Abstract
Purpose.
Photochemical cross-linking of corneal collagen is an evolving treatment for keratoconus and other ectatic disorders. We evaluated collagen cross-linking by rose bengal plus green light (RGX) in rabbit eyes and investigated factors important for clinical application.
Methods.
Rose bengal (RB, 0.1%) was applied to deepithelialized corneas of enucleated rabbit eyes for 2 minutes. The diffusion distance of RB into the stroma was measured by fluorescence microscopy on frozen sections. RB-stained corneas were exposed to green (532-nm) light for 3.3 to 9.9 minutes (50–150 J/cm2). Changes in the absorption spectrum during the irradiation were recorded. Corneal stiffness was measured by uniaxial tensiometry. The spatial distribution of the stromal elastic modulus was assessed by Brillouin microscopy. Viable keratocytes were counted on H&E-stained sections 24 hours posttreatment.
Results.
RB penetrated approximately 100 μm into the corneal stroma and absorbed >90% of the incident green light. RGX (150 J/cm2) increased stromal stiffness by 3.8-fold. The elastic modulus increased in the anterior approximately 120 μm of stroma. RB was partially photobleached during the 2-minute irradiation, but reapplication of RB blocked light transmission by >70%. Spectral measurements suggested that RGX initiated cross-linking by an oxygen-dependent mechanism. RGX did not decrease keratocyte viability.
Conclusions.
RGX significantly increases cornea stiffness in a rapid treatment (≅12 minutes total time), does not cause toxicity to keratocytes and may be used to stiffen corneas thinner than 400 μm. Thus, RGX may provide an attractive approach to inhibit progression of keratoconus and other ectatic disorders.
Keywords: collagen cross-linking, corneal stiffness, ectatic disorder, keratoconus, photochemistry
Cross-linking corneal collagen using rose bengal and short exposure to green light substantially increases corneal stiffness without causing keratocyte toxicity. Localization of cross-links near the anterior surface allows treatment of thin corneas in ectatic disorders.
Introduction
Keratoconus is characterized by protrusion of the corneal surface due to thinning and weakening of the corneal stroma. The cornea assumes a progressively conical shape at or around the visual axis, resulting in severe astigmatism and poor visual acuity. Keratoconus typically begins in puberty and may stabilize at 30 to 40 years or continue to progress. Structural changes in the cornea include thinning of the central stroma and breaks in Bowman's layer as well as a decreased number of collagen lamellae.1 The mechanisms underlying keratoconus are poorly understood and may involve an inheritable genetic factor, increased protease activity, oxidative stress, and environmental factors such as eye rubbing.2
Management of keratoconus revolves around visual rehabilitation as well as slowing the disease progression. Eye glasses and soft and hard contact lenses are the traditional modalities to improve visual acuity. As the disease progresses, other treatments may be needed such as scleral contact lenses or intracorneal ring segments.3 Lamellar or penetrating keratoplasty is the final resort if progression continues. More recently, collagen cross-linking using riboflavin-5-phosphate (riboflavin) and ultraviolet A (UVA) light has been used to stiffen the stromal collagen and shown to be effective for decreasing progression in keratoconus.4–6 Covalent cross-links formed between collagen molecules in collagen fibers increase the tensile strength of the stroma, making the cornea more able to resist the intraocular pressure (IOP) that distorts the normal curvature.7,8 Despite its usefulness, collagen cross-linking with riboflavin has been associated with drawbacks including cytotoxicity to keratocytes,9–11 a long procedure time,12 and limitation to only treating corneas greater than 400 μm thick.10,13,14 Recent technical developments, however, allow treatment of thinner corneas using hypoosmotic riboflavin15 and a shorter irradiation time using a commercially available light source (KXL System; Avedro, Waltham, MA).
We have developed a collagen cross-linking technology that uses green light to activate rose bengal (RB), a well-known diagnostic agent for ocular surface damage. We have previously demonstrated that this technology can be used to seal wounds in cornea, to bond amniotic membrane to the corneal surface, and for applications in many other tissues.16–21 In this study, we investigated whether photochemical cross-linking of cornea stromal collagen using RB and green light, a process called RGX, increases corneal stiffness. We investigated the processes involved and factors important for clinical application.
Materials and Methods
Materials
Rose bengal sodium salt (Sigma-Aldrich, St. Louis, MO) was prepared as a 0.1% weight/volume solution in phosphate buffered saline (PBS). Riboflavin-5-monophosphate (Sigma-Aldrich) was prepared as a 0.1% weight/volume solution in PBS containing 20% dextran (≈500 kDa; Sigma-Aldrich). Fresh young rabbit eyes were purchased from a commercial supplier (Pel-Freez Biologicals, Rogers, AR) and received on ice 17 to 24 hours after enucleation. Previously frozen eyes were also purchased and were thawed in PBS. Eyes were also obtained immediately after euthanasia from New Zealand white rabbits using an IACUC-approved protocol and in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. The corneal epithelium was removed by brief treatment with 30% ethanol and scraping.
Light Sources
A cw KTP-frequency doubled solid state laser (Oculight OR; IRIDEX Corporation, Mountain View, CA) provided green light (532 nm), which is strongly absorbed by RB (absorption coefficient at 532 nm: ≈25,000 M−1 cm−1 in PBS; Fig. 1A). The laser light from an optical fiber was expanded to a 1.2-cm-diameter beam and was delivered with an irradiance of 0.25 W/cm2. Riboflavin-treated corneas were exposed to UVA radiation from a filtered high-intensity UV lamp (Blak-Ray Lamp, Model B-100A; Ted Pella Inc., Redding, CA) with output at 365 ± 3 nm as measured with a spectroradiometer (SPR-01, 235–850 nm; Luzchem Research Inc., Gloucester, Ontario, Canada). The power of each light source was measured with a power meter (NOVA; Ophir Optronics Ltd., North Andover, MA).
Figure 1.
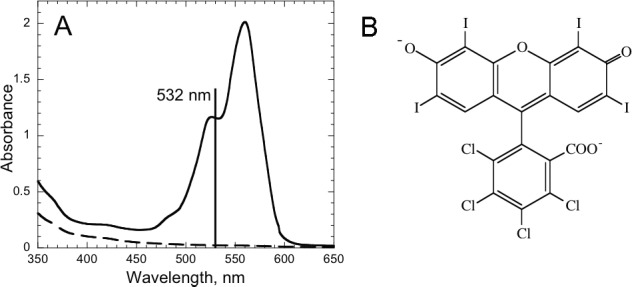
Rose bengal. (A) Absorption spectrum of RB (0.1% in PBS) applied to rabbit cornea for 2 minutes. Dashed line is spectrum of cornea without RB. (B) Structure of RB.
Absorption Spectra
After application of RB (0.1% in PBS) to deepithelialized cornea and removal of excess RB by brief washing with PBS, a 4-mm central cornea disc was placed on a glass coverslip for measuring the spectrum using a plate reader (Spectramax M5; Molecular Devices, Sunnyvale, CA). Spectra after varying irradiation times were measured similarly.
Fluorescence Microscopy
After removing the epithelial layer, the cornea was immersed in 0.1% RB solution for 2 minutes before a 4-mm disc of central cornea was removed. The RB-stained corneal disc was placed in 1 μM nuclear stain (TO-PRO-3; Life Technologies, Grand Island, NY) in PBS for 15 minutes to stain cell nuclei. The disc was bisected and the halves were placed in optical cutting temperature compound. Vertically cut frozen sections (5 μm) were dried on slides and washed three times with water, then coverslipped. The RB fluorescence was excited at 559 nm and emission detected at 575 to 620 nm (Olympus FluoView 1000 confocal microscope; Olympus America Inc., Center Valley, PA). Fluorescence (TO-PRO-3) was excited at 635 nm and emission was detected in the range 665 to 755 nm. The RB intensity profile was determined by averaging the signal along 20 lines drawn perpendicular to the stroma surface using ImageJ software (available in the public domain at http://rsbweb.nih.gov/ij/index.html; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD).
Collagen Photo-Cross-Linking
After application of 0.1% RB (8 drops over 2 minutes), excess RB was removed by brief washing with PBS and corneas were irradiated with 532-nm light with an irradiance of 0.25 W/cm2. In an initial study using previously frozen eyes, the RB-stained cornea was irradiated for 3.3, 6.6, or 10 minutes to deliver fluences of 50, 100, or 150 J/cm2, respectively. In subsequent studies using fresh commercially available rabbit eyes stained with RB, an additional 4 drops of 0.1% RB were applied after 3.3 and 6.6 minutes. For comparison, deepithelialized corneas pretreated with 0.1% riboflavin in 20% dextran solution every 5 minutes for 30 minutes were exposed to UVA light for 30 minutes (3 mW/cm2; 5.4 J/cm2) with 0.1% riboflavin solution added every 5 minutes. This procedure mimics the frequently used clinical protocol.22
Uniaxial Tensiometry
After removing the cornea, the central thickness was measured with a spring-loaded micrometer with 10-μm gradations (No. 1010; Starrett Co., Athol, MA). A 2-mm-wide strip through the central cornea was cut on the horizontal axis with parallel blades and trimmed to approximately 15-mm length. Corneal strips were kept on moistened gauze in a covered dish and measured within 1 hour of treatment. A total of four or five samples representing different groups were produced and tested in a batch. Each strip was mounted in the jaws of a testing machine (Micro EP Miniature; Admet, Norwood, MA) with a 10 N load cell. The initial distance between the jaws was increased until a load of 0.01 N was detected and the separation at this load was used for calculating the length changes. Cornea strips were conditioned by applying uniaxial tension (three load cycles between 0.1 and 0.3 N) before loading to failure at a stretch rate of 1 mm/min. The force generated during the test was recorded in Newtons (N) along with the distance between the jaws (using an MTEST Quattro controller; Admet). Stiffness of the sample was calculated by dividing the force by the displacement (in mm) at 10% extension. Young's modulus was calculated by dividing the stress (force/cross-sectional area) by the strain (10% extension).
Brillouin Optical Microscopy
Brillouin optical microscopy is a recently developed technique for 3-dimensional mechanical imaging that has been used to assess the biomechanical properties of cornea in situ at high resolution.23,24 The longitudinal elastic modulus of a material is related to the frequency shift of the Brillouin-scattered light. The cornea from a freshly enucleated eye was placed in 0.1% RB for 2 minutes, then the cornea was exposed to 150 J/cm2 green light. The control eye was treated with PBS only. The Brillouin-scattered light was collected as described previously.23,24 A Brillouin image of the cornea is produced by plotting the measured Brillouin frequency shifts over space with color encoding.
Keratocyte Viability
The deepithelialized cornea of freshly enucleated rabbit eyes were placed in 0.1% RB for 2 minutes. One-half of each cornea was shielded from light, and the other half was exposed to either 100 or 200 J/cm2 green light. The cornea and a rim of sclera were removed and cultured for 24 hours, which increased the thickness by 2.4-fold (RB-treated) and 2.0-fold (riboflavin/dextran-treated).25 Corneas were fixed in 10% phosphate buffered formalin and paraffin-embedded. Corneas treated with riboflavin/dextran + UVA were positive controls since this protocol causes keratocyte toxicity.9,11 A fluence of 14.4 J/cm2 was delivered in 30 minutes (8 mW/cm2). Cell nuclei were counted on 5-μm H&E-stained sections (at least nine fields/eye for each treatment and three eyes/treatment). Cells in the most anterior 200 μm were counted on RB-treated cornea, which corresponded to approximately 85 μm in unswollen cornea and to a depth of 500 μm on riboflavin/dextran-treated cornea, which corresponded to 250 μm. The field width was 500 μm.
Statistics
A two-tailed, unpaired Student's t-test was performed to assess differences between groups. Significance was set at P < 0.05.
Results
Light Absorption by Rose Bengal on Rabbit Cornea
Absorption spectra were measured on cornea of commercially obtained fresh rabbit eyes after application of 0.1% RB in PBS. The spectra have a maximum at 562 nm and a shoulder from 520 to 535 nm (Fig. 1A). At the irradiation wavelength, 532 nm, the absorption is usually approximately 1.0, indicating that RB within the stroma absorbs approximately 90% of the incident light.
Diffusion of Rose Bengal Into Rabbit Cornea
Since RGX can occur only in the stromal region containing RB to absorb the green light, we determined the depth that RB diffused into the stroma. Corneas of previously frozen eyes were RB-stained as described for RGX, then the cornea was removed and stained with a long-wavelength absorbing/fluorescing nuclear stain (TO-PRO-3). Fluorescence observed on frozen sections showed that RB localizes near the anterior surface (Fig. 2A). Measurement of the RB fluorescence intensity on these images as a function of depth indicated that the intensity decreased to 10% of the maximum value by approximately 150 μm (Fig. 2B). Correcting this value for the increase in thickness due to freezing (to ≈750 μm from ≈500 μm), indicates that RB actually diffuses approximately 100 μm into the stroma. The depth of RB fluorescence increased only very slightly when observed over 4 hours by confocal microcopy on intact cornea, suggesting that RB was strongly bound to molecules in the anterior stroma. This suggestion was supported by agitating RB-stained corneas in PBS for varying lengths of time and measuring the RB remaining in the cornea. Approximately 20% of the RB was released over 40 minutes but the remaining 80% remained at least up to 160 minutes (Fig. 2C).
Figure 2.
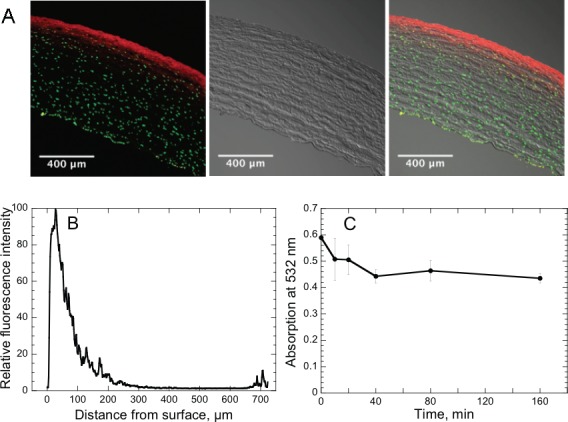
Diffusion of RB into corneal stroma. (A) Frozen section of rabbit cornea stained on anterior surface with RB, then with a nuclear stain (TO-PRO-3), on anterior and posterior surfaces. Left: fluorescence of RB (red) and TO-PRO-3 (green). Center: brightfield image. Right: merged image. (B) RB fluorescence intensity as a function of distance from stroma anterior surface; measured on frozen section shown in (A). (C) Retention of RB by rabbit cornea. RB-stained rabbit corneas were incubated in PBS and absorption spectra of the cornea were measured at times up to 160 minutes. Absorption at 532 nm is shown.
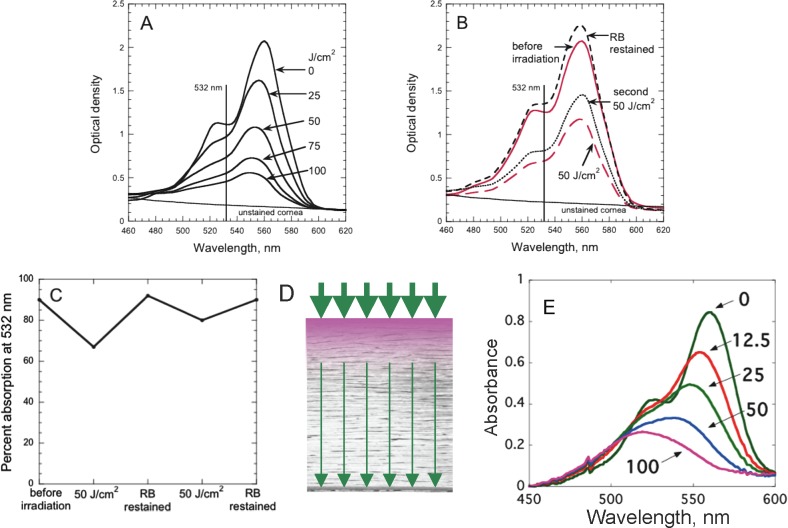
Rose Bengal Photobleaching
Absorption of 532-nm light by RB-stained cornea decreased after irradiation (i.e., RB photobleached), using fluences of 25, 50, 75, and 100 J/cm2 green light (1.7, 3.3, 5, and 6.7 minutes; Fig. 3A) on previously frozen eyes. The peak absorbance (optical density corrected for spectrum of unstained cornea) decreased in a fluence-dependent manner. To maximize the absorption of green light (and consequently cross-linking) by the RB-stained cornea during RGX, RB solution was added after fluences of 50 and 100 J/cm2. Figure 3B shows that 50 J/cm2 decreases the RB absorption at 532 nm by approximately 50%. Subsequent restaining with RB restores the full absorption and during the second 50 J/cm2 (total fluence = 100 J/cm2), photobleaching occurs again. Restaining with RB and irradiation with an additional 50 J/cm2 to reach a total fluence of 150 J/cm2 showed similar changes in the absorption spectra (results not shown). The absorption measurements were converted into the percentage absorption of 532-nm light as shown in Figure 3C. At all time points, RB absorbed between 70 and 90% of the incident light. The absorption of green light by RB in the cornea is shown schematically in Figure 3D.
Figure 3.
Absorption of green light by RB during irradiation. (A) Absorption spectra of RB on cornea before irradiation and after 25, 50, 75, and 100 J/cm2 irradiation with 532-nm green light without additional RB drops. (B) Restaining cornea with RB during irradiation. Absorption spectra were taken before irradiation (red solid line) and after 50 J/cm2 (red long dashed line). Cornea was then restained with RB and absorption spectrum measured (black short dashed line), then irradiated a second time with 50 J/cm2 and absorption measured (black dotted line). (C) Percentage of green light at 532 nm that is absorbed by RB-stained cornea before irradiation, after 50 J/cm2 irradiation, after subsequent restaining with RB, and a second 50 J/cm2 irradiation, then a second restaining with RB. (D) Schematic diagram showing absorption of green light by RB in the cornea. The incident light (thick green arrows) is diminished due to absorption by RB near the anterior surface; much less light, thin green arrows, reaches the posterior surface. (E) Absorption spectra of RB-stained cornea irradiated in the absence of oxygen. Numbers are the fluences (J/cm2) of 532-nm light delivered to the cornea surface.
The decrease in RB absorption without a change in the absorption maximum (Fig. 3B) is consistent with a photobleaching mechanism in which singlet oxygen, that is known to be formed by energy transfer from the RB triplet excited state to oxygen,26 reacts with RB in the tissue to destroy the xanthene chromophore. To evaluate this concept, oxygen diffusion into the cornea was blocked by sandwiching the RB-stained cornea between two microscope slides and surrounding it with silicone sealant before the irradiation. As shown in Figure 3E, the RB absorption decreased and the spectrum maximum shifted from 562 nm to a shorter wavelength (≈520 nm), indicating that the products formed with minimal oxygen present differ from those formed by photobleaching in the presence of oxygen.
Stiffening Cornea Using RGX
Cornea stiffness was evaluated by uniaxial extensiometry. A pilot study using previously frozen rabbit eyes stained with 0.1% RB indicated that fluences of 100 and 150 J/cm2 produced an increase in stromal stiffness. Next, corneas in commercially obtained fresh rabbit eyes (n = 7–9/group) were untreated or stained with 0.1% RB and kept in the dark or irradiated with green light. RGX using 150 J/cm2 decreased the mean central stromal thickness by 13% (Table). Uniaxial tensiometry measurements were used to calculate values of the corneal stiffness (the resistance force exerted by the full thickness of the cornea divided by the change in length of the cornea strip at 10% extension). Stiffness was chosen as the most informative parameter for clinical application because it is a property of the full thickness of the stroma, which in the intact eye is responsible for resisting the IOP. RB-stained, but not irradiated, corneas were 1.9-fold stiffer than untreated control corneas (Table). Irradiating RB-stained corneas with 100 J/cm2 (additional RB after 3.3 minutes) increased the average stiffness by 2.3-fold compared with untreated control corneas, whereas using 150 J/cm2 (additional RB after 2.7 and 6.7 minutes) the increase was even more substantial, 3.8-fold. The values for Young's modulus, an intrinsic property of the material tested, show a similar pattern with a 4.4-fold increase using 150 J/cm (Table).
Table.
Stiffening Rabbit Cornea by Photo-Cross-Linking
|
Treatment |
n |
Stromal Thickness, mm* |
Average Stromal Stiffness, N/mm* |
Average Young's Modulus, MPa* |
| Control, no treatment | 8 | 0.565 ± 0.051 | 0.772 ± 0.380 | 3.72 ± 1.69 |
| Rose bengal, 0.1% in PBS | ||||
| No light | 7 | 0.538 ± 0.074† | 1.49 ± 0.34‡ | 6.86 ± 3.58† |
| 100 J/cm2 green light | 9 | 0.474 ± 0.040‡ | 1.80 ± 0.42‡ | 10.2 ± 2.88‡ |
| 150 J/cm2 green light | 6 | 0.494 ± 0.040‡ | 2.92 ± 0.60‡ | 16.3 ± 4.08‡ |
| Riboflavin, 0.1% in 20% dextran | ||||
| No light | 7 | 0.429 ± 0.049‡ | 1.71 ± 0.47‡ | 11.9 ± 2.89‡ |
| 5.4 J/cm2 UVA | 7 | 0.335 ± 0.029‡ | 4.24 ± 2.50‡ | 31.7 ± 17.5‡ |
Mean ± SD.
Indicates P < 0.01 compared with no treatment control group.
Indicates P < 0.001 compared with no treatment control group.
Similar measurements were made on corneas treated with riboflavin in 20% dextran using a sequence mimicking a clinical protocol (see the Materials and Methods section).12 The 41% decrease in cornea thickness by photo-cross-linking using 5.4 J/cm2 UVA was greater than observed after RGX (Table). The average stiffness after irradiation increased by a factor of 5.5-fold compared with the untreated controls and Young's modulus increased by 8.5-fold, showing the effect of the substantial decrease in stromal thickness on calculation of Young's modulus.
The shallow diffusion distance of RB into the stroma (Fig. 2) suggests that collagen cross-links, and therefore increased stiffness, will be localized close to the anterior surface. Brillouin microscopy, a recently introduced technology for measuring elasticity of materials, was used to measure the elastic modulus as a function of distance from the anterior surface.24 In a normal fresh rabbit cornea the elastic modulus is highest (i.e., the cornea is stiffest) in the most anterior region.27 Brillouin microscopy measurements confirmed this observation.24 After RGX (0.1% RB, 150 J/cm2) using freshly enucleated rabbit eyes, the stroma showed an even higher elastic modulus near the anterior surface than the untreated cornea (Fig. 4). The elasticity returns to the values in a normal cornea at 100 to 120 μm from the surface, for an approximately 450-μm-thick stroma, mimicking the penetration depth of RB in the stroma.
Figure 4.
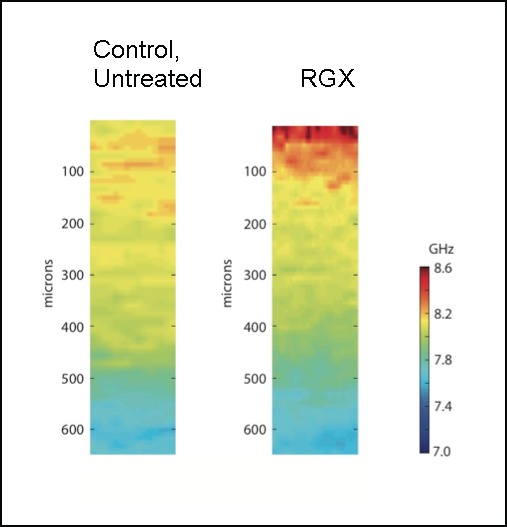
Cross-sectional images of Brillouin scattering of corneas. Anterior surface of deepithelialized fresh rabbit cornea is at top and distance into cornea is indicated. Cornea thickness is 450 to 500 μm. Left: untreated; right: treated with RB and 150 J/cm2 green (532 nm) light. Higher-frequency values (GHz) indicate higher elastic modulus and greater corneal stiffness.
Effect of RGX on Keratocyte Viability
Although RB is known to be phototoxic to cultured cells,28,29 we have previously shown that photo-cross-linking with RB and green light to seal skin wounds did not cause phototoxicity to dermal cells.30 Consequently, cell viability was measured 24 hours after RGX using cornea of freshly enucleated eyes. After RB application one-half of each cornea was shielded from light and the other half irradiated with 100 or 200 J/cm2. As shown in Figures 5A, 5B, the density of nuclei on H&E-stained vertical sections appeared to be the same in irradiated and nonirradiated portions of the cornea. Previous studies have shown that treatment with riboflavin plus UVA cross-linking causes keratocyte apoptosis9–11 and our study confirmed this finding (Figs. 5C, 5D). Riboflavin and UVA produced loss of cells in the irradiated cornea half. Counting nuclei in the irradiated and unirradiated areas of RB and riboflavin/dextran-treated corneas confirmed these observations (Fig. 5E).
Figure 5.
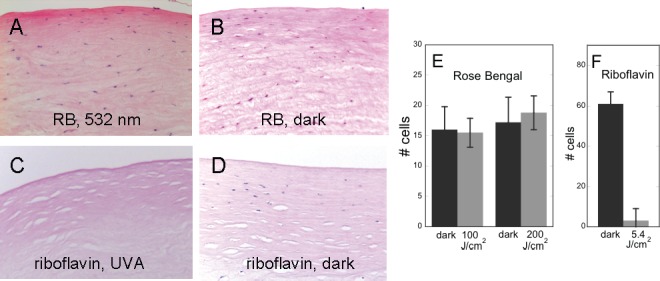
Keratocytes in cornea after photo-cross-linking. (A, B) RB was applied to cornea and exposed to 100 or 200 J/cm2 green light (A) or kept in dark (B). (C, D) Riboflavin/dextran was applied to cornea and exposed to 8.7 J/cm2 UVA (C) or kept in dark (D). Corneas were cultured for 24 hours then fixed, paraffin-embedded, and H&E-stained. (E) Cells in stroma of corneas treated with RB, then with 100 or 200 J/cm2 green light or kept in dark. Only cells in most anterior stroma (200 μm) on section were counted. (F) Cells in corneas treated with riboflavin, then with 8.7 J/cm2 UVA or kept in dark. Cells to a depth of 500 μm on the sections were counted. n = 3 to 4 cornea for each treatment condition.
Discussion
The results of this study suggest that cross-linking corneal collagen with RB and green light (RGX) may provide a novel approach to inhibiting progression of keratoconus and other ectatic disorders. After a brief application to deepithelialized cornea, RB penetrates approximately 100 μm into the corneal stroma, where it absorbed >90% of the incident green light. Irradiation (<10 minutes) increased the elastic modulus selectively in the anterior stroma, leading to a 3.8-fold increase in stromal stiffness. Significantly, RGX did not decrease keratocyte viability.
A key aspect of RGX is the relatively short distance that RB diffuses into the corneal stroma. Fluorescence microscopy showed that RB localizes in an approximately 100-μm-wide band adjacent to the anterior surface (Figs. 2A, 2B) where it absorbs most of the incident green light (Fig. 3). The exact reason for the limited diffusion of RB into the stroma is not clear. Since RB is tightly bound to the stroma (Fig. 3C), we hypothesize that complexes formed between negatively-charged RB (Fig. 1B) and the many positively charged lysines, hydroxylysines, and arginines in collagen may inhibit entry of additional RB into the stroma. Complex formation between RB and other proteins has been reported.31 The RB/collagen complexes at the surface of the cornea neutralize the positive charges on collagen, and thus the surface layer becomes strongly negatively charged due to the high concentration of negatively charged glycosaminoglycans. Additional RB molecules may not enter the stroma due to ionic repulsion, leading to the observed band of RB at the surface. This hypothesis requires investigation.
Tight association of RB with collagen may also be a reason that RB is not phototoxic to keratocytes during RGX (Fig. 5). Although both RB and riboflavin are phototoxic to cultured cells,10,29,32 only riboflavin is phototoxic during photo-cross-linking, causing loss of keratocytes by apoptosis and necrosis to the same distance from the anterior surface as UVA penetration.9,11 Formation of RB/collagen complexes may greatly reduce the amount of free RB available to enter cells, whereas riboflavin, which rapidly diffuses in cornea,33 enters the keratocytes, where it absorbs UVA and initiates toxicity.
The localization of RB in the most anterior approximately 100 μm of stroma limits the depth to which collagen cross-links are formed by RGX, but interestingly, the increases in stromal stiffness are substantial (Table). The formation of collagen cross-links in the upper layers of the cornea by RGX was confirmed by Brillouin microscopy measurements of the spatial distribution of the elastic modulus in the stroma before and after RGX (Fig. 4). Values of the Brillouin frequency shift, which are related to the elastic modulus, are higher in the RGX-treated cornea at depths up to 100 to 120 μm. This contrasts with the greater depth of cornea stiffening by riboflavin cross-linking as measured by Brillouin microscopy and optical microscopy.24,34 A significant implication of the stiffening effect of RGX near the anterior cornea surface is that corneas thinner than 400 μm can be cross-linked with RGX. A disadvantage of riboflavin cross-linking is that the stroma must be at least 400 μm thick to allow accumulation of sufficient riboflavin to block UVA that may damage endothelial cells.10,13,14,35 Since corneal thickness is significantly decreased in keratoconus patients a subset of patients cannot be treated.
Association of RB with collagen may account for the increase in stromal stiffness of corneas treated with RB but not irradiated (Table). RB may bridge between collagen molecules or microfibrils, as well as change the ionic environment as described above, to alter the molecular organization of the stoma sufficiently to increase its stiffness. This hypothesis is consistent with the observation that RB (no light) decreases the stromal thickness (Table). Interestingly, application of riboflavin/dextran without UVA irradiation also increased stromal stiffness in this study (Table) and in a study using Brillouin microscopy to assess the stromal modulus,36 although not in an earlier, seminal study.37 Riboflavin/dextran application substantially decreases the stromal thickness8,38 due to dehydration, which is also known to increase corneal stiffness.39
The mechanism for protein–protein cross-linking by RGX is initiated by light absorption by RB molecules, which are promoted to excited singlet, then excited triplet states. When sufficient oxygen is present, the RB triplet state transfers energy to oxygen, generating singlet oxygen.26 Previous studies have shown that singlet oxygen reacts with certain amino acids, mainly histidine, to initiate covalent bonds between protein molecules.40,41 The photobleaching results shown in Figure 3B indicate that sufficient oxygen is present during RGX because RB is photobleached without the formation of the short-wavelength–absorbing products that are formed in the absence of oxygen (shown in Fig. 3E). This result suggests that the cross-links are formed in an oxygen-dependent process, consistent with our previous report of oxygen-dependent RB sensitized photo-cross-linking of amniotic membrane to the cornea surface.21 In contrast, photo-cross-linking during riboflavin/UVA treatment of cornea appears to be a nearly oxygen-independent process.33
For application of RGX to keratoconus eyes or other cornea conditions, the safety of the treatment must be considered. Since RB does not penetrate through the stroma, photosensitized damage to the endothelial layer is not a concern. However, some green light will reach the retina through the pupil. We calculated that RGX would deliver an irradiance of 3.47 mW/cm2 on the retina when a light delivery device is used that produces a diverging beam with a 12-mm image on the retina. This irradiance is at more than a factor of 20 below the thresholds for 532-nm light set by the American National Standard Institute of 75.5 and 159 mW/cm2 for thermal and photochemical damage.42,43 This calculation allowed 50% of the light to be transmitted to the retina, which is greater than the maximum observed in the photobleaching studies (Fig. 3C).
In summary, RGX significantly increases corneal stiffness in a rapid treatment (approximately 12 minutes total time) by an oxygen-dependent mechanism, does not cause toxicity to stromal keratocytes and may be used to stiffen corneas thinner than 400 μm. Thus, RGX may provide an attractive approach to stiffen corneas for inhibiting progression of keratoconus and other ectatic disorders.
Acknowledgments
The authors thank colleagues Conor Evans, Robert Webb, Francois Delori, and Charles Lin for enlightening discussions of this work, and Kenneth Bujold and Jie Zhao for technical assistance.
Disclosure: D. Cherfan, None; E.E. Verter, None; S. Melki, None; T.E. Gisel, None; F.J. Doyle Jr, None; G. Scarcelli, None; S.H. Yun, None; R.W. Redmond, P; I.E. Kochevar, P
References
- 1. Ambekar R, Toussaint KC Jr. Wagoner Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011; 4: 223–236. [DOI] [PubMed] [Google Scholar]
- 2. Sugar J, Macsai MS. What causes keratoconus? Cornea. 2012; 31: 716–719. [DOI] [PubMed] [Google Scholar]
- 3. Pinero DP, Alio JL. Intracorneal ring segments in ectatic corneal disease—a review. Clin Exp Ophthalmol. 2010; 38: 154–167. [DOI] [PubMed] [Google Scholar]
- 4. Tomkins O, Garzozi HJ. Collagen cross-linking: strengthening the unstable cornea. Clin Ophthalmol. 2008; 2: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006; 17: 356–360. [DOI] [PubMed] [Google Scholar]
- 6. Caporossi A, Mazzotta C, Baiocchi S, et al. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012; 31: 227–231. [DOI] [PubMed] [Google Scholar]
- 7. Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009; 87: 48–51. [DOI] [PubMed] [Google Scholar]
- 8. Kling S, Remon L, Perez-Escudero A, Merayo-Lloves J, Marcos S. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest Ophthalmol Vis Sci. 2011; 51: 3961–3968. [DOI] [PubMed] [Google Scholar]
- 9. Wollensak G, Spoerl E, Wilsch M, Seiler T. Keratocyte apoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea. 2004; 23: 43–49. [DOI] [PubMed] [Google Scholar]
- 10. Wollensak G, Spoerl E, Reber F, Seiler T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye. 2004; 18: 718–722. [DOI] [PubMed] [Google Scholar]
- 11. Kruger A, Hovakimyan M, Ramirez Ojeda DF, et al. Combined nonlinear and femtosecond confocal laser-scanning microscopy of rabbit corneas after photochemical cross-linking. Invest Ophthalmol Vis Sci. 2011; 52: 4247–4255. [DOI] [PubMed] [Google Scholar]
- 12. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135: 620–627. [DOI] [PubMed] [Google Scholar]
- 13. Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003; 29: 1786–1790. [DOI] [PubMed] [Google Scholar]
- 14. Wollensak G, Sporl E, Reber F, Pillunat L, Funk R. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res. 2003; 35: 324–328. [DOI] [PubMed] [Google Scholar]
- 15. Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009; 35: 621–624. [DOI] [PubMed] [Google Scholar]
- 16. Gu C, Ni T, Verter EE, et al. Photochemical tissue bonding: a potential strategy for treating limbal stem cell deficiency. Lasers Surg Med. 2011; 43: 433–442. [DOI] [PubMed] [Google Scholar]
- 17. Henry FP, Cote D, Randolph MA, et al. Real-time in vivo assessment of the nerve microenvironment with coherent anti-Stokes Raman scattering microscopy. Plast Reconstr Surg. 2009; 123: 123S–130S. [DOI] [PubMed] [Google Scholar]
- 18. O'Neill AC, Winograd JM, Zeballos JL, et al. Microvascular anastomosis using a photochemical tissue bonding technique. Lasers Surg Med. 2007; 39: 716–722. [DOI] [PubMed] [Google Scholar]
- 19. Proano CE, Mulroy L, Jones E, et al. Photochemical keratodesmos for bonding corneal incisions. Invest Ophthalmol Vis Sci. 2004; 45: 2177–2181. [DOI] [PubMed] [Google Scholar]
- 20. Tsao S, Yao M, Tsao H, et al. Light-activated tissue bonding for excisional wound closure: a split-lesion clinical trial. Br J Dermatol. 2012; 166: 555–563. [DOI] [PubMed] [Google Scholar]
- 21. Verter EE, Gisel TE, Yang P, et al. Light-initiated bonding of amniotic membrane to cornea. Invest Ophthalmol Vis Sci. 2011; 52: 9470–9477. [DOI] [PubMed] [Google Scholar]
- 22. Snibson GR. Collagen cross-linking: a new treatment paradigm in corneal disease—a review. Clin Exp Ophthalmol. 2010; 38: 141–153. [DOI] [PubMed] [Google Scholar]
- 23. Scarcelli G, Yun SH. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat Photonics. 2007; 2: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci. 2012; 53: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foreman DM, Pancholi S, Jarvis-Evans J, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp Eye Res. 1996; 62: 555–564. [DOI] [PubMed] [Google Scholar]
- 26. Kochevar IE, Redmond RW. Photosensitized production of singlet oxygen. Methods Enzymol. 2000; 319: 20–28. [DOI] [PubMed] [Google Scholar]
- 27. Hennighausen H, Feldman ST, Bille JF, McCulloch AD. Anterior–posterior strain variation in normally hydrated and swollen rabbit cornea. Invest Ophthalmol Vis Sci. 1998; 39: 253–262. [PubMed] [Google Scholar]
- 28. Schieke SM, von Montfort C, Buchczyk DP, et al. Singlet oxygen-induced attenuation of growth factor signaling: possible role of ceramides. Free Radic Res. 2004; 38: 729–737. [DOI] [PubMed] [Google Scholar]
- 29. Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000; 275: 25939–25948. [DOI] [PubMed] [Google Scholar]
- 30. Yao M, Yaroslavsky A, Henry FP, Redmond RW, Kochevar IE. Phototoxicity is not associated with photochemical tissue bonding of skin. Lasers Surg Med. 2011; 42: 123–131. [DOI] [PubMed] [Google Scholar]
- 31. Alarcon E, Edwards AM, Aspee A, Borsarelli CD, Lissi EA. Photophysics and photochemistry of rose bengal bound to human serum albumin. Photochem Photobiol Sci. 2009; 8: 933–943. [DOI] [PubMed] [Google Scholar]
- 32. Maguire A, Morrissey B, Walsh JE, Lyng FM. Medium-mediated effects increase cell killing in a human keratinocyte cell line exposed to solar-simulated radiation. Int J Radiat Biol. 2011; 87: 98–111. [DOI] [PubMed] [Google Scholar]
- 33. Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012; 53: 2360–2367. [DOI] [PubMed] [Google Scholar]
- 34. Chai D, Gaster RN, Roizenblatt R, et al. Quantitative assessment of UVA-riboflavin corneal cross-linking using nonlinear optical microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 4231–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007; 26: 385–389. [DOI] [PubMed] [Google Scholar]
- 36. Scarcelli G, Kling S, Quijano E, et al. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013; 54: 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998; 66: 97–103. [DOI] [PubMed] [Google Scholar]
- 38. Hayes S, Kamma-Lorger CS, Boote C, et al. The effect of riboflavin/UVA collagen cross-linking therapy on the structure and hydrodynamic behaviour of the ungulate and rabbit corneal stroma. PLoS One. 2013; 8: e52860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jayasuriya AC, Scheinbeim JI, Lubkin V, Bennett G, Kramer P. Piezoelectric and mechanical properties in bovine cornea. J Biomed Mater Res A. 2003; 66: 260–265. [DOI] [PubMed] [Google Scholar]
- 40. Shen HR, Spikes JD, Kopeckova P, Kopecek J. Photodynamic crosslinking of proteins. II. Photocrosslinking of a model protein-ribonuclease A. J Photochem Photobiol B. 1996; 35: 213–219. [DOI] [PubMed] [Google Scholar]
- 41. Verweij H, Van Steveninck J. Model studies on photodynamic cross-linking. Photochem Photobiol. 1982; 35: 265–267. [Google Scholar]
- 42. American National Standard for Safe Use of Lasers (ANSI 136.1-2000). Orlando, FL: The Laser Institute of America; 2000. [Google Scholar]
- 43. Delori F, Webb R, Sliney D. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J Opt Soc Am A Opt Image Sci Vis. 2007; 24: 1250–1265. [DOI] [PubMed] [Google Scholar]