Abstract
Background
Gap junctional intercellular communication (GJIC) is typically decreased in malignant tumors. Gap junction is not presented between hematopoietic cells but occurred in bone marrow stromal cells (BMSCs). Connexin 43 (Cx43) is the major gap junction (GJ) protein; our previous study revealed that Cx43 expression and GJIC were decreased in acute leukemic BMSCs. All-trans retinoic acid (ATRA) increases GJIC in a variety of cancer cells and has been used to treat acute promyelocytic leukemia, but the effects of ATRA on leukemic BMSCs is unknown. In this study, we evaluated the potential effects of ATRA on cell cycle, proliferation, and apoptosis of leukemic BMSCs. Effects of ATRA on Cx43 expression and GJIC were also examined.
Methods
Human BMSCs obtained from 25 patients with primary acute leukemia, and 10 normal healthy donors were cultured. Effects of ATRA on cell cycle, cell proliferation, and apoptosis were examined with or without co-treatment with amphotericin-B. Cx43 expression was examined at both the mRNA and protein expression levels. GJIC was examined by using a dye transfer assay and measuring the rate of fluorescence recovery after photobleaching (FRAP).
Results
ATRA arrested the cell cycle progression, inhibited cell growth, and increased apoptosis in leukemic BMSCs. Both Cx43 expression and GJIC function were increased by ATRA treatment. Most of the observed effects mediated by ATRA were abolished by amphotericin-B pretreatment.
Conclusions
ATRA arrests cell cycle progression in leukemic BMSCs, likely due to upregulating Cx43 expression and enhancing GJIC function.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-015-0212-7) contains supplementary material, which is available to authorized users.
Keywords: All-trans retinoic acid, Bone marrow stromal cells, Connexin 43, Gap junctional intercellular communication, Leukemia BMSCs
Background
Gap junctions (GJs) are specialized structures consisting of highly conserved trans-membrane proteins connexins and allow passage of small molecule (<1 kD) between the cytoplasm of two adjacent cells [1, 2]. A total of 21 human connexin subtypes have been identified up to date, with each one displaying unique tissue expression pattern [3]. Decreased expression of connexins and thus compromised gap junctional intercellular communication (GJIC) is noted in a variety of malignant tumors and contributes to their uncontrolled proliferation [1, 3–5]. Increased connexin expression and restored GJIC using molecular approach have been shown to inhibit the growth of solid tumors [6–9], decrease invasive potential, and jeopardize neovascularization [10, 11].
GJs do not exist between hematopoietic cells but are present between bone marrow stromal cells (BMSCs) and hematopoietic cells [12, 13]. Communication between BMSCs and hematopoietic cells is an important part of the microenvironment for establishment and growth of tumor cells [14–16]. For GJs at these sites, connexin 43 (Cx43) is the major subtype [12]. Functional GJIC in BMSCs has direct impact on the proliferation and differentiation of hematopoietic stem/progenitor cells [17]. Our previous studies revealed decreased Cx43 expression and GJIC function of acute leukemic BMSCs, which could recover after adopting effective chemotherapy or transfection with Cx43 gene [18, 19]. These findings suggested that Cx43 expression and GJIC function in BMSCs are likely to be a dynamic process and regulated by many factors in vivo and in vitro. This hypothesis has been verified by some studies which demonstrated that some hormones, cytokines, and drugs could affect the expression of Cxs between coupling cells [20–23].
All-trans retinoic acid (ATRA) is a natural derivative of vitamin A and could regulate various physiological events, including cell cycle, embryonic development, and cellular differentiation [24]. ATRA could inhibit malignant transformation and produce anti-proliferative effect in several types of tumor cells, possibly through enhancing GJIC function in tumor cells [25–27]. ATRA has been used in the treatment of acute promyelocytic leukemia (APL) [28] and other hematologic diseases [29, 30] based on induction of differentiation and inhibition of growth. However, whether ATRA affects GJIC in leukemic BMSCs remains unknown.
In this study, we examined the potential effects of ATRA on cell cycle progression of leukemic BMSCs from 25 patients with acute leukemia. Increased Cx43 expression by ATRA was verified with mRNA and Western blot. Enhanced GJIC function was verified with fluorescent dye dispersion as well as a bleaching assay. The experiments revealed arrested cell cycle progression, inhibited growth, and increased apoptosis upon ATRA exposure. These findings suggest that GJIC function is implicated in the therapeutic action of ATRA for leukemia.
Results
ATRA increases Cx43 mRNA
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis showed that treatment with ATRA (10 uM) increased Cx43 mRNA levels in leukemic BMSCs by >50 % (p < 0.01) to a level closed to normal BMSCs (p < 0.05, Fig. 1). Pretreatment with amphotericin-B did not affect the ATRA action on Cx43 mRNA.
Fig. 1.
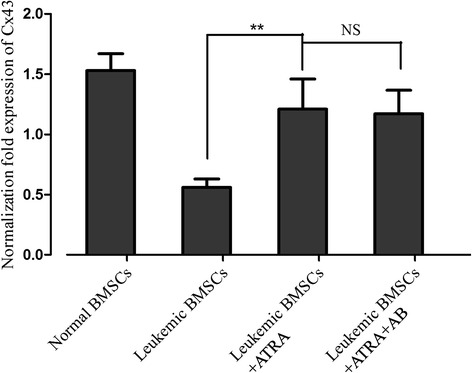
Effects of ATRA and amphotericin-B pretreatment on Cx43 mRNA level in leukemic BMSCs. Cx43 mRNA (normalized against β-actin) was 1.53 ± 0.14 in healthy controls (N = 10), 0.56 ± 0.07 leukemic BMSCs (N = 10) treated with DMSO, 1.21 ± 0.36 in leukemic BMSCs (N = 25) exposed to ATRA (10 uM), and 1.17 ± 0.196 in leukemic BMSCs exposed to ATRA + amphotericin-B. Data are presented as mean ± SD. Double asterisks indicate P < 0.01
ATRA increases Cx43 protein expression
Western blot analysis showed that ATRA (10 uM) increased Cx43 protein levels by approximately 70 % (p < 0.01) to a level not statistically different from normal BMSCs (Fig. 2a, b). Moreover, treatment with amphotericin-B did not affect Cx43 protein expression levels mediated by ATRA.
Fig. 2.
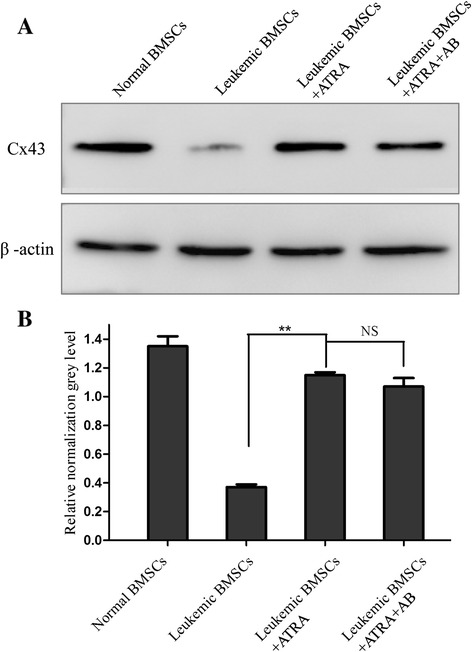
Effects of ATRA and amphotericin-B pretreatment on Cx43 protein level in leukemic BMSCs. a Cx43 protein (normalized against β-actin) was 1.35 ± 0.17 in healthy controls (N = 10), 0.37 ± 0.02 in leukemic BMSCs (N = 25) treated with DMSO, 1.15 ± 0.03 in leukemic BMSCs exposed to ATRA (10 uM) (N = 25), and 1.07 ± 0.02 in leukemic BMSCs treated with both ATRA and amphotericin-B (N = 25); b relative normalization gray level of Cx43 Western blotting band in health controls, leukemic BMSCs, leukemic BMSCs + ATRA, leukemic BMSCs + ATRA + AB. Data are presented as mean ± SD. Double asterisks indicate P < 0.01
ATRA enhances GJIC
Dye transfer assay was a rapid, sensitive method to detect GJIC function between cells [31]. In this study, Lucifer yellow (LY) fluorescence in leukemic BMSCs dispersed to only 1–2 cell lines surrounding the wounded edge (Fig. 3a). After ATRA treatment, LY fluorescence dispersed to 4–5 cell lines (Fig. 3b). Pretreatment with AB could block the effect mediated by ATRA (Fig. 3c).
Fig. 3.
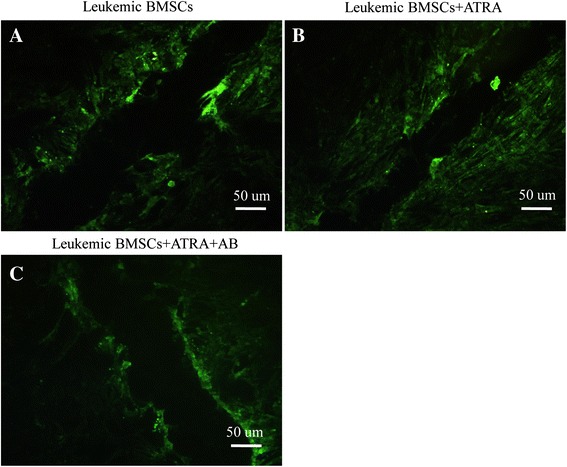
Effects of dye transfer between BMSCs by using laser confocal microscopy. a Leukemic BMSCs not exposed to ATRA; b leukemic BMSCs exposed to ATRA (10 M); c leukemic BMSCs treated with both ATRA and amphotericin-B. Scale bar = 50 um
The fluorescence recovery after photobleaching (FRAP) assay showed 20 % recovery of fluorescent signal in leukemic BMSCs at 1 min after photobleaching. The maximal recovery percentage was 36.25 % (±2.14); the average recovery rate was 4.82 % (±0.78) min−1 (Fig. 4d–f). ATRA treatment promoted the recovery of fluorescent signal after photobleaching (Fig. 4a–c): 35 % of cells could be recovered in 1 min and 80.94 % (±1.46) could be recovered within 30 min. The average recovery rate was increased to 15.03 % (±1.27) min−1 (Fig. 4g, h). Pretreatment with amphotericin-B blocked the effects of ATRA.
Fig. 4.
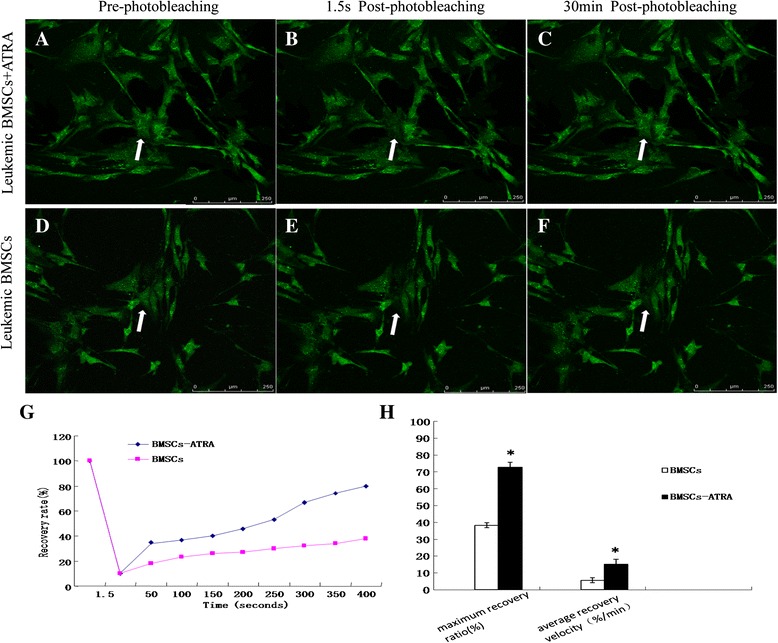
FRAP assay in BMSCs. a Pre-photobleaching of leukemic BMSCs exposed to ATRA; b 1.5 s post-photobleaching of leukemic BMSCs exposed to ATRA; c 30 min post photobleaching of leukemic BMSCs exposed to ATRA. d–f Leukemic BMSCs without ATRA treatment (d pre-photobleaching of leukemic BMSCs without ATRA treatment; e 1.5 s post-photobleaching of leukemic BMSCs without ATRA treatment; f 30 min post photobleaching of leukemic BMSCs without ATRA treatment). g Effects of ATRA on fluorescence redistribution in leukemic BMSCs. h Effects of ATRA on GJIC in leukemic BMSCs. Arrow symbols photobleached cells. *p < 0.01 vs. leukemic BMSCs. Images were taken under a laser confocal microscope at ×400 magnification
ATRA changes biologic characteristics of BMSCs through GJIC
ATRA (10 uM for 48 h) inhibited the cell growth of leukemic BMSCs by 40 % (p < 0.05; Fig. 5), and pretreatment with amphotericin-B blocked the ATRA effects.
Fig. 5.
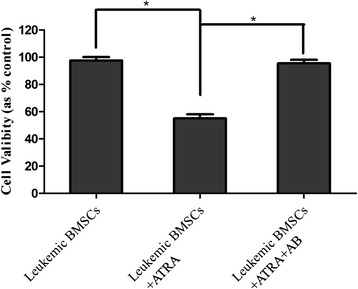
Effects of ATRA on the cell viability of BMSCs (MTT assay). Cell viability was evaluated by MTT assay in leukemic BMSCs, leukemic BMSCs treated with ATRA, and leukemic BMSCs treated with both ATRA and amphotericin-B. Data are presented as mean ± SD. Single asterisk indicates significant difference (P < 0.05)
Flow cytometric analysis showed that ATRA treatment led to decrease the percentage of the cells in S phase from 29.5 to 7.96 % and led to increase the percentage of cells in the G0/G1 phase from 61.1 to 77.2 % (Additional file 1: Figure S1A–C). Such effects were abolished by amphotericin-B pretreatment (Fig. 6).
Fig. 6.
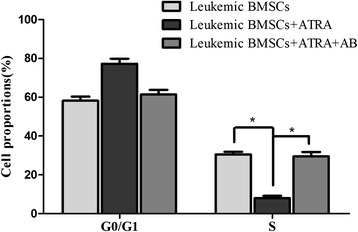
Effects of ATRA on the cell cycle (FCM assay). Cell cycle was examined with FCM assay in leukemic BMSCs, leukemic BMSCs treated with ATRA, and leukemic BMSCs treated with both ATRA and amphotericin-B. Data are presented as mean ± SD. Single asterisk indicates significant difference (P < 0.05)
ATRA treatment increased apoptosis rate from 5.16 ± 2.35 to 9.78 ± 4.88 % (p < 0.05) (Additional file 2: Figure S2A–C). Such effects were attenuated by amphotericin-B pretreatment (p < 0.05; Fig. 7).
Fig. 7.
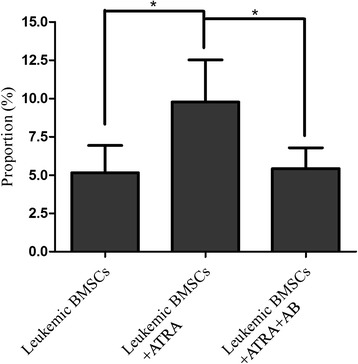
Effects of ATRA on the apoptosis of BMSCs (FCM assay). Cell apoptosis was examined with FCM assay in leukemic BMSCs, leukemic BMSCs treated with ATRA, and leukemic BMSCs treated with both ATRA and amphotericin-B. Data are presented as mean ± SD. Single asterisk indicates significant difference (P < 0.05)
Discussion
Abnormal expression of connexin genes in tumor cells is the result of downregulation rather than genomic deletion or mutation, and thus could be rescued by pharmacological treatment [32, 33]. Increasing the expression of connexin genes could restore the function of gap junctions [3, 34]. Recent studies showed that ATRA could improve GJIC function in several types of tumor cells [28, 35].
In the current study, we extended previous findings to leukemic BMSCs. ATRA treatment increased the expression of Cx43 at both mRNA and protein levels and resulted in increased intercellular communication. The mechanisms of such action remain obscure, but based on the secondary structure of Cx43 [36, 37], it is reasonable to speculate that ATRA could bind to retinoic acid nuclear receptors (i.e., RARs and RXRs) and activate the PKC pathways, which in turn phosphorylate proteins that bind to the phosphorylation site of the Cx43 mRNA. The resulting instability due to AQUA repeated sequences decrease the degradation of Cx43 mRNA. Alternatively, ATRA could activate Cx43 gene transcription and translation more directly through binding to factors such as AP-1.
In a previous study from this laboratory [18], we demonstrated that GJIC function is dysfunctional between leukemic BMSCs. Such a finding is consistent with the reported decrease in GJIC in several solid tumors [8, 38, 39]. In the current study, we showed that GJIC between leukemic BMSCs is enhanced by ATRA treatment. It is noteworthy to mention the discrepancy of the reported action of ATRA on connexin gene expression in tumors in previous studies. For example, Wageenblatt et al. [40] reported inhibited expression of several connexin genes, including Cx43, by ATRA in carcinoma of urinary bladder. Based on such a finding, the authors argued that connexin gene upregulation provides a “growth advantage” in certain type(s) of tumor cells.
The results of the current study clearly showed that ATRA could arrest cell cycle progression in leukemic BMSCs. Consistently, cell proliferation was decreased and apoptosis was enhanced. These effects could be abolished by amphotericin-B pretreatment, and thus could reasonably attributed to GJICs.
The connection between increased GJIC and BMSC biological properties is now unknown. Potential mechanisms could include purinergic signaling via extracellular adenosine triphosphate (ATP), change of Ca2+ internal flow from extracellular through gap junction, and downregulation of monocyte chemotactic protein 1 expression [41–44]. Nevertheless, the current study extended previous findings of ATRA in solid tumors [11, 45] to leukemia.
Conclusions
ATRA treatment could arrest cell cycle progression in leukemic BMSCs, likely due to increasing Cx43 expression and enhancing GJICs in human leukemic BMSCs. These findings encourage future exploration of using ATRA in the treatment of hematopoietic malignancies.
Materials
Sample source
Samples of acute leukemic bone marrow were obtained from 25 patients with primary acute leukemia from the Department of Hematology of Xinqiao Hospital between April 2014 and February 2015. Patients included 14 males and 11 females, with a median age of 33. Among the patients were 14 patients with ALL, 3 patients with AML-M2, 3 patients with AML-M3, 3 patients with AML-M5, and 2 patients with AML-M6. The diagnosis was established on the basis of clinical data, tissue/cell morphology, and immunophenotyping. Bone marrow samples from ten normal healthy donors were used as healthy controls. The research was approved by the Ethics Committee of Xinqiao Hospital, and all subjects gave written informed consent.
Cell culture and treatment
Human BMSCs were isolated as described previously [46] and were plated in T75 flasks for continuous passage in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) supplemented with 12.5 % FBS (Gibco, USA), 12.5 % HS (Gibco, USA), and 0.1 mM hydrocortisone (Sigma, USA). Medium was changed twice weekly, and cells were passaged into fresh culture flasks at a ratio of 1:4 upon reaching confluence by using trypsin (Sigma, USA). The cultures were incubated at 37 °C in a humidified incubator with 5 % CO2.
ATRA and amphotericin-B (both from Sigma) stock solutions were prepared in dimethyl sulfoxide (DMSO; Sigma, USA) and stored at −20 °C prior to use. The final concentration of DMSO was <0.1 %. BMSCs at passage 3 (reach to 80 % confluence) were used for experiments.
qRT-PCR
Total RNA was extracted from BMSCs by using a commercial kit from Stratagene (USA). cDNA synthesis and amplification were carried out using 2 μg mRNA with RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, USA) and GeneAmp PCR System 2700 Thermal Cycler (Applied Biosystems, USA). qRT-PCR conditions were as follows: 95 °C for 15 min, then 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Primers were as follows: Cx43 (NM_000165), forward 5′-AACCTGGTTGTGAAAATGTC-3′; reverse 5′-GCAAGTGTAAACAGCACTCA-3′; β-actin (NM_001101), forward 5′-CCTGTGGCATCCACGAAACT-3′; reverse 5′-GAGCAATGATCCTGATCTTC-3′. Results were normalized against β-actin.
Western blot
Western blot analysis was performed using whole-cell extract. Protein concentration of the samples was determined using a BCA method. Proteins (20 μg per lane) were separated using 12 % SDS-PAGE gel, transferred to nitrocellulose membrane, and blocked with 5 % skim milk in PBS. The membrane was incubated with a rabbit polyclonal anti-Cx43 antibody (Sigma, USA), and then with a secondary antibody linked to horseradish peroxidase prior to ECA visualization (Amersham Biosciences AB, Uppsala, Sweden). Blots were washed, reprobed with an anti-GAPDH antibody (Sigma, USA), and then developed in an identical manner for assessment of GAPDH to ensure even loading.
Dye transfer assay
Transfer assay was carried out as previously described [47]. Briefly, cultured cells were rinsed thoroughly with Hanks’ balanced salt solution (HBSS) (with calcium and magnesium) containing 1 % BSA. A 27-gauge needle was used to create multiple scratches (minimum distance between scratches 1 cm) in the cell monolayer in the presence of PBS containing 0.5 % Lucifer yellow (Gibco, USA). After exactly 1 min, the culture was rinsed with HBSS and then incubated for 1–10 min in the saved culture medium to allow the loaded dye to transfer to adjoining cells. The cells were then rinsed and fixed with 4 % paraformaldehyde and immediately analyzed under a laser confocal scanning microscope (LCSM; Leica, German) at 485 nm. GJIC was defined as the average distance of dye travel (μm) at six different sites in each sample.
FRAP assay
FRAP assay was carried out in living cells as described previously [48]. Briefly, cells cultured on glass cover slips were loaded with C-FDA (10 μmol/L, 15 min; Sigma) at 37 °C and washed thoroughly with DMEM medium. A cell with direct contact with 4–5 neighboring cells within a cluster was selected under the LCSM. A pre-bleach image of the whole field was taken using low laser intensity (acousto-optic tunable filter (AOTF) = 10 %, zoom = ×2). Then, laser intensity was increased by ×50 (AOTF = 50 %, zoom = ×20), and the target BMSCs was photobleached for 1 s. Transfer of fluorescent dye from neighboring cells was examined by scanning at 50 s intervals for a total period of 30 min. The rate of fluorescence recovery index was calculated as: R = (It − I0) / (I − I0) × 100 %, where I0 is the intensity of the photo-bleached fluorescence and I is the intensity of pre-bleached fluorescence.
MTT assay
Cell proliferation was examined using a methyl thiazolyl tetrazolium (MTT) assay (Sigma, USA) at 610 nm. Data were averaged from three independent experiments.
Flow cytometry
Flow cytometry (FCM) assay was carried out as described previously [49]. BMSCs were washed, fixed in 70 % ethanol, and resuspended in 10 mL PBS. Cells were stained with propidium iodide (5 μL 10 mg/mL) and DNAse-free RNase (200 μg/mL) for 20 min prior to FACS analysis using a FACSVantage flow cytometer (Becton Dickinson, USA) and analyzed by CellQuest software. At least 1 × 104 cells were analyzed for each sample.
Apoptosis
Apoptosis was determined by Annexin V-FITC (Gibco, USA) and FCM analyses. After washing with PBS, 106 BMSCs were resuspended in binding buffer containing Annexin V-FITC (1 mg/mL). The mixture was incubated for 10 min in the dark under room temperature and then analyzed with FACSVantage flow cytometer and CellQuest software.
Statistical analysis
Data are represented as mean with standard deviation and analyzed with Student’s t test, except for GJIC (Pearson’s chi-squared test). Statistical significance was set at p < 0.05.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81370594, 81070388, 81270569), Chongqing Key Discipline of Natural Science (No. 2009BA5056), and Youth Innovation Project of Military Medicine of Chinese People’s Liberation Army (No. 13QNP116).
Abbreviations
- AOTF
acousto-optic tunable filter
- ATP
adenosine triphosphate
- ATRA
all-trans retinoic acid
- BMSCs
bone marrow stromal cells
- Cx43
connexin 43
- DMSO
dimethyl sulfoxide
- FCM
flow cytometry
- FRAP
fluorescence recovery after photobleaching
- GJIC
gap junctional intercellular communication
- LCSM
laser confocal scanning microscope
- LY
Lucifer yellow
- MTT
methyl thiazolyl tetrazolium
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
Additional files
The original flow plots of cell cycle in leukemic BMSCs using FCM assay. (A) Leukemic BMSCs; (B) leukemic BMSCs exposed to ATRA; (C) leukemic BMSCs treated with both ATRA and amphotericin-B.
The original flow plots of cell apoptosis in leukemic BMSCs using FCM assay. Leukemic BMSCs were treated DMSO, ATRA, and ATRA + AB, then cells were stained with Annexin V-FITC and propidium iodide (PI), followed by analysis on a flow cytometer. (A) Leukemic BMSCs; (B) leukemic BMSCs exposed to ATRA; (C) leukemic BMSCs treated with both ATRA and amphotericin-B.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YL, XZ, and QW carried out the cell culture and qRT-PCR, participated in the Western blot and dye transfer assay, and drafted the manuscript. XLC, SJY, LG, and CZ carried out the Western blot and FRAP assay. LG, JLL, XXX, KW, and XHC participated in the MTT assay and FCM and sample collection. XZ and JFZ participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Contributor Information
Yao Liu, Email: yl749@foxmail.com.
Qin Wen, Email: qiqi105@sina.com.
Xue-lian Chen, Email: chenxuelian9@gmail.com.
Shi-jie Yang, Email: xyzysj102@163.com.
Lei Gao, Email: gaolei7765@163.com.
Li Gao, Email: gaotiantiantiger@163.com.
Cheng Zhang, Email: chengzhang2006@163.com.
Jia-li Li, Email: l135940@aliyun.com.
Xi-xi Xiang, Email: cherry_xiangxixi@163.com.
Kai Wan, Email: wankai1111@163.com.
Xing-hua Chen, Email: xhchen888@aliyun.com.
Xi Zhang, Phone: +86 023 68755609, Email: zhangxxi@sina.com.
Jiang-fan Zhong, Email: jzhong@usc.edu.
References
- 1.Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12:225–56. doi: 10.1615/CritRevOncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 2.Cotrina ML, Lin JH, Nedergaard M. Adhesive properties of connexin hemichannels. Glia. 2008;56:1791–8. doi: 10.1002/glia.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oshima A. Structure and closure of connexin gap junction channels. FEBS Lett. 2014;588:1230–7. doi: 10.1016/j.febslet.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Khan Z, Yaiw KC, Wilhelmi V, Lam H, Rahbar A, Stragliotto G, et al. Human cytomegalovirus immediate early proteins promote degradation of connexin 43 and disrupt gap junction communication: implications for a role in gliomagenesis. Carcinogenesis. 2014;35:145–54. doi: 10.1093/carcin/bgt292. [DOI] [PubMed] [Google Scholar]
- 5.Anand RJ, Hackam DJ. The role of gap junctions in health and disease. Crit Care Med. 2005;33:S535–8. doi: 10.1097/01.CCM.0000194035.40266.B2. [DOI] [PubMed] [Google Scholar]
- 6.Mehta PP, Perez-Stable C, Nadji M, Mian M, Asotra K, Roos BA. Suppression of human prostate cancer cell growth by forced expression of connexin genes. Dev Genet. 1999;24:91–110. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<91::AID-DVG10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Ignatenko NA, Zhang H, Watts GS, Skovan BA, Stringer DE, Gemer EW. The chemopreventive agent alpha-difluoromethylornithine blocks Ki-ras-dependent tumor formation and specific gene expression in Caco-2 cells. MolCarcinog. 2004;39:221–33. doi: 10.1002/mc.20008. [DOI] [PubMed] [Google Scholar]
- 8.Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng DZ, et al. Aberrant expression of Cx43 is associated with the peritoneal metastasis of gastric cancer and Cx43-mediated gap junction enhances gastric cancer cell diapedesis from peritoneal mesothelium. PLoS One. 2013;8:e74527. doi: 10.1371/journal.pone.0074527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao K, Wang W, Guan C, Cai J, Wang P. Inhibition of gap junction channel attenuates the migration of breast cancer cells. MolBiol Rep. 2012;39:2607–13. doi: 10.1007/s11033-011-1013-x. [DOI] [PubMed] [Google Scholar]
- 10.Qin H, Shao Q, Belliveau DJ, Laird DW. Aggregated DsRed-tagged Cx43 and over-expressed Cx43 are targeted to lysosomes in human breast cancer cells. Cell CommunAdhes. 2001;8:433–9. doi: 10.3109/15419060109080766. [DOI] [PubMed] [Google Scholar]
- 11.Zhu D, Caveney S, Kidder GM, Naus CC. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci U S A. 1991;88:1883–7. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Darley RL, Hallett M, Evans WH. Low connexin channel-dependent intercellular communication in human adult hematopoietic progenitor/stem cells: probing mechanisms of autologous stem cell therapy. Cell Commun Adhes. 2009;16:138–45. doi: 10.3109/15419061003653763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosendaal M, Krenacs TT. Regulatory pathways in blood-forming tissue with particular reference to gap junctional communication. Pathol Oncol Res. 2006;6:243–9. doi: 10.1007/BF03187326. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Wang S, Zhao R. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 16.Ploemacher RE, Mayen AE, De Koning AE, Krenacs T, Rosendaal M. Hematopoiesis: gap junction intercellular communication is likely to be involved in regulation of stroma-dependent proliferation of hemopoietic stem cells. Hematology. 2000;5:133–47. doi: 10.1080/10245332.2000.11746498. [DOI] [PubMed] [Google Scholar]
- 17.Hurtado SP, Balduino A, Bodi EC, El-Cheikh MC, de Carvalho AC C, Borojevic R. Connexin expression and gap-junction-mediated cell interactions in an in vitro model of haemopoieticstroma. Cell Tissue Res. 2004;316:65–76. doi: 10.1007/s00441-003-0829-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zhang X, Li ZJ, Chen XH. Up-regulation of Cx43 expression and GJIC function in acute leukemia bone marrow stromal cells post-chemotherapy. Leuk Res. 2010;34:631–40. doi: 10.1016/j.leukres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liu Y, Si YJ, Chen XH, Li ZJ, Gao L, et al. Effect of Cx43 gene-modified leukemic bone marrow stromal cells on the regulation of Jurkat cell line in vitro. Leuk Res. 2012;36:198–204. doi: 10.1016/j.leukres.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Bodi E, Hurtado SP, Carvalho MA, Borojevic R, Carvalho AC. Gap junctions in hematopoietic stroma control proliferation and differentiation of blood cell precursors. An Acad Bras Cienc. 2004;76:743–56. doi: 10.1590/S0001-37652004000400009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, et al. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–95. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 22.Alves LA, Nihei OK, Fonseca PC, Carvalho AC, Savino W. Gap junction modulation by extracellular signaling molecules: the thymus model. Braz J Med Biol Res. 2000;33:457–65. doi: 10.1590/s0100-879x2000000400012. [DOI] [PubMed] [Google Scholar]
- 23.Tan XY, He JG. The remodeling of connexin in the hypertrophied right ventricular in pulmonary arterial hypertension and the effect of a dual ET receptor antagonist (bosentan) Pathol Res Pract. 2009;205:473–82. doi: 10.1016/j.prp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Di Francesco AM, Ubezio P, Torella AR, Meco D, Pierri F, Barone G, et al. Enhanced cell cycle perturbation and apoptosis mediate the synergistic effects of ST1926 and ATRA in neuroblastoma preclinical models. Invest New Drugs. 2012;30:1319–30. doi: 10.1007/s10637-011-9689-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang KZ, Zhang QB, Zhang QB, Sun HC, Ao JY, Chai ZT, et al. Arsenic trioxide induces differentiation of CD133+ hepatocellular carcinoma cells and prolongs posthepatectomy survival by targeting GLI1 expression in a mouse model. J Hematol Oncol. 2014;7:28. doi: 10.1186/1756-8722-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Yan C, Hou J, Pu J, Ouyang J, Wen D. ATRA enhances bystander effect of suicide gene therapy in the treatment of prostate cancer. Urol Oncol. 2008;26:397–405. doi: 10.1016/j.urolonc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Qin SK, Wu Q, Wang ZS, Zheng RS, Tong XH, et al. Connexin-dependent gap junction enhancement is involved in the synergistic effect of sorafenib and all-trans retinoic acid on HCC growth inhibition. Oncol Rep. 2014;31:540–50. doi: 10.3892/or.2013.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci. 2004;101:5328–35. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Chen Q-S, Xu P-P, Qian Y, Wang A-H, Xiao D, et al. Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARαoncoprotein degradation. J Hematol Oncol. 2014;7:75. doi: 10.1186/s13045-014-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda Y, Yamaguchi T, Hijikata Y, Tanaka M, Hirase C, Takai S, et al. Clinical efficacy of all-trans retinoic acid for treating adult T cell leukemia. J Cancer Res Clin Oncol. 2008;134:673–7. doi: 10.1007/s00432-007-0334-6. [DOI] [PubMed] [Google Scholar]
- 31.el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–30. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 32.Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: biochemical activities and anti-tumor effects. Biochim Biophys Acta. 2014;1845:20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Matesic DF, Sidorova TS, Burns TJ, Bell AM, Tran PL, Ruch RJ, et al. p38 MAPK activation, JNK inhibition, neoplastic growth inhibition, and increased gap junction communication in human lung carcinoma and Ras-transformed cells by 4-phenyl-3-butenoic acid. J Cell Biochem. 2012;113:269–81. doi: 10.1002/jcb.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Ren Z, Zuo J, Su C, Wang R, Chang Y, et al. The effect of all-trans retinoic acid on gap junctional intercellular communication and connexin 43 gene expression in glioma cells. Chin Med Sci J. 2002;17:22–6. [PubMed] [Google Scholar]
- 36.Geimonen E, Jiang W, Ali M, Fishman GI, Garfield RE, Andersen J. Activation of protein kinase C in human uterine smooth muscle induces connexin-43 gene transcription through an AP-1 site in the promoter sequence. J Biol Chem. 1996;271:23667–74. doi: 10.1074/jbc.271.39.23667. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan R, Ruangvoravat C, Joo D, Morgan J, Wang BL, Wang XK, et al. Structure, sequence and expression of the mouse Cx43 gene encoding connexin 43. Gene. 1993;130:191–9. doi: 10.1016/0378-1119(93)90419-4. [DOI] [PubMed] [Google Scholar]
- 38.Gotow T, Shiozaki M, Higashi T, Yoshimura K, Shibata M, Kominami E, et al. Hepatic gap junctions in the hepatocarcinogen-resistant DRH rat. Histochem Cell Biol. 2008;130:583–94. doi: 10.1007/s00418-008-0473-0. [DOI] [PubMed] [Google Scholar]
- 39.Lamiche C, Clarhaut J, Strale PO, Crespin S, Pedretti N, Bernard FX, et al. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. ClinExp Metastasis. 2012;29:111–22. doi: 10.1007/s10585-011-9434-4. [DOI] [PubMed] [Google Scholar]
- 40.Hotz-Wagenblatt A, Shalloway D. Gap junctional communication and neoplastic transformation. Crit Rev Oncog. 1993;4:541–58. [PubMed] [Google Scholar]
- 41.Qian MX, Wen J, Zhu X, Jia XH, Yang XW, Du YZ, et al. Structurally differentiated Cis-elements that interact with PU.1 are functionally distinguishable in acute promyelocyticleukemia. J Hematol Oncol. 2013;6:25. doi: 10.1186/1756-8722-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makarenkova HP, Shestopalov VI. The role of pannexin hemichannels in inflammation and regeneration. Front Physiol. 2014;25:1–8. doi: 10.3389/fphys.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xianrong Z, Yipeng Q. Role of intramolecular interaction in connexin 50: mediating the Ca2+-dependent binding of calmodulin to gap junction. Arc BiocBiop. 2005;2:111–7. doi: 10.1016/j.abb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Akinleye A, Avvaru P, Furqan M, Song YP, Liu DL. Phosphatidylinositol 3-kinase(PI3K) inhibitors as cancer therapeutics. J HematolOncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czyz J, Szpak K, Madeja Z. The role of connexins in prostate cancer promotion and progression. Nat Rev Urol. 2012;5:274–82. doi: 10.1038/nrurol.2012.14. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Suh H, Jo SA, Kim HW, Lee JM, Kim EH, et al. In vivo bone formation by human marrow stromal cells in biodegradable scaffolds that release dexamethasone and ascorbate-2-phosphate. BiochemBiophys Res Commun. 2005;332:1053–60. doi: 10.1016/j.bbrc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 47.Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. MolBiol Cell. 2008;19:2631–41. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–8. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 49.Katarzyna AD, Wang Y, Li JM, Wayne AC, Cynthia RG, Huang CZ, et al. Host bone marrow-derived IL-12 enhances donor T cell engraftment in a mouse model of bone marrow transplantation. J Hematol Oncol. 2014;7:16. doi: 10.1186/1756-8722-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


