Highlights
-
•
The adolescent brain processes rewards differently than in adults.
-
•
These differences occur even when behavior is similar between age groups.
-
•
DS was the locus of substantial developmental differences in reward activity.
-
•
Surprisingly, differences were not as pronounced in VS.
-
•
These differences may have implications for adolescent psychiatric vulnerability.
Keywords: Adolescent, Reward, Electrophysiology, Striatum, Dopamine, Rat
Abstract
Immaturities in adolescent reward processing are thought to contribute to poor decision making and increased susceptibility to develop addictive and psychiatric disorders. Very little is known; however, about how the adolescent brain processes reward. The current mechanistic theories of reward processing are derived from adult models. Here we review recent research focused on understanding of how the adolescent brain responds to rewards and reward-associated events. A critical aspect of this work is that age-related differences are evident in neuronal processing of reward-related events across multiple brain regions even when adolescent rats demonstrate behavior similar to adults. These include differences in reward processing between adolescent and adult rats in orbitofrontal cortex and dorsal striatum. Surprisingly, minimal age related differences are observed in ventral striatum, which has been a focal point of developmental studies. We go on to discuss the implications of these differences for behavioral traits affected in adolescence, such as impulsivity, risk-taking, and behavioral flexibility. Collectively, this work suggests that reward-evoked neural activity differs as a function of age and that regions such as the dorsal striatum that are not traditionally associated with affective processing in adults may be critical for reward processing and psychiatric vulnerability in adolescents.
1. Introduction
Current research on psychiatric disorders has placed a strong emphasis on early detection and treatment. Many symptoms of schizophrenia, mood disorders and addiction first manifest during the adolescent period (Adriani and Laviola, 2004, Casey et al., 2008, Schramm-Sapyta et al., 2009, Mitchell and Potenza, 2014). Accordingly, it is critical to elucidate the biological and environmental risk factors that render adolescents highly vulnerable to these disorders. Such mechanistic knowledge is necessary for the development of interventions to prevent or attenuate the emergence of disease.
Previous preclinical research on brain development and disease has primarily assessed morphological changes or alterations at the receptor level. These studies have yielded critical information about adolescent biology and behavior. There is little known, however, about real-time dynamics of neuronal activity during behavior. This information is particularly relevant in light of recent theories positing that dysfunctional neuronal network activity is a critical contributor to the etiology of disease (Uhlhaas and Singer, 2012, Moghaddam and Wood, 2014). To fully understand how behaviorally relevant neuronal network activity is altered in vulnerable individuals, we must first understand how individual neurons and neural ensembles encode salient events in healthy adolescents and adults.
Changes in affect, motivation, and motivational processing during adolescence are among the first observed behaviors predictive of schizophrenia and other psychiatric illnesses in high risk individuals (Ernst et al., 2006, Gladwin et al., 2011, Juckel et al., 2012). To understand the development of symptoms during this vulnerable developmental period, it is essential to quantify the basic neural mechanisms underlying adolescent reward processing. Recent data accumulated in our lab using adolescence rats suggest substantial age-related differences in reward-induced neuronal activity. These differences are manifested even when (1) measurable behavior is equivalent between adolescent and adult subjects, and (2) baseline levels of neuronal activity are equivalent between age groups. Thus, reward-evoked neuronal activity may, in some instances, be more effective than behavioral measures of motivation or baseline activity as a marker of early vulnerability to disease. In this review, we summarize adolescent reward-processing data acquired from a rat model across multiple brain regions, and discuss the implications of these differences for adolescent behavior and disease vulnerability.
2. Adolescent reward processing differs from adults across multiple regions
The technique focused in this review is single-unit extracellular recording where neuronal activity of multiple neurons can be measured in real-time in behaving animals (Sturman and Moghaddam, 2011b). For this method, multiwire electrode arrays are implanted in specific brain regions and electrical signals are amplified and high pass filtered to isolate high frequency neuronal activity, such as action potentials or local field potential oscillations (Buzsaki, 2004, Sturman and Moghaddam, 2011b, Wood et al., 2012). Measuring neural activity in awake-behaving adolescent rats is a challenging endeavor, as the adolescent window only spans approximately between postnatal days 28–55 (Spear, 2000). After accounting for the required time for electrode implantation surgery, recovery and habituation, the brief remaining time window precludes the use of complex behavioral paradigms with electrophysiology. Therefore, behavioral tasks that do not require long training times must be used to measure reward processing in adolescent rats. Our lab utilizes a rewarded instrumental task in which rats learn to nose poke into a lit port to receive a single sugar pellet, while neural activity is recorded from electrode arrays implanted into specific brain regions (Fig. 1). Importantly, the task is simple enough that learning and performance of the primary components of the task are comparable between adults and adolescents (Sturman et al., 2010), thus any differences in neuronal activity are indicative of reward processing differences, rather than a product of behavioral asymmetry between groups. Each of these behavioral events can be synchronized with measures of neural activity with sub-second long temporal resolution, allowing assessment of neural activity associated with reward-related cues, goal-directed actions, and reward anticipation and delivery. Using variants of this task, we recorded from orbitofrontal cortex, dorsal and ventral striatum, and ventral tegmental area in adult and adolescent rats. We then discuss how these differences in reward-processing may be related to reward-related cognitive traits observed during adolescence, including impulsivity, risk taking and behavioral flexibility.
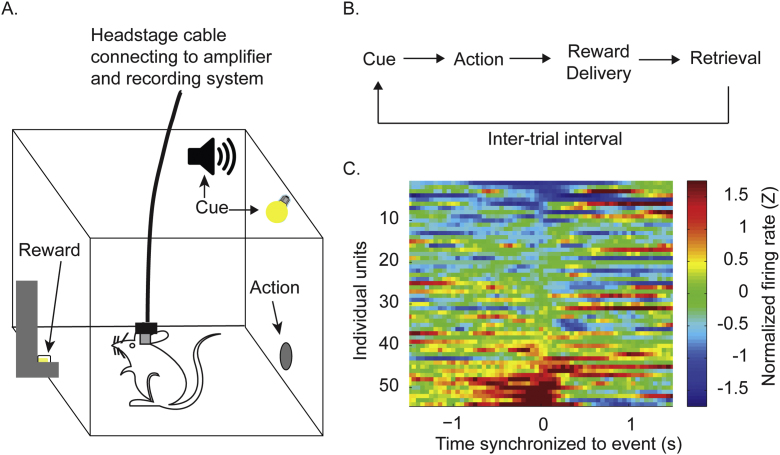
Fig. 1.
(A) Single unit electrophysiology was performed with awake-behaving adolescent and adult rats during reward-related behavior. Rats were implanted with microwire arrays and placed into an operant chamber equipped with a nose poke port, food trough that delivered sugar pellet rewards, and a cue light used to signal reward availability. It should be noted that the identity of the cue was a light, a tone, or a compound cue consisting of both. (B) The instrumental tasks utilized began with illumination of the light cue, during which performance of a nose-poke (action) caused delivery of a pellet reward. After the rat collected the reward, a variable inter-trial interval was initiated, then the next trial began. (C) This heat plot shows sample data demonstrating the typical response of individual neurons to a reward-associated event. A subset of neurons demonstrate increased firing rate surrounding the event (bottom), others demonstrate suppressed firing rate during the event (top), and others are unresponsive (middle).
2.1. Prefrontal cortex
Prefrontal cortex (PFC) undergoes substantial development throughout adolescence, and this development has been implicated in adolescent behavioral tendencies, particularly the ability to regulate and inhibit motivated behaviors (Brenhouse et al., 2010, Geier et al., 2010, Sturman and Moghaddam, 2011a, Ernst, 2014). PFC is divided into multiple functionally distinct subregions with different implications for adolescent behavior and disease vulnerability. Orbitofrontal cortex (OFC) is a lateral prefrontal-cortical region that receives input from sensory regions and is extensively connected with limbic areas (Price, 2007, Rolls and Grabenhorst, 2008). Accordingly, OFC is ideally suited to integrate physical aspects of rewarding and aversive outcomes with emotional information, and then utilize this affective information to guide behavior. Neuronal activity in OFC has been associated with the representation of rewarding outcomes (van Duuren et al., 2007, Balleine et al., 2011, Schoenbaum et al., 2011), and has been implicated in multiple facets of impulsive behavior (Berlin et al., 2004, Winstanley et al., 2010, Zeeb et al., 2010), which is elevated in humans and rats during adolescence (Green et al., 1994, Adriani and Laviola, 2003, Burton and Fletcher, 2012, Doremus-Fitzwater et al., 2012, Mitchell and Potenza, 2014). Because OFC (along with other prefrontal regions) has been shown to be underdeveloped in human adolescents (Sowell et al., 1999, Galvan et al., 2006), OFC is a logical target for probing for age-related differences in reward processing.
Single unit extracellular recording was used to measure task-evoked activity in individual neurons. In adults, OFC population neuronal activity decreased during reward retrieval (Fig. 1B). In contrast, the adolescent OFC population activity was increased during retrieval (Sturman and Moghaddam, 2011b). This profound difference in activity occurred despite similar baseline firing rate between groups, and comparable neuronal inhibition during the performance phase of the instrumental action that lead to reward delivery. These data suggest that reward processing in OFC can be an effective biomarker of age-related differences, even when baseline neuronal activity and behavior are equivalent between groups.
Although baseline firing rate was similar between age groups, an alternate analysis of firing patterns revealed further distinctions. Adolescent OFC showed increased variability compared to adults in firing rate across multiple trials, as assessed by fano factor, which provides a measure of normalized variability and can be calculated as cross-trial variance divided by cross-trial mean (Churchland et al., 2010). This variability may be indicative of inefficient neural coding of reward-related events, as spike variability undermines effective inter-regional communication through spike-field coherence (Fries, 2005, Churchland et al., 2010). Importantly, this finding suggests that measures beyond simple firing rate may be necessary to detect functional differences in neural processing between age groups, and possibly between healthy controls and diseased or at-risk patients.
OFC plays a modulatory role in impulsive choice, defined as a preference for immediate rewards/gratification (Winstanley, 2007). Adolescent humans and rats have increased preference for immediate gratification compared to adult humans and rats, and this has been implicated in adolescent drug abuse and maladaptive behavior (Adriani and Laviola, 2003, Doremus-Fitzwater et al., 2012, Mitchell and Potenza, 2014, Stanis and Andersen, 2014). Impulsive decision-making is associated with several psychiatric disorders (Bechara et al., 2001, Ahn et al., 2011, Nolan et al., 2011), and is both a predictor of drug abuse and a consequence of long-term exposure to drugs of abuse (Simon et al., 2007, Perry et al., 2008, Anker et al., 2009, de Wit, 2009, Mendez et al., 2010). Thus, a feed forward condition may develop in which individuals with psychiatric vulnerabilities involving aberrant impulsive regulation are highly likely to abuse drugs, which then exacerbates trait impulsivity (Garavan and Stout, 2005, Setlow et al., 2009). Our data suggest that age differences in impulsivity may be, in part, due to neuronal processing differences in the OFC, as OFC encodes information about reward-associated delays (Roesch and Olson, 2005, Roesch et al., 2006). The highly variable neural processing throughout task performance (as assessed by fano factor) and hyperactive reward-evoked response observed in adolescent OFC may, therefore, be related to unstable representations of reward-related events. Our observation may also be related to a suboptimal ability to bridge long delays between actions and outcomes, a function associated with OFC neurons (Roesch et al., 2006). This in turn would facilitate persistent choice of immediate over delayed gratification.
Age-related differences also are observed in infralimbic and prelimbic regions of the medial PFC, which are implicated in behavioral planning and feedback, attention, and response inhibition (Goldman-Rakic, 1995, Fuster, 2001, Killcross and Coutureau, 2003, Magno et al., 2006, Peters et al., 2008, Burgos-Robles et al., 2013, Pezze et al., 2014). While neuronal activity has not yet been recorded in these regions in behaving adolescent animals, developmental correlates of reward processing have been revealed by quantifying immediate early genes. After heroin self-administration, adolescents showed an attenuated increase in Fos positive neurons in prelimbic and infralimbic cortices compared to adults, indicative of reduced activation of adolescent medial PFC by drug reward seeking (Doherty et al., 2013). Reports of nicotine-evoked activity are conflicting, demonstrating either enhanced increase in Arc or similar changes in cfos in adolescent compared to adult medial PFC (Leslie et al., 2004, Schochet et al., 2005). Finally, cocaine exposure caused increase c-fos expression in adolescent PFC (Cao et al., 2007). While these studies provide useful data, direct measurements of neural processing of both drug and natural rewards in adolescent medial PFC will yield temporally specific information about adolescent medial PFC function.
Dopamine receptor expression in prelimbic cortex peaks during adolescence (Andersen et al., 2000). D1 dopamine receptors, in particular, have been linked to adolescent motivated behavior. Adolescent rats demonstrate increased vulnerability to drug-associated cues compared to adult rats (Leslie et al., 2004, Brenhouse and Andersen, 2008, Brenhouse et al., 2008, Kota et al., 2011); blocking D1 receptors in adolescent prelimbic cortex decreases sensitivity to these cues (Brenhouse et al., 2008). In addition, overexpressing D1 receptors in adult prelimbic cortex recapitulated adolescent behavioral tendencies, including impulsivity and increased sensitivity to drug-associated cues (Sonntag et al., 2014). D1 receptor manipulation also modulates behavioral sensitivity to amphetamine to a greater degree in adolescents than adults (Mathews and McCormick, 2012).
2.2. Striatum
Neural development during adolescence is ongoing in the striatum (Sowell et al., 1999, Ernst et al., 2006, Casey et al., 2008, Geier et al., 2010, Somerville et al., 2011). Striatum is involved with learning, reward processing and movement, and has been strongly implicated in psychiatric disorders including schizophrenia and addiction (Kalivas and Volkow, 2005, Everitt et al., 2008, Horga and Abi-Dargham, 2014). Both ventral and dorsal striatum receive dense dopaminergic projections from the midbrain, and dopamine transmission has repeatedly been shown to differ between adulthood and adolescence (Adriani and Laviola, 2004, Volz et al., 2009, McCutcheon et al., 2012). While there is a wealth of data from animal models describing neuroanatomical and pharmacological differences in striatum between adolescent and adult rodents (Andersen et al., 1997, Bolanos et al., 1998, Tarazi et al., 1998), there are considerably less data describing age-related differences in neural activity. The majority of the neural imaging studies performed in human adolescent subjects have focused on ventral striatum (VS) in particular the nucleus accumbens (NAc), which is implicated in motivation, learning and cue processing (Robbins and Everitt, 1996, Kelley, 2004, Ernst et al., 2006, Galvan et al., 2006, Geier et al., 2010, Hart et al., 2014). However, dorsal striatum (DS), which is implicated in learning, action-selection and habit formation (Packard and White, 1990, Balleine et al., 2007, Kimchi et al., 2009), has been largely overlooked as a locus of developmental differences. To quantify and compare neural correlates of reward processing in both striatal regions, our lab recorded single-unit extracellular activity in both DS and NAc of adult and adolescent rats during goal-directed behavior.
Somewhat surprisingly, task-evoked activity in NAc did not differ substantially between adult and adolescent rats (Sturman and Moghaddam, 2012). Robust age-related differences, however, were observed in DS. Adolescent neurons were activated just prior to a reward-seeking action, whereas adult neurons did not respond until after action completion (Fig. 1B). Adolescent neurons in DS also were activated prior to reward retrieval, while adult neurons were inhibited by reward (Fig. 1B). This demonstrated that the adolescent brain recruits DS circuitry both earlier and to a greater degree than adults during reward-retrieval.
While adolescent DS neurons are hyper-responsive to rewards, amphetamine-evoked dopamine release is attenuated compared to adults in this region. Lower levels of amphetamine-evoked dopamine efflux in the DS, but, again, not the NAc of adolescent rats compared to adults (Matthews et al., 2013). Interestingly, the opposite effect has been observed with dopaminergic drugs that act as uptake inhibitors, such as cocaine and methylphenidate, which cause enhanced dopamine efflux in adolescent compared to adult DS (Walker and Kuhn, 2008, Walker et al., 2010). As with amphetamine, this age-related cocaine effect was more pronounced in DS than NAc (Frantz et al., 2007, Walker and Kuhn, 2008). This difference between DS dopamine release may be a function of baseline dopamine availability, as reduced dopamine availability in projection dopamine neurons would likely affect drugs that facilitate dopamine release (such as amphetamine) to a greater degree than drugs that maintain dopamine in the synapse (such as cocaine). Accordingly, tyrosine hydroxylase, an enzyme involved with the synthesis of dopamine, was reduced in adolescent DS but not NAc (Matthews et al., 2013). This reduction in evoked-dopamine neurotransmission suggests that dopamine projections to DS, which arise from substantia nigra pars compacta (Ungerstedt, 1971, Lynd-Balta and Haber, 1994), may be hypoactive during adolescence. Dopamine has an inhibitory influence on medium spiny neurons in the striatum (Kreitzer and Malenka, 2008). A hypoactive dopamine neurotransmission in adolescence DS may, therefore, contribute to our observed enhanced reward-evoked activity in DS neurons. Future studies recording from dopamine projections to the adolescent DS will directly address this mechanism.
The area of striatum traditionally associated with attributing value and motivation to cues and rewards is VS (Robbins and Everitt, 1996, Kelley, 2004, Cooper and Knutson, 2008, Flagel et al., 2011). Accordingly, many theories of adolescent illness and behavioral vulnerability hinge on aberrant reward-related motivated behavior and responsivity of reward-related brain circuitry (Bjork et al., 2004, Galvan et al., 2006, Geier et al., 2010, Van Leijenhorst et al., 2010). The previous data, on the other hand, suggest that age-related differences to rewards may be even greater in DS (Sturman and Moghaddam, 2012, Matthews et al., 2013). While these do not preclude the role of the developing VS in adolescent behavioral and disease vulnerability, they suggest that DS may also play a substantial role in adolescent behavioral tendencies.
The DS is strongly associated with learning and the physical manifestation of locomotive behavior (Robbins and Everitt, 1992, Packard and Knowlton, 2002, Gittis and Kreitzer, 2012). In particular the dorsomedial striatum (DMS), or associative striatum region of the DS is implicated in linking actions to rewarding outcomes, as lesions of DMS abolish the learning and expression of goal-directed behavior (Yin and Knowlton, 2004, Ragozzino, 2007), and DMS activity also has been implicated in the encoding of flexible response patterns (Kimchi and Laubach, 2009). Conversely, dorsolateral striatum (DLS) is involved with the consolidation and expression of habitual behavior, during which actions are no longer dependent on outcome representation (Yin et al., 2004, Yin et al., 2009). The studies of adolescent neuronal activity and dopamine release detailed in this review (Sturman and Moghaddam, 2012, Matthews et al., 2013) were both localized to DMS, underscoring the importance of this region in development toward the adolescent behavioral phenotype and illness vulnerability. In line with this idea, several differences have been observed in instrumental behavior between adult and adolescent rats, with adolescents demonstrating differences in instrumental behavior, including differences in appetitive motivation, reduced extinction, attenuated response inhibition and impaired ability to adjust to changes in action-outcome contingencies (Friemel et al., 2010, Sturman et al., 2010, Andrzejewski et al., 2011, Spear, 2011, Burton and Fletcher, 2012, Naneix et al., 2012). In addition, adolescents exhibit reduced ability to quickly initiate an appropriate response after a stop signal (Simon et al., 2013), similar to the effect observed after lesions of DMS (Eagle and Robbins, 2003).
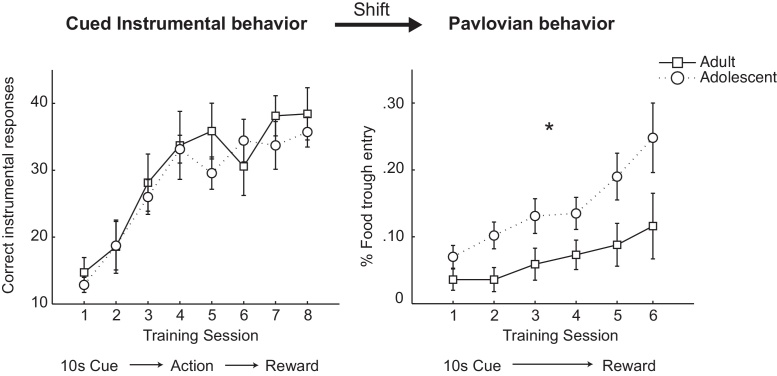
In contrast to adolescent DMS, the presence of developmental differences in DLS is less clear. During the expression of goal-directed behavior, actions are initially tightly linked to outcome representation. After overtraining, however, actions become less influenced by outcome representation, and more automated (“habitual”) (Dickinson, 1985). Plasticity related to this habit learning occurs in DLS (Yin et al., 2009, Balleine and O’Doherty, 2010, Thorn et al., 2010), and the shift from goal-directed to habitual behavior is mediated in part by dopamine transmission in DS (Packard and White, 1991, Belin and Everitt, 2008). There are conflicting data on the development of habit formation in adolescent vs. adult rats. Adolescent rats demonstrate an inability to adjust responding to changes in contingency, as well as increased habitual behavior in a reinforcer devaluation task (Naneix et al., 2012, Hammerslag and Gulley, 2014). There is evidence for either behavioral rigidity or flexibility in adolescent rats on a set shifting task compared to adults, based on task design and parameters (Leslie et al., 2004, Newman and McGaughy, 2011, Snyder et al., 2014). More complex tasks appear to consistently yield greater levels of flexibility in adolescents. A four-choice reversal task, which requires a greater cognitive load than the standard two-choice set shifting design, revealed greater flexibility in adolescent compared to adult mice (Johnson and Wilbrecht, 2011). In addition, recent data demonstrate that, after learning to withhold an action in the presence of a cue, adolescent rats acquire that cue more quickly as a Pavlovian-conditioned stimulus predictive of reward, as assessed by an increase in reward approach behavior. This suggested that adolescents are able to rapidly adjust the value of a cue that was previous salient (which differs from reversal tasks, which typically involve attributing value to a previously unrewarded cue). A recent experiment in our lab tested this ability to adjust to changes in cue identity further by training rats in a cued instrumental paradigm, during which a 10 s cue (light or tone) was presented, and a nose poke into a lit port resulted in food pellet delivery. No difference in correct responses between adults and adolescents was observed in this task (F(1,12) = .23, p = .64; n = 7/age group; Fig. 2). In the second phase of this experiment, the instrumental cue was shifted in modality to a 10 s Pavlovian cue. After the shift in cue-outcome relationship, adolescents showed a higher percentage of Pavlovian approach during this cue than adults, as assessed by time spent in the food trough during the cue (F(1,12) = 6.96, p = .023; Fig. 2). In a control experiment, adolescent and adult rats acquired Pavlovian approach to a novel cue at an equal rate, indicating that this effect was not related to an age related difference in the general ability to learn or perform Pavlovian conditioning (F(1,12) = .26, p = .62). These data, therefore, indicate that, when a cue acts as either a stop or go signal within an instrumental context, changes in cue-outcome relationships can be flexibly acquired by adolescent rats more quickly than adults. This characteristic of the adolescent brain would allow it to adjust to changes in value of previously salient cues or environments more efficiently than an adult brain. This is an interesting finding because much of the research on adolescents focuses on maladaptive behaviors, whereas behavioral flexibility is generally suggested to be an advantageous characteristic.
Fig. 2.
(A) Adult and adolescent rats learned to perform an instrumental action for reward following cue presentation. (B) The same cue was shifted to a Pavlovian cue, during which reward was no longer contingent on a response, but was always delivered as the cue terminated. Adolescent rats acquired a Pavlovian response to the cue (defined as time spent in the food trough anticipating reward during the cue) more quickly than adults.
The summarized data suggest that adolescent rats may encode relationships between cues and outcomes in which cues were previously meaningful more flexibly than adults (Simon et al., 2013; Fig. 2), or in situations with a higher cognitive load (Johnson and Wilbrecht, 2011). The hyper-responsivity observed in adolescent DMS during reward-related events (Sturman and Moghaddam, 2012) may promote increased ability to alter behavioral strategies (Kimchi and Laubach, 2009). It would be of interest to record from adolescent DLS, which is involved with the learning and expression of habitual behavior, to observe if this region is hypoactive compared to adults. Accelerated habit formation is proposed to promote addiction, as habitual drug seeking behavior is less sensitive to the negative consequences of drug abuse and addiction (Everitt et al., 2008, Hogarth et al., 2013). Thus, ongoing study of the role of the developing DS in habit formation is highly relevant toward the preponderance of adolescent drug addiction.
Both DS and VS are involved with risky decision-making (Cardinal, 2006, Simon et al., 2011, Kohno et al., 2013, Mitchell et al., 2014), defined as a preference for risky over safe rewards. Risky behavior is a hallmark of adolescence, and is linked to drug abuse (Bornovalova et al., 2005, Balogh et al., 2013). Moreover, recent evidence from a rat model of risky decision-making demonstrates that risky behavior in adolescents predicts cocaine self-administration (Mitchell et al., 2014), which may facilitate the drug abuse and addiction vulnerability during adolescence (Adriani and Laviola, 2004, Merline et al., 2004, Doremus-Fitzwater et al., 2010). Reduced dopamine receptor availability in both striatal regions is predictive of higher levels of risky decision-making in rats, and local infusion of selective dopamine agonists either systemically or into adolescent striatum reduces risky behavior (Simon et al., 2011, Mitchell et al., 2014). Accordingly, adolescent rats demonstrate reduced dopamine responsivity and TH expression in DS (Matthews et al., 2013), which may provide a partial mechanism for adolescent risky behavior. Risky decision-making also is associated with neuronal activity and dopamine receptor expression in OFC (Eshel et al., 2007, Van Leijenhorst et al., 2010, Simon et al., 2011, O’Neill and Schultz, 2013). It is possible that the hyperactive reward responses in both OFC and DS (Sturman and Moghaddam, 2011b, Sturman and Moghaddam, 2012) are related to the excessive and occasionally maladaptive risky decision-making during adolescence. Further study of this circuitry could yield interesting data and therapeutic options for the early stages of diseases characterized by risky behavior that manifest during adolescence, including addiction, schizophrenia and depression (Ludewig et al., 2003, Bornovalova et al., 2005, Taylor Tavares et al., 2007).
2.3. Ventral tegmental area
Dopamine neurons, especially those localized in ventral tegmental area (VTA), are involved with reward processing, associative learning, and the pathophysiology of addiction, mood disorders, and schizophrenia (Wise and Bozarth, 1985, Schultz, 1998, Wise, 2004, Sesack and Grace, 2010, Howes et al., 2012). The dopamine system has been implicated in adolescent behavioral and illness vulnerabilities (Luciana et al., 2012, Matthews et al., 2013, Niwa et al., 2013), and aspects of dopamine transmission and VTA activity are different in adults and adolescents (Robinson et al., 2011, McCutcheon et al., 2012, Matthews et al., 2013). In addition, dopamine neurons in VTA project to prefrontal cortex and ventral striatum, regions undergoing development during adolescence. Little is known, however, about how adolescent VTA neurons process reward related events compared to adults. Recent preliminary recording of extracellular activity from VTA neurons in adult and adolescent rats indicates that these neurons have similar basal firing rate and responding to reward related cues (Kim and Moghaddam, 2012), and work is ongoing that will assess adolescent reward processing in this, and other, dopaminergic regions.
2.4. Reward processing circuitry summary
Adolescents demonstrate enhanced impulsive behavior, risk taking, cue salience, drug and reward seeking, and behavioral flexibility compared to adults. As detailed above, single unit electrophysiology revealed age-related differences in reward processing that are likely involved with these behavioral tendencies. Adolescents demonstrate hyper-activation to reward relative to adults in both OFC and DS (Fig. 3). The OFC directly projects to the DS, at least in adult rodents, suggesting that immature OFC-DS connectivity also may contribute to these observed effects (Berendse et al., 1992, Reep et al., 2003). Dopaminergic neurons projecting from the substantia nigra also project to DS (Voorn et al., 2004), and aberrant reward-evoked activity in these neurons may contribute to hyperactive DS reward processing in adolescence. The reduced dopamine efflux observed in DS following amphetamine exposure suggests that these neurons may indeed be hyperactive compared to adults, although further experiments are necessary to confirm this functional difference. Reward-evoked activity in DLS, which receives the strongest dopaminergic input from substantia nigra (Groenewegen, 2003, Voorn et al., 2004), also is likely to differ between adults and adolescents, as the development of behavioral habits varies across the lifespan (Johnson and Wilbrecht, 2011, Newman and McGaughy, 2011, Simon et al., 2013, Snyder et al., 2014).
Fig. 3.
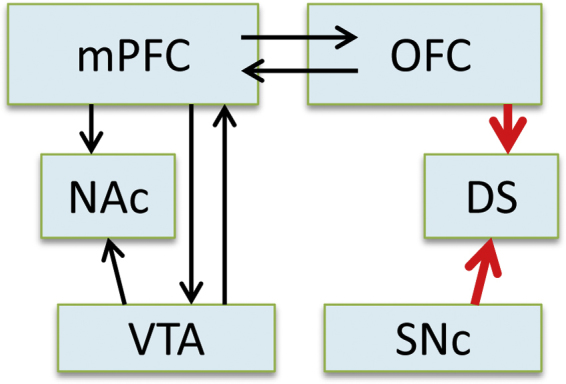
A modified reward circuit for the adolescent brain. Connections of the common “reward circuits” are depicted in black and involve nucleus accumbens (NAc), ventral tegmental area (VTA), and medial prefrontal cortex (mPFC). Our findings in adolescents identify a complementary reward processing pathway depicted in red. We find that dopamine projections to the dorsal striatum (DS), which arise from substantia nigra (SNc) may be hypoactive in adolescents (Matthews et al., 2013) while orbitofrontal cortex (OFC) and DS neurons of adolescents are hyper-responsive to reward compared to adults (Sturman and Moghaddam, 2011a, Sturman and Moghaddam, 2011b, Sturman and Moghaddam, 2012). On the other hand, NAc dopamine release and reward-evoked activity, and baseline firing of dopamine neurons in the ventral tegmental area (VTA) are comparable between adults and adolescents (Kim and Moghaddam, 2012, Matthews et al., 2013).
Interestingly, no substantial age-related differences were observed in NAc reward processing, despite VS being a prominent factor in models of adolescent behavioral vulnerability (Ernst et al., 2009, Geier et al., 2010). This similar neural activity between age groups is consistent with reports of no age related differences in drug-evoked dopamine efflux in NAc (Frantz et al., 2007, Matthews et al., 2013), although studies about dopamine receptor expression in NAc are conflicting (Teicher et al., 1995, Tarazi and Baldessarini, 2000). The lack of differences in NAc reward-processing does not preclude the influence of the developing adolescent NAc on behavioral and psychopathology vulnerabilities; however, the observed differences in motivational processes during adolescence (Spear, 2011) may arise from functional neural activity in DS and PFC regions to a greater extent than NAc. Collectively, these finding suggest that the traditional brain reward circuitry should be modified for adolescents (Fig. 3).
3. Conclusion
The findings reviewed here inform future adolescent research in two ways: (1) baseline activity or response to sensory stimuli such as reward predicting cues are unaffected, or less affected, than neuronal processing around the time of reward. Thus, a focus on reward response may provide the ideal biomarker for early vulnerability to disorders of motivation and affect. (2) Robust neuronal responses were observed in regions that are not typically associated with reward processing in adults. Thus, the dynamic circuitry of motivated behavior may be different than our adult models and involve cortical and basal ganglia regions that are not classically associated with reward processing. Future emphasis on regions such as the DS may greatly enhance our knowledge of this dynamic circuitry and its contribution to disease vulnerability in at-risk individuals.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by DA 035050 (NWS) and MH048404-23 (BM).
Footnotes
Available online 22 November 2014
References
- Adriani W., Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav. Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W., Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav. Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ahn W.-Y., Rass O., Fridberg D.J., Bishara A.J., Forsyth J.K., Breier A., Busemeyer J.R., Hetrick W.P., Bolbecker A.R., O’Donnell B.F. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J. Abnorm. Psychol. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Dumont N.L., Teicher M.H. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Thompson A.T., Rutstein M., Hostetter J.C., Teicher M.H. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andrzejewski M.E., Schochet T.L., Feit E.C., Harris R., Mc Kee B.L., Kelley A.E. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav. Neurosci. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Anker J.J., Perry J.L., Gliddon L.A., Carroll M.E. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol. Biochem. Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., O’Doherty J.P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Delgado M.R., Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Leung B.K., Ostlund S.B. The orbitofrontal cortex, predicted value, and choice. Ann. N. Y. Acad. Sci. 2011;1239:43–50. doi: 10.1111/j.1749-6632.2011.06270.x. [DOI] [PubMed] [Google Scholar]
- Balogh K.N., Mayes L.C., Potenza M.N. Risk-taking and decision-making in youth: relationships to addiction vulnerability. J. Behav. Addict. 2013;2:1–9. doi: 10.1556/JBA.2.2013.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Dolan S., Denburg N., Hindes A., Anderson S.W., Nathan P.E. Decision-making deficits, linked to dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Belin D., Everitt B.J. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berendse H.W., Galis-de Graaf Y., Groenewegen H.J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berlin H.A., Rolls E.T., Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos C.A., Glatt S.J., Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res. Dev. Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bornovalova M.A., Daughters S.B., Hernandez G.D., Richards J.B., Lejuez C.W. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp. Clin. Psychopharmacol. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adoelscent rats, compared to adults. Behav. Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient d1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Dumais K., Andersen S.L. Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;169:628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Bravo-Rivera H., Quirk G.J. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLOS ONE. 2013;8:e57575. doi: 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C.L., Fletcher P.J. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav. Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Cao J., Lotfipour S., Loughlin S.E., Leslie F.M. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2007;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M.M. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat. Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.C., Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioral autonomy. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1985;308:67–78. [Google Scholar]
- Doherty J.M., Cooke B.M., Frantz K.J. A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2013;38:446–454. doi: 10.1038/npp.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Varlinskaya E.I., Spear L.P. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cognit. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Barretto M., Spear L.P. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behav. Neurosci. 2012;126:735–741. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle D.M., Robbins T.W. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav. Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cognit. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol. Biochem. Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Belin D., Economidou D., Pelloux Y., Dalley J.W., Robbins T.W. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. B: Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel S.B., Clark J.J., Robinson T.E., Mayo L., Czuj A., Willuhn I., Akers C.A., Clinton S.M., Phillips P.E.M., Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz K.J., O’Dell L.E., Parsons L.H. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Friemel C.M., Spanagel R., Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Front. Behav. Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. The prefrontal cortex – an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Stout J.C. Neurocognitive insights into substance abuse. Trends Cognit. Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis A.H., Kreitzer A.C. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012;35:557–564. doi: 10.1016/j.tins.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin T.E., Figner B., Crone E.A., Wiers R.W. Addiction, adolescence, and the integration of control and motivation. Dev. Cognit. Neurosci. 2011;1:364–376. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Green L., Fry A.F., Myerson J. Discounting of delayed rewards: a life-span comparison. Psychol. Sci. 1994;5:33–36. [Google Scholar]
- Groenewegen H.J. The basal ganglia and motor control. Neural Plast. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag L.R., Gulley J.M. Age and sex differences in reward behavior in adolescent and adult rats. Dev. Psychobiol. 2014;56:611–621. doi: 10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G., Leung B.K., Balleine B.W. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol. Learn. Mem. 2014;108:104–118. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L., Balleine B.W., Corbit L.H., Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Horga G., Abi-Dargham A. The striatum and dopamine: a crossroad of risk for schizophrenia. JAMA Psychiatry. 2014;71:489–491. doi: 10.1001/jamapsychiatry.2014.191. [DOI] [PubMed] [Google Scholar]
- Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev. Cognit. Neurosci. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Friedel E., Koslowski M., Witthaus H., Ozgurdal S., Gudlowski Y., Knutson B., Wrase J., Brune M., Heinz A., Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley A.E. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Killcross S., Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb. Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kim Y., Moghaddam B. Society for Neuroscience Abstracts 704.11/DDD73. 2012. VTA neural responses are different between adolescent and adult rats during appetitive instrumental learning and extinction. [Google Scholar]
- Kimchi E.Y., Laubach M. Dynamic encoding of action selection by the medial striatum. J. Neurosci. 2009;29:3148–3159. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi E.Y., Torregrossa M.M., Taylor J.R., Laubach M. Neuronal correlates of instrumental learning in the dorsal striatum. J. Neurophysiol. 2009;102:475–489. doi: 10.1152/jn.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M., Ghahremani D.G., Morales A.M., Robertson C.L., Ishibashi K., Morgan A.T., Mandelkern M.A., London E.D. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht218. Advance Access published August 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D., Sanjakdar S., Marks M.J., Khabour O., Alzoubi K., Damaj M.I. Exploring behavioral and molecular mechanisms of nicotine reward in adolescent mice. Biochem. Pharmacol. 2011;82:1008–1014. doi: 10.1016/j.bcp.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A.C., Malenka R.C. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie F.M., Loughlin S.E., Wang R., Perez L., Lotfipour S., Belluzzia J.D. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann. N. Y. Acad. Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- Luciana M., Wahlstrom D., Porter J.N., Collins P.F. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev. Psychol. 2012;48:844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K., Paulus M.P., Vollenweider F.X. Behavioural dysregulation of decision-making in deficit but not nondeficit schizophrenia patients. Psychiatry Res. 2003;119:293–306. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E., Haber S.N. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59:625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Magno E., Foxe J.J., Molholm S., Robertson I.H., Garavan H. The anterior cingulate and error avoidance. J. Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews I.Z., McCormick C.M. Role of medial prefrontal cortex dopamine in age differences in response to amphetamine in rats: locomotor activity after intra-mPFC injections of dopaminergic ligands. Dev. Neurobiol. 2012;72:1415–1421. doi: 10.1002/dneu.22000. [DOI] [PubMed] [Google Scholar]
- Matthews M., Bondi C., Torres G.E., Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38:1344–1351. doi: 10.1038/npp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J.E., Conrad K.L., Carr S.B., Ford K.A., McGehee D.S., Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J. Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I.A., Simon N.W., Hart N., Mitchell M.R., Nation J.R., Wellman P.J., Setlow B. Self-administered cocaine causes lasting increases in impulsive choice in a delay discounting task. Behav. Neurosci. 2010;124:470–477. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline A.C., O’Malley P.M., Schulenberg J.E., Bachman J.G., Johnston L.D. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am. J. Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.R., Potenza M.N. Addictions and personality traits: impulsivity and related constructs. Curr. Behav. Neurosci. Rep. 2014;1:1–12. doi: 10.1007/s40473-013-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.R., Weiss V.G., Beas B.S., Morgan D., Bizon J.L., Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:955–962. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Wood J. Teamwork matters: coordinated neuronal activity in brain systems relevant to psychiatric disorders. JAMA Psychiatry. 2014;71:197–199. doi: 10.1001/jamapsychiatry.2013.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F., Marchand A.R., Di Scala G., Pape J.-R., Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L.A., McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev. Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M., Jaaro-Peled H., Tankou S., Seshadri S., Hikida T., Matsumoto Y., Cascella N.G., Kano S.-i., Ozaki N., Nabeshima T., Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K.A., D’Angelo D., Hoptman M.J. Self-report and laboratory measures of impulsivity in patients with schizophrenia or schizoaffective disorder and healthy controls. Psychiatry Res. 2011;187:301–303. doi: 10.1016/j.psychres.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M., Schultz W. Risk prediction error coding in orbitofrontal neurons. J. Neurosci. 2013;33:15810–15814. doi: 10.1523/JNEUROSCI.4236-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M.G., Knowlton B.J. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard M.G., White N.M. Lesions of the caudate nucleus selectively impair reference memory acquisition in the radial maze. Behav. Neural Biol. 1990;53:39–50. doi: 10.1016/0163-1047(90)90780-a. [DOI] [PubMed] [Google Scholar]
- Packard M.G., White N.M. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav. Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Perry J.L., Nelson S.E., Carroll M.E. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp. Clin. Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Peters J., LaLumiere R.T., Kalivas P.W. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze M., McGarrity S., Mason R., Fone K.C., Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J. Neurosci. 2014;34:7931–7946. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Ragozzino M.E. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. N. Y. Acad. Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Reep R.L., Cheatwood J.L., Corwin J.V. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. Functions of dopamine in the dorsal and ventral striatum. Semin. Neurosci. 1992;4:119–127. [Google Scholar]
- Robbins T.W., Everitt B.J. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson D.L., Zitzman D.L., Smith K.J., Spear L.P. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch M.R., Olson C.R. Neuronal activity in primate orbitofrontal cortex reflects the value of time. J. Neurophysiol. 2005;94:2457–2471. doi: 10.1152/jn.00373.2005. [DOI] [PubMed] [Google Scholar]
- Roesch M.R., Taylor A.R., Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog. Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schochet T.L., Kelley A.E., Landry C.F. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience. 2005;135:285–297. doi: 10.1016/j.neuroscience.2005.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Takahashi Y., Liu T-L., McDannald M.A. Does the orbitofrontal cortex signal value? Ann. N. Y. Acad. Sci. 2011;9:1287–1299. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta N.L., Walker Q.D., Caster J.M., Levin E.D., Kuhn C.M. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berlin) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Grace A.A. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Mendez I.A., Mitchell M.R., Simon N.W. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav. Pharm. 2009;20:380–389. doi: 10.1097/FBP.0b013e3283305eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N.W., Mendez I.A., Setlow B. Cocaine exposure causes long term increases in impulsive choice. Behav. Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N.W., Montgomery K.S., Beas B.S., Mitchell M.R., LaSarge C.L., Mendez I.A., Banuelos C., Vokes C.M., Taylor A.B., Haberman R.P., Bizon J.L., Setlow B. Dopaminergic modulation of risky decision-making. J. Neurosci. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N.W., Gregory T.A., Wood J., Moghaddam B. Differences in response inhibition and behavioral flexibility between adolescent and adult rats. Behav. Neurosci. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K.P., Barry M., Valentino R.J. Cognitive impact of social stress and coping strategy throughout development. Psychopharmacology (Berlin) 2014 doi: 10.1007/s00213-014-3654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cognit. Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag K.C., Brenhouse H.C., Freund N., Thompson B.S., Puhl M., Andersen S.L. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology (Berlin) 2014;231:1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev. Cognit. Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis J., Andersen S. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology. 2014;231:1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci. Biobehav. Rev. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J. Neurosci. 2011;31:1471–1478. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proc. Natl. Acad. Sci. 2012;109:1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Mandell D.R., Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav. Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi F.I., Baldessarini R.J. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int. J. Dev. Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi F.I., Tomasini E.C., Baldessarini R.J. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci. Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares J.V., Clark L., Cannon D.M., Erickson K., Drevets W.C., Sahakian B.J. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol. Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Hostetter J.C., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev. Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Thorn C.A., Atallah H., Howe M., Graybiel A.M. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66:781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol. Scand. Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- van Duuren E., Escamez F.A.N., Joosten R.N.J.M.A., Visser R., Mulder A.B., Pennartz C.M.A. Neural coding of reward magnitude in the orbitofrontal cortex of the rat during a five-odor olfactory discrimination task. Learn Mem. 2007;14:446–456. doi: 10.1101/lm.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Volz T.J., Farnsworth S.J., Rowley S.D., Hanson G.R., Fleckenstein A.E. Age-dependent differences in dopamine transporter and vesicular monoamine transporter-2 function and their implications for methamphetamine neurotoxicity. Synapse. 2009;63:147–151. doi: 10.1002/syn.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P., Vanderschuren L.J., Groenewegen H.J., Robbins T.W., Pennartz C.M. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Walker Q.D., Kuhn C.M. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol. Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Q.D., Morris S.E., Arrant A.E., Nagel J.M., Parylak S., Zhou G., Caster J.M., Kuhn C.M. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J. Pharmacol. Exp. Ther. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann. N. Y. Acad. Sci. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- Winstanley C.A., Zeeb F.D., Bedard A., Fu K., Lai B., Steele C., Wong A.C. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav. Brain Res. 2010;210:263–272. doi: 10.1016/j.bbr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise R.A., Bozarth M.A. Brain mechanisms of drug reward and euphoria. Psychiatric Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Wood J., Kim Y., Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J. Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J., Balleine B.W. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin H.H., Mulcare S.P., Hilario M.R., Clouse E., Holloway T., Davis M.I., Hansson A.C., Lovinger D.M., Costa R.M. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb F., Floresco S., Winstanley C. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology. 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]