Abstract
Personality disorders have been associated with a wide swath of adverse health outcomes and correspondingly high costs to healthcare systems. To date, however, there has not been a systematic review of the literature on health conditions among individuals with personality disorders. The primary aim of this article is to review research documenting the associations between personality disorders and health conditions. A systematic review of the literature revealed 78 unique empirical English-language peer-reviewed articles examining the association of personality disorders and health outcomes over the past 15 years. Specifically, we reviewed research examining the association of personality disorders with sleep disturbance, obesity, pain conditions, and other chronic health conditions. In addition, we evaluated research on candidate mechanisms underlying health problems in personality disorders and potential treatments for such disorders. Results underscore numerous deleterious health outcomes associated with PD features and PD diagnoses, and suggest potential biological and behavioural factors that may account for these relations. Guidelines for future research in this area are discussed.
Keywords: Systematic review, personality disorders, medical conditions
Nearly 10% of people in the general population suffer from personality disorders (PDs), based on epidemiological studies (Samuels, 2011). PDs are complex mental health problems with high costs to society (Frankenburg & Zanarini, 2004; van Asselt, Dirksen, Arntz, & Severens, 2007), due, in part, to the frequent co-occurrence of mental and medical health problems (Samuels, 2011). Although literature suggests that the co-occurrence of mental health problems among those with PDs is the rule rather than the exception (Zanarini et al., 1998; Zanarini, Frankenburg, Hennen, Reich, & Silk, 2004), PDs are also associated with medical health problems such as cardiovascular disease (Moran et al., 2007; Powers & Oltmanns, 2013), sleep problems (Asaad, Okasha, & Okasha, 2002; Kamphuis, Karsten, de Weerd, & Lancel, 2013), arthritis, obesity (Powers & Oltmanns, 2013) and chronic pain (Fishbain et al., 2007).
Existing reviews of literature underscore a link between PDs and health conditions. For instance, reviews emphasize the association of borderline personality disorder (BPD) with sleep disturbance (Hafizi, 2013). Furthermore, researchers have identified consistent, robust relations between chronic pain conditions and PDs (Conrad, Wegener, Geiser, & Kleiman, 2013). Researchers have also highlighted the high rates of co-occurrence between PDs and other disorders, such as depression, eating disorders, anxiety and substance use behaviours (Samuels, 2011; Zimmerman, Rothschild, & Chelminski, 2005). As such, it is likely that there is an interplay of medical and mental health problems among those with PDs.
Although there has been a recent surge in literature examining the association between health-related outcomes and PDs, to date, there is no comprehensive review and synthesis of this literature. Despite a large literature base on the adverse health problems associated with other mental health disorders, such as depression (Mavrides & Nemeroff, 2013), bipolar (Krishnan, 2005), and panic disorder (Smitherman, Kolivas, & Bailey, 2013); research on PDs and health-related issues lags behind these other areas. Further research in this area is particularly important due to the high societal cost and healthcare utilization associated with PDs (Frankenburg & Zanarini, 2004) and chronic conditions. The combination of PD traits or diagnoses, in addition to chronic health conditions, poses a particularly heavy burden on social health care systems (Frankenburg & Zanarini, 2004). PD features may present unique challenges for standard medical treatment, which is generally not designed to address complex combinations of mental and medical health problems. A systematic review of the link between PDs and health conditions would help to (a) highlight current gaps and future directions for research, (b) identify important areas for clinicians to assess and attend to (suggesting the importance of coordination of care across both psychosocial and medical health), and (c) suggest important targets for medical intervention among those with PDs.
The purpose of the current work is to provide a systematic review of recent empirical studies examining the association between PDs and health conditions. Specifically, this review focuses on sleep, obesity, chronic pain, other chronic health conditions. The bio-psychosocial (BPS) model (Engel, 1977) theorizes that adverse health problems result from a transactional relationship between psychological, biological and social factors, rather than emerging from genetic or physiological factors alone. Thus, we also aimed to identify potential mechanisms (biological, behavioural, and environmental) underlying these associations. The objectives of the present review are to: (1) provide a summary of the extant literature examining relations between PDs and health conditions, (2) examine the scientific rigor of the reviewed studies, and (3) provide recommendations for future research and clinical practice.
Methods
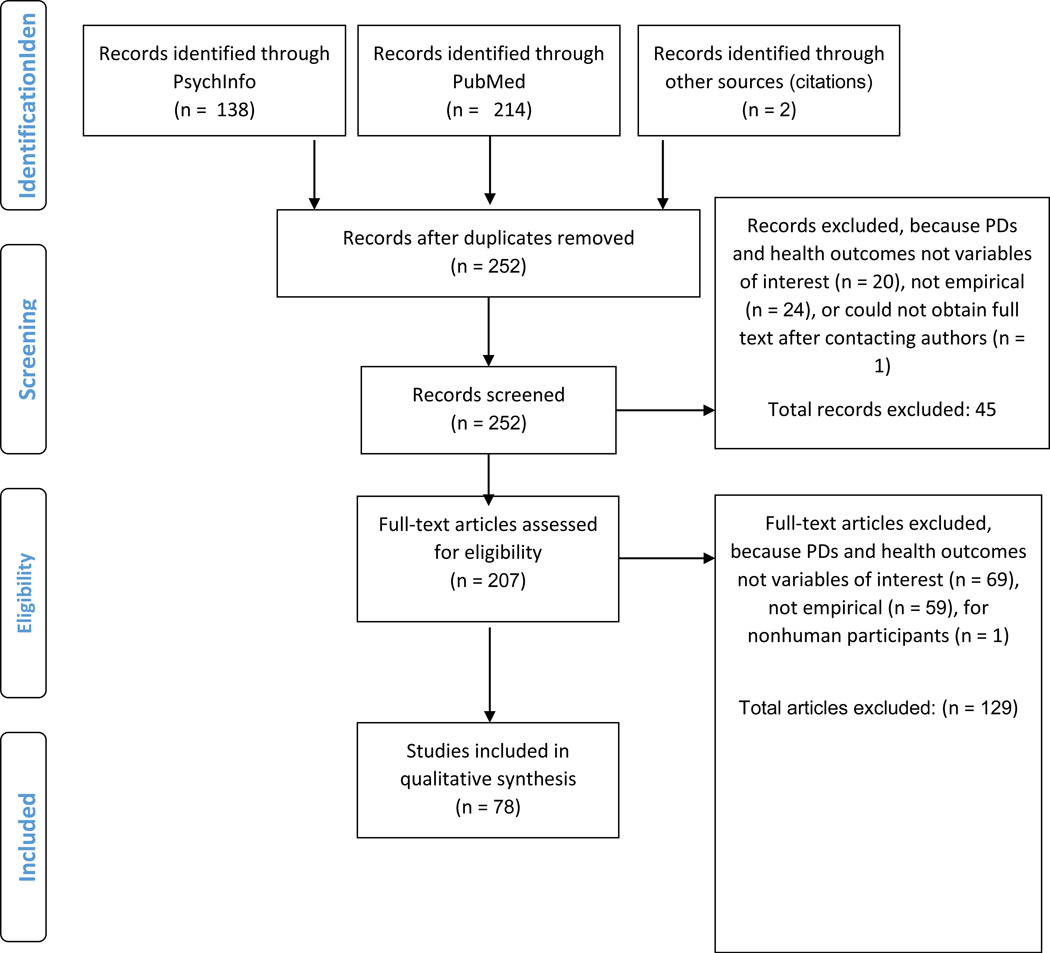
We conducted a search of two databases, PsychInfo and PubMed, for relevant research related to PDs and health outcomes. Our search terms were: [(“personality disorder”) AND (“medical condition” or “medical illness” or “chronic pain” or “fibromyalgia” or “migraine” or “sleep disorder” or headache or “sleep disturbance” or “insomnia” or “surgery” or “irritable bowel syndrome” or “Crohn’s disease” or “cardiovascular disease” or “obesity” or “immune” or immun*)]. We also identified articles that were cited within any reviewed full-text to ensure comprehensive coverage of this area. To be included in this review, research needed to be (1) an empirical primary source, (2) peer-reviewed literature, (3) written or translated into the English language, (4) published from January 1, 2000 to the date the most recent search was conducted for the present manuscript (October 7, 2014), (5) conducted in human samples, and (6) pertaining to the association between PDs and health-related outcomes. For the purposes of the present study, consistent with the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-5; American Psychiatric Association, 1987, 2013), we refer to the 10 distinct personality disorders in DSM-5, organized within three clusters: Cluster A PDs, characterized by odd or eccentric features, including schizoid, paranoid, and schizotypal PDs; Cluster B PDs, characterized by dramatic and impulsive patterns of behaviour, including antisocial PD, BPD, narcissistic and histrionic PDs; and Cluster C PDs, characterized by anxious or fearful patterns of behaviour, including avoidant, dependent, obsessive compulsive PDs, passive aggressive and depressive PDs, as delineated in DSM-III, and PD-not otherwise specified (PD-NOS), as it is one of the most frequently diagnosed PDs (Verheul, Bartak, & Widiger, 2007). In the wake of recent debates regarding the most appropriate conceptualization for PDs (Trull, Distel, & Carpenter, 2011; Trull, Widiger, Lynam, & Costa, 2003; Zimmerman, Chelminski, Young, Dalrymple, & Martinez, 2013), the most recent revision of the DSM (American Psychiatric Association, 2013) has included an alternative trait-based model of PDs. Consistent with the bulk of the literature in this area to date, however, we have opted to review the PDs above. Please see Figure 1 for our selection process and excluded articles. A total of 78 articles are summarized below.
Figure 1.
Selection Process Diagram
Results
The studies included in the final review are presented in Table 1. The majority of the studies were cross-sectional (n = 48), with 14 using epidemiological data (n = 3 from the National Comorbidity Survey Replication [NCS-R]-II, n = 2 Saint Louis, MO, n =2 from Norway, n = 4 National Epidemiologic Survey on Alcohol and Related Conditions [NESARC]-I, n = 2 NESARC-II, and n = 1 from the United Kingdom). Of the studies identified, only 11 studies were primarily longitudinal, with 6 drawing from the same larger study sample (McLean Study of Adult Development [MSAD]). In addition, 16 studies included a laboratory component. Our search identified 2 treatment studies. We have organized this literature broadly by health outcome area, into work examining the relation of PDs with sleep disorders, headache and pain disorders, obesity, other chronic health conditions, potential mechanisms, and treatment studies.
Table 1.
Empirical Studies of Personality Disorders and Adverse Health Outcomes Identified from Systematic Review
| Study | Sample(s) | PDs | Assessment | %Female | Age | Methods | Relevant Findings |
|---|---|---|---|---|---|---|---|
| Sleep (n = 8) | |||||||
| (Asaad et al., 2002) | N = 60; BPD (n = 20); depressed (n = 20); healthy controls (n = 20) |
BPD | ICD-10 symptom checklist |
66.7% | 27.6 | Self-report, 1- night polysomnography |
BPD > controls in sleep latency, sleep wave deficits, and REM abnormalities; BPD < controls in sleep efficiency MDD > BPD sleep latency, number of arousals, REM density; MDD < BPD sleep efficiency, REM latency |
| (Bastien et al., 2008) | N = 57; BPD (n = 12); primary insomnia (n = 15); primary paradoxical insomnia (n = 15); good sleepers (n = 15) |
BPD | DIB-R | 82.5% | 36.4 | Self-report, 3-night Polysomnography, 2-week daily diary |
-On self-reported sleep disturbance, BPD ∼= good-sleepers < sleep-disordered -On objective measures, BPD ∼= sleep-disordered > good- sleepers in sleep latency -BPD ∼= sleep-disordered < good-sleepers in sleep time, sleep efficiency -BPD > paradoxical insomniacs in stage 4 |
| (Kamphuis, Karsten, de Weerd, & Lancel, 2013b) | N = 110 forensic psychiatric patients |
All | Chart review | 2.7% | 36.6 | Cross-sectional self-report and interview, chart review |
-The presence of a PD and antisocial traits was associated with poorer sleep quality |
| (Plante et al., 2013a) | N = 223 patients with BPD; recovered (n = 105); non-recovered (n = 118) |
BPD | DIPD; DIB- R |
81.2% | 42.8 | MSAD | -Recovered < non-recovered sleep onset latency, dysfunction associated with sleep disturbance, even controlling for age, sex, and psychopathology |
| (Plante et al., 2013b) | N = 223 patients with BPD; recovered (n = 105); non-recovered (n = 118) |
BPD | DIPD; DIB- R |
81.2% | 42.8 | MSAD | -Recovered < non-recovered in terms of maladaptive sleep related cognitions (i.e., worry about sleep, beliefs that medication was necessary, helplessness) |
| (Ruiter et al., 2012) | N = 84 patients with chronic insomnia and hypnotic dependence |
All DS M |
SCID-II-SQ | 76.2% | 52.6 | Self-report, 3- night polysomnography, 2-week daily diary |
-Most prevalent PDs were OCPD (n = 39), AvPD (n = 10), PPD (n = 9), NPD (n = 9), BPD (n = 9) -OCPD and APD associated with greater insomnia-related impairment -SzPD and StPD associated with greater insomnia severity |
| (Selby, 2013) | N = 5692 | BPD | IPDE-SQ | - | - | Epidemiological survey – NCS-R |
-Prevalence of ≥ 1 sleep problem in BPD = 63% -BPD > non-BPD in sleep problem duration (19.9 weeks vs. 8.9 weeks) -BPD was predictor of (1) delayed sleep onset, (2) amount of time awake after sleep onset, (3) early morning awakenings, and (4) daytime sleepiness and fatigue (AORs = 1.8–2.2), -BPD ∼= other axis I disorders (e.g., GAD, MDD, PTSD) characterized by sleep disturbance |
| (Semiz et al., 2008) | N = 188; BPD patients (n = 88); age- gender-matched controls (n = 100) |
BPD | SCID-II (Turkish version) |
44.1% | 22.0 | Cross-sectional interview and self- report |
-BPD > control: nightmare disorder (49% vs. 7%), self- reported poor sleepers (95.5% vs. 12%) |
| Pain (n = 22) | |||||||
| (Atasoy et al., 2005) | N = 117 headache patients; migraine patients (n = 50), chronic tension headache patients (n = 28), episodic tension headache patients (n = 31) |
Any repor ted in chart |
Chart review | 85.5% | 41.4 | Retrospective chart review |
-PD prevalence = 81.8% -OCPD more common in migraine patients vs. chronic tension headache patients (31.0% vs. 10.7%), episodic tension headaches were not different from other groups (19.3%) |
| (Braden & Sullivan, 2008) | N = 5692; with self- reported pain (n = 3391); without self- reported pain (n = 2298) |
AsP D, BPD |
IPDE-SQ | - | - | Epidemiological survey – NCS-R |
-Self-reported pain history > no pain in positive screens for BPD or ASPD traits (27.4% vs. 18.6%) -Those with “other chronic pain” > “chronic back/neck pain” in positive screens for BPD or ASPD |
| (Breckenridge & Clark, 2003) | N = 200 Veteran Affairs back pain patients; patients who received nonsteroidal anti-inflammatory drugs (NSAIDs; n = 100); patients who received opioid therapy (n = 100) |
PD vs. no PD |
Chart review | 5.5% | 61.7 | Retrospective chart review |
-Opioid > NSAID in proportion of PD diagnoses (14 vs. 1, adjusted OR = 18.61) |
| (Conrad et al., 2007) | N = 312; chronic pain patients (n = 207); pain-free controls (n = 105) |
All | SCID-II (German version) |
43.9% | 46.7 | Cross-sectional interview and self- report |
-Pain > control in overall PD presence (41% vs. 7%), PPD (12% vs. 0%), SzPD (4% vs. 0%), BPD (11% vs. 0%), AvPD (8% vs. 1%), OCPD (6% vs. 1%), PAPD (8% vs. 0%) -No significant group differences in StPD (3% vs. 0%), ASPD (2% vs. 0%), NPD (3% vs. 0%), DPD (5% vs. 2%), DvPD (5% vs. 1%) |
| (Egloff, Maecker, Stauber, Sabbioni, Tunklova, & von Kanel, 2012) | N = 180 chronic pain patients; with nondermatomal somatosensory deficits (NDSDs; n = 90), and without NDSDs (n = 90) |
PD vs. no PD |
Semi- structured clinical interview of ICD-10 criteria |
53.4% | 45.4 | Cross-sectional interview and self- report |
-Chronic pain with NDSDs < without NDSDs in rates of PDs (3.3% vs. 25.6%) |
| (Ericsson et al., 2002) | N = 184 Swedish chronic pain patients |
PD vs. no PD |
SCID-II-SQ (Swedish version) |
72.8% | 43.4 | Long-term follow- up of records of disability status following baseline evaluation (M = 1358 days later) |
-PD presence was not a significant predictor of disability status (OR = 0.57), whereas depression was (OR = 2.60) |
| (Fischer-Kern et al., 2011) | N = 43 Viennese chronic pain patients |
All | SCID-II (German version) |
81.4% | 51.5 | Cross-sectional interview and self- report |
-PD prevalence = 62.8% -OCPD was most prevalent (27.9%), followed by BPD (25.6%) |
| (Fishbain et al., 2007) | N = 221 chronic pain patients; cigarette smokers (n = 79); nonsmokers (n = 140) |
Not state d |
Diagnosis based on DSM flow chart by psychiatrist |
42.1% | 41.1 | Cross-sectional interview and self-report |
-Smokers > nonsmokers in HPD (61.7% vs. 38.3%) -Smokers < nonsmokers in OCPD (22.8% vs. 77.2%), DPD (35.1% vs. 64.9%) |
| (Loder & Geweke, 2002) | N = 90 callers accounted for 165 calls to a headache clinic |
Any in chart revie w |
Chart review | - | - | Chart review | -Most calls (87%) were placed by a caller with a PD -53% of repeat calls involved a PD patient -All calls characterized as emergencies (n = 11) were placed by those with a PD diagnosis |
| (McWilliams & Higgins, 2013) | N = 5692 | BPD | IPDE-SQ | - | - | Epidemiological survey – NCS-R Part II |
History of each (arthritis, headaches, spinal pain, other) pain condition (remitted or past year) was predictive of higher levels of BPD symptoms -Past-year > remitted headaches in BPD symptoms, controlling for demographic variables and past-year Axis I disorders -Past-year ∼= remitted headaches in BPD symptoms |
| (Manlick et al., 2012) | N = 352; first-degree relatives of Obsessive compulsive disorder patients (n = 168); healthy controls (n = 184) |
All | SIDP | 41.4% | 43.9 | Cross-sectional interview and self- report |
-Those with migraines > those without in rates of PDs (42.9% vs. 15.5%; OR = 3.31) -Migraine > no migraine in most common PD, PaPD (16.3% vs. 5.9%) |
| (Meeks et al., 2008) | N = 148 depressed geriatric psychiatric inpatients; with chronic pain (n = 92); without chronic paint (n = 56) |
PD vs. no PD |
Chart review | 60.1% | 80 | Retrospective chart review |
-Chronic pain > no pain in PD presence (13% vs. 0%) |
| (Olssøn & Dahl, 2009) | N = 2214 Norwegians; with PD pathology (n = 369); age- and gender- matched controls (n = 1845) |
PD vs. no PD |
IPDS | 52% | ∼43.7 | Large-sale population survey |
-PD pathology > no PD in asthma (11% vs. 8%), fibromyalgia (4% vs. 2%), muscular pain ≥ 3 mo (33% vs. 22%), frequent use of nonprescribed analgesics (12% vs. 7%), self-rated poor health (18% vs. 9%), frequent use of GP (19% vs. 12%), physiotherapist (14% vs. 10%), and dissatisfaction with last GP visit (57% vs. 44%) - PD ∼= no PD in bronchitis (3% vs. 2%), diabetes (1% each), cardiovascular disease (<1% each), prescribed analgesics (5% vs. 3%) |
| (Olssøn & Dahl, 2012) | N = 1680 Norwegians; with AvPD pathology (n = 280); age- and gender-matched controls (n = 1400) |
AvP D patho log y vs. no AvP D path olog y |
IPDS | 65% | - | Large-sale population survey |
-AvPD > no AvPD in self-rated poor health (50% vs. 16%), somatic conditions (30% vs. 19%), impairing musculoskeletal pain (37% vs. 20%), insomnia (43% vs. 15%), frequent use non-prescribed analgesics (29% vs. 12%), frequent GP visits (36% vs. 15%) |
| (Proctor et al., 2013) | N = 216 pain and injury patients |
All | Comprehens ive assessment and Psychologic al Evaluation (CAAPE) |
51.9% | 47.8 | Cross-sectional interview and self- report |
-OCPD prevalence = 62.5% -Other PDs accounted for 5% of the sample |
| (Rothrock et al., 2007) | N = 150 migraine patients; with BPD (n = 50); no BPD (n = 50); age- and gender- matched no BPD (n = 50) |
BPD | MSI-BPD, chart review |
- | - | Pre-post treatment study |
-BPD ∼= non-BPD in average headache days BPD > matched non-BPD in functionally incapacitating headache days (18.1 vs. 5.9), and medication overuse headaches (74% vs. 62%) -matched non-BPD > BPD in positive treatment response (58% vs. 14%) -BPD > matched non-BPD in unscheduled headache treatment 6 mo. later (3.1 vs. 0.6) |
| (Sansone et al., 2009) | N = 114 patients in opiate detoxification (reported 111 completed all study measures) |
BPD | PDQ-4 BPD scale |
46.5% | 32.8 | Cross-sectional self-report |
Migraine headaches associated with BPD symptoms (r = .24) but not chronic fatigue, irritable bowel syndrome, arthritis, fibromyalgia, asthma, chemical sensitivity, chronic pain, obesity, dermatitis, multiple sclerosis, allergies, or temporomandibular joint problems (rs < .17). |
| (Sansone, Whitecar, et al., 2001) | N = 17 chronic pain patients |
BPD | PDQ, DIB, self harm inventory |
70.6% | 15.6 | Cross-sectional interview and self- report |
-23.5% screened for BPD on one measure -23.5% screened for BPD on two measures -17.6% screened for BPD on three measures -64.7% screened for BPD on any measure -BPD predicted somatic preoccupation |
| (Sansone et al., 2006) | N = 87 medical outpatients |
BPD | SHI | 59% | 43 | Cross-sectional study using self-report |
-19.5% of patients scored above the clinical cut-off for BPD -SHI scores were not predictive of pain disorders |
| (Tragesser et al., 2010) | N = 777 pain or injury rehabilitation patients |
BPD | The Battery for Health Improvemn t 2 (BHI 2) |
56.2% | 38.5 | Cross-sectional self-report |
-Controlling for gender and payment type, BPD features predicted pain complaints, somatic complaints, lowest/highest pain in past mo. -This relationship became nonsignificant when controlling for depression, anxiety, hostility |
| (Wilsey et al., 2008) | N = 113 ER patients seeking opiates for chronic pain |
PD vs. no PD |
PDQ-4 | 42.5% | ∼46.1 | Cross-sectional interview and self- report |
-18% of ER patients seeking opiates for pain screened positive for PD -PD positive screens was associated with opiate abuse propensity (r = .45) |
| (Wright et al., 2004) | N = 74 patients with acute jaw pain or facial discomfort |
Clus ters A, B, C |
SCID-II | 81.1% | 37.5 | Cross-sectional interview and self- report |
-High risk for progression to chronic pain vs. low risk had higher rates of PDs (59.6% vs. 27.3%) -High risk vs. low risk had higher rates of clusters A (11.5% vs. 0%), B (11.5% vs. 4.5%), and C (59.6% vs. 27.3%) |
| Obesity (n = 17) | |||||||
| (Black et al., 2003) | N = 44 obese patients who completed pre- gastroplasty interviews for psychopathology |
All | PDQ | 77.3% | 37.7 | Longitudinal | -PD prevalence = 56% -Most common PDs were cluster A (49%), followed by B (29%) and C (5%) -No PD predicted of 6-month post-operative weight, when controlling for baseline BMI |
| (Carpiniello et al., 2009) | N = 150 patients attending an obesity treatment center |
All | SCID-II | 24.0% | 44.6 | Cross-sectional interview and self- report |
PD prevalence = 30.5% for females and 18.8% males -The most common PD among women was NOS (14.4%), followed by cluster C (10.2%), followed by cluster B (2.5%), followed by cluster A (1.7%) -The most common PD among men was NOS (6.3%), followed by cluster C (3.1%), followed by cluster A (3.1%), followed by cluster B (0.0%) |
| (Frankenburg & Zanarini, 2006a) | N = 264 patients with BPD who completed a 6-year follow-up |
BPD | DIPD; DIB- R |
81.2% | 42.8 | MSAD | -At baseline 17.4% of participants were obese -At 6-year follow-up 28.0% were obese -Obese patients > not obese in chronic medical conditions (diabetes, hypertension, asthma) -Obese status was associated with PTSD, lack of exercise, family history, and taking 3+ psychotropic medications |
| (Frankenburg & Zanarini, 2011b) | N = 210 patients with BPD who completed a 10-year follow-up |
BPD | DIPD; DIB- R |
81.2% | 42.8 | MSAD | -Increases in BMI were associated with BPD symptoms (self- mutilation; z = 2.62; suicide attempts; z = 1.86), poorer psychosocial outcomes, with each 5-unit increase conferring heightened risk of having no partner/spouse (z = 3.10; 23%), poor work/school history (z = 3.30; 14%), receiving disability benefits (z = 5.10; 55%), having a low global functioning score (z = 4.31; GAF= < 60), and low (<$10000) income (z = 8.68; 27%) -Each 5-unit increase in BMI was associated with multiple weight-related medical conditions (z = 5.48; 60%), having an ER visit (z = 8.68; 27%) or medical hospitalization (z = 4.46; 35%) |
| (Goldstein et al., 2008) | N = 41654 nationally representative participants in the United States |
ASP D |
AUDADIS | - | - | Epidemiological survey - NESARC |
-AsPD prevalence = 3.7% -5.6% of men and 1.9% of women met criteria for AsPD -Rates of obesity and extreme obesity higher in antisocial behavioral syndromes (ASPD, non-diagnosable antisocial personality traits, and childhood CD only) -In women, highest rates of extreme obesity were found in AsPD and childhood CD -In men, highest rates of extreme obesity in ASPD |
| (Iacovino et al., 2014) | N = 1064 community based participants |
BPD | SIDP-IV | 62% | 59.5 | Baseline data from a representative community sample |
-BPD pathology was associated with higher BMI -BPD-BMI relation mediated by self-reported impulsivity -Facets of impulsiveness and lack of deliberation were mediators |
| (Johnson, Cohen, Kasen, & Brook, 2006b) | N = 658 community participants |
All | PDQ; SCID- II |
53% | 32.9 | Longitudinal | PD prevalence ranged from 10.1%-14.7% -Any PD > no PD by early adulthood at risk for age 33 binge eating, dietary restriction, obesity, and eating disorders -PD status did not increase risk for those with early adulthood eating/weight problems -BPD, HPD, SzPD predicted eating disorders -ASPD, BPD, DPD, DvPD, HPD, PAPD, StPD predicted binge eating -BPD, HPD, StPD predicted purging -DvpPD predicted restriction -AsPD, SzPD, and StPD predicted obesity |
| (Lier et al., 2011) | N = 141 patients referred for bariatric surgery |
BPD | SCID-II (Norwegian version) |
73.0% | 42.0 | Cross-sectional self-report preceding treatment study |
-PD prevalence = 26.0% -Most prevalent was AvPD (17.0%), followed by PPD (5.7%), OCPD (5.0%), BPD(3.5%), DPD (.7%), SzPD (.7%), and ASPD (1.4%). -Patients with AvPD more likely to refuse to participate in pre- and post-operative counseling groups |
| (Masheb & Grilo, 2008) | N= 75 patients with BED |
All | DIPD-IV | 81 | 46 | Treatment study | -24% of sample had co-morbid PD -Patients with PD > patients without PD on post-treatment eating pathology, negative affect |
| (Mather et al., 2008) | N = 41654 nationally representative participants in the United States |
ASP D |
AUDADIS | 56.4% | ∼45.8 | Epidemiological survey - NESARC |
-Obese individuals were more likely to have a cluster A PD -Extremely obese individuals were more likely to have cluster B PD, specifically AsPD and AvPD -Underweight individuals were more likely to have SzPD -Overweight men were more likely to meet 2+PDs and PPD -Overweight women were more likely to have 2+ PDs or AsPD -Obese women were more likely to have PPD -Extremely obese women were more likely to have 2+ PDs, AsPD, AvPD |
| (Mauri et al., 2008) | N = 282 Italian obese candidates for bariatric surgery |
All | SCID-II | 79.8% | 42.0 | Cross-sectional interview and self- report |
-PD prevalence = 19.5% -Most common cluster C (18.8%) -Cluster C was more common among those in the severe/very severe BMI class -Only PD associated with eating disorders was AvPD (OR = 3.57) -PDs only predicted poorer quality of life when co-occurring with an Axis I disorder |
| (Petry et al., 2008) | N = 41654 nationally representative participants in the United States |
All | AUDADIS | 49.6% | ∼45.4 | Epidemiological survey - NESARC |
-Mood, anxiety, alcohol, or PDs was associated with heightened BMI (ORs = 1.01–1.03) -AsPD (OR = 1.03), AvPD (OR = 1.04), OCPD (OR = 1.02), PPD (OR = 1.03), SzPD (OR = 1.03) associated with heightened BMI -DPD and HPD not associated with BMI (ORs = 1.03 and 1.01, respectively) -PDs more likely to be obese (BMI = 30.0–39.9; ORs = 1.31– 1.69) or extremely obsess (BMI 40.0+; ORs = 1.61–2.55) |
| (Pickering et al., 2007) | N = 43093 nationally representative participants in the United States |
All | AUDADIS | - | - | Epidemiological survey - NESARC |
-Adjusted for age, racial/ethnic background, marital status, education, income, region, urbanicity, 12-month physical conditions and stressful events, lifetime substance use, PDs were not associated with BMI category (healthy, overweight, obese, or extremely obese) for males (ORs = .6–1.2) -In women, AvPD associated with extremely obese category (OR =1.7) -AsPD associated with overweight category (OR = 1.5) or extremely obese (OR = 1.9) |
| (Sansone et al., 2008) | N = 121 patients seeking gastric surgery for obesity |
BPD | SHI, PDQ, MSI-BPD |
85.9% | 44.6 | Cross-sectional interview and self- report |
-14% > SHI BPD cut off -14% > PDQ-R BPD cut of -7.4% > MSI-BPD BPD cut off -24.8% > BPD cutoff on any measure -BPD on any measure ∼= no BPD in BMI -Discrepancy between highest and lowest adult weight was negatively correlated with PDQ-R and MSI-BPD scores, indicating a lack of weight fluctuation and BPD symptoms |
| (Sansone, Wiederman, & Monteith, 2001) | N = 48 women | BPD | PDQ | 100% | 33.0 | Cross-sectional self-report |
-BPD pathology correlated with BMI (r = .44), body dissatisfaction (r = .35), and inversely associated with self- rated attractiveness (rs = -.42-.48) -Obese > not obese women in BPD pathology (64.7% vs. 19.4% exceeding clinical cutoff score) |
| (Sansone et al., 2012) | N = 238 consecutive patients undergoing cardiac stress testing |
BPD | PDQ; SHI | 53.4% | 58.2 | Cross-sectional self-report and laboratory exam |
-8.4% > BPD clinical cut off on either measure -Current or highest BMI not associated with BPD |
| (van Hanswijck de Jonge et al., 2003) | N = 87; patients with bulimia nervosa (n = 35); binge eating disorder (n = 15); non-binge eating obesity (n = 37) |
All | IPDE | 100% | 35.19 | Cross-sectional interview and self- report |
-Patients with bulimia > binge eating or obesity in AvPD (21.6% vs. 2.9% and 13.3%) -Patients with binge eating > bulimia or obesity in BPD (13.3% vs. 0% and 2.7%) -Patients with bulimia > binge eating or obesity in PPD (25.7% vs. 0% and 0%) -Patients with obesity < binge eating = bulimia in PPD, StPD, AsPD continuous criteria -Patients with obesity < binge eating < bulimia in BPD continuous criteria -Patients with obesity < bulimia in AvPD, OCPD continuous criteria |
| Chronic Health Conditions (n = 17) | |||||||
| (Bennett et al., 2009) | N = 58301 psych emergency patients; with HIV+ status (n = 1178) and without (n = 57123) |
PD, BPD |
Chart review | 38.2% | 38.8 | Chart review | -HIV+ ∼= HIV- in PD (8.6% vs. 8.0%), and BPD (4.7% vs. 4.5%) -Controlling for gender, race, and age, HIV+ > HIV- in BPD (OR = 2.1) |
| (Black et al., 2001) | N = 18 patients with multiple chemical sensitivities (representing 69% of original study) |
All | PDQ | 89% | 60 | 9-year follow-up study |
-39% screened positive for a PD -The most common PD at follow up was StPD (27%), followed by SzPD, PPD, HPD, NPD, OCP (13% each), BPD, DPD, AvPD, PaPD (7% each) |
| (Chattopadhyay et al., 2012) | N = 66; patients with Graves disease (n = 36); age- and gender- matched controls (n = 30) |
PD vs. no PD |
Clinical interview |
∼89% | 36.0 | Treatment | -High rates of psychiatric disorders (n = 31) -Low rates of PDs in Graves patients (n = 2; 5.6%) -At the end of 1 year of Graves treatment, anxiety and depression was reduced |
| (Edelstein et al., 2012) | N = 70 college graduates |
NPD | California Adult Q-Sort |
100% | 53 | Longitudinal study |
-Women higher on the hypersensitive facet of narcissism reported more physical health problems concurrently as well as 10 years later |
| (El-Gabalawy et al., 2010) | N = 34653 nationally representative participants in the United States |
BPD | AUDADIS | 52.1% | ∼49.8 | Epidemiological survey - NESARC – Wave 2 |
-Prevalence of BPD was 5.9% -Controlling for demographic and Axis I and II disorders, the likelihood of a BPD diagnosis was associated with hypertension, hepatic disease, cardiovascular disease, gastrointenstinal disease, sexually transmitted infection, or any medical condition (ORs = 1.46–2.80) -Diabetes, stroke, and obesity were not associated with BPD with covariates in model |
| (Frankenburg & Zanarini, 2004) | N = 264 BPD patients; ever- remitted (n = 200); never-remitted (n = 64) |
BPD | DIPD; DIB- R |
80.7% | 33.0 | Subset of the longitudinal MSAD |
-Never-remitted BPD > ever-remitted in chronic medical conditions (obesity, diabetes, arthritis, hypertension, back pain, incontinence, multiple medical conditions), unhealthy lifestyle choices (smoking, alcohol use, lack of exercise, pain medication), and medical services utilized |
| (Frankenburg, Fitzmaurice, & Zanarini, 2014) | N = 327; patients with BPD (n = 264); patients with another PD (n = 63) |
All | DIPD; DIB- R |
78.0% | 33.1 | Subset of the longitudinal MSAD |
-Opiate medication use among BPD patients was associated with cancer (z = 2.17), back pain (z = 5.54), osteoarthritis (z = 6.31), and fibromyalgia (z = 5.08). |
| (Gish et al., 2001) | N= 61 liver transplant patients with alcohol dependence who completed follow-up |
Not speci fied |
Not specified |
33% | 47 | Long-term follow- up of live transplant patients |
-Prevalence rate of PDs was 18.0% Personality disorder predicted recidivism over the median 6.9 year follow-up (relative hazard ratio 6.0) |
| (Hansen et al., 2003) | N = 268 HIV+ bereaved adults who had lost a loved one to AIDS |
All | SCID-II-SQ | 35.1% | - | Cross-sectional self-report preceding treatment study |
-Males > females in ASPD symptoms (8% vs 2.1% females) -Females > males in BPD symptoms (29.8% vs. 18.4% males) -PD symptoms were associated with lower health-related quality of life |
| (Keuroghlian et al., 2013) | N = 264 patients with BPD |
BPD | DIPD; DIB- R |
80.7% | 33.0 | Subset of the longitudinal MSAD |
-Recovered BPD < Nonrecovered in rates of chronic medical condition, obesity, osteoarthritis, diabetes, urinary incontinence, or multiple medical conditions, smoking, alcohol use, lack of exercise, use of sleep medication, overuse of pain medication, ER visits, hospitalizations |
| (Lopes et al., 2012) | N = 34653 | PD vs. no PD |
AUDADIS | 58.0% | ∼46.5 | Epidemiological survey - NESARC – Wave 2 |
-HIV+ males > HIV- males in the presence of PDs (43.17% vs. 23.28%; OR = 2.50) -HIV+ males > HIV- males specifically in terms of StPD (16.2% vs. 4.2%), NPD (15.2% vs. 7.7%), and BPD (19.1% vs. 5.6%) -HIV+ females ∼= HIV- females in the presence of PDs (19.0% vs. 20.0%; OR = 1.28) |
| (Marquine et al., 2014) | N = 136; participants with HIV-positive status (n = 64); and HIV-negative status (n = 72) |
AsP D |
DIS | 21.2% | 39.3 | Cross-sectional self-report |
-Participants with methamphetamine dependence > no dependence in AsPD (35.7% and 45.8% vs. 11.1% and 6.4%), regardless of HIV+ or HIV- status, respectively |
| (Moran et al., 2007) | N = 8580 United Kingdom residents |
All | SCID-II-SQ | 50.0% | 42.6 | National survey | -Controlling for potential confounds (age, sex, occupation, self-reported hypertension, diabetes, smoking, alcoholism), positive PD screen predicted increased rates of stroke (OR = 1.9), ischemic heart disease (OR = 1.4) -Controlling for confounds, AvPD, OCPD, BPD associated with stroke (ORs =4.0, 2.9, and 8.5, respectively) -AvPD, PPD, StPD, SzPD, BPD associated with ischemic heart disease (ORs = 2.2, 2.1, 3.6, 1.6, and 7.2, respectively) |
| (Palmer et al., 2003) | N = 107 methadone maintained HIV+ patients with a psychiatric disorder |
AsP D; BPD |
SCID-II | 53.3% | 43.1 | Cross-sectional interview and self- report |
-BPD prevalence = 37% -AsPD prevalence = 56% -BPD < non-BPD in HIV medication adherence (39% adherent BPD vs. 65% adherent non-BPD) |
| (Powers & Oltmanns, 2013) | N = 1051 Saint Louis residents |
All | SIDP, MAPP |
52.5 | 59.4 | Epidemiological survey and interview |
-BPD features associated with arthritis (OR = 2.67) and obesity (OR = 2.61) -Pattern remained when controlling for demographics, Axis I disorders, and other PDs (ORs = 2.64 and 2.94) -MAPP BPD features associated with physical health conditions - arthritis, obesity, and heart disease (ORs = 1.54, 1.48, and 2.22) -With covariates, only the association with heart disease remained -Association between BPD and arthritis was statistically mediated by BMI |
| (Vamanshankar et al., 2013) | N = 100 South Indian adults; patients with allergic rhinitis (n = 50); healthy individuals (n = 50) |
All | IPDE-SQ | 38.0% | 33.1 pt group |
Cross-sectional self-report and laboratory exam |
-Cluster C symptoms associated with more antigens -Control > allergic patients in Cluster A PD (48% vs. 20%) -Allergic patients > controls in Cluster C PD (68% vs. 50%) |
| (Woody et al., 2003) | N = 487 cocaine- dependent patients; 330 completed 6- month measures |
All | SCID-II | 23% | 34 | Longitudinal in the context of a treatment study |
AsPD prevalence = 14% (n = 46) -AsPD ∼= nonAsPD in HIV risk behaviors |
| Candidate Mechanisms (n = 12) | |||||||
| (Coccaro, Lee, Liu, et al., 2012) | N = 60; participants with a=>=1 PD (n = 40); participants with no history of psychopathology (n = 20) |
All | SCID-II | 21.7% | 32.5 | Cross-sectional self-report and laboratory exam |
-PD > controls in NPY-LI immunoreactivity, but not after controlling for self-reported impulsive aggression -NPY-LI higher in patients with intermittent explosive disorder, but did not differ as a function of BPD or ASPD |
| (Coccaro, Lee, Owens, et al., 2012) | N = 38 patients with PDs |
Not speci fied |
SCID-II | 23.7% | 32.5 | Cross-sectional self-report and laboratory examination |
-Basal lumbar cerebrospinal fluid samples were obtained -Substance P like immunoreactivity was associated with aggression and impulsivity |
| (Greggersen et al., 2011) | N = 75; women with BPD (n = 47); age- matched healthy women (n = 28) |
BPD | SCID-II | 100% | 31.5 | Interview and laboratory exam |
-BPD > non-BPD in intima-media thickness (M = .41 mm, SD = .11 vs. M = .34 mm, SD = .11), controlling for BMI and physical activity |
| (Kahl, Bens, et al., 2006) | N = 24; patients with MDD+BPD (n = 12); healthy controls (n = 12) |
BPD | SCID-II (German version) |
100% | 26.0 | Cross-sectional self-report and laboratory exam |
MDD+BPD > controls in serum cortisol, TNF-alpha, IL-6, cortisol/DHEA -Findings held even after controlling for BMI and activity level. |
| (Kahl, Bester, et al., 2005) | N = 68; patients with MDD (n = 18); patients with MDD and BPD (n = 18); patients with BPD (n = 12); healthy controls (n = 20) |
BPD | SCID-II (German version) |
100% | 28.4 | Cross-sectional self-report and laboratory exam |
-MDD with and without BPD > BPD alone and controls in visceral fat -MDD with and without BPD > controls and -MDD with BPD > BPD and controls in fasting insulin and tumor necrotic alpha |
| (Kahl et al., 2013) | N = 1144 German adults; adult psychiatric inpatients with BPD (n = 135); primary care patients (n = 1009) |
BPD | SCID-II (German version) |
68.7% | 48.0 | Cross-sectional self-report and laboratory examination |
-BPD ∼= controls in BMI (26.3 vs. 26.8) -BPD > controls in metabolic syndrome (23.3% vs. 10.6%) -In the BPD group metabolic syndrome was associated with higher weight, BMI, less physical activity, and MetS criteria. -Metabolic syndrome was associated with body max index, treatment with second generation antipsychotics, and other psychiatric disorders |
| (Kahl, Greggersen, et al., 2006) | N = 83; patients with MDD (n = 24); patients with MDD+BPD (n = 23); patients with BPD (n = 16); healthy controls (n = 20) |
BPD | SCID-II (German version) |
100% | 23.5 | Cross-sectional self-report and laboratory examination |
-MDD+BPD < BPD, younger MDD in bone mineral density -MDD > BPD and controls in markers of bone turnover and cytokines |
| (Kahl, Rudolf, et al., 2005) | N = 58; patients with MDD and BPD (n = 22); patients with BPD (n = 16); healthy controls (n = 20) |
BPD | SCID-II (German version) |
100% | 26.9 | Cross-sectional self-report and laboratory examination |
-BPD+MDD > BPD or controls in tumor necrosis alpha, interleukin, and markers of bone turnover |
| (Kahl et al., 2009) | N = 24; patients with MDD+BPD (n = 12); healthy controls (n = 12) |
BPD | SCID-II (German version) |
100% | 26.0 | Cross-sectional self-report and laboratory examination |
-MDD+BPD > controls in angiogenic cytokines (VEGF and FGF-2) |
| (Mamabolo et al., 2012) | N = 113 psychiatric patients |
Any in chart |
Chart review | 32% | 38 | Cross-sectional questionnaire and chart review |
-PD diagnosis (p < .1) was associated with one risk factor for HIV, which is a history of sexual assault |
| (Martin et al., 2013) | N = 303 HIV- positive individuals |
NPD | SNAP | 15.5% | 43.7 | Cross-sectional self-report |
-NPD features associated with unprotected sex, a risk factor for HIV, even controlling for substance and demographic features -high in NPD features (n = 150) > low NPD in higher risk factors (in terms of multiple sex partners, number of risky sexual behaviors |
| (Roepke et al., 2010) | N= 31 patients with BPD and N = 30 healthy controls |
BPD | SCID-II and ZAN-BPD |
100 | 29 | Cross-sectional clinical interview, self-report, and pelvic ultrasound examination study |
-30.4% of BPD patients had PCO compared to 6.9% of healthy controls -BPD patients also had higher serum androgen concentrations of testosterone, FT, androstenedione, and 17-OHP compared to health controls |
| Treatment (n = 2) | |||||||
| (Bellino et al., 2010) | N = 14 patients with BPD (4 discontinued study) |
BPD | SCID-II; BPDSI |
64.3 | 29.6 | Treatment study of 12 weeks of duloxetine |
-12 weeks of duloxetine associated with improvements in BPD, depressive symptoms, and somatic symptoms -4 patients (22.2%) discontinued treatment in first 4 weeks due to noncompliance |
| (Zonneveld et al., 2012) | N = 162 patients with unexplained physical symptoms |
All | IPDE-SQ (Dutch version) |
- | - | Treatment study – participants randomized to CBT or waitlist |
-59 patients dropped out of treatment -47 out of remaining 103 patients had ≥1 PD -PD features predicted worse short-term outcome in cognitive behavior therapy – no differences in long-term outcomes |
Note. ASPD = antisocial personality disorder; AvPD = avoidant personality disorder; BPD = borderline personality disorder; BPDSI = BPD severity index; CBT = cognitive behavior therapy; DSM = diagnostic and statistical manual; DIB-R = diagnostic interview for borderline – revised; DIPD = diagnostic interview for personality disorders; DIS = diagnostic interview schedule; DPD = dependent personality disorder; DvPD = depressive personality disorder; GP = general practitioner; HPD = histrionic personality disorder; IPDE = International Personality Disorder Examination; IPDE-SQ = IPDE screening questionnaire; IOWA = Iowa personality disorder screen; NPD = narcissistic personality disorder; OCPD = obsessive compulsive personality disorder; MAPP = multisource assessment of personality pathology; MDD = major depressive disorder; MSI-BPD = McLean screening instrument for BPD; MSAD = McLean Study of Adult Development; NCS-R = National Comorbidity Survey-Replication; NESARC = National Epidemiologic Survey on Alcohol and Related Conditions; NPI = narcissism personality inventory; PDQ = personality diagnostic questionnaire; PaPD = passive aggressive personality disorder; PD = personality disorder; PDQ = Personality Diagnostic Questionnaire; PPD = paranoid personality disorder; PTSD = posttraumatic stress disorder; SCID = Structured Clinical Interview for DSM; SCID-II = SCID for Axis II personality disorders; SCID-II-SQ = SCID-II screening questionnaire; SHI = self harm inventory; SNAP = schedule for nonadaptive and adaptive personality personality; SIDP = structured interview for DSM-III personality; StPD = schizotypal personality disorder; SzPD = schizoid personality disorder;
Where not provided, age was estimated as the midpoint of age ranges provided.
Sleep
Eight studies assessed relations between PD features and sleep. Of these, one examined group differences sleep patterns between patients with sleep conditions and patients with BPD, one assessed sleep patients for the presence of PDs, five assessed sleep difficulties in patients with PDs, and one used an epidemiological sample.
PDs were relatively common among patients suffering from sleep disorders (Ruiter, Lichstein, Nau, & Geyer, 2012). Specifically, in a sample of adults with chronic insomnia and hypnotic dependence (N = 84), PD features were assessed with the SCID-II screening questionnaire. The most prevalent PDs in this sample were Cluster C PDs, constituting nearly half of the sample (50%). In terms of specific PDs, the most common was obsessive compulsive (46%), followed by avoidant (12%), paranoid, narcissistic PDs, and BPD (11% each). Both obsessive compulsive and avoidant PD features were associated with more influence of insomnia on daytime functioning. Of note, the SCID-II screening questionnaire is an instrument with yes/no questions rather than a comprehensive diagnostic tool, designed to facilitate the more efficient completion of the structured clinical interview for PDs, in which interviewers query only those items rated “yes” on the screening questionnaire. Although these findings suggest high rates of PDs in patients with sleep problems, research in this area is at a preliminary stage.
Five studies have investigated sleep problems among individuals with BPD (Asaad, Okasha, & Okasha, 2002; Bastien, Guimond, St-Jean, & Lemelin, 2008; Plante, Frankenburg, Fitzmaurice, & Zanarini, 2013b; Semiz, Basoglu, Ebrinc, & Cetin, 2008). Compared to age- and sex-matched participants without psychopathology (n = 100), BPD patients (n = 88) reported worse subjective sleep quality (Semiz et al., 2008). Indeed, the vast majority (95.5%) of BPD patients described themselves as poor sleepers, compared to only 12% of the control group. Furthermore, BPD patients reported higher self-reported dream anxiety (e.g., nightmares, autonomic hyperactivity, and difficulty falling asleep) than the control group, and within the BPD sample, those who had nightmare disorder were more clinically severe (e.g., child abuse history, unemployment, self-harm, and lower education) than BPD patients without a nightmare disorder. In research using subjective and objective measures to compare BPD patients (n = 20) to a clinical control group of depressed patients (n = 20), however, BPD patients evidenced comparable, and on some indices slightly better, sleep patterns than the clinical controls (Asaad et al., 2002). In fact, the depressed participants (n = 20) evidenced significantly longer sleep latency, lower sleep efficiency, higher number of nighttime awakenings, shorter REM latency and higher REM density than BPD participants. Also drawing on subjective (daily monitoring and questionnaires) and objective measures of sleep, researchers compared BPD patients (n = 12) to patients with insomnia (n = 30) and self-reported good sleepers without insomnia (n = 15; (Bastien et al., 2008). Patients with BPD reported comparable sleep disturbance, and also evidenced similar levels of sleep onset latency, sleep time and sleep efficiency in the laboratory relative to individuals with insomnia. These groups diverged with respect to stage four sleep, however, with BPD patients evidencing a greater proportion and amount of time in this stage of sleep than paradoxical insomniacs. In a study based on epidemiological data (from the NCS-R; N = 5692) BPD symptoms were significantly associated with self-reported difficulty initiating sleep, difficulty maintaining sleep, and waking earlier than desired, as well as negative consequences of poor sleep (Selby, 2013). These sleep problems were similar to those reported by individuals with Axis I disorders (Selby, 2013). Using 16-year longitudinal follow-up data from the MSAD, recovered BPD patients (n = 105) reported shorter sleep onset latency, less fatigue-related dysfunction (Plante, Frankenburg, Fitzmaurice, & Zanarini, 2013a), and less severe maladaptive sleep related cognitions relative to non-recovered (n = 118) BPD patients (Plante et al., 2013b). These data suggest that as BPD symptoms improve, sleep problems may also improve.
Preliminary research also points to sleep disturbance in other PDs. Among 110 forensic psychiatric patients, most of whom (83%) had a PD (60% antisocial, 27% BPD, 28% narcissistic PD), 29% a self-reported sleep disorder (Kamphuis, Karsten, de Weerd, & Lancel, 2013a). Thus, the prevalence of sleep disorders in PD samples may exceed that of sleep disorders (i.e., insomnia) in other populations, with rates from 6–10% (Roth, 2007). In addition, 49% of those with PDs exceeded clinical cut offs for poor sleep quality on self-report measures. Antisocial PD was the only disorder that significantly predicted self-reported poor sleep quality.
Overall, these findings suggest that sleep impairments are relatively common among those with BPD and possibly antisocial PD. Nevertheless, these impairments are comparable to those associated with other psychopathology or sleep disorders. The research on sleep disturbance in BPD included subjective and objective indices, as well as clinical comparison groups. Given preliminary evidence suggesting that Cluster C PDs may be overrepresented among those suffering from sleep disorders, further research should conduct comprehensive assessments for PDs in order to compare rates of sleep disorders across diverse PDs.
Obesity
Seventeen studies investigated the relation between PDs and obesity via a number of methods (e.g., self-report and laboratory measures) as well as across a variety of samples (e.g., clinical, epidemiological, community). Specifically, nine studies used clinical patient samples, five studies used epidemiological data (n = 4 NCS/NCS-R, n = 1 Saint Louis) and three studies used longitudinal community-based samples (n = 2 MSAD, n = 1 Children in the Community [CIC]). Within studies examining clinical patient samples, four of the eight used samples of patients who were candidates for bariatric surgery. Overall, studies support a positive association between obesity and the presence of and/or symptoms of PDs.
Four studies investigated rates of PD among patients seeking or referred for bariatric surgery (Black, Goldstein, & Mason, 2003; Lier, Biringer, Stubhaug, Eriksen, & Tangen, 2011; Mauri et al., 2008; Sansone, Schumacher, Wiederman, & Routsong-Weichers, 2008). A substantial proportion (19.5–56%) of patients in these studies evidenced PD symptoms or diagnoses well above the rates of PDs typically seen in the general population. For example, the prevalence of PDs (diagnosed via structured clinical interviews) among consecutively admitted obese (BMI ≥ 30 kg/m2) candidates for bariatric surgery (n = 282; 79.8% female) was 19.5% (Mauri et al., 2008). Of these, nearly all of the PDs detected were Cluster C disorders (avoidant, dependent, and obsessive compulsive, n = 53). Similarly, avoidant was the most common PD in a different sample (n = 141) of obese bariatric surgery patients (Lier et al., 2011). In a smaller patient sample (n = 44), Cluster A disorders (49%) were the most prevalent PD (Black et al., 2003). Thus, although the rates of PD are relatively high among obese patients seeking surgery, there appears to be some discrepancy regarding the most prevalent types of PD.
Five additional studies investigated symptoms of PD in patient samples not seeking bariatric surgery (Carpiniello et al., 2009; Sansone, Hahn, Dittoe, & Wiederman, 2012; Sansone, Wiederman, Sansone, & Monteith, 2001; van Hanswijck de Jonge, van Furth, Lacey, & Waller, 2003). Rates of PD in these samples ranged from 8–65%. For example, among patients undergoing cardiac stress testing (n = 238), 8% scored above BPD clinical cut off on a self-report measure (Sansone et al., 2012). Among obesity treatment centre patients (n = 150), 31% of female and 19% of male patients had a PD based on a clinical interview (Carpiniello et al., 2009). Similarly, overweight patients with binge eating disorder (N = 75) in a trial of self-help interventions demonstrated high rates of PDs, with nearly one-fourth (24%) meeting criteria for a PD (Masheb & Grilo, 2008). The most common PDs in this sample were Cluster C. Furthermore, PD presence predicted negative affect and eating disorder symptoms post-treatment.
Evidence also supports an association between PDs and obesity. Drawing from the NESARC study, four papers examined obesity and PD (Goldstein et al., 2008; Mather, Cox, Enns, & Sareen, 2008; Petry, Barry, Pietrzak, & Wagner, 2008; Pickering, Grant, Chou, & Compton, 2007). Results revealed that antisocial (OR = 1.03), avoidant (OR = 1.04), obsessive compulsive (OR = 1.02), paranoid (OR = 1.03), and schizoid (OR = 1.03) PDs were associated with heightened BMI (e.g., obesity or extreme obesity), whereas depressive and histrionic PD were not, ORs = 1.03 and 1.01, respectively (Petry et al., 2008). BPD was not assessed in this sample. In a different analysis however, after adjusting for demographic and physical health variables, PDs were not associated with BMI in men (Pickering et al., 2007). Among women, avoidant PD was associated with higher likelihood of being in the extremely obese category (OR = 1.7), and among women, antisocial PD was associated with higher likelihood of being classified as overweight (OR = 1.5) or extremely obese (OR = 1.9). In another study, controlling for Axis I diagnoses and other relevant demographic variables, obese individuals had higher odds of meeting criteria for at least one Cluster A PD (Mather et al., 2008). Extremely obese individuals had higher odds of having at least one Cluster B PD, such as antisocial and avoidant PD. Whereas overweight men were less likely to meet criteria for multiple PDs, overweight women were more likely to meet criteria for antisocial or multiple PDs. In this data set, 3.7% of the sample met criteria for antisocial PD (Goldstein et al., 2008). The relation of antisocial PD and obesity or extreme obesity among women remained (ORs = 1.4–3.2), even after controlling for relevant demographic factors, substance use and medical conditions. In another sample of 1064 community participants in Saint Louis, higher BMI was associated with greater BPD severity, as assessed by a clinical interview (Iacovino, Powers, & Oltmanns, 2014).
Longitudinal studies have suggested an association of PDs with later obesity. In the MSAD studies, of the 264 patients with BPD at the 6-year follow-up assessment, 74 (28%) patients were obese, whereas at baseline, only 46 (17%) patients had been obese (Frankenburg & Zanarini, 2006b), although it is unclear how these compare to rates seen in the general population. A subset of these patients (N = 210) completed the 10-year follow-up (Frankenburg & Zanarini, 2011a) and, among these participants, BMI was associated with BPD symptoms (self-mutilation; z = 2.62; suicide attempts; z = 1.86) at baseline, and predicted poorer psychosocial outcomes across time. Each 5-unit increase in BMI was associated with a greater likelihood of multiple weight-related medical conditions (z = 5.48; 60%), visiting the emergency room (z = 8.68; 27%), or being hospitalized for medical reasons (z = 4.46; 35%). Similarly, in another longitudinal project of 658 community participants (Johnson, Cohen, Kasen, & Brook, 2006a), the prevalence of any PD ranged from 10.1%-14.7%. Furthermore, the presence of a PD by early adulthood predicted greater risk for binge eating, dietary restriction, obesity, and eating disorders at age 33. Further, specific PDs demonstrated unique associations with health-related behaviour problems. Controlling for demographics, several PDs predicted eating disorders (BPD, histrionic, schizoid PDs), binge eating (antisocial, BPD, dependent, depressive, histrionic, passive aggressive, schizotypal PDs), obesity (antisocial, schizoid, schizotypal PDs), purging (BPD, histrionic, schizotypal PDs) and restricting behaviours (depressive PD).
This set of findings indicates that there are high rates of PD among obese individuals seeking bariatric surgery and those not seeking surgery, suggesting that obesity and PDs tend to co-occur. In large-scale epidemiological or longitudinal community samples, there appear to be concurrent relations between several PDs and obesity, as well as evidence suggesting that PDs prospectively predict greater obesity and the onset of eating problems, including binge eating.
Chronic Pain/Headaches
Twenty-two studies investigated the relation between PDs and chronic pain. Chronic pain included self-reported pain intensity, interference, and self-reported or provider-identified pain conditions such as headache, back pain or arthritis. Five studies assessed relations between headache and PDs, whereas 17 studies looked at chronic pain generally. Fifteen studies examined PD prevalence in pain patient samples, 3 studies used psychiatric patient samples, and 4 studies examined PD-pain associations using epidemiological data. Four studies used chart review, and one study compared patients pre- and post-treatment.
Data from epidemiological studies broadly suggest a positive relation between chronic pain and PDs. Drawing from the NCS-R part II (N = 5692), individuals with versus without chronic pain were more likely to screen positive using the IPDE for antisocial and/or BPD traits (Braden & Sullivan, 2008). This pattern did not emerge, however, when examining specific pain issues involving chronic back and neck problems. Also from the NCS-R sample, findings revealed that a history of self-reported pain conditions (arthritis, headaches, spinal pain, other) predicted higher levels of BPD symptoms specifically, even after controlling for relevant demographic variables and symptoms of psychopathology (McWilliams & Higgins, 2013). Population-based studies in Norway compared individuals (n = 369) with any positive endorsement on the Iowa Personality Disorder Screen (IPDS; a short form containing five items, each corresponding to a PD), to age- and gender-matched controls (n = 1845) who endorsed no items on this measure (Olssøn & Dahl, 2009). Results suggest that participants that endorsed PD items were more likely to rate their health poorly (18% vs. 9%), suffer from fibromyalgia (4% vs. 2%), suffer from recent musculoskeletal pain (33% vs. 22%), and report dissatisfaction with their last visit to their general practitioner (57% vs. 44%). In other work using the same sample, individuals endorsing the avoidant PD item on the IPDS (n = 280), compared with those who did not, were more likely to rate their health poorly (50% vs. 16%), suffer from a somatic condition (30% vs. 19%), have impairing musculoskeletal pain (37% vs. 20%), and frequent visits to a general practitioner (36% vs. 15%; Olssøn & Dahl, 2012). The large, nationally-representative nature of the samples used in these studies is a strength; however, the restricted assessment of PDs or lack of comparison across PDs limits our ability to draw conclusions about specific associations between or among PDs in conferring risk for pain conditions.
Studies using clinical pain patient samples (n = 13) yielded similar findings to the epidemiological studies. Patients with chronic back pain (Breckenridge & Clark, 2003), jaw pain/facial discomfort (Wright et al., 2004), headaches (Atasoy, Atasoy, Unal, Emre, & Sumer, 2005; Loder & Geweke, 2002; Rothrock et al., 2007) and other chronic pain conditions (Conrad et al., 2007; Egloff, Maecker, Stauber, Sabbioni, Tunklova, & von Känel, 2012; Fischer-Kern et al., 2011; Fishbain et al., 2007; Proctor, Estroff, Empting, Shearer-Williams, & Hoffmann, 2013; Sansone, Whitecar, Meier, & Murry, 2001; Sansone, Whitecar, & Wiederman, 2009; Tragesser, Bruns, & Disorbio, 2010; Wilsey et al., 2008) evidenced high rates of PDs or PD features (13–28%), particularly when compared to rates in the general population (approximately 10%; (Samuels, 2011). The methods used to assess PD symptoms was highly variable across these studies, ranging from structured diagnostic interviews to self-reported symptoms, with some studies relying on assessments of specific PDs and others assessing multiple PDs.
In psychiatric samples, PDs were associated with chronic pain. Among depressed geriatric psychiatric inpatients (n = 148), 13% of those with chronic pain (n = 92) versus 0% without chronic pain (n = 56) had a PD documented in their medical chart (Meeks et al., 2008). In addition, symptoms of migraine were assessed among first-degree relatives of individuals with obsessive compulsive disorder (n = 168) and healthy controls (n = 184; Manlick, Black, Stumpf, McCormick, & Allen, 2012). The odds of meeting criteria for at least one PD on a structured diagnostic interview were significantly higher (OR = 3.31) among those with migraines (Manlick et al., 2012). When examining specific PDs, migraines were associated with paranoid PD (OR = 3.18) and mixed PDs (OR = 5), although other PDs were not assessed.
Research has identified an association between BPD and chronic pain. For instance, studies have found that BPD symptoms are correlated with migraine headaches (Sansone et al., 2009), somatic preoccupation among pain patients (Sansone, Whitecar, et al., 2001), and are highly prevalent (20%) in medical outpatients (n = 87; Sansone, Pole, Dakroub, & Butler, 2006). Furthermore, among migraine patients (n = 100), BPD diagnoses (derived via chart review and self-report) were associated with migraine severity and disability; BPD participants (n = 50) reported three-fold greater functional incapacity due to migraine symptoms (self-reported as ‘disabling’) compared to migraine sufferers without BPD (Rothrock et al., 2007). Nevertheless, these studies did not assess multiple PDs, so it is not possible to determine whether BPD specifically or PDs more generally, are uniquely associated with pain and related impairment.
There appears to be some support for obsessive compulsive PD being uniquely related to chronic pain. One study found that obsessive compulsive PD (28%) and BPD (26%) were the most commonly diagnosed PDs in a sample of chronic pain (n = 43) patients (Fischer-Kern et al., 2011). In a larger sample of chronic pain patients (n = 216), 62.5% met criteria for obsessive compulsive PD based on a structured interview, whereas only 5% met criteria for other PDs (Proctor et al., 2013). Likewise, among patients with medication overuse headaches, those that also had pre-existing migraines (n = 50; vs. episodic tension headaches, n = 31) had higher rates of obsessive compulsive PD diagnosed via structured clinical interviews (Atasoy et al., 2005). Among jaw pain patients (n = 43), Cluster C PDs generally, and obsessive compulsive PD specifically, conferred higher risk for pain to persist and become chronic (Wright et al., 2004).
Beyond conferring risk for pain conditions, PDs may be associated with higher rates of service utilization among pain patients (e.g. Olsson & Dahl, 2012). In one study, authors tracked calls to a headache practitioner’s office over one month (Loder & Geweke, 2002). Of the 165 calls from 90 callers, most callers (n = 77) had a PD documented in their chart, 91% of repeat callers had a PD, and all 11 emergency calls came from an individual with a PD. The authors suggest that patients with PDs place a disproportionate burden on telephone practice as 24 ½ hours of staff time went toward answering those calls during the month. Migraine sufferers with BPD (n = 50) also had more unscheduled visits for acute headache treatment over the treatment period (an average of 3.1 visits vs. < 1 in non-PD migraine patients; Rothrock et al., 2007). Conversely, in a study of Swedish chronic pain patents (n = 184), depression (OR = 2.60), but not PDs (OR = 0.57) were found to be a significant predictor of disability status across one year (Ericsson et al., 2002). The results from this study suggest that examining the co-occurrence of PDs with other psychiatric disorders may be crucial in aiding our understanding of what may be unique in the relations between pain and PD above and beyond other disorders.
Taken together, these results suggest that individuals with chronic pain have high rates of PDs, those with chronic pain and PD have high rates of service utilization, and that pain conditions and PDs tend to co-occur in the general population.
Chronic Health Conditions
Seventeen studies investigated the relation between PD and chronic health conditions. Chronic health conditions were assessed by self-report and providers. Eight studies used clinical patient samples, four studies used epidemiological data (two of which were described in the Chronic Pain Section) and four studies used longitudinal community-based samples. Three of these four longitudinal community based studies used the same sample (MSAD). Overall, studies support a positive relation between chronic health conditions and PDs.
PD symptoms are prevalent among patients with a variety of chronic health conditions. In particular, PDs were prevalent in samples of 61 liver transplant patients (18%; Gish et al., 2001), patients (n = 18) with multiple chemical sensitivities (39%; Black, Okiishi, & Schlosser, 2001), patients (n = 64) with HIV-seropositive status (11–36%; Marquine et al., 2014), and patients (n = 50) suffering from allergic rhinitis (68%; Vamanshankar et al., 2013). Only one study failed to find a high PD prevalence rate in chronic health condition patients – only a small subset (6%) of patients (n = 36) with Graves disease evidenced PDs (Chattopadhyay, Chakrabarti, & Ghosh, 2012). Several of these studies only assessed for the presence of one PD (e.g., ASPD; Marquine et al., 2014; Woody et al., 2003), but of those that assessed multiple PDs, the most common PDs were schizotypal (Black et al., 2001), and Cluster C PDs generally (Vamanshankar et al., 2013). Among bereaved HIV-seropositive adults (N = 268), antisocial PD was more prevalent among males (8% vs. 2.1% females), whereas BPD was more prevalent among females (29.8% vs. 18.4% males; Hansen, Wang, Stage, & Kragh-Sorensen, 2003). Likewise, in a sample (n = 107) of methadone-maintained HIV-seropositive psychiatric patients, both BPD and antisocial PD were prevalent (37% and 56%, respectively; other PDs not assessed) (Palmer, Salcedo, Miller, Winiarski, & Arno, 2003). In a chart review of HIV status and psychiatric symptoms among psychiatric emergency services patients (N = 28301), a subsample (n = 1178) of these patients were HIV-positive (Bennett, Joesch, Mazur, & Roy-Byrne, 2009). Controlling for gender, race, and age, HIV-positive patients were more likely to carry a diagnosis of BPD (OR = 2.1). In addition, chronic health problems in PD patients is associated with greater treatment utilization and clinical severity. For instance, patients with non-remitted BPD have higher medical services utilization (Frankenburg & Zanarini, 2004), and were more likely to use prescription opioid medication (Frankenburg, Fitzmaurice, & Zanarini, 2014). Further, cancer, back pain, osteoarthritis and fibromyalgia increased the likelihood of opioid use by patients with BPD.
Epidemiological studies support the conclusions drawn from studies with smaller patient samples, indicating that PDs generally, and BPD specifically, have high rates of comorbidity with several chronic health conditions. For example, in an epidemiologically-based sample (n = 1051) of Saint Louis residents, BPD features were associated with the presence of arthritis and obesity (ORs = 2.67 and 2.61), even when controlling for demographic characteristics, Axis I disorders, and any PD diagnoses other than BPD (ORs = 2.64 and 2.94; Powers & Oltmanns, 2013). Similar findings emerged from the NESARC (N = 34,653; El-Gabalawy, Katz, & Sareen, 2010). The presence of hypertension/atherosclerosis, hepatic disease, cardiovascular disease, gastrointestinal disease, arthritis, venereal disease, or other medical condition was associated with a greater likelihood of having BPD, even after adjusting for demographic characteristics and psychopathology. The NESARC data also revealed that PDs were associated with self-reported HIV status (Lopes et al., 2012). Compared to their HIV-negative sex-matched counterparts, HIV-positive men were more likely to have a PD (43.17% vs. 23.28%; OR = 2.50). Likewise, in a national survey of 8580 adults in the United Kingdom (Moran et al., 2007), after controlling for demographic and health-related confounds, avoidant, obsessive compulsive PDs, and BPD diagnoses were associated with stroke (ORs = 4.0, 2.9, and 8.5, respectively); these PDs in addition to paranoid, schizotypal, and schizoid were also associated with ischemic heart disease (ORs = 1.6–7.2). In addition to documenting an association between PDs and pain, the aforementioned large-scale Norwegian population survey (Olsson & Dahl, 2009) revealed that participants endorsing PD criteria were more likely than those not endorsing PD criteria to rate their health poorly (18% vs. 9%), and suffer from asthma (11% vs. 8%). These epidemiological studies provide some support for unique links between BPD and chronic health conditions, as findings held even after controlling for the presence of other PDs.
Longitudinal research has suggested a prospective association of certain PDs and PD features with chronic medical conditions. In a sample of female college students (n = 70), certain features associated with clinical diagnoses of narcissistic PD (i.e., hypersensitivity) predicted physical health problems concurrently and ten years later (Edelstein et al., 2012). In the longitudinal MSAD data set, researchers compared 200 patients who had, at one point, demonstrated remission from BPD, to 64 never-remitted patients with BPD, indicating that never-remitted patients were more likely to have chronic medical conditions (obesity, diabetes, arthritis, hypertension, back pain, incontinence, multiple medical conditions; Frankenburg & Zanarini, 2004). In the 16-year follow-up of this sample, patients who did not recover from BPD were more likely to report being diagnosed with a chronic medical condition compared to patients with BPD who had ever recovered (Keuroghlian, Frankenburg, & Zanarini, 2013).
Together, results suggest high rates of PD among individuals with chronic health conditions. There also appears to be relatively strong support for the concurrent associations between BPD and chronic health conditions in large-scale community and longitudinal work.
Potential Mechanisms
Twelve additional studies, as well as some of the research previously discussed, have examined potential biological and behavioural mechanisms that might explain the associations between PDs and health problems. These studies have used both clinical samples of patients with PDs and subthreshold PDs, as well as laboratory and behavioural approaches. This is an important next step in the field that will facilitate improved treatment and care of a population that, as previously mentioned, places a heavy burden on health care systems.
Several potential mechanisms, transacting in a dynamic manner, likely account for elevated rates of health problems among persons with PDs. Guided by the findings from the literature review and consistent with the BPS framework (Engel, 1977) for understanding health, these mechanisms were conceptualized in terms of biological vulnerabilities, behavioural (psychological) risk factors, and environmental (social) factors. Biological vulnerabilities might include the possibility that PDs are linked with the propensity to experience certain types of health difficulties, such as metabolic problems and inflammatory disorders, as discussed below. Behavioural (psychological) factors likely exacerbate these biological vulnerabilities and include health risk behaviours that directly impact, for example, the metabolic and cardiovascular systems (such as overeating, smoking, and drug use). Finally, environmental (social) factors can include a range of variables, such as insufficient medical care (partly due to stigma and poor understanding of these problems), and poor social and occupational functioning.
Apart from the likely behavioral factors influencing the health problems in PD, the existing literature pinpoints several biological vulnerabilities associated with PDs. One condition implicated in the association between PDs and health problems is metabolic syndrome. This syndrome is characterized by obesity, hypertension, and elevated fasting blood glucose. In one study, psychiatric inpatients with BPD (n = 135) had twice the rate of metabolic syndrome compared to adult primary care (n = 1009) subjects (Kahl et al., 2013). Metabolic syndrome was also associated with use of second-generation antipsychotics among BPD patients. Relatedly, visceral fat (measured via lumbar spine puncture) and insulin-resistance was highest among those with co-occurring BPD and depression (n = 18) compared with BPD alone or healthy controls (Kahl, Bester, et al., 2005). While a biological vulnerability to metabolic syndrome might characterize PDs (and BPD in particular), it is just as possible that health-related behaviours associated with PDs elevate the risk of this syndrome (e.g., impulsive eating, difficulty establishing or sustaining an exercise regimen, substance use problems).
Another potential biological vulnerability factor identified in existing research is related to androgen dysfunction. Specifically, Roepke and colleagues (Roepke et al., 2010) examined androgen dysfunction in persons with BPD and the presence of polycystic ovaries, which has been linked with obesity and other negative health conditions. Inpatients with BPD (n = 31) and healthy controls completed pelvic ultrasounds and hormone assays. Results revealed a heightened proportion of patients with BPD had polycystic ovaries (30.4%, vs. 6.9%). Furthermore, BPD patients had higher BMI, and higher levels of free testosterone, adrostenedione (A) and prolactin compared with controls. The BPD patients with polycystic ovaries did not differ from BPD patients without polycystic ovaries in terms of psychopathology or BMI. Whether BPD is associated with a pre-existing biological vulnerability to hormonal issues that confer vulnerability to polycystic ovaries is unclear, but this possibility should be explored, as should the relative contribution of specific behavioural problems known to contribute to obesity, related hormonal issues (such as disordered eating).
Other markers of poor health may constitute additional biological vulnerability factors in populations suffering from PDs. Findings from one study revealed a higher prevalence of proinflammatory cytokines (TNF-alpha serum concentrations and osteoclastic markers) in patients with co-occurring BPD and depression compared to patients with depression and BPD alone (Kahl, Greggersen, et al., 2006). Similarly, patients with co-occurring depression and BPD (n = 12) evidenced higher concentrations of factors associated with neurogenesis and angiogenesis (VEGF, fibroblast growth factor 2) that have associations with negative health conditions, such as diabetic retinopathy (Kahl et al., 2009) and factors associated with endocrine and immune dysfunction (interleukin-6; Kahl, Bens, et al., 2006). Likewise, 22 female patients with BPD and co-occurring depression were compared to 16 patients with BPD and 20 healthy controls in terms of bone mineral density. Patients with BPD and MDD had higher levels of tumour necrosis alpha, interleukin, and markers of bone turnover than patients with BPD only or controls (Kahl, Rudolf, et al., 2005). In another study, patients with a PD (n = 40) evidenced more markers of immunoreactivity than healthy controls (n = 20), as assessed via lumbar puncture (Coccaro, Lee, Liu, & Mathé, 2012).This association did not remain after controlling for self-reported impulsive aggression. Indeed, indices of immunoreactivity (substance P) have demonstrated associations with aggression and impulsivity in PD patients (n = 38) (Coccaro, Lee, Owens, Kinkead, & Nemeroff, 2012). This finding bears importance as the field moves toward a more dimensional view of PDs, suggesting that certain dimensions of PD features, traits, and behaviors may be contribute to or exacerbate specific biological vulnerability factors.
Additional biological vulnerabilities that may account for some adverse health outcomes in PDs are BMI and other cardiovascular factors. One study found that women with BPD (n = 47) compared with age-matched healthy controls show greater intima-media thickness of common carotid arteries, a marker of atherosclerosis and cardiovascular risk, via ultrasound (Greggersen et al., 2011). Importantly, other findings suggest that BMI may account for this association of BPD with heart disease, arthritis and obesity (Powers & Oltmanns, 2013).
Behavioural (psychological) factors might also account for deleterious health outcomes in PDs. In particular, individuals with PDs often by definition (e.g., in the case of BPD, where diagnostic criteria capture impulsive, self-damaging and suicidal/self-injurious behaviours) engage in a variety of behaviours that result in negative interpersonal, intrapersonal, and adverse health outcomes. Data from several studies suggest that PDs are associated with high rates of health risk behaviours. Specifically, smoking, alcohol use, lack of exercise, overuse of medication and overuse of pain medication, poor health-related lifestyle choices and medical service utilization are all higher among non-recovered BPD patients compared to recovered BPD patients (Frankenburg & Zanarini, 2004; Keuroghlian et al., 2013). Among patients with HIV-seropositive status (N = 303), those high in narcissistic PD features had more HIV risk factors in terms of risky sexual behaviours (Martin, Benotsch, Lance, & Green, 2013). In addition, antisocial PD was associated with HIV risk behaviours among patients receiving treatment for cocaine dependence (Woody et al., 2003). Moreover, in a community sample (n = 1064), impulsive behaviors accounted for the relation between BPD severity and BMI (Iacovino et al., 2014). Thus, deficits in self-care and engagement in impulsive, risky behaviors among persons with PDs may directly and indirectly (such as via BMI) lead to adverse health consequences.
Environmental and social risk factors might include adverse or traumatic events, other environmental stressors, poor access to or quality of healthcare, difficulties associated with poor occupational or social functioning, and so on. Few studies have addressed these factors in the context of PDs and associated health conditions. In the present review, one article indicated that a diagnosis of PD was marginally associated with an environmental risk factor for HIV (history of sexual assault) in a sample (n = 113) of psychiatric patients (Mamabolo, Magagula, Krüger, & Fletcher, 2012). In the studies reviewed, there was no evidence of greater difficulties accessing health services among persons with remitted versus non-remitted BPD (Frankenburg & Zanarini, 2004; Keuroghlian et al., 2013), although non-remitted BPD patients were more likely to have lost or limited employment due to health problems. As such, it is possible that PDs and associated health problems may lead to greater environmental barriers to accessing health care.
Although in its infancy, research in this area has yielded promising data pointing to potential behavioural and biological mechanisms underlying the PD-medical condition relations, although less is known about environmental vulnerabilities in these populations. Given the complex nature of the interrelations of these factors, with reciprocal influences within each level (for instance, with sleep problems and obesity negatively affecting each other), and across levels, future studies are needed to pinpoint how these factors transact in PD samples.
Treatment
Only two studies have examined the impact of treatments on PDs or health related concerns. These studies have examined the influence of PD treatment on physical symptoms, as well as the influence of PD features on treatments of physical symptoms. The first study the impact of 12 weeks of duloxetine (a dual serotonin and norepinephrine reuptake inhibitor) among patients with BPD (n = 18) in an open trial (Bellino, Paradiso, Bozzatello, & Bogetto, 2010). Duloxetine led to improvements in BPD and depressive symptoms, and self-reported somatic symptoms, suggesting that treatment of PDs yields improvement in physical symptoms. In the second study, PD features were associated with poorer short-term CBT outcomes in a randomized trial for patients (n = 162) with unexplained physical symptoms (Zonneveld et al., 2012). Thus, PDs may complicate the treatment of medical problems. Although the scant research in this area precludes any conclusions, it seems likely that effective treatments incorporate a BPS approach, and include medical, behavioural, and psychosocial interventions.
Discussion
The past decades have heralded a surge in research on health outcomes associated with PDs. In particular, the existing literature suggests that PDs are linked with sleep disturbances, with strong evidence to suggest that sleep disturbances in BPD are comparable to that of other psychiatric and sleep disordered populations. There is also robust evidence for particularly high levels of chronic pain and obesity in PD samples. Indeed, myriad health conditions are associated with PDs. Although preliminary, research on mechanisms underlying the association of health problems with PDs highlights potential biological, behavioural, and environmental factors.
Despite the aggregated empirical support for the relation between PD and poor health outcomes, there are several characteristics of the extant literature and the present review that limit the conclusions we can draw regarding these associations. First, as with other reviews, this review is limited by potential publication and outcome reporting bias (Higgins & Green, 2011). Second, our search string was not exhaustive, and therefore relevant articles may have escaped the scope of this review. Our results must be interpreted with these caveats in mind.
Third, in many cases, the primary aim of the studies reviewed was not to examine the association of PDs with various health outcomes. The PDs assessed were sometimes specific to the study’s hypotheses, and convenience samples were often used. For example, studies by Sansone and colleagues (e.g., Sansone & Hawkins, 2004; Sansone et al., 2006; Sansone, Wiederman, & Monteith, 2001) only assessed selected symptoms of BPD. Conversely, in other studies, patients with one specific chronic condition, such as chronic pain, were assessed for the presence of a specific PD. In fact, most work did not assess the full scope of PDs or co-occurring psychiatric conditions, and in many cases, the rationale for the focus on a limited set of PDs was unclear. Furthermore, it is not clear from many of the studies as to whether there are unique associations of PDs with the medical conditions examined, or whether these associations are accounted for by co-occurring PDs or other psychopathology.
Mirroring the limitation associated with assessing only a subset of PDs, many studies only assessed a small subset of health conditions. Research on health conditions supports co-occurrence and reciprocal associations between conditions, such as poor sleep and obesity (Wolk, Shamsuzzaman, & Somers, 2003). Only a handful of the studies reviewed, however, assessed co-occurrence of health problems. Furthermore, the studies in the present review often did not account for higher use of psychotropic medication in PD samples. In light of demonstrated associations between such medications (i.e., antipsychotics) and health concerns (Kahl et al., 2013), attention to medications is needed.
This body of literature is further hindered by inconsistencies in the assessment of PDs. There were numerous approaches to assessment of PDs or PD symptoms, and many were specific to one particular PD. Furthermore, few articles mentioned PD-NOS, which is the most common PD (Verheul et al., 2007). Each measure has strengths and weaknesses for assessing PDs; however, the inconsistent assessment makes drawing firm conclusions challenging. Indeed, there are qualitative differences between forms of PD assessment. For instance, whereas the clinical diagnostic threshold likely identifies those who are more severe and impaired as a result of their symptoms, the use of PD screening instruments or self-report questionnaires is likely to result in high rates of false positives.
Perhaps most intriguing is the lack of a developmental approach in the existing work. All literature included in this review studied adults over age 18. Despite our thorough and exhaustive literature search, no longitudinal studies of youth meeting our inclusion criteria were detected. The closest examination of these constructs in younger samples included adolescent patients (n = 2) with BPD (Seng, Graham-Bermann, Clark, McCarthy, & Ronis, 2005). The authors reported on two female adolescent patients with BPD from the much larger sample, and found a high likelihood of physical problems in those patients. This is particularly problematic, as many of the health conditions (such as obesity) included in the literature reviewed above begin well before adulthood (Deckelbaum & Williams, 2001). Furthermore, the literature on PDs has expanded to include support for the earlier diagnosis and treatment of these conditions (Westen, Shedler, Durrett, Glass, & Martens, 2003). As a result of the lack of longitudinal research and lack of samples including children and adolescents, we know very little about the nature of the relationships studied: Which condition (health-related or PD) precedes the other? What are the developmental transactions that occur between symptoms of PDs and health conditions? These questions, and others, will be important to address to move research in this area forward.
This existing literature has the potential to inform future steps in this line of inquiry. This area could be further advanced with comprehensive and strategic selection of PDs of interest, increasing the scope of PDs assessed in order to identify which PDs may be uniquely associated with adverse health outcomes. In line with newly proposed models of PDs (American Psychiatric Association, 2013), future research should examine how specific dimensions of PDs may confer risks for specific health outcomes. In addition, future research in this area would be strengthened by the incorporation of assessments of health-related comorbidity and other factors (e.g., medication, healthy lifestyle). Furthermore, researchers should attend to the interrelations among biological, behavioural, and environmental risk factors for negative health outcomes in PD samples. A longitudinal, developmental perspective may be particularly important in this regard, serving to pinpoint the temporal relations of risk factors to health outcomes in PD samples.
Taken together, researchers have amassed a rich literature delineating a strong association between PDs and numerous adverse health outcomes. Chronic conditions place a high burden on the individual (van Asselt et al., 2007) and lead to increased utilization of health care systems (Frankenburg & Zanarini, 2004). Thus, further research in this area is of great public health importance. This body of research would be facilitated by comprehensive, theory driven assessment of PDs and more rigorous assessments of the multiple, interacting health concerns present in this population.
Acknowledgments
Dr. Whalen’s effort on this manuscript was supported by grant T32 MH100019 to Drs. Deanna Barch and Joan Luby from the National Institutes of Health.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd, revis. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Asaad T, Okasha T, Okasha A. Sleep EEG findings in ICD-10 borderline personality disorder in Egypt. Journal of Affective Disorders. 2002;71(1–3):11–18. doi: 10.1016/s0165-0327(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Atasoy HT, Atasoy N, Unal AE, Emre U, Sumer M. Psychiatric comorbidity in medication overuse headache patients with pre-existing headache type of episodic tension-type headache. European Journal of Pain. 2005;9(3):285–291. doi: 10.1016/j.ejpain.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Guimond S, St-Jean G, Lemelin S. Signs of insomnia in borderline personality disorder individuals. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 2008;4(5):462–470. [PMC free article] [PubMed] [Google Scholar]
- Bellino S, Paradiso E, Bozzatello P, Bogetto F. Efficacy and tolerability of duloxetine in the treatment of patients with borderline personality disorder: A pilot study. Journal of Psychopharmacology. 2010;24(3):333–339. doi: 10.1177/0269881108095715. [DOI] [PubMed] [Google Scholar]
- Bennett WRM, Joesch JM, Mazur M, Roy-Byrne P. Characteristics of HIV-positive patients treated in a psychiatric emergency department. Psychiatric Services. 2009;60(3):398–401. doi: 10.1176/ps.2009.60.3.398. [DOI] [PubMed] [Google Scholar]
- Black DW, Goldstein RB, Mason EE. Psychiatric diagnosis and weight loss following gastric surgery for obesity. Obesity Surgery. 2003;13(5):746–751. doi: 10.1381/096089203322509327. [DOI] [PubMed] [Google Scholar]
- Black DW, Okiishi C, Schlosser S. The Iowa follow-up of chemically sensitive persons. Annals of the New York Academy of Sciences. 2001;933:48–56. doi: 10.1111/j.1749-6632.2001.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Braden JB, Sullivan MD. Suicidal thoughts and behavior among adults with self-reported pain conditions in the national comorbidity survey replication. The Journal of Pain: Official Journal of the American Pain Society. 2008;9(12):1106–1115. doi: 10.1016/j.jpain.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge J, Clark JDD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. Journal of Pain. 2003;4(6):344–350. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- Carpiniello B, Pinna F, Pillai G, Nonnoi V, Pisano E, Corrias S, … Loviselli A. Obesity and psychopathology: A study of psychiatric comorbidity among patients attending a specialist obesity unit. Epidemiologia E Psichiatria Sociale. 2009;2:119–127. [PubMed] [Google Scholar]
- Chattopadhyay C, Chakrabarti N, Ghosh S. An assessment of psychiatric disturbances in Graves disease in a medical college in eastern India. Nigerian Journal of Clinical Practice. 2012;15(3):276–279. doi: 10.4103/1119-3077.100620. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Liu T, Mathé AA. Cerebrospinal fluid neuropeptide Y-like immunoreactivity correlates with impulsive aggression in human subjects. Biological Psychiatry. 2012;72(12):997–1003. doi: 10.1016/j.biopsych.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Owens MJ, Kinkead B, Nemeroff CB. Cerebrospinal fluid substance P-like immunoreactivity correlates with aggression in personality disordered subjects. Biological Psychiatry. 2012;72(3):238–43. doi: 10.1016/j.biopsych.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Conrad R, Schilling G, Bausch C, Nadstawek J, Wartenberg HC, Wegener I, … Liedtke R. Temperament and character personality profiles and personality disorders in chronic pain patients. Pain. 2007;133(1-3):197–209. doi: 10.1016/j.pain.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Conrad R, Wegener I, Geiser F, Kleiman A. Temperament, character, and personality disorders in chronic pain. Current Pain and Headache Reports. 2013;17(3) doi: 10.1007/s11916-012-0318-3. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obesity Research. 2001;9(Suppl 4):239S–243S. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- Egloff N, Maecker F, Stauber S, Sabbioni ME, Tunklova L, von Kanel R. Nondermatomal somatosensory deficits in chronic pain patients: are they really hysterical? Pain. 2012;153(9):1847–1851. doi: 10.1016/j.pain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Egloff N, Maecker F, Stauber S, Sabbioni ME, Tunklova L, von Känel R. Nondermatomal somatosensory deficits in chronic pain patients: Are they really hysterical? Pain. 2012;153(9):1847–1851. doi: 10.1016/j.pain.2012.05.006. http://silk.library.umass.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2012-21322-040&site=ehost-live&scope=site Retrieved from. [DOI] [PubMed] [Google Scholar]
- El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosomatic Medicine. 2010;72(7):641–647. doi: 10.1097/PSY.0b013e3181e10c7b. [DOI] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: A challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. http://www.ncbi.nlm.nih.gov/pubmed/847460 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Ericsson M, Poston WSCII, Linder J, Taylor JE, Haddock CK, Foreyt JP. Depression predicts disability in long-term chronic pain patients. Disability and Rehabilitation. 2002;24(6):334–340. doi: 10.1080/09638280110096241. [DOI] [PubMed] [Google Scholar]
- Fischer-Kern M, Kapusta ND, Doering S, Horz S, Mikutta C, Aigner M. The relationship between personality organization and psychiatric classification in chronic pain patients. Psychopathology. 2011;44(1):21–26. doi: 10.1159/000317271. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Lewis JE, Cole B, Cutler RB, Rosomoff HL, Rosomoff RS. Variables associated with current smoking status in chronic pain patients. Pain Medicine (Malden, Mass.) 2007;8(4):301–311. doi: 10.1111/j.1526-4637.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Fitzmaurice GM, Zanarini MC. The use of prescription opioid medication by patients with borderline personality disorder and axis II comparison subjects: a 10-year follow-up study. The Journal of Clinical Psychiatry. 2014;75(4):357–61. doi: 10.4088/JCP.13m08557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illness, poor health-related lifestyle choices, and costly forms of health care utilization. Journal of Clinical Psychiatry. 2004;65:1660–1665. doi: 10.4088/jcp.v65n1211. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. Obesity and obesity-related illnesses in borderline patients. Journal of Personality Disorders. 2006a;20(1):71–80. doi: 10.1521/pedi.2006.20.1.71. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. Personality disorders and medical comorbidity. Current Opinion in Psychiatry. 2006b;19(4):428–431. doi: 10.1097/01.yco.0000228766.33356.44. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. Relationship between cumulative BMI and symptomatic, psychosocial, and medical outcomes in patients with borderline personality disorder. Journal of Personality Disorders. 2011a;25(4):421–431. doi: 10.1521/pedi.2011.25.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. Relationship between cumulative BMI and symptomatic, psychosocial, and medical outcomes in patients with borderline personality disorder. Journal of Personality Disorders. 2011b;25(4):421–431. doi: 10.1521/pedi.2011.25.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish RG, Lee A, Brooks L, Leung J, Lau JY, Moore DH., 2nd Long-term follow-up of patients diagnosed with alcohol dependence or alcohol abuse who were evaluated for liver transplantation. Liver Transplantation. 2001;7(7):581–587. doi: 10.1053/jlts.2001.25455. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Stinson FS, Ruan WJ, Chou SP, Pickering RP, Grant BF. Antisocial behavioral syndromes and body mass index among adults in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Comprehensive Psychiatry. 2008;49(3):225–237. doi: 10.1016/j.comppsych.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggersen W, Rudolf S, Brandt P-W, Schulz E, Fassbinder E, Willenborg B, … Schweiger U. Intima-media thickness in women with borderline personality disorder. Psychosomatic Medicine. 2011;73(7):627–632. doi: 10.1097/PSY.0b013e3182231fe2. [DOI] [PubMed] [Google Scholar]
- Hafizi S. Sleep and borderline personality disorder: A review. Asian Journal of Psychiatry. 2013;6(6):452–459. doi: 10.1016/j.ajp.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Hansen PEB, Wang AG, Stage KB, Kragh-Sorensen P. Comorbid personality disorder predicts suicide after major depression: A 10-year follow-up. Acta Psychiatrica Scandinavica. 2003;107(6):436–440. [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2011. www.cochrane-handbook.org (Version 5.) The Cochrane Collaboration. Retrieved from. [Google Scholar]
- Iacovino JM, Powers AD, Oltmanns TF. Impulsivity mediates the association between borderline personality pathology and body mass index. Personality and Individual Differences. 2014;56:100–104. doi: 10.1016/j.paid.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Brook JS. Personality disorder traits evident by early adulthood and risk for eating and weight problems during middle adulthood. International Journal of Eating Disorders. 2006a;39:184–192. doi: 10.1002/eat.20223. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Brook JS. Personality disorder traits evident by early adulthood and risk for eating and weight problems during middle adulthood. International Journal of Eating Disorders. 2006b;39(3):184–192. doi: 10.1002/eat.20223. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Dibbelt L, Kordon A, Schweiger U. Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biological Psychiatry. 2006;59(7):667–71. doi: 10.1016/j.biopsych.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34(3):353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bester M, Greggersen W, Rudolf S, Dibbelt L, Stoeckelhuber BM, … Schweiger U. Visceral fat deposition and insulin sensitivity in depressed women with and without comorbid borderline personality disorder. Psychosomatic Medicine. 2005;67(3):407–412. doi: 10.1097/01.psy.0000160458.95955.f4. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Greggersen W, Rudolf S, Stoeckelhuber BM, Bergmann-Koester CU, Dibbelt L, Schweiger U. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosomatic Medicine. 2006;68(5):669–674. doi: 10.1097/01.psy.0000237858.76880.3d. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Greggersen W, Schweiger U, Cordes J, Correll CU, Frieling H, … Moebus S. Prevalence of the metabolic syndrome in patients with borderline personality disorder: Results from a cross-sectional study. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(3):205–213. doi: 10.1007/s00406-012-0339-2. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Rudolf S, Stoeckelhuber BM, Dibbelt L, Gehl H, Hohagen F, Schweiger U. Bone mineral density, markers of bone turnover , and cytokines in young women with borderline personalty disorder with and without comorbid major depressive disorder. American Journal of Psychiatry. 2005;162:168–174. doi: 10.1176/appi.ajp.162.1.168. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Karsten J, de Weerd A, Lancel M. Sleep disturbances in a clinical forensic psychiatric population. Sleep Medicine. 2013a;14(11):1164–1169. doi: 10.1016/j.sleep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Karsten J, de Weerd A, Lancel M. Sleep disturbances in a clinical forensic psychiatric population. Sleep Medicine. 2013b;14(11):1164–1169. doi: 10.1016/j.sleep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Frankenburg FR, Zanarini MC. The relationship of chronic medical illnesses, poor health-related lifestyle choices, and health care utilization to recovery status in borderline patients over a decade of prospective follow-up. Journal of Psychiatric Research. 2013;47(10):1499–1506. doi: 10.1016/j.jpsychires.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KRR. Psychiatric and medical comorbidities of bipolar disorder. Psychosomatic Medicine. 2005;67(1) doi: 10.1097/01.psy.0000151489.36347.18. [DOI] [PubMed] [Google Scholar]
- Lier HO, Biringer E, Stubhaug B, Eriksen HR, Tangen T. Psychiatric disorders and participation in pre- and postoperative counselling groups in bariatric surgery patients. Obesity Surgery. 2011;21(6):730–737. doi: 10.1007/s11695-010-0146-7. [DOI] [PubMed] [Google Scholar]
- Loder E, Geweke L. Volume and nature of telephone calls in a specialty headache practice. Headache. 2002;42(9):883–887. doi: 10.1046/j.1526-4610.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- Lopes M, Olfson M, Rabkin J, Hasin DS, Alegría AA, Lin K-H, … Blanco C. Gender, HIV status, and psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2012;73(3):384–391. doi: 10.4088/JCP.10m06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamabolo M, Magagula T, Krüger C, Fletcher L. A survey of risk behaviour for contracting HIV among adult psychiatric patients. A South African study - Part 1. African Journal of Psychiatry. 2012;15(5):329–334. doi: 10.4314/ajpsy.v15i5.40. [DOI] [PubMed] [Google Scholar]
- Manlick CF, Black DW, Stumpf A, McCormick B, Allen J. Symptoms of migraine and its relationship to personality disorder in a non-patient sample. Journal of Psychosomatic Research. 2012;73(6):479–480. doi: 10.1016/j.jpsychores.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Marquine MJ, Iudicello JE, Morgan EE, Brown GG, Letendre SL, Ellis RJ, … Heaton RK. “Frontal systems” behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Research. 2014;215(1):208–16. doi: 10.1016/j.psychres.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Benotsch EG, Lance SP, Green M. Transmission risk behaviors in a subset of HIV-positive individuals: The role of narcissistic personality features. Personality and Individual Differences. 2013;54(2):256–260. [Google Scholar]
- Masheb RM, Grilo CM. Examination of predictors and moderators for self-help treatments of binge-eating disorder. Journal of Consulting and Clinical Psychology. 2008;76(5):900–904. doi: 10.1037/a0012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather AA, Cox BJ, Enns MW, Sareen J. Associations between body weight and personality disorders in a nationally representative sample. Psychosomatic Medicine. 2008;70(9):1012–1019. doi: 10.1097/PSY.0b013e318189a930. [DOI] [PubMed] [Google Scholar]
- Mauri M, Rucci P, Calderone A, Santini F, Oppo A, Romano A, … Cassano GB. Axis I and II disorders and quality of life in bariatric surgery candidates. Journal of Clinical Psychiatry. 2008;69(2):295–301. doi: 10.4088/jcp.v69n0216. [DOI] [PubMed] [Google Scholar]
- Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression and Anxiety. 2013;30(4):328–341. doi: 10.1002/da.22051. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Higgins KS. Associations between pain conditions and borderline personality disorder symptoms: Findings from the National Comorbidity Survey Replication. Clinical Journal of Pain. 2013;29(6):527–532. doi: 10.1097/AJP.0b013e31826ab5d0. [DOI] [PubMed] [Google Scholar]
- Meeks TW, Dunn LB, Kim DS, Golshan S, Sewell DD, Atkinson JH, Lebowitz BD. Chronic pain and depression among geriatric psychiatry inpatients. International Journal of Geriatric Psychiatry. 2008;23(6):637–642. doi: 10.1002/gps.1954. [DOI] [PubMed] [Google Scholar]
- Moran P, Stewart R, Brugha T, Bebbington P, Bhugra D, Jenkins R, Coid JW. Personality disorder and cardiovascular disease: Results from a national household survey. Journal of Clinical Psychiatry. 2007;68(1):69–74. doi: 10.4088/jcp.v68n0109. [DOI] [PubMed] [Google Scholar]
- Olssøn I, Dahl AA. Personality problems are considerably associated with somatic morbidity and health care utilisation. European Psychiatry. 2009;24(7):442–449. doi: 10.1016/j.eurpsy.2009.05.004. http://silk.library.umass.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2009-13696-001&site=ehost-live&scope=site Retrieved from. [DOI] [PubMed] [Google Scholar]
- Olssøn I, Dahl AA. Avoidant personality problems--their association with somatic and mental health, lifestyle, and social network. A community-based study. Comprehensive Psychiatry. 2012;53(6):813–821. doi: 10.1016/j.comppsych.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care and STDs. 2003;17(12):635–644. doi: 10.1089/108729103771928690. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic Medicine. 2008;70(3):288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Pickering RP, Grant BF, Chou SP, Compton WM. Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. Journal of Clinical Psychiatry. 2007;68(7):998–1009. doi: 10.4088/jcp.v68n0704. [DOI] [PubMed] [Google Scholar]
- Plante DT, Frankenburg FR, Fitzmaurice GM, Zanarini MC. Relationship between maladaptive cognitions about sleep and recovery in patients with borderline personality disorder. Psychiatry Research. 2013a;210(3):975–979. doi: 10.1016/j.psychres.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Frankenburg FR, Fitzmaurice GM, Zanarini MC. Relationship between sleep disturbance and recovery in patients with borderline personality disorder. Journal of Psychosomatic Research. 2013b;74(4):278–282. doi: 10.1016/j.jpsychores.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AD, Oltmanns TF. Borderline personality pathology and chronic health problems in later adulthood: The mediating role of obesity. Personality Disorders: Theory, Research, and Treatment. 2013;4(2):152–159. doi: 10.1037/a0028709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Estroff TW, Empting LD, Shearer-Williams S, Hoffmann NG. Prevalence of substance use and psychiatric disorders in a highly select chronic pain population. Journal of Addiction Medicine. 2013;7(1) doi: 10.1097/ADM.0b013e3182738655. [DOI] [PubMed] [Google Scholar]
- Roepke S, Ziegenhorn A, Kronsbein J, Merkl A, Bahri S, Lange J, … Lammers C-H. Incidence of polycystic ovaries and androgen serum levels in women with borderline personality disorder. Journal of Psychiatric Research. 2010;44(13):847–852. doi: 10.1016/j.jpsychires.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Roth T. Insomnia: Definition, prevalence, etiology, and consequences. Journal of Clinical Sleep Medicine. 2007;3(5 Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- Rothrock J, Lopez I, Zweilfer R, Andress-Rothrock D, Drinkard R, Walters N. Borderline personality disorder and migraine. Headache. 2007;47(1):22–26. doi: 10.1111/j.1526-4610.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- Ruiter ME, Lichstein KL, Nau SD, Geyer JD. Personality disorder features and insomnia status amongst hypnotic-dependent adults. Sleep Medicine. 2012;13(9):1122–1129. doi: 10.1016/j.sleep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J. Personality disorders: Epidemiology and public health issues. International Review of Psychiatry. 2011;23(3):223–233. doi: 10.3109/09540261.2011.588200. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Hahn HS, Dittoe N, Wiederman MW. The relationship between borderline personality symptoms and body mass index in a consecutive sample of cardiac stress test patients. Eating and Weight Disorders. 2012;17(2):e128–e131. doi: 10.1007/BF03325336. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Hawkins R. Fibromyalgia, borderline personality, and opioid prescription. General Hospital Psychiatry. 2004;26(5):415–416. doi: 10.1016/j.genhosppsych.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Pole M, Dakroub H, Butler M. Childhood trauma, borderline personality symptomatology, and psychophysiological and pain disorders in adulthood. Psychosomatics: Journal of Consultation and Liaison Psychiatry. 2006;47(2):158–162. doi: 10.1176/appi.psy.47.2.158. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Schumacher D, Wiederman MW, Routsong-Weichers L. The prevalence of binge eating disorder and borderline personality symptomatology among gastric surgery patients. Eating Behaviors. 2008;9(2) doi: 10.1016/j.eatbeh.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Whitecar P, Meier BP, Murry A. The prevalence of borderline personality among primary care patients with chronic pain. General Hospital Psychiatry. 2001;23(4):193–7. doi: 10.1016/s0163-8343(01)00148-7. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Whitecar P, Wiederman MW. Psychophysiological disorders among buprenorphine patients. International Journal of Psychiatry in Clinical Practice. 2009;13(4):338–340. doi: 10.3109/13651500903094575. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Wiederman MW, Monteith D. Obesity, borderline personality symptomatology, and body image among women in a psychiatric outpatient setting. International Journal of Eating Disorders. 2001;29(1):76–79. doi: 10.1002/1098-108x(200101)29:1<76::aid-eat12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Wiederman MW, Sansone LA, Monteith D. Obesity and borderline personality symptomatology: Comparison of a psychiatric versus primary care sample. International Journal of Obesity and Related Metabolic Disorders. 2001;25(2) doi: 10.1038/sj.ijo.0801514. [DOI] [PubMed] [Google Scholar]
- Selby EA. Chronic sleep disturbances and borderline personality disorder symptoms. Journal of Consulting and Clinical Psychology. 2013;81(5):941–947. doi: 10.1037/a0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiz UB, Basoglu C, Ebrinc S, Cetin M. Nightmare disorder, dream anxiety, and subjective sleep quality in patients with borderline personality disorder. Psychiatry and Clinical Neurosciences. 2008;62(1):48–55. doi: 10.1111/j.1440-1819.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Seng JS, Graham-Bermann SA, Clark MK, McCarthy AM, Ronis DL. Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service-use data. Pediatrics. 2005;116(6):e767–e776. doi: 10.1542/peds.2005-0608. [DOI] [PubMed] [Google Scholar]
- Smitherman TA, Kolivas ED, Bailey JR. Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache. 2013;53(1):23–45. doi: 10.1111/head.12004. [DOI] [PubMed] [Google Scholar]
- Tragesser SL, Bruns D, Disorbio JM. Borderline personality disorder features and pain: The mediating role of negative affect in a pain patient sample. Clinical Journal of Pain. 2010;26(4):348–353. doi: 10.1097/AJP.0b013e3181cd1710. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Distel Ma, Carpenter RW. DSM-5 Borderline personality disorder: At the border between a dimensional and a categorical view. Current Psychiatry Reports. 2011;13(1):43–49. doi: 10.1007/s11920-010-0170-2. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Widiger TA, Lynam DR, Costa PT. Borderline personality disorder from the perspective of general personality functioning. Journal of Abnormal Psychology. 2003;112:193–202. doi: 10.1037/0021-843x.112.2.193. [DOI] [PubMed] [Google Scholar]
- Vamanshankar H, Hegde KS, Chaturvedi J, Pratibha CB, Ross A, Nayar RC, Parameshwaran S. Do patients with allergic rhinitis have a particular personality trait? The Journal of Laryngology and Otology. 2013;127(4):378–382. doi: 10.1017/S0022215113000170. [DOI] [PubMed] [Google Scholar]
- Van Asselt ADI, Dirksen CD, Arntz A, Severens JL. The cost of borderline personality disorder: Societal cost of illness in BPD-patients. European Psychiatry. 2007;22:354–361. doi: 10.1016/j.eurpsy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Van Hanswijck de Jonge P, van Furth EF, Lacey JH, Waller G. The prevalence of DSM-IV personality pathology among individuals with bulimia nervosa, binge eating disorder and obesity. Psychological Medicine. 2003;33(7):1311–1317. doi: 10.1017/s0033291703007505. [DOI] [PubMed] [Google Scholar]
- Verheul R, Bartak A, Widiger T. Prevalence and construct validity of Personality Disorder Not Otherwise Specified (PDNOS) Journal of Personality Disorders. 2007;21(4):359–370. doi: 10.1521/pedi.2007.21.4.359. [DOI] [PubMed] [Google Scholar]
- Westen D, Shedler J, Durrett C, Glass S, Martens A. Personality diagnoses in adolescence: DSM-IV Axis II diagnoses and an empirically derived alternative. American Journal of Psychiatry. 2003 May;160:952–966. doi: 10.1176/appi.ajp.160.5.952. [DOI] [PubMed] [Google Scholar]
- Wilsey BL, Fishman SM, Tsodikov A, Ogden C, Symreng I, Ernst A. Psychological comorbidities predicting prescription opioid abuse among patients in chronic pain presenting to the emergency department. Pain Medicine. 2008;9(8):1107–1117. doi: 10.1111/j.1526-4637.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Wolk R, Shamsuzzaman ASM, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42(6):1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- Woody GE, Gallop R, Luborsky L, Blaine J, Frank A, Salloum IM, … Crits-Christoph P. HIV risk reduction in the National Institute on Drug Abuse Cocaine Collaborative Treatment Study. Journal of Acquired Immune Deficiency Syndromes. 2003;33(1):82–87. doi: 10.1097/00126334-200305010-00012. [DOI] [PubMed] [Google Scholar]
- Wright AR, Gatchel RJ, Wildenstein L, Riggs R, Buschang P, Ellis E., 3rd Biopsychosocial differences between high-risk and low-risk patients with acute TMD-related pain. Journal of the American Dental Association. 2004;135(4):474–483. doi: 10.14219/jada.archive.2004.0213. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, Reynolds V. Axis I comorbidity of borderline personality disorder. American Journal of Psychiatry. 1998;155:1733–1739. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. Axis I comorbidity in patients with borderline personality disorder: 6-year follow-up and prediction of time to remission. American Journal of Psychiatry. 2004;161:2108–2114. doi: 10.1176/appi.ajp.161.11.2108. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Young D, Dalrymple K, Martinez J. Is dimensional scoring of borderline personality disorder important only for subthreshold levels of severity? Journal of Personality Disorders. 2013;27(2):244–51. doi: 10.1521/pedi.2013.27.2.244. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorder in psychiatric outpatients. American Journal of Psychiatry. 2005;162:1911–1918. doi: 10.1176/appi.ajp.162.10.1911. [DOI] [PubMed] [Google Scholar]
- Zonneveld LNL, van Rood YR, Kooiman CG, Timman R, van ‘t Spijker A, Busschbach JJV. Predicting the outcome of a cognitive-behavioral group training for patients with unexplained physical symptoms: A one-year follow-up study. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-848. [DOI] [PMC free article] [PubMed] [Google Scholar]