Abstract
Diabetes results from a reduction of pancreatic β-cells. Stimulating replication could normalize β-cell mass. However, adult human β-cells are recalcitrant to proliferation. We identified osteoprotegerin, a bone-related decoy receptor, as a β-cell mitogen. Osteoprotegerin was induced by and required for lactogen-mediated rodent β-cell replication. Osteoprotegerin enhanced β-cell proliferation in young, aged, and diabetic mice. This resulted in increased β-cell mass in young mice and significantly delayed hyperglycemia in diabetic mice. Osteoprotegerin stimulated replication of adult human β-cells, without causing dedifferentiation. Mechanistically, osteoprotegerin induced human and rodent β-cell replication by modulating CREB and GSK3 pathways, through binding Receptor Activator of NF-κB (RANK) Ligand (RANKL), a brake in β-cell proliferation. Denosumab, an FDA-approved osteoporosis drug, and RANKL-specific antibody, induced human β-cell proliferation in vitro, and in vivo, in humanized mice. Thus, osteoprotegerin and Denosumab prevent RANKL/RANK interaction to stimulate β-cell replication, highlighting the potential for repurposing an osteoporosis drug to treat diabetes.
Keywords: Denosumab, diabetes, human islet transplant, human pancreatic β-cell proliferation, lactogens, Osteoprotegerin, Receptor activator of NF-κB ligand
INTRODUCTION
Type 1 (T1D) and Type 2 (T2D) diabetes result from a loss of functional pancreatic β-cell mass caused by β-cell death and dysfunction (Padgett et al., 2013; Weir et al., 2013). Patients with long-standing diabetes retain residual β-cells despite this loss (Oram et al., 2014). Therefore, a primary focus for the treatment of diabetes is to normalize β-cell homeostasis by reducing loss, recovering function, and enhancing regeneration of remnant β-cells. There is evidence in rodents that β-cell replication can be induced in response to metabolic demand, such as pregnancy, obesity, or insulin resistance (Dor et al., 2004; Rieck et al., 2010; Sachdeva et al., 2009). This suggests that external stimuli could be used to further induce endogenous β-cell replication. However, the adult human β-cell has a low rate of basal proliferation and is highly refractory to stimulation (Parnaud et al., 2008; Perl et al., 2010).
Multiple studies have demonstrated the ability of lactogenic hormones, prolactin (PRL) and placental lactogen (PL), to enhance rodent β-cell function, proliferation, and survival acting through a common PRL receptor (Guthalu et al., 2010; Vasavada et al., 2006). Transgenic (TG) mice expressing mouse PL-1 (mPL-1) in the β-cell, under the rat insulin promoter (RIP), display hyperinsulinemia, hypoglycemia, β-cell hyperplasia due to increased replication, with a resultant increase in β-cell mass, and resistance to streptozotocin (STZ)-induced diabetes and β-cell death (Fujinaka, et al., 2004; Vasavada et al., 2000). Lactogens protect rodent and human β-cells against cell death inducers relevant to T1D and T2D (Fujinaka et al., 2007; Guthalu, et al., 2012). PRL-R signaling is also required for normal β-cell growth and function in development, and for the adaptive β-cell response to the metabolic demands of pregnancy (Freemark et al., 2002; Huang, et al., 2009). Although lactogens have therapeutic and physiological relevance, how they modulate β-cell proliferation is not fully understood.
To determine the molecular pathways involved in β-cell replication, microarray analysis performed on islets from three distinct models of β-cell expansion, pregnancy, obesity/insulin resistance, and β-cell regeneration, found Osteoprotegerin (OPG) as one of only two common genes upregulated in islets from all three models (Rieck et al., 2009). OPG is expressed in rodent insulinoma cells, in rodent and human islets, and importantly, in human β-cells (Rieck et al., 2009; Kutlu et al., 2009; Schrader, et al., 2007). However, whether OPG is involved in mediating β-cell proliferation is not known.
OPG is an unusual member of the Tumor Necrosis Factor (TNF) Receptor Superfamily (TNFRSF), in that it lacks a transmembrane domain, and hence is a soluble decoy receptor. OPG (TNFRSF11B) is expressed in numerous tissues, but was initially discovered for its role in skeletal metabolism. It inhibits osteoclast differentiation and activation, thereby enhancing bone formation. OPG acts by modulating two specific ligands, Receptor Activator of NF-κB (RANK; TNFRSF11A) ligand (RANKL; TNFSF11) and TNF-related apoptosis-inducing ligand (TRAIL). It binds to them and thus inhibits interactions with their respective receptors, RANK and the death receptor (DR) (Hanada, et al., 2010; Kearns et al., 2008). In vitro competition and functional studies show that the RANKL/RANK pathway is more sensitive to interference from OPG than the TRAIL/DR pathway (Vitovski et al., 2007). Denosumab (DMB), a humanized monoclonal antibody that specifically recognizes human RANKL, acts as a partial functional equivalent of OPG, as it inhibits only the RANKL/RANK and not the TRAIL/DR interaction. DMB is an FDA-approved drug for osteoporosis (Miller et al., 2009).
Using an unbiased analysis we found OPG expression was induced by lactogens in rodent islets and insulinoma cells. We hypothesized that OPG could mediate lactogen-induced β-cell proliferation, and that OPG may directly enhance replication of rodent and human β-cells. Indeed, the current report identifies OPG as a downstream mediator of PRL-induced proliferation in rodent β-cells in vivo. OPG by itself enhances rodent β-cell replication and mass in young mice, and also increases β-cell replication in conditions relevant to diabetes: aged and STZ-treated mice. Importantly, OPG stimulates human β-cell proliferation in vitro. Mechanistically, OPG modulates two proliferative pathways in rodent and human islets; it inhibits glycogen synthase kinase-3 (GSK3) and stimulates cAMP response element-binding protein (CREB). We identified the RANKL/RANK, an OPG target, as a brake for β-cell replication in rodent and human β-cells. DMB, an osteoporosis drug, and a RANKL specific antibody, counteracts this brake to induce human β-cell proliferation in vitro, and more importantly, in vivo in humanized mice. Thus, a pathway originally characterized in bone may have therapeutic potential to normalize β-cell homeostasis in diabetes.
RESULTS AND DISCUSSION
OPG is a target of lactogens in β-cells and is required for lactogen-induced rodent β-cell proliferation
Lactogens are therapeutically and physiologically important for the β-cell (Guthalu et al., 2010; Vasavada et al., 2006). To identify novel downstream targets of lactogens in β-cells, we used PCR-array to examine the differential expression of genes in islets from normal and RIP-mPL1 TG mice. OPG was upregulated in TG versus normal islets by PCR-array; this was confirmed by real-time PCR (Figures S1A-B). As islets are a composite of many cell types, we treated the rodent insulinoma cell line, INS-1, with PRL, to determine if acute lactogen treatment can induce OPG in β-cells. Indeed, OPG mRNA was induced by PRL-treatment in INS-1 cells (Figure S1C). The increase in OPG mRNA expression with lactogens translated to an increase in OPG protein in PRL-treated INS-1 cells and in TG islets (Figureure S1D). To examine our hypothesis that OPG mediates lactogen-induced β-cell proliferation in vivo, whole body OPG knockout (KO) and wild-type (WT) male mice were infused with PRL or saline for 7 days. Although basal β-cell proliferation was similar in saline-treated WT and OPG-KO mice, PRL induced a 2.5-fold increase in β-cell replication in WT but not in OPG-KO mice, compared to saline-treated controls (Figure 1A). This indicates that OPG is required for PRL-stimulated β-cell proliferation in vivo. Taken together, these results uncover OPG as a downstream effector of PRL-induced proliferation in rodent β-cells in vivo, implying that like lactogens, OPG may influence physiological β-cell growth and expansion. In that context, OPG is increased in islets from pregnant mice (Rieck et al., 2009), as well as in the circulation of pregnant women (Hong et al., 2005).
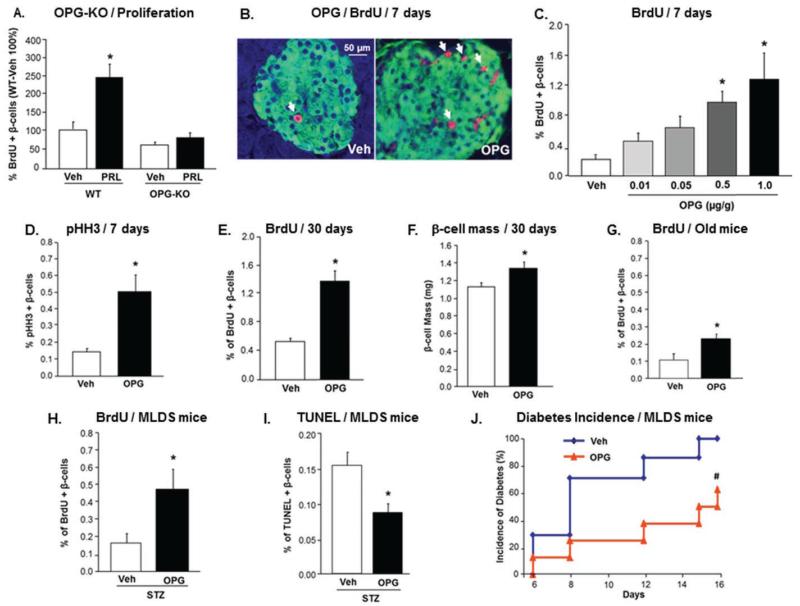
Figure 1. OPG is required for PRL-induced rodent β-cell proliferation, and enhances rodent β-cell replication in young, aged, and STZ-treated mice.
(A) Percent BrdU-positive β-cells in male OPG KO and WT mice infused with Veh or PRL for 7 days. β-cell proliferation in WT-Veh mice is depicted as 100% (n=4-5 mice/group); *p<0.05 vs other three groups; (See also Figure S1). (B) Representative images of pancreatic sections stained for insulin (green) and BrdU (red) from mice injected daily for 7 days with vehicle (Veh) and 1.0μg mOPG-Fc/g body weight. Arrows indicate BrdU- and insulin-positive cells. (C) Percentage of BrdU-positive β-cells in 10-week old mice injected daily for 7 days with Veh or different doses of mOPG-Fc; (n=5-10 mice/group, >1000 β-cells counted/mouse; See also Figure S2). (D) Percentage of pHH3-positive β-cells in mice treated as in (B). (E) Percentage of BrdU-positive β-cells in mice injected every alternate day with Veh or 1.0μg/g mOPG-Fc/g for 30 days; (n=5 mice/group; See also Figure S3). (F) β-cell mass in mice treated as in (E); *p=0.05 vs Veh. (G) Percent BrdU-positive β-cells in one-year old mice injected daily with Veh or 1.0μg/g mOPG-Fc for 7 days (n=6-7 mice/group). (H) Percent BrdU-positive β-cells in MLDS mice injected daily with Veh or 1.0μg/g mOPG-Fc for 16 days (n=7-8 mice/group). (I) Percent TUNEL-positive β-cells in mice treated as in (H). (J) Diabetes incidence, defined as blood glucose >250mg/dl, in mice treated as in (H); #p<0.05 vs Veh by chi-square test. All values are presented as mean ± SEM. *p<0.05 vs Veh, except where specified otherwise.
OPG enhances rodent β-cell proliferation in young, aged, and STZ-treated mice
As OPG is increased in three models of β-cell expansion (Rieck et al., 2009) and was required for lactogen-induced β-cell proliferation (Figure 1A), we tested if OPG can stimulate rodent β-cell replication. C57BL/6 male mice injected daily with mouse recombinant OPG (mOPG-Fc) at 0.01-1.0μg/g body weight for 7 days were assessed for β-cell proliferation by co-staining for insulin and BrdU (Figure 1B). OPG at lower doses (0.01-0.05μg/g) induced a 2-3-fold increase, and at the higher doses (0.5-1.0μg/g) induced a significant 5-6-fold increase in β-cell replication relative to saline-treated mice (Figure 1C). This was validated by staining for an endogenous proliferation marker, phospho-Histone H3 (pHH3), which confirmed the increase in β-cell replication observed with 1.0μg/g of mOPG-Fc (Figure 1D). Body weight, blood glucose, or basal rate of β-cell death did not change in these mice (Figures S2A-C). Glucose homeostasis, as measured by plasma insulin and intraperitoneal glucose tolerance test (IPGTT), significantly improved in mice treated with 1.0μg/g of mOPG-Fc (Figures S2D-F).
To determine whether OPG-induced β-cell proliferation yields increased β-cell mass, C57BL/6 mice were treated every alternate day for 30 days with 1.0μg/g of mOPG-Fc. Indeed, β-cell replication was increased at day 30 (Figure 1E), and there was a significant ~20% increase in β-cell mass (Figure 1F) in mice receiving mOPG-Fc relative to controls. However, body weight, blood glucose, β-cell death, IPGTT and insulin tolerance test (ITT) did not significantly differ between mOPG-Fc-treated versus control mice with the longer treatment (Figure S3A-F).
Thus, OPG induces rodent β-cell replication in vivo in young mice within a span of 1-4 weeks, causing a transient improvement in glucose homeostasis with the shorter treatment. An increase in cell proliferation markers does not necessarily equate to an increase in cell number, depending on whether cells complete the cell cycle or whether there is a concomitant increase in cell death. However, the significant increase in β-cell mass observed with the prolonged treatment together with no increase in β-cell death, indeed suggests that OPG-induced proliferation results in an increase in β-cell number, at least in vivo.
Contrary to our findings, a recent report found that injecting human OPG at low doses (1.0μg/mouse) and infrequent intervals in mice caused islet inflammation and β-cell death, resulting in hypoinsulinemia and hyperglycemia (Toffoli et al., 2011). Injecting human OPG in rats generates antibodies against the human peptide (Mochizuki et al., 2002). Thus, use of species-incompatible OPG, together with the extremely low-dose and intermittent regimen, could likely lead to the negative effects observed in the study (Toffoli et al., 2011). In contrast, studies using two distinct transgenic rodent models of OPG overexpression, with systemic elevation of rodent OPG at levels 5-200-fold over endogenous, did not find changes in blood glucose levels even at one year of age, suggesting that chronic high levels of OPG do not negatively impact glucose homeostasis (Ominsky et al., 2009; Simonet et al., 1997).
A relevant question in the context of diabetes is whether OPG can induce β-cell proliferation under conditions of increased metabolic demand. We tested the effect of OPG: i) in aged mice, known to have insulin resistance and low β-cell replication (Teta et al., 2005); and ii) in the multiple low dose STZ (MLDS)-induced diabetes model, with increased β-cell death and hyperglycemia (Leiter, 1982). One-year old C57BL/6 mice treated with 1.0μg/g of mOPG-Fc for 7 days showed a significant two-fold increase in β-cell proliferation compared to control mice (Figure 1G). Injection of mOPG-Fc in MLDS-treated mice caused a significant increase in β-cell proliferation (Figure 1H), and reduction in β-cell death (Figure 1I) by 16 days, compared to vehicle-injected MLDS mice. Importantly, this resulted in a significant delay in the incidence of diabetes (defined as blood glucose >250mg/dl) (Figure 1J), in OPG-treated versus vehicle-treated MLDS mice. These findings indicate that OPG is beneficial to the β-cell not only in young normal mice, but is efficacious also under conditions of increased metabolic demand.
OPG stimulates rodent and human β-cell proliferation in vitro without causing dedifferentiation
To examine if OPG can directly stimulate β-cell proliferation, mouse islet cells were treated with mOPG-Fc in culture for 24h. OPG increased mouse β-cell replication in vitro (Figure 2A), suggesting a direct effect of OPG on the β-cell. A major challenge in this field is inducing replication of adult human β-cells. Therefore, we tested if human OPG-Fc (hOPG-Fc) could stimulate adult human β-cell proliferation. In fact, 0.05 and 0.1μg/ml of hOPG-Fc caused a significant 3-fold increase in human β-cell replication in vitro by 24h, as assessed by BrdU-insulin co-staining (Figure 2B-C). We verified this by staining for a different proliferation marker, Ki67, which showed a similar 3-fold increase in human β-cell replication with 0.1μg/ml of hOPG-Fc at 24h and 72h (Figure 2D). Inducing human β-cell proliferation in vitro can lead to dedifferentiation of β-cells (Beattie et al., 1999). To determine if OPG affected differentiation of human β-cells, gene expression of hormones, a glucose sensor, and transcription factors (Figure S4A; Table S2), and insulin content (Figure S4B) were measured in human islets treated with hOPG-Fc for 24h. There was no significant change in these parameters in OPG-versus vehicle-treated islets. To further ensure at the cellular level that OPG does not induce dedifferentiation of proliferating cells, OPG-treated human islet cells (Figure S4C) and mouse pancreatic sections (Figure S4E) were co-stained for the proliferation marker Ki67 or BrdU and the β-cell transcription factor Nkx6.1 or Pdx1. Clear co-staining of the proliferation marker and the transcription factor was observed in human and mouse islets (Figure S4C,E). There was a significant increase in the double-positive cells in OPG-treated versus vehicle-treated human and rodent islets (Figure S4D,F).
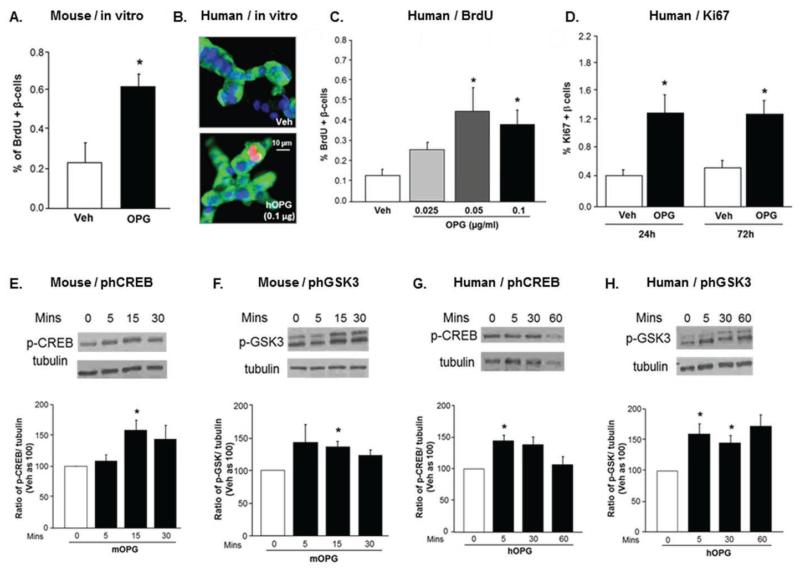
Figure 2. OPG stimulates β-cell proliferation, and increases phosphorylation of CREB and GSK3 in rodent and human islets in vitro.
(A) Percent BrdU-positive β-cells in mouse islet cell cultures treated with Veh or 0.1μg/ml of mOPG-Fc for 24h in the presence of BrdU (n=3). (B) Representative confocal images of human islet cell cultures treated with Veh or 0.1μg/ml of hOPG-Fc and BrdU for 24h, and stained for insulin (green), BrdU (red), and DAPI (blue). (C) Percent BrdU-positive human β-cells treated with Veh or different doses of hOPG-Fc for 24 or 72h; (n=4-6 human islet preps; See also Figure S4). (D) Percent Ki67-positive human β-cells as treated in (C). (E) Representative western blot analysis and quantitation of phCREB/tubulin ratio in mouse islets treated with Veh or mOPG-Fc for varying times. (F) Analysis as in (E) of phGSK3/tubulin ratio. (G) Representative western blot analysis and quantitation of phCREB/tubulin ratio in human islets treated with Veh or hOPG-Fc for varying times. (H) Analysis as in (G) of phGSK3/tubulin ratio. (n=3-6 islet preps). All values are presented as mean ± SEM. *p<0.05 vs Veh.
A major limitation and therefore a focus in diabetes research, is to find ways to normalize human β-cell homeostasis under conditions of stress. This study ascribes OPG, originally described in bone, with a role in stimulating human β-cell proliferation. The recalcitrance of adult human β-cells to proliferate is well known (Parnaud et al., 2008; Perl et al., 2010). The average age of the human islet donors for these studies was 46±2.5 years, ranging from 18-62 years (Table S1), indicating that OPG can stimulate replication of adult human β-cells. An important question in this field is whether a 2-3-fold increase in the very low basal level of human β-cell proliferation is sufficient to cause a clinically meaningful difference in β-cell mass. In rodents, a comparable increase in β-cell proliferation leads to measurable increase in β-cell mass within a few weeks (Figure 1F; Williams et al., 2011). Since OPG-induced β-cell proliferation did not cause β-cell dedifferentiation in human islets, this suggests that a consistent but low increase in human β-cell replication may be adequate to restore sufficient functional β-cell mass over time.
OPG increases phosphorylation of CREB and GSK3 in rodent and human islets
To investigate the signaling pathways through which OPG could mediate its proliferative effects in β-cells, mouse and human islets were treated for varying times with species-compatible OPG-Fc and assessed by western blot analysis for modulation of signaling pathways known to be important in β-cell proliferation (Kulkarni et al., 2012). OPG did not affect the phosphorylation of extracellular-signal-regulated kinases 1/2 (ERK1/2; not shown), but significantly enhanced phosphorylation of CREB and GSK3 in rodent islets (Figure 2E-F) and in human islets (Figure 2G-H). Thus, OPG induced phosphorylation of two pathways previously shown to be important regulators of β-cell proliferation (Hussain et al., 2005; Kulkarni et al., 2012; Liu et al., 2009). OPG inactivated the negative-regulator GSK3, and activated the positive-regulator CREB in both rodent and human islets.
OPG induces human β-cell proliferation by interfering with RANKL/RANK, a brake for β-cell replication
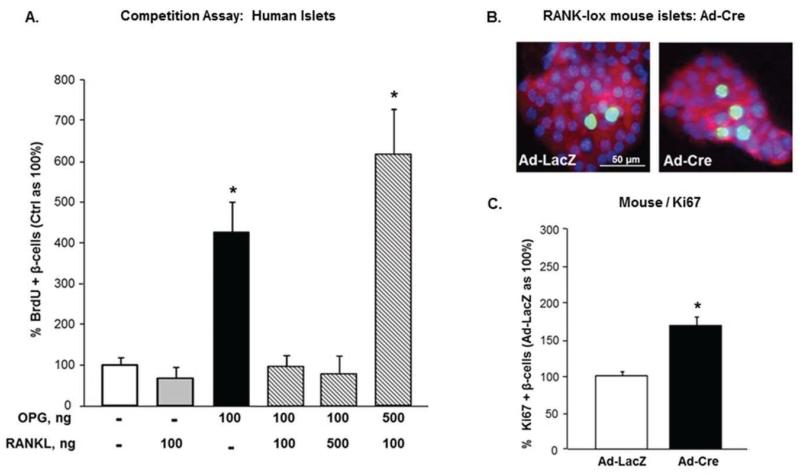
We hypothesized that OPG induces β-cell replication through inhibiting the RANKL/RANK pathway, as it is more sensitive to inhibition by OPG than TRAIL/DR interactions (Vitovski et al., 2007). The receptor for this pathway, RANK, and RANKL are expressed in human islets and human β-cells (Kutlu et al., 2009; Schrader, et al., 2007). We used a competition assay to assess the role of the RANKL/RANK pathway in OPG-induced human β-cell proliferation. Human islet cell cultures were treated with vehicle, 0.1μg/ml of either hOPG-Fc, or human RANKL (hRANKL), or the combination of the two peptides at a 1:1 ratio or an excess of each peptide at a 5:1 ratio, and β-cell replication quantified after 24h. As expected, hOPG-Fc alone stimulated human β-cell proliferation; whereas hRANKL alone did not further decrease the low rate of basal human β-cell replication (Figure 3A). The combination of hRANKL/hOPG-Fc at a 1:1 or 5:1 ratio completely blocked OPG-induced human β-cell replication (Figure 3A); however, increasing OPG concentration 5-fold, reversed the inhibition by RANKL, implying that OPG induces human β-cell proliferation by interrupting RANKL/RANK interaction. If RANKL/RANK is indeed a brake for β-cell proliferation, we hypothesized that deletion of RANK will induce β-cell replication. To examine this, we genetically deleted RANK by transducing islet cells from RANK-lox mice with adenovirus (Ad)-Cre recombinase, or Ad-LacZ as control, and measured β-cell proliferation. Cre-transduced islets displayed a significant two-fold increase in β-cell replication compared to LacZ-transduced islets (Figures 3B-C) indicating that deletion of RANK induces β-cell proliferation. Thus, using two complementary approaches, competition assay and genetic deletion, we demonstrate that the RANKL/RANK pathway is a brake for β-cell proliferation in rodent and human islets, which can be overcome by OPG.
Figure 3. OPG induces human β-cell proliferation by interfering with RANKL/RANK, which acts as a brake in β-cell replication.
(A) Competition assay in which human islet cell cultures treated with either Veh (−), 0.1μg/ml of hOPG-Fc, or hRANKL, or the combination of hRANKL/hOPG-Fc at a 1:1, 1:5, or 5:1 ratio, and BrdU for 24h, were assessed for percent BrdU-positive β-cells (Veh as 100%; n=3-8 human islet preps); *p<0.05 vs the four remaining groups. (B) Representative images of islet cell cultures from RANK-lox mice transduced with Ad-LacZ or Ad-Cre for 72h, and stained for insulin (red), Ki67 (green), and DAPI (blue). (C) Percent Ki67-positive β-cells in Ad-LacZ (as 100%) and Ad-Cre transduced RANK-lox islet cells (n=3); *p<0.05 vs Ad-LacZ. All values are presented as mean ± SEM.
FDA-approved osteoporosis drug, Denosumab (DMB), increases human β-cell proliferation in vitro and in vivo
If RANKL/RANK is a negative modulator of human β-cell replication then DMB, an osteoporosis drug that specifically inhibits hRANKL (Miller et al., 2009), should stimulate human β-cell replication. Indeed, DMB significantly increased human β-cell proliferation in vitro as assessed by two different methods: BrdU-insulin (Figure 4A), and Ki67-insulin (Figures 4B-C) co-staining at 24h and 72h. This further validates the RANKL/RANK pathway as a brake for human β-cell proliferation.
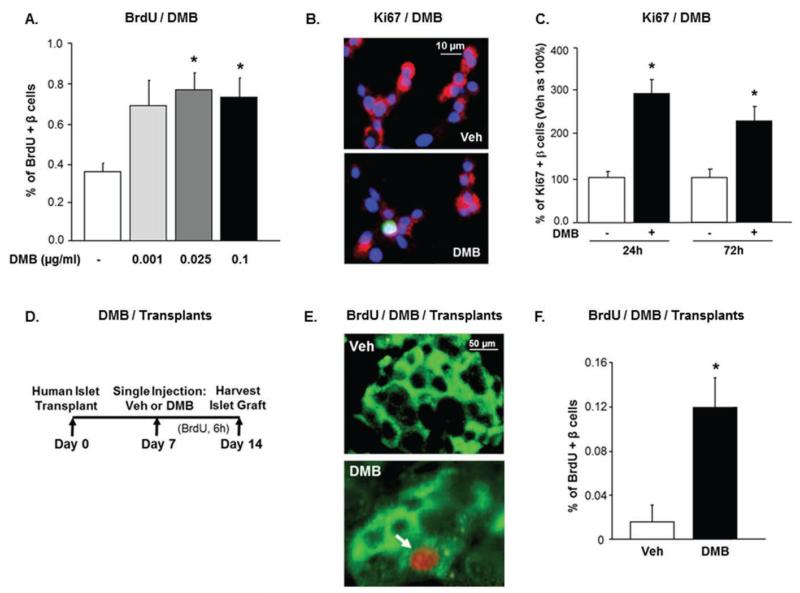
Figure 4. DMB increases human β-cell proliferation in vitro and in vivo.
(A) Percent BrdU-positive β-cells in human islet cell cultures treated with Veh (−) or different concentrations of DMB, and BrdU for 24h. (B) Human islet cell cultures treated with Veh or 0.1μg/ml of DMB for 24h, and stained for insulin (red), Ki67 (green), and DAPI (blue). (C) Percent Ki67-positive human β-cells treated with Veh (−) (as 100%) or 0.1μg/ml of DMB for 24 or 72h; (n=3-5 human islet preps). (D) Experimental design of human islets transplanted under the kidney capsule of male euglycemic NOD/SCID mice. (E) Representative images of human islet grafts in the kidney capsule of Veh or DMB (1μg/g) treated mice stained for insulin (green) and BrdU (red). Arrow represents BrdU-positive β-cell. (F) Percent BrdU-positive β-cells in human islet grafts of Veh and DMB-treated mice (n=5 human islet preps; 1427±121 β-cells counted/prep). All values are presented as mean ± SEM. *p<0.05 vs Veh.
An essential aspect in the treatment of diabetes is the ability to stimulate adult human β-cell replication in vivo. To test whether DMB can achieve this, human islets were transplanted under the kidney capsule of euglycemic immunodeficient non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. One week post-transplant the mice were given a single injection of either vehicle or 1μg/g of DMB. The DMB regimen was based on the dose used in humans and in humanized RANKL mice to treat osteoporosis (Kostenuik et al., 2009; Miller et al., 2009). The kidney bearing the human islet graft was harvested a week later (Figure 4D), to assess human β-cell death and proliferation. β-cell death was negligible in the islet grafts of vehicle- or DMB-treated mice (not shown). Human β-cell replication measured by BrdU-insulin co-staining (Figure 4E) was absent or extremely low in the islet graft of vehicle-treated mice. In contrast, DMB significantly increased human β-cell proliferation in the human islet grafts in vivo (Figure 4F) in a humanized mouse model, validating our observations that the RANKL/RANK pathway is a brake for human β-cell replication. It is possible that injecting DMB earlier at the time of islet transplant, or prolonging the treatment beyond seven days, may further enhance β-cell proliferation in the islet graft. Future studies will determine if either hOPG-Fc or DMB will have beneficial effects on human islets transplanted into diabetic mice to normalize hyperglycemia.
A recent study in postmenopausal women analyzing glucose homeostasis and diabetes incidence in post hoc analyses of randomized, placebo-controlled trials in women taking DMB versus placebo for three years did not find any significant changes in these parameters (Schwartz et al., 2013). However, whether DMB will have any effect on glucose homeostasis in patients with diabetes has not been examined. In a prospective population-based study, high serum concentration of soluble RANKL was a significant and independent risk predictor of T2D, whereas increase in circulating OPG which occurred later was unrelated to T2D risk (Kiechl et al., 2013).
These findings imply that inhibiting the RANKL/RANK pathway can have therapeutic potential for the β-cell. This raises an important issue regarding the specificity of targeting this pathway in the β-cell. The RANKL/RANK pathway has roles in many tissues, including bone (Hanada, et al., 2010; Kearns et al., 2008). Therefore, systemic inhibition of this pathway through OPG or DMB could likely enhance bone formation. There is evidence that patients with T1D and T2D, including children, have an increase in the incidence of bone fractures due to decreased bone strength (Isidro et al., 2010; Saito, et al., 2014). In that case, the off-target effect of these molecules on bone may actually be beneficial. A recent report found that treatment with OPG, or genetic disruption of the RANKL/RANK pathway, led to improved insulin sensitivity in the insulin-resistant ob/ob mouse, indicating an advantageous off-target effect of OPG in the context of diabetes (Kiechl et al., 2013). Importantly, long-term increase in systemic OPG levels in transgenic rodents did not affect immune or mammary gland function, two other known targets of RANKL action (Kearns et al., 2008). Of clinical relevance, DMB is currently an osteoporosis drug, a testament to its relative safety, despite its multi-organ actions.
The current study uncovers a link in gene expression and function between OPG and lactogen signaling in the β-cell, implying that OPG, like lactogens, is important in β-cell physiology. It identifies RANKL/RANK as a brake in β-cell replication. OPG and DMB counteract this brake to stimulate human β-cell proliferation, indicating that they could regulate β-cell homeostasis under stress. The proliferative effect of DMB on human β-cells in vivo suggests that there is potential for repurposing this osteoporosis drug for diabetes.
EXPERIMENTAL PROCEDURES
Peptides
Recombinant (r) hOPG-Fc and rmOPG-Fc were procured from R&D Systems (Minneapolis, MN); rhRANKL from Enzo Life Sciences (Farmingdale, NY); Denosumab (Prolia) from Amgen (Thousand Oaks, CA); and ovine PRL from Sigma (St. Louis, MO). The vehicle to reconstitute these peptides was PBS with 0.1%BSA, saline, or DMB vehicle (Prolia).
Treatment in mice
C57BL/6 8-10 weeks old or one-year old male mice (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously (sc) with saline or different doses (0.01-1.0 g/g body weight) of mOPG-Fc, either daily for 7 days, or every alternate day for 30 days. In the MLDS model, 10-week old C57BL/6 male mice injected daily for 5 days with 50mg/kg of STZ, were administered vehicle or mOPG-Fc (1.0μg/g body weight) daily for 16 days, with blood glucose measured every alternate day. 12-14 week old male OPG-KO (Jackson Labs) and WT littermates were infused with either saline or oPRL (0.175μg/h) for 7 days using Alzet micro-osmotic pumps (DURECT Corporation, Cupertino, CA) implanted sc between scapulae. Pancreata were harvested 6h after an intraperitoneal BrdU (50μg/g, Sigma) injection. All animal studies were performed with the approval of, and in accordance with, guidelines established by the University of Pittsburgh, and the Icahn School of Medicine at Mount Sinai, and principles of laboratory animal care were followed.
Glucose homeostasis
Body weight and blood glucose were measured twice a week; IPGTT was done on days 5 and 25; and ITT was done on day 21. Blood glucose was measured on tail snips using a portable glucometer (Alphatrak, Alameda, CA). IPGTT was performed in mice fasted for 16-18h injected with 2g glucose/kg body weight. ITT was performed in mice injected sc with 1.5U/kg of human insulin, humulin (Lilly, Indianapolis, IN). Plasma insulin was measured on blood drawn at the end of the study, using an insulin ELISA kit (Mercodia, Uppsala, Sweden (Williams et al., 2011).
INS-1 cells; Islets; Ad-transduction
Rat insulinoma cell line INS-1 was a generous gift from Dr. Doris Stoffers (University of Pennsylvania School of Medicine) and cultured as previously indicated (Fujinaka et al., 2007). Human islets were obtained from the Integrated Islet Distribution Program (IIDP; Table S1). Mouse islets were isolated by collagenase digestion and histopaque gradient separation. Islets were kept in complete media (RPMI with 5.5mM glucose, 10% fetal calf serum, and 1% penicillin-streptomycin) for at least 24h, before they were hand-picked under the microscope for treatment and analysis. Mouse islets were isolated from 5-6 month old RIP-mPL1 TG and normal littermates for gene expression, 8-20 week old C57BL/6 or CD1 mice for signaling pathway analysis, 8-10 week and one year old C57BL/6 male mice, and 8-12 week old RANK-lox mice for β-cell proliferation analysis. Recombinant Ad were generated and purified as described earlier. Trypsinized RANK-lox islet cell cultures were transduced with Ad-LacZ or Ad-Cre recombinase (Gene Transfer Vector Core, Iowa City, IA) at a multiplicity of infection of 100 in a volume of 50μl of complete media for 2h, after which 1ml of media was added. After 72h cells were fixed in 2% paraformaldehyde for 30min (Guthalu et al., 2012; Williams et al., 2011).
β-cell histomorphometry and immunostaining
Pancreata were weighed, fixed in Bouin’s (Sigma, St Louis, MO), and paraffin embedded. Histomorphometry for β-cell mass was performed in a blinded way on 5-7 insulin-stained pancreatic sections per animal separated by 50μm each, using the Optimas software package. β-cell mass was quantified per animal as the ratio of the insulin-positive to total pancreatic area, multiplied by the pancreas weight and averaged for all the sections/mouse. β-cell proliferation was quantified as percentage of BrdU-insulin or pHH3-insulin to total insulin-positive cells. Pancreatic sections were stained with antibodies against insulin (Dako) and BrdU (1:200 dilution, Abcam, Cambridge, MA) or pHH3 (1:500, Millipore, Billerica, MA), using an immunofluorescence secondary antibody (Williams et al., 2011). Mouse pancreatic sections were co-stained for BrdU and Pdx1 (1:300, EMD Millipore), and DAPI. β-cell death was quantified as percentage of TUNEL-insulin to total insulin-positive cells on pancreatic sections stained with antibodies against insulin and TUNEL (Promega, Indianapolis, IN) (Williams et al., 2011). In human islet transplant studies, mice were injected with BrdU on day 14, and 6h later the kidney with the islet graft was harvested, fixed in Bouins, and β-cell proliferation was analyzed on at least 3-4 BrdU-insulin co-stained sections/islet graft, and β-cell death on TUNEL-insulin co-stained sections.
For in vitro studies mouse and human islets were trypsinized and cultured on glass coverslips in complete media for 24h. Subsequently the cells were cultured in serum-free medium containing either vehicle or species-compatible OPG-Fc, or DMB at specific concentrations, with BrdU (10μg/ml) added in the last 24h of treatment, after which they were fixed in 2% paraformaldehyde for 30min. β-cell proliferation was quantified using either BrdU or Ki-67 co-staining with insulin (Guthalu et al., 2010a). Human islet cells were co-stained with Ki67 (1:300, Thermo Scientific, Somerset, NJ) and Nkx6.1 (1:300, DSHB at the University of Iowa, Iowa City, IA) and DAPI.
mRNA and protein analysis
Gene expression in RIP-mPL1 TG and normal islets incubated in serum-free medium for 24h, in INS-1 cells treated with 0.2μg/ml of PRL or vehicle in serum-free medium for 24h, and human islets treated with vehicle or 0.1μg/ml of hOPG-Fc in serum-free medium for 24h, was analyzed by PCR-array (apoptosis for mouse, SA Biosciences, Valencia, CA), or by real time PCR (ABI 7300; Life Technologies, Carlsbad, CA), after mRNA isolation and cDNA synthesis using specific primers indicated in Table S2. Western blot analysis of islet extracts (20-30μg) from RIP-mPL1 TG and normal islets, and INS1 cells treated with PRL or vehicle in serum-free medium for 24h, was done using antibodies to OPG (Santa Cruz Biotech, Dallas, TX), and tubulin (EMD Millipore-Calbiochem, Billerica, MA). For analysis of cell signaling, protein extracts (15-20μg) from mouse or human islets pre-treated overnight in serum-free medium and subsequently treated with species-compatible OPG-Fc (0.2μg/ml) for varying times were analyzed for phospho-CREB, phospho-GSK3α/β, phospho-ERK1/2 (Cell Signaling, Beverly, MA), and tubulin by western blot analysis. Quantitative densitometry of digitalized blots was performed using the Image J program (National Institute of Health) (Williams et al., 2011). Insulin content in human islets treated with vehicle or 0.1μg/ml of hOPG-Fc in serum-free medium for 24h was analyzed on acid-ethanol extracts by RIA for human insulin (Linco Research, St. Louis, MO).
Human islet transplants
Two 8-12 week old male euglycemic NOD/SCID mice (Jackson Labs) were transplanted under the kidney capsule with 1000 islet equivalents (IEQ; 1 IEQ=125μm diameter) of each human islet prep (Rao et al., 2005). Mice were injected sc with vehicle or DMB (1μg/g body weight) at day 7 post-transplant. On day 14 the kidney with the islet graft was harvested for β-cell proliferation and β-cell death analysis. Ages of donors of human islets range from 46-61 years (Table S1).
Statistical analysis
Data are expressed as means ± SEM. Statistical significance was considered at p≤0.05, determined by unpaired two-tailed Student’s t-test for comparison between two groups; by One-way Analysis of Variance (ANOVA) with Tukey’s post-hoc HSD (http://statistica.mooo.com) for comparison between more than two groups, and by chi-square test for diabetes incidence.
Supplementary Material
HIGHLIGHTS.
RANKL/RANK is a brake for rodent and human β-cell proliferation
Osteoprotegerin induces human β-cell replication by inhibiting RANKL
FDA-approved osteoporosis drug Denosumab enhances human β-cell replication in vivo
Osteoprotegerin is required for lactogen-induced rodent β-cell proliferation
ACKNOWLEDGEMENTS
We are grateful to Drs. A. Stewart, D. Scott, and N. Taesch, for their critical input; to Dr. D. Scott for manuscript editing; to C. Chin, T. Buch, A. Amlani, A. Otero, and Dr. J F López Acosta for experimental assistance; to the NIDDK-supported Integrated Islet Distribution Program (IIDP) for providing human islets; and to the microscopy cores at the University of Pittsburgh and the Icahn School of Medicine at Mount Sinai for their assistance and use of their facilities.
FUNDING: J.P. is supported by advanced ERC grant and Innovator award by Era of Hope/DoD. This work was supported by grants from the National Institutes of Health (R01DK072264; DK102893) and the Juvenile Diabetes Research Foundation (17-2012-37) to R. C. Vasavada.
ABBREVIATIONS
- Ad
adenovirus
- CREB
cAMP response element-binding protein
- DMB
Denosumab
- DR
death receptor
- GSK3
glycogen synthase kinase-3
- hOPG
human Osteoprotegerin
- IPGTT
intraperitoneal glucose tolerance test
- ITT
insulin tolerance test
- KO
knockout
- MLDS
multiple low dose streptozotocin
- mOPG
mouse Osteoprotegerin
- mPL
mouse Placental lactogen
- NOD/SCID
non-obese diabetic/severe combined immunodeficient
- pHH3
phospho-Histone H3
- PRL
Prolactin
- RANK
Receptor activator of NF-κB
- RANKL
Receptor activator of NF-κB ligand
- RIP
rat insulin promoter
- sc
subcutaneously
- STZ
streptozotocin
- TNF
Tumor Necrosis Factor
- TNFRSF
Tumor Necrosis Factor Receptor Superfamily
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TG
transgenic
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- Veh
vehicle
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DUALITY OF INTEREST: R.C.V. and N.G.K. are named inventors on a patent application involving OPG and the β-cell.
AUTHOR CONTRIBUTIONS: N.G.K. designed experiments, researched/analyzed data, wrote/reviewed/edited manuscript; R.F., I.P., researched/analyzed data, reviewed/edited manuscript; A.G.O. contributed to discussion, reviewed/edited manuscript; M.O., J.P. contributed RANK-lox mice, reviewed/edited manuscript; and R.C.V. conceptualized/designed experiments, researched/analyzed data, wrote/reviewed/edited manuscript.
REFERENCES
- Beattie GM, Itkin-Ansari P, Cirulli V, Leibowitz G, Lopez AD, Bossie S, Mally MI, Levine F, Hayek A. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes. 1999;48:1013–1019. doi: 10.2337/diabetes.48.5.1013. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Sipula D, Garcia-Ocaña A, Vasavada RC. Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic beta cell survival. Diabetes. 2004;53:3120–3130. doi: 10.2337/diabetes.53.12.3120. [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- Guthalu NK, Mozar A, Otero A, Chin C, Garcia-Ocaña A, Vasavada RC. Lactogens protect rodent and human beta cells against glucolipotoxicity-induced cell death through Jak2/Stat5 signaling. Diabetologia. 2012;55:1721–1732. doi: 10.1007/s00125-012-2501-9. [DOI] [PubMed] [Google Scholar]
- Guthalu NK, Zhang XY, Williams K, Mozar A, Vasavada RC. Growth factor mediated regulation of beta cell survival. The Open Endocrine J. 2010;4:80–95. [Google Scholar]
- Hanada R, Hanada T, Penninger JM. Physiology and pathophysiology of the RANKL/RANK system. Biol Chem. 2010;391:1365–1370. doi: 10.1515/BC.2010.149. [DOI] [PubMed] [Google Scholar]
- Hong JS, Santolaya-Forgas J, Romero R, Espinoza J, Gonçalves LF, Kim YM, Edwin S, Yoon BH, Nien JK, Hassan S, Mazor M. Maternal plasma osteoprotegerin concentration in normal pregnancy. Am J Obstet Gynecol. 2005;193:1011–1015. doi: 10.1016/j.ajog.2005.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, Wondisford FE. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidro ML, Ruano B. Bone disease in diabetes. Curr Diabetes Rev. 2010;6:144–155. doi: 10.2174/157339910791162970. [DOI] [PubMed] [Google Scholar]
- Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, Moschen AR, Muscogiuri G, Sorice GP, Kireva T, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu B, Burdick D, Baxter D, Rasschaert J, Flamez D, Eizirik DL, Welsh N, Goodman N, Hood L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics. 2009;2:3. doi: 10.1186/1755-8794-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA. 1982;79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA, McDaniel ML. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58:663–672. doi: 10.2337/db07-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PD. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7:18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Fujise N, Higashio K, Tsuda E. Osteoclastogenesis inhibitory factor/osteoprotegerin ameliorates the decrease in both bone mineral density and bone strength in immobilized rats. J Bone Miner Metab. 2002;20:14–20. doi: 10.1007/s774-002-8441-x. [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Stolina M, Li X, Corbin TJ, Asuncion FJ, Barrero M, Niu QT, Dwyer D, Adamu S, Warmington KS, et al. One year of transgenic overexpression of osteoprotegerin in rats suppressed bone resorption and increased vertebral bone volume, density, and strength. J Bone Miner Res. 2009;24:1234–1246. doi: 10.1359/jbmr.090215. [DOI] [PubMed] [Google Scholar]
- Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, Tisdale JF. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Roccisana J, Takane KK, Bottino R, Zhao A, Trucco M, García-Ocaña A. Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic SCID mice. Diabetes. 2005;54:1664–1675. doi: 10.2337/diabetes.54.6.1664. [DOI] [PubMed] [Google Scholar]
- Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva MM, Stoffers DA. Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol. 2009;23:747–758. doi: 10.1210/me.2008-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Kida Y, Kato S, Marumo K. Diabetes, collagen, and bone quality. Curr Osteoporos Rep. 2014;12:181–188. doi: 10.1007/s11914-014-0202-7. [DOI] [PubMed] [Google Scholar]
- Schrader J, Rennekamp W, Niebergall U, Schoppet M, Jahr H, Brendel MD, Hörsch D, Hofbauer LC. Cytokine-induced osteoprotegerin expression protects pancreatic beta cells through p38 mitogen-activated protein kinase signalling against cell death. Diabetologia. 2007;50:1243–1247. doi: 10.1007/s00125-007-0672-6. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Schafer AL, Grey A, Vittinghoff E, Palermo L, Lui LY, Wallace RB, Cummings SR, Black DM, Bauer DC, et al. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J Bone Miner Res. 2013;28:1348–1354. doi: 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2267. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- Toffoli B, Bernardi S, Candido R, Sabato N, Carretta R, Corallini F, Secchiero P, Zauli G, Fabris B. Osteoprotegerin induces morphological and functional alterations in mouse pancreatic islets. Mol Cell Endocrinol. 2011;331:136–142. doi: 10.1016/j.mce.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch NM, Cozar I, Garcia-Ocaña A. Growth factors and pancreatic beta cell proliferation. International J of Cell Biol and Biochem. 2006;38:931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Vitovski S, Phillips JS, Sayers J, Croucher PI. Investigating the interaction between osteoprotegerin and receptor activator of NF-kappaB or tumor necrosis factor-related apoptosis-inducing ligand: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J Biol Chem. 2007;282:31601–31609. doi: 10.1074/jbc.M706078200. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Abanquah D, Joshi-Gokhale S, Otero A, Lin H, Guthalu NK, Zhang XY, Mozar A, Bisello A, Stewart AF, et al. Systemic and acute administration of parathyroid hormone-related peptide 1-36 stimulates endogenous beta cell proliferation while preserving function in adult mice. Diabetologia. 2011;54:2867–2877. doi: 10.1007/s00125-011-2260-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.