Abstract
Background
High tidal volume ventilation has shown to cause ventilator-induced lung injury (VILI), possibly contributing to concomitant extrapulmonary organ dysfunction. The present study examined whether left ventricular (LV) function is dependent on tidal volume size and whether this effect is augmented during lipopolysaccharide(LPS)-induced lung injury.
Methods
Twenty male Wistar rats were sedated, paralyzed and then randomized in four groups receiving mechanical ventilation with tidal volumes of 6 ml/kg or 19 ml/kg with or without intrapulmonary administration of LPS. A conductance catheter was placed in the left ventricle to generate pressure-volume loops, which were also obtained within a few seconds of vena cava occlusion to obtain relatively load-independent LV systolic and diastolic function parameters. The end-systolic elastance / effective arterial elastance (Ees/Ea) ratio was used as the primary parameter of LV systolic function with the end-diastolic elastance (Eed) as primary LV diastolic function.
Results
Ees/Ea decreased over time in rats receiving LPS (p = 0.045) and high tidal volume ventilation (p = 0.007), with a lower Ees/Ea in the rats with high tidal volume ventilation plus LPS compared to the other groups (p < 0.001). Eed increased over time in all groups except for the rats receiving low tidal volume ventilation without LPS (p = 0.223). A significant interaction (p < 0.001) was found between tidal ventilation and LPS for Ees/Ea and Eed, and all rats receiving high tidal volume ventilation plus LPS died before the end of the experiment.
Conclusions
Low tidal volume ventilation ameliorated LV systolic and diastolic dysfunction while preventing death following LPS-induced lung injury in mechanically ventilated rats. Our data advocates the use of low tidal volumes, not only to avoid VILI, but to avert ventilator-induced myocardial dysfunction as well.
Keywords: Left ventricle, Mechanical ventilation, Tidal volume, Contractility, Lipopolysaccharide, Lung injury
Background
Pneumosepsis resulting in acute lung injury frequently requires mechanical ventilation to ensure adequate gas exchange. However, mechanical ventilation itself can instigate ventilator-induced lung injury (VILI) and aggravate respiratory failure through excessive lung distention at peak inspiration, and repetitive opening and closure of lung units. The use of high tidal volume ventilation has shown to be an important contributing factor of VILI with increased morbidity and mortality [1].
The effect of changes in tidal volume size on stroke volume are in part dependent on right ventricular (RV) preload, contractility and afterload which in turn are affected by lung volume, lung compliance and chest wall compliance among others [2]. These complex interactions make an accurate prediction of the change in RV stroke volume upon increased tidal volume size challenging. Moreover, possible deleterious effects of high tidal volumes on left ventricular (LV) function are largely unknown, partly because of the difficulty of measuring myocardial function independent of changes in loading conditions [3]. Commonly employed indices of LV systolic and diastolic function, the peak pressure change in time during isovolumetric contraction (dP/dtmax) and isovolumetric relaxation (dP/dtmin) respectively, are both affected by loading conditions. Alternatively, intraventricular derived pressure-volume loops using a conductance catheter can assess LV contractility and compliance relatively load-independent by constructing end-systolic and end-diastolic pressure-volume relationships [4–6]. Furthermore, LV afterload can be assessed and used for the determination of the relationship between LV contractility and afterload which has been used in various experimental and clinical conditions as the primary parameter for LV function [7–11].
Experimental studies have suggested that high tidal volume ventilation may induce various inflammatory mediators that leak into the circulation causing injury to distant organs including the heart [12, 13], with this effect being amplified in the setting of acute lung injury [14–16]. We therefore hypothesized that low tidal volume ventilation may ameliorate a decline in LV function measured in vivo in a model of lipopolysaccharide (LPS)-induced lung injury.
Methods
Animal experiments
The study protocol was approved by the Institutional Animal Care and Use Committee (VU University Medical Center, Amsterdam, the Netherlands) and applied with the Guide for Care and Use for laboratory animals of the National Institutes of Health. Twenty Wistar rats, all males, were intraperitoneal anaesthetized with 85 μg/g ketamine (Alfasan, Woerden, the Netherlands) and 12.5 μg/g midazolam (Pharmachemie BV, Haarlem, the Netherlands), followed by continuous intravenous sedation with 20 μg/g/h ketamine and 1.2 μg/g/h midazolam. The animals were paralyzed with continuous administration of 0.6 μg/g/h pancuronium (Organon, Oss, the Netherlands) to facilitate controlled mechanical ventilation (Avea, Care Fusion, Houten, the Netherlands) after placement of a 14G tracheostoma. A stable body temperature of 37 °C was maintained using a heating pad.
The left carotid artery was used for arterial blood gas analysis and, after zero-referencing against ambient air pressure, arterial blood pressure was continuously measured from which heart rate was derived. A balloon catheter (12060 2.0 F, Edwards Life Sciences, Santa Ana, CA, USA) was positioned through the right internal jugular vein in the inferior vena cava to allow occlusion by means of balloon inflation. A Millar pressure-volume conductance catheter system (1.4 F, Millar instruments, Houston, TX, USA) was inserted through the right carotid artery and placed in the left ventricle. This multi-electrode catheter generates an electric field and measures continuously segmental conductance from which LV relative volume units can be determined. With the addition of a micro manometer on the 1.4 F conductance catheter, LV pressure-volume relationships can be generated even in small animals allowing continuous hemodynamic evaluation of LV systolic and diastolic function including contractile and relaxation parameters that are relatively independent of loading conditions [17, 18].
Study protocol
During at least 10 min animals were allowed to stabilize following preparation after which baseline measurements were obtained. Animals were then randomized for ventilation with tidal volumes of 6 ml/kg or 19 ml/kg and to receive either 2 μg/g saline-dissolved LPS (L2880, LPS from E. coli 055:B5, Sigma-Aldrich, Buchs, Switzerland) intrapulmonary through a miniature nebulizer (Penn-Century, Wyndmoor, PA, USA) or no LPS. Subsequently, four groups (low tidal volumes (LTV) without LPS; LTV with LPS; high tidal volumes (HTV) without LPS; HTV with LPS) of each 5 animals were studied, all receiving a fraction of inspired oxygen (FiO2) of 40 % with an inspiration : expiration ratio of 1 : 2. Positive end-expiratory pressure (PEEP) was set at 5 cmH2O with a respiratory rate of 45/min in the low tidal group, while PEEP was set at 1 cmH2O with a respiratory rate of 20/min in the high tidal group to acquire equal mean airway pressure and minute ventilation respectively. The respiratory rate and FiO2 were only adjusted if necessary to maintain normocapnia or prevent hypoxia respectively. All other ventilatory settings and drug doses remained unaltered during the 4 h entailing experimental protocol. At the end of the experiment, the animals were sacrificed and myocardial function was measured in an ex vivo study [14].
Heart rate, arterial blood pressure, body temperature and ventilatory pressures, including mean and plateau pressure, were continuously measured. Similarly, LV systolic and diastolic parameters were continuously derived from the pressure-volume conductance catheter. Effective arterial elastance (Ea, mmHg/relative volume units) was defined as end-systolic pressure divided by stroke volume and represents LV afterload with a strong correlation with the gold standard aortic input impedance [7, 19]. The maximum rate of pressure development (dP/dtmax, mmHg/s) was determined during isovolumetric contraction while the maximum rate of pressure decline (dP/dtmin, mmHg/s) was measured during isovolumetric relaxation. Averages of the aforementioned LV systolic and diastolic parameters were recorded during steady state and calculated during 3 s covering approximately 15 heart cycles. Arterial blood gas analysis was performed every hour and withdrawn blood was replaced by equal volumes of 0.9 % saline. At every hour the balloon of the catheter positioned in the vena cava was inflated for a maximum of 30 s to diminish venous return and subsequently cardiac preload to enable the construction of the LV end-systolic pressure-volume relationship representing LV end-systolic elastance (Ees). Ees was obtained within a few seconds of vena cava occlusion to prevent sympathetic reflexes that increase ventricular inotropy. This way, Ees describes the maximum pressure that can be developed by the ventricle at any given volume and has shown to be a sensitive index of contractility relatively independent of loading conditions in contrast to dP/dtmax [20]. As primary parameter of LV systolic function, the Ees/Ea ratio was derived resembling the relationship between LV contractility and afterload [21]. Finally, the end-diastolic elastance (Eed) was calculated using the LV end-diastolic pressure-volume relationship during vena cava occlusion representing LV compliance as primary parameter of LV diastolic function.
Statistical analysis
We considered 5 animals per group, with 5 measurements in each animal, as a sufficient number for this physiologic study taken into account that no prior study investigated LV hemodynamics with a pressure-volume conductance catheter in the setting of changes in tidal volume combined with LPS administration. The respiratory, hemodynamic and LV parameters were continuously acquired with PowerLab 16/30 (ADInstruments Ltd, Oxford, UK) displayed using Chart version 5.5.6 (ADInstruments Ltd, Oxford, UK) and analyzed offline with PVAN 3.6 (Millar instruments, Houston, TX, USA). The comparison of baseline measurements between the 4 groups was performed using one-way analysis of variance (ANOVA). The pressure-volume loops during vena cava occlusion were obtained hourly during the 4 h entailing experimental protocol. Responses between the 4 groups were compared by general estimated equations (GEE) with tidal volume ventilation and LPS as factors and time as within-subject variable. To analyze an interaction between tidal volume ventilation and LPS on LV parameters, the interaction term “tidal volume ventilation * LPS” was added to the GEE model. Correlations were calculated using Spearman’s rank correlation coefficients. Analyses were performed using SPSS Statistics version 20.0 (IBM Corporation, New York, NY, USA). Data are presented as means ± SD. A two-sided p-value < 0.05 was considered statistically significant. Exact p-values are given unless p < 0.001.
Results
Baseline characteristics
The average weight of the twenty rats was 327 ± 12 g with an average tidal volume of 1.95 ± 0.08 ml in the low tidal volume ventilation group vs. 6.27 ± 0.18 ml in the high tidal volume ventilation group (p < 0.001). There were no further differences in respiratory, hemodynamic and LV systolic and diastolic parameters at baseline between the 4 groups except for a higher central venous pressure in rats receiving low tidal volume plus LPS (Table 1).
Table 1.
Baseline measurements in the rats before randomization (n = 20)
| Variable | LTV | LTV + LPS | HTV | HTV + LPS | P-value |
|---|---|---|---|---|---|
| Respiration | |||||
| pH | 7.31 ± 0.02 | 7.31 ± 0.03 | 7.30 ± 0.02 | 7.32 ± 0.05 | 0.66 |
| PaCO2 (torr) | 40.3 ± 4.3 | 42.5 ± 4.1 | 42.6 ± 3.6 | 40.7 ± 3.1 | 0.68 |
| PaO2/FiO2 (torr) | 564 ± 60 | 538 ± 45 | 510 ± 31 | 525 ± 49 | 0.36 |
| Pmean (cmH2O) | 7.6 ± 0.5 | 7.8 ± 0.4 | 7.4 ± 0.5 | 7.8 ± 0.8 | 0.70 |
| Hemodynamics | |||||
| MAP (mmHg) | 100 ± 12 | 122 ± 23 | 105 ± 16 | 115 ± 23 | 0.28 |
| CVP (mmHg) | 1.4 ± 0.8 | 2.9 ± 0.9a | 1.5 ± 1.2 | 1.3 ± 0.4 | 0.045 |
| HR (beats/min) | 343 ± 35 | 407 ± 46 | 360 ± 26 | 378 ± 49 | 0.12 |
| LV parameters | |||||
| ESP (mmHg) | 151 ± 16 | 157 ± 16 | 189 ± 36 | 174 ± 30 | 0.13 |
| EDP (mmHg) | 21 ± 9 | 16 ± 13 | 21 ± 5 | 18 ± 8 | 0.78 |
| dP/dtmax (mmHg/s) | 8043 ± 1689 | 8928 ± 2729 | 9912 ± 2709 | 8809 ± 2150 | 0.63 |
| dP/dtmin (mmHg/s) | −10080 ± 1609 | −11917 ± 2954 | −9741 ± 2230 | −11716 ± 2221 | 0.35 |
| Ees/Ea | 1.74 ± 0.64 | 2.03 ± 0.56 | 1.88 ± 0.89 | 1.74 ± 0.80 | 0.91 |
LTV low tidal volume ventilation, LPS lipopolysaccharide, HTV high tidal volume ventilation, CVP central venous pressure, dP/dtmax maximum rate of pressure development, dP/dtmin maximum rate of pressure decline, EDP end-diastolic pressure, ESP end-systolic pressure, Ees/Ea end-systolic elastance/effective arterial elastance ratio, HR heart rate, MAP mean arterial pressure, PaCO2 arterial partial pressure of carbon dioxide, PaO2/FiO2 arterial partial pressure of oxygen/fraction of inspired oxygen ratio, Pmean mean airway pressure
aCVP was higher in the LTV + LPS group compared to the other groups. Data are mean values ± SD
Respiratory and hemodynamic parameters
The mean pH throughout the 4 h entailing experiment was lower in LPS-treated rats compared to non-LPS treated rats, with a higher PaCO2 in rats receiving low tidal volume plus LPS compared to the other groups (Table 2). The mean airway pressure was higher in rats subjected to high tidal volume ventilation, with a lower PaO2/FiO2 ratio in the rats with high tidal volume ventilation plus LPS compared to the other groups. The mean arterial pressure and central venous pressure was lower in the rats receiving high tidal volume ventilation, while heart rate was lower in the rats with high tidal volume ventilation without LPS treatment compared to the other groups.
Table 2.
Respiratory, hemodynamic and left ventricular measurements throughout the experiment in the rats (n = 20)
| Variable | LTV | LTV + LPS | HTV | HTV + LPS |
|---|---|---|---|---|
| Respiration | ||||
| pH | 7.31 ± 0.01 | 7.26 ± 0.02a,c | 7.36 ± 0.01a | 7.28 ± 0.02a,c |
| PaCO2 (torr) | 39.1 ± 1.7 | 47.0 ± 3.0a,c,d | 35.8 ± 1.3 | 37.8 ± 1.8 |
| PaO2/FiO2 (torr) | 534 ± 36 | 501 ± 23 | 485 ± 33 | 324 ± 28a,b,c |
| Pmean (cmH2O) | 7.6 ± 0.3 | 7.8 ± 0.2 | 8.3 ± 0.2a,b | 8.9 ± 0.2a,b,c |
| Hemodynamics | ||||
| MAP (mmHg) | 115 ± 8 | 116 ± 6 | 104 ± 6a,b | 98 ± 8a,b |
| CVP (mmHg) | 1.9 ± 0.3 | 2.2 ± 0.6 | 1.1 ± 0.3a,b | 0.7 ± 0.3a,b |
| HR (beats/min) | 393 ± 13 | 413 ± 9 | 362 ± 12a,b,d | 403 ± 9 |
| LV parameters | ||||
| ESP (mmHg) | 184 ± 12b,d | 154 ± 10 | 167 ± 12 | 146 ± 14 |
| EDP (mmHg) | 20 ± 4 | 17 ± 5 | 18 ± 2 | 17 ± 3 |
| dP/dtmax (mmHg/s) | 11740 ± 1114 | 11763 ± 1251 | 10343 ± 1000 | 9014 ± 682a,b |
| dP/dtmin (mmHg/s) | −13482 ± 1485 | −13072 ± 1103 | −11267 ± 990 | −8555 ± 1005a,b,c |
| Ees/Ea | 1.67 ± 0.33 | 1.45 ± 0.16 | 1.33 ± 0.13 | 0.86 ± 0.11a,b,c |
LTV low tidal volume ventilation, LPS lipopolysaccharide, HTV high tidal volume ventilation, CVP central venous pressure, dP/dtmax maximum rate of pressure development, dP/dtmin maximum rate of pressure decline, EDP end-diastolic pressure, ESP end-systolic pressure, Ees/Ea end-systolic elastance/effective arterial elastance ratio, HR heart rate, MAP mean arterial pressure, PaCO2 arterial partial pressure of carbon dioxide, PaO2/FiO2 arterial partial pressure of oxygen/fraction of inspired oxygen ratio, Pmean mean airway pressure
aP < 0.05 compared to LTV group. bP < 0.05 compared to LTV + LPS group. cP < 0.05 compared to HTV group. dP < 0.05 compared to HTV + LPS group. Data are mean values ± SE
Left ventricular systolic and diastolic function
LV systolic and diastolic parameters could successfully be derived from the pressure-volume loops in the rats during steady state (Fig. 1a) and during balloon inflation of the vena cava catheter (Fig. 1b). Ees/Ea decreased over time in rats receiving LPS compared to non-LPS treated rats (p = 0.045), as well as in rats subjected to high tidal volume ventilation vs. low tidal volume ventilation (p = 0.007) (Fig. 2a). Mean Ees/Ea was lower in the rats with high tidal volume ventilation plus LPS compared to the other groups (p < 0.001) (Table 2). Furthermore, mean dP/dtmax was lower in the rats with high tidal volume ventilation plus LPS compared to the rats with low tidal volume ventilation. Mean end-systolic pressure was higher in the rats receiving low tidal volume ventilation without LPS compared to LPS-treated rats.
Fig. 1.
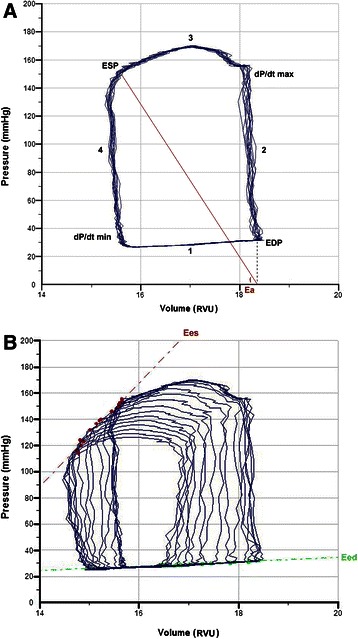
Pressure-volume loops obtained during three seconds showing left ventricular (LV) hemodynamics during steady state (a) and vena cava occlusion (b). a LV function is described during 4 phases of the cardiac cycle: 1) diastolic filling, 2) isovolumetric contraction, 3) ejection and 4) isovolumetric relaxation. EDP = end-diastolic pressure, ESP = end-systolic pressure, Ea = effective arterial elastance which slope is calculated by end-systolic pressure divided by stroke volume, dP/dtmax = maximum rate of pressure development during isovolumetric contraction, dP/dtmin = maximum rate of pressure decline during isovolumetric relaxation. b By decreasing preload through vena cava occlusion, the pressure-volume loops move to the left and become smaller, enabling the measurement of end-systolic elastance (Ees) by the pressure-volume relationship at end-systole. Simultaneously, end-diastolic elastance (Eed) can be measured by the pressure-volume relationship at end-diastole. A representative sample trace as displayed and analyzed by PVAN 3.6 is shown
Fig. 2.
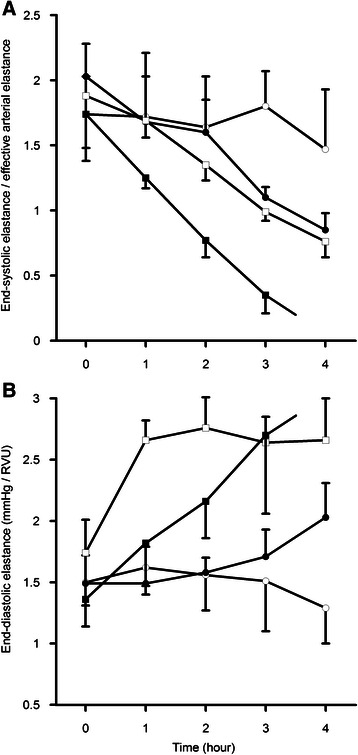
Course of left ventricular end-systolic elastance/effective arterial elastance (Ees/Ea) (A) and end-diastolic elastance (Eed) (B) over time in the 4 rat groups. (○: low tidal volume (LTV), ●: LTV + lipopolysaccharide (LTV + LPS), □: high tidal volume (HTV), ■: HTV + LPS. a Ees/Ea decreased over time in all groups (p < 0.001) except for the LTV group (p = 0.428). A decrease in Ees/Ea was observed in the rats subjected to high tidal volume ventilation vs. low tidal volume ventilation (p = 0.007), as well in the rats receiving LPS vs. non-LPS (p = 0.045). b Eed remained unchanged over time in the LTV group (p = 0.223), but increased in the LTV + LPS (p = 0.036), HTV (p < 0.001) and HTV + LPS (p = 0.001) group. An interaction between tidal ventilation and LPS was found for Ees/Ea and Eed (p < 0.001) and all the rats receiving HTV + LPS died before the end of the experiment. A p-value < 0.05 was considered statistically significant. Error bars represent ± SE
Eed increased over time in the rats receiving LPS with low tidal volume ventilation (p = 0.036) and during high tidal volume ventilation (p = 0.001) with an increase in the Eed slope representing decreased LV compliance (Fig. 2b). In the rats without LPS administration, Eed remained unchanged over time during low tidal volume ventilation (p = 0.223), but increased during high tidal volume ventilation (p < 0.001). No differences in mean end-diastolic pressure was observed, yet mean dP/dtmin was lower in the rats subjected to high tidal volume ventilation plus LPS compared to the other groups (Table 2). Tidal volume was significantly correlated with Ees/Ea and Eed (r2 = −0.34; p = 0.001 and r2 = 0.39; p < 0.001 respectively), as were the correlations between plateau pressure and Ees/Ea as well as Eed (r2 = −0.47; p < 0.001 and r2 = 0.42; p < 0.001 respectively).
Survival
A significant interaction (p < 0.001) was found between tidal ventilation and LPS for Ees/Ea and Eed. Three rats receiving high tidal volume ventilation plus LPS did not survive beyond 2,5 h, while the 2 remaining rats from that group died at 3.5 h. All rats from the other groups survived till the end of the experiment.
Discussion
We investigated whether tidal volume size affects LV function in a two-hit animal model of high tidal volumes and lung injury induced by intrapulmonary administered LPS. Our data show that high tidal volume ventilation resulted in a progressive decline in LV systolic and diastolic function over time, especially during LPS-induced lung injury, yet LV function remained unchanged in rats receiving low tidal volume ventilation without LPS. An interaction between tidal volume ventilation and LPS was observed for LV contractile and relaxation parameters implying that the LPS-induced lung injury exerted an additional detrimental effect on LV function possibly contributing to the death of all the rats subjected to high tidal volume ventilation plus LPS administration. Therefore, low tidal volumes may be advocated to prevent VILI as well as to ameliorate LV dysfunction.
Conventional hemodynamic parameters have shown to poorly reflect LV function in sepsis [22, 23]. Even during elevated cardiac output, LV dysfunction is frequently present [24]. Furthermore, cardiac output may not predict mortality in sepsis [25], whereas myocardial depression is thought to characterize the fatal course of septic shock [26, 27]. This paradox may in part be explained by the fact that cardiac output can remain unaltered despite LV systolic dysfunction when a concomitant decreased LV afterload is present [28]. Therefore, LV systolic function should be evaluated in relationship with LV afterload, explaining the common use of Ees/Ea as primary parameter for LV systolic function [7–11]. A mean Ees/Ea of > 1.6 has been associated with a normal LV systolic function while a ratio < 1.0 correlated with a severely impaired LV systolic function [10]. LV systolic function appears to deteriorate at the early phase of septic shock [29]. We observed a progressive decrease in Ees/Ea with a ratio < 1.0 in the rats subjected to high tidal volume ventilation plus LPS, with a lower dP/dtmax compared to rats receiving low tidal volume ventilation. Furthermore, end-systolic pressure was lower in LPS-treated rats compared to non-LPS treated rats receiving low tidal volume ventilation.
Besides evidence of systolic dysfunction during sepsis, there are also several reports in the literature demonstrating diastolic dysfunction [30–32]. In our study, LV diastolic function as assessed by Eed was progressively impaired over time in rats subjected to high tidal volume ventilation and/or LPS administration, with the lowest dP/dtmin during high tidal volume ventilation plus LPS. Nevertheless, end-diastolic pressure remained unaltered corresponding with a previous described preservation of normal filling pressures during sepsis [28].
Surprisingly, in comparison to the well-known deleterious effects on RV function [33, 34], little is known about the effect of tidal volume size on LV function. Therefore the contribution of LV dysfunction in the observed decrease in cardiac output during high tidal volume ventilation in previous studies remains largely unknown [35, 36]. To our knowledge, the only study specifically studying LV function during changes in tidal volume was performed in pigs by Renner et al. [37]. In this study no decrease in LV function, measured by echocardiography-derived myocardial performance index, was seen during low tidal volume ventilation with 5 ml/kg in contrast to high tidal volume ventilation with 10 ml/kg and 15 ml/kg. In accordance, in our non-LPS treated rats no decrease in relatively load-independent LV systolic and diastolic function was observed during low tidal volume ventilation with 6 ml/kg in contrast to high tidal volume ventilation with 19 ml/kg. The concept that LV function can deteriorate during high tidal ventilation in a load-independent manner is enhanced by the fact that positive end-expiratory pressure, while affecting ventricular loading conditions as well, does not directly alter LV function [38, 39].
A possible explanation for the decrease in LV function upon high tidal ventilation may be the ventilation-induced inflammation that may not only damage the lung, but has shown to injure distant organs as well contributing to morbidity and mortality [40–42]. We used intrapulmonary administration of nebulized LPS which, together with the application of high tidal volume ventilation, created a severe model of acute lung injury with a decrease in PaO2/FiO2 ratio. The observed aggravation of acidosis in the LPS-treated animals could be explained by the induction of sepsis, which has repeatedly shown to decrease ventricular contractility load-independently assessed by pressure-volume loops [43–45]. Moreover, extrapulmonary organ dysfunction induced by VILI is thought to depress myocardial function rather similar to sepsis [46], such that a cumulative effect on the heart can be expected. We indeed observed an aggravation of myocardial dysfunction induced by high tidal volume ventilation during LPS-induced lung injury potentially contributing to the death of all rats before the end of the experiment. Interestingly, these findings are confirmed ex vivo where high tidal volumes were applied in combination with intrapulmonary LPS [14], in contrast to where LPS was administered intraperitoneal [47], supporting the additional detrimental effect of high tidal volume ventilation in the setting of acute lung injury.
Injurious lung effects of high tidal volume ventilation are increasingly recognized since a large ARDS Network trial showed an absolute reduction in mortality of 10 % when lower tidal volumes were compared with conventional (i.e., high) tidal volumes [1]. Our data show a correlation between tidal volumes and LV systolic and diastolic function suggesting that low tidal volume ventilation may mitigate LV dysfunction beyond its protective effects against VILI. Plateau pressure, another key component of lung protective ventilation, correlated even slightly better with LV function. One could therefore argue that the survival benefit seen in prior lung protective ventilation trials may be explained not only by a reduction in VILI, but in addition by a reduction in concomitant “VIMD”: Ventilator-Induced Myocardial Dysfunction.
Several limitations in our study should be discussed. We looked at LV systolic and diastolic function, but did not measure RV function which could have shed light on the overall effect on cardiac function. Although RV dysfunction induced by high tidal volume ventilation could have contributed to changes in LV loading conditions, this could not fully explain the decrease in LV systolic and diastolic function parameters that are relatively load-independent. It has already been documented that myocardial depression during sepsis affects both ventricles simultaneously [24]. We focused on LV systolic and diastolic parameters as little is known about the effect of high tidal volume ventilation on LV function in comparison to the notorious effects on RV function. Mean airway pressure became higher during the experiment in the rats subjected to high tidal volume ventilation despite application of lower PEEP compared to the low tidal volume ventilation group. Therefore the observed LV systolic and diastolic dysfunction may in part be explained by mechanical ventilator-induced lung injury due to increased airway pressure. Detrimental effects of low PEEP on lung volume as well as an increase in lung injury from greater dynamic strain cannot be excluded [48], although PEEP has predominantly failed to demonstrate a decrease in LV function in the literature [3, 38, 39]. While the rats ventilated with 6 ml/kg showed amelioration of LV dysfunction, the ideal tidal volume size cannot be determined from our data. Left ventricular hemodynamics were measured intraventricular with a pressure-volume conductance catheter as has been previously done in animal models during either changes in tidal volumes or during LPS administration, although not during both interventions [49, 50]. The pressure-volume conductance catheter, when zeroed to atmospheric pressure, provides absolute pressure values in millimeters of mercury, but offers volumes in relative volume units unless external calibration is performed for which the thermodilution technique can be used. Unfortunately, simultaneous thermodilution measurements proved to be infeasible in our rats with already extensive instrumentation. Nevertheless, the ratio of Ees/Ea would not be influenced by the usage of relative volume units while the other systolic (dP/dtmax and end-systolic pressure) and diastolic (dP/dtmin and end-diastolic pressure) parameters were all pressure derived. However, the Eed value is subjective to the usage of relative volume units, so only the Eed trend over time and not the absolute values could be analyzed. Heart rates and respiratory rates were naturally greater in our small animal model then seen in human, but with a fairly comparable 7:1 heart-lung interaction ratio. Nonetheless, further research in humans need to be undertaken to confirm the findings of our study.
Conclusions
High tidal volume ventilation decreases LV function, which is aggravated during LPS-induced lung injury in mechanically ventilated rats. Previously, the use of low tidal volumes was advocated to reduce ventilator-induced lung injury. Our data suggests that low tidal volumes preserve LV systolic and diastolic function as well during acute lung injury, ameliorating so-called ventilator-induced myocardial dysfunction.
Key messages
In mechanically ventilated rats, both high tidal volume ventilation as well as lung injury induced by intrapulmonary administered LPS decreased LV systolic and diastolic function.
High tidal volume ventilation exerted an additional detrimental effect on LV function in the LPS-treated rats, none surviving the experiment in contrast to low tidal volume ventilation.
Low tidal volumes may be advocated to ameliorate ventilator-induced myocardial dysfunction besides ventilator-induced lung injury.
Acknowledgements
We would like to thank Carolien Paes for her assistance with the figure preparations.
No institutional funds or financial support was used for the study.
Abbreviations
- VILI
Ventilator-induced lung injury
- LV
Left ventricular
- RV
Right ventricular
- dP/dtmax
Peak pressure change in time during isovolumetric contraction
- dP/dtmin
Peak pressure change in time during isovolumetric relaxation
- LPS
Lipopolysaccharide
- LTV
Low tidal volumes
- HTV
High tidal volumes
- FiO2
Fraction of inspired oxygen
- PEEP
Positive end-expiratory pressure
- Ea
Effective arterial elastance
- Ees
End-systolic elastance
- Eed
End-diastolic elastance
- PaCO2
Arterial partial pressure of carbon dioxide
- PaO2
Arterial partial pressure of oxygen
- VIMD
Ventilator-induced myocardial dysfunction
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ABJG designed the concept of the experiments; LS and TGVC performed the experiments; TGVC, AH and ABJG analyzed the data of the experiments; TGVC and ABJG interpreted the results of the experiments; TGVC prepared the figures; TGVC, WKL, MJS and ABJG drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Thomas GV Cherpanath, Phone: +31 20 56 63307, Email: t.g.cherpanath@amc.uva.nl.
Lonneke Smeding, Email: l.smeding@amc.uva.nl.
Alexander Hirsch, Email: a.hirsch@amc.uva.nl.
Wim K. Lagrand, Email: w.k.lagrand@amc.uva.nl
Marcus J. Schultz, Email: marcus.j.schultz@gmail.com
AB Johan Groeneveld, Email: a.b.j.groeneveld@erasmusmc.nl.
References
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJ, et al. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interaction. Crit Care Med. 2014;42:1983–90. doi: 10.1097/CCM.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 3.Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care. 2005;9:607–21. doi: 10.1186/cc3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–22. doi: 10.1161/01.RES.32.3.314. [DOI] [PubMed] [Google Scholar]
- 5.Sagawa K. The end-systolic pressure-volume relation of the ventricle: definition, modifications and clinical use. Circulation. 1981;63:1223–7. doi: 10.1161/01.CIR.63.6.1223. [DOI] [PubMed] [Google Scholar]
- 6.LeWinter MM, Osol G. Normal physiology of the cardiovascular system. In: Fuster V, Alexander RW, O’Rouke RA, editors. Hurst’s The Heart. 10. New York: McGraw-Hill; 2001. pp. 63–98. [Google Scholar]
- 7.Sunagawa K, Maughan WL, Burkhoff D, Sugawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–80. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 8.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J. 1993;125:1659–66. doi: 10.1016/0002-8703(93)90756-Y. [DOI] [PubMed] [Google Scholar]
- 9.Steendijk P, Tulner SA, Bax JJ, Oemrawsingh PV, Bleeker GB, van Erven L, et al. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation. 2006;113:1295–304. doi: 10.1161/CIRCULATIONAHA.105.540435. [DOI] [PubMed] [Google Scholar]
- 10.Remmelink M, Sjauw KD, Henriques JP, Vis MM, van der Schaaf RJ, Koch KT, et al. Acute left ventricular dynamic effects of primary percutaneous coronary intervention: from occlusion to reperfusion. J Am Coll Cardiol. 2009;53:1498–502. doi: 10.1016/j.jacc.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 11.Remmelink M, Sjauw KD, Yong ZY, Haeck JD, Vis MM, Koch KT, et al. Coronary microcirculatory dysfunction is associated with left ventricular dysfunction during follow-up after STEMI. Neth Heart J. 2013;21:238–44. doi: 10.1007/s12471-013-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nin N, Penuelas O, de Paula M, Lorente JA, Fernández-Segoviano P, Esteban A. Ventilation-induced lung injury in rats is associated with organ injury and systemic inflammation that is attenuated by dexamethasone. Crit Care Med. 2006;34:1093–8. doi: 10.1097/01.CCM.0000205663.92384.E7. [DOI] [PubMed] [Google Scholar]
- 13.Brander L, Sinderby C, Lecomte F, Leong-Poi H, Bell D, Beck J, et al. Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and non-pulmonary organ dysfunction in rabbits with acute lung injury. Intensive Care Med. 2009;35:1979–89. doi: 10.1007/s00134-009-1626-x. [DOI] [PubMed] [Google Scholar]
- 14.Smeding L, Kuiper JW, Plötz FB, Kneyber MC, Groeneveld AJ. Aggravation of myocardial dysfunction by injurious mechanical ventilation in LPS-induced pneumonia in rats. Respir Res. 2013;14:92. doi: 10.1186/1465-9921-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuiper JW, Plötz FB, Groeneveld AJ, Haitsma JJ, Jothy S, Vaschetto R, et al. High tidal volume mechanical ventilation-induced lung injury in rats is greater after acid instillation than after sepsis-induced acute lung injury, but does not increase systemic inflammation: an experimental study. BMC Anesthesiol. 2011;11:26. doi: 10.1186/1471-2253-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelosi P, D’Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48–56. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 17.Van der Velde ET, van Dijk AD, Steendijk P, Diethelm L, Chagas T, Lipton MJ, et al. Left ventricular segmental volume by conductance catheter and Cine-CT. Eur Heart J. 1992;13:15–21. doi: 10.1093/eurheartj/13.suppl_E.15. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Takaki M, Yamaguchi H, Tachibana H, Suga H. Left ventricular volumetric conductance catheter for rats. Am J Physiol. 1996;270:1509–14. doi: 10.1152/ajpheart.1996.270.4.H1509. [DOI] [PubMed] [Google Scholar]
- 19.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–21. doi: 10.1161/01.CIR.86.2.513. [DOI] [PubMed] [Google Scholar]
- 20.Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. New York: Oxford University Press; 1988. [Google Scholar]
- 21.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions and applications. Ann Biomed Eng. 1992;20:41–62. doi: 10.1007/BF02368505. [DOI] [PubMed] [Google Scholar]
- 22.Ellrodt AG, Riedinger MS, Kimchi A, Berman DS, Maddahi J, Swan HJ, et al. Left ventricular performance in septic shock: reversible segmental and global abnormalities. Am Heart J. 1985;110:402–9. doi: 10.1016/0002-8703(85)90163-2. [DOI] [PubMed] [Google Scholar]
- 23.Raper RF, Sibbald WJ, Driedger AA, Gerow K. Relative myocardial depression in normotensive sepsis. J Crit Care. 1989;4:9–18. doi: 10.1016/0883-9441(89)90086-5. [DOI] [Google Scholar]
- 24.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–90. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 25.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–8. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent JL, Gris P, Coffernils M, Leon M, Pinsky M, Reuse C, et al. Myocardial depression characterizes the fatal course of septic shock. Surgery. 1992;111:660–7. [PubMed] [Google Scholar]
- 27.Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–5. doi: 10.1097/01.CCM.0000114827.93410.D8. [DOI] [PubMed] [Google Scholar]
- 28.Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011;1:6. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubuel C, Mebazaa A. Septic shock: a heart story since the 1960s. Intensive Care Med. 2006;32:799–807. doi: 10.1007/s00134-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 30.Jafri SM, Lavine S, Field BE, Bahorozian MT, Carlson RW. Left ventricular diastolic function in sepsis. Crit Care Med. 1990;18:709–14. doi: 10.1097/00003246-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Munt B, Jue J, Gin K, Fenwick J, Tweeddale M. Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med. 1998;26:1829–33. doi: 10.1097/00003246-199811000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby JJ. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med. 2008;36:766–74. doi: 10.1097/CCM.0B013E31816596BC. [DOI] [PubMed] [Google Scholar]
- 33.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33:444–7. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 35.Cheifetz IM, Craig DM, Quick G, McGovern JJ, Cannon ML, Ungerleider RM. Increasing tidal volumes and pulmonary overdistention adversely affect pulmonary vascular mechanics and cardiac output in a pediatric swine model. Crit Care Med. 1998;26:710–6. doi: 10.1097/00003246-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Mesquida J, Kim HK, Pinsky MR. Effect of tidal volume, intrathoracic pressure, and cardiac contractility on variations in pulse pressure, stroke volume, and intrathoracic blood volume. Intensive Care Med. 2011;37:1672–9. doi: 10.1007/s00134-011-2304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renner J, Cavus E, Gruenewald M, Steinfath M, Scholz J, Lutter G. Myocardial performance index during rapidly changing loading conditions: impact of different tidal ventilation. Eur J Anaesthesiology. 2008;25:217–23. doi: 10.1017/S0265021507002967. [DOI] [PubMed] [Google Scholar]
- 38.Crottogini AJ, Willshaw P, Barra JG, Breitbart GJ, Pichel RH. End-systolic pressure-volume relationships in dogs during ventilation with PEEP. Am J Physiol. 1988;254:664–70. doi: 10.1152/ajpheart.1988.254.4.H664. [DOI] [PubMed] [Google Scholar]
- 39.Johnston WE, Vinten-Johansen J, Santamore WP, Case LD, Little WC. Mechanism of reduced cardiac output during positive end-expiratory pressure in the dog. Am Rev Respir Dis. 1989;140:1257–64. doi: 10.1164/ajrccm/140.5.1257. [DOI] [PubMed] [Google Scholar]
- 40.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol. 2001;91:1836–44. doi: 10.1152/jappl.2001.91.4.1836. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: From the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 43.Herbertson MJ, Werner HA, Goddard CM, Russell JA, Wheeler A, Coxon R, et al. Anti-tumor necrosis factor-alpha prevents decreased ventricular contractility in endotoxemic pigs. Am J Respir Crit Care Med. 1995;152:480–8. doi: 10.1164/ajrccm.152.2.7633696. [DOI] [PubMed] [Google Scholar]
- 44.Davani EY, Boyd JH, Dorscheid DR, Wang Y, Meredith A, Chau E, et al. Cardiac ICAM-1 mediates leukocyte-dependent decreased ventricular contractility in endotoxemic mice. Cardiovasc Res. 2006;72:134–42. doi: 10.1016/j.cardiores.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Tokunaga C, Bateman RM, Boyd J, Wang Y, Russell JA, Walley KR. Albumin resuscitation improves ventricular contractility and myocardial tissue oxygenation in rat endotoxemia. Crit Care Med. 2007;35:1341–7. doi: 10.1097/01.CCM.0000260242.77637.57. [DOI] [PubMed] [Google Scholar]
- 46.Villar J, Blanco J, Zhang H, Slutsky AS. Ventilator-induced lung injury and sepsis: two sides of the same coin? Minerva Anestesiol. 2011;77:647–53. [PubMed] [Google Scholar]
- 47.Smeding L, Plötz FB, Lamberts RR, van der Laarse WJ, Kneyber MC, Groeneveld ABJ. Mechanical ventilation with high tidal volumes attenuates myocardial dysfunction by decreasing cardiac edema in a rat model of LPS-induced peritonitis. Respir Res. 2012;13:23. doi: 10.1186/1465-9921-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med. 2003;167:1633–40. doi: 10.1164/rccm.200210-1215OC. [DOI] [PubMed] [Google Scholar]
- 49.Szwarc RS, Ball HA. Simultaneous LV and RV volumes by conductance catheter: effects of lung insufflation on parallel conductance. Am J Physiol. 1998;275:653–61. doi: 10.1152/ajpheart.1998.275.2.H653. [DOI] [PubMed] [Google Scholar]
- 50.Barraud D, Faivre V, Damy T, Welschbillig S, Gayat E, Heymes C, et al. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med. 2007;35:1376–82. doi: 10.1097/01.CCM.0000261889.18102.84. [DOI] [PubMed] [Google Scholar]


