therapies for ANCA-associated vasculitis and lupus nephritis. Clin J Am Soc Nephrol 2014; 9: 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan SL, Nachman PH, Wilkman AS, et al. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7: 23–32 [DOI] [PubMed] [Google Scholar]
- 16.Falk RJ, Jennette JC. ANCA disease: where is this field heading? J Am Soc Nephrol 2010; 21: 745–752 [DOI] [PubMed] [Google Scholar]
- 17.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37: 187–192 [DOI] [PubMed] [Google Scholar]
- 18.Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2006; 55: 146–149 [DOI] [PubMed] [Google Scholar]
- 19.Allenbach Y, Seror R, Pagnoux C, et al. High frequency of venous thromboembolic events in Churg-Strauss syndrome, Wegener's granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: a systematic retrospective study on 1130 patients. Ann Rheum Dis 2009; 68: 564–567 [DOI] [PubMed] [Google Scholar]
- 20.Merkel PA, Lo GH, Holbrook JT, et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med 2005; 142: 620–626 [DOI] [PubMed] [Google Scholar]
- 21.Stassen PM, Derks RP, Kallenberg CG, et al. Venous thromboembolism in ANCA-associated vasculitis--incidence and risk factors. Rheumatology (Oxford) 2008; 47: 530–534 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 23.Wall N, Harper L. Complications of long-term therapy for ANCA-associated systemic vasculitis. Nat Rev Nephrol 2012; 8: 523–532 [DOI] [PubMed] [Google Scholar]
- 24.Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener's granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43: 1021–1032 [DOI] [PubMed] [Google Scholar]
- 25.Lai QY, Ma TT, Li ZY, et al. Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a study of 398 Chinese patients. J Rheumatol 2014; 41: 1849–1855 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients [see comments]. Ann Intern Med 1992; 116: 488–498 [DOI] [PubMed] [Google Scholar]
- 27.Flossmann O, Berden A, de GK, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011; 70: 488–494 [DOI] [PubMed] [Google Scholar]
- 28.Stegeman CA, Cohen Tervaert JW, Manson WL, et al. Chronic nasal carriage of Staphylococcal aureus in Wegener's granulomatosis identifies a subgroup of patients more prone to relapse. Ann Intern Med 1994; 120: 12–17 [DOI] [PubMed] [Google Scholar]
- 29.McGregor JG, Hogan SL, Hu Y, et al. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol 2012; 7: 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lightstone L. Minimising steroids in lupus nephritis—will B cell depletion pave the way? Lupus 2013; 22: 390–399 [DOI] [PubMed] [Google Scholar]
- 31.Tadema H, Abdulahad WH, Lepse N, et al. Bacterial DNA motifs trigger ANCA production in ANCA-associated vasculitis in remission. Rheumatology (Oxford) 2011; 50: 689–696 [DOI] [PubMed] [Google Scholar]
- 32.Tadema H, Abdulahad WH, Stegeman CA, et al. Increased expression of Toll-like receptors by monocytes and natural killer cells in ANCA-associated vasculitis. PLoS One 2011; 6: e24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallenberg CG, Stegeman CA, Abdulahad WH, et al. Pathogenesis of ANCA-associated vasculitis: new possibilities for intervention. Am J Kidney Dis 2013; 62: 1176–1187 [DOI] [PubMed] [Google Scholar]