A PARADIGM SHIFT TO LONG-TERM OUTCOMES
We propose in this chapter a paradigm shift from a focus on short-term outcomes to long-term benefits after regional anesthesia. In the context of new models of health care delivery,1 we discuss the clinical evidence favoring regional anesthesia for long-term patient-centered outcomes.2 We define these as beneficial patient-centered outcomes that are the result of providing local or regional anesthesia and that persist for several months after the surgical intervention.
Bundled Capitated Payments Change the Way We Practice
Dramatic changes are underway to contain rising healthcare costs, which currently consume about one-fifth of the gross domestic product of the United States.1 In this changing healthcare system, anesthesiologists are confronted with a shift from fee-for-service to bundled capitated payments; anesthesia providers can no longer bill the insurance company directly for individual services.3 Instead, lump payouts go to the institution. Providing additional services, such as a regional block, does not increase these capitated payments. The payments are to be shared between many providers including anesthesiologists, surgeons, internists, administrators, rehabilitation specialists, and nurses, all working under the same roof and competing for their fair share of the reimbursement. Regional anesthesia services require additional resources and training, and may work best in dedicated systems such as orthopedic hospitals or specialized ambulatory facilities.4 However, we will increasingly have to justify to all stakeholders, even more than we do already, the additional manpower, resources, and time needed for regional anesthesia. Other stakeholders have their own interests. They include:
Providers (surgeons)
Payers (inside and outside our institution)
Patients (and their relatives)
The public/regulators (especially Congress)
Beyond the well-researched and -documented short-term benefits of regional anesthesia, we need to convince everyone of the sustained and meaningful difference that extended perioperative nociceptive blockade can make in the lives of our patients long after surgery. To be effective patient advocates, we must support our arguments with the best available clinical evidence.5
Patient-centered Sustained Outcomes Will Dominate Resource Allocation
Abstract, population-based average effects or biomarkers as evidence of meaningful improvement in care are unconvincing in this day and age. Outcomes suitable as arguments for the sustained value of regional anesthesia should instead be patient centered.2 Patient preferences, shared decision-making, and individualized tailored care are the hallmarks of this new paradigm in outcomes research, differentiating it from prior concepts of comparative effectiveness research. Much needs to be done to define and investigate patient-centered outcomes in anesthesiology and pain medicine, especially long-term outcomes.6 Pay for performance is another emerging concept, forcing us to emphasize our unique contribution to the quality of patient outcomes.7 What is the added value that anesthesiologists providing patients regional anesthesia contribute in the long run in the perioperative surgical home, where these anesthesiology subspecialists serve as the shepherds guiding the individualized perioperative recovery process?
Pain, Function, and Cognition as Cornerstones of Meaningful Long-term Recovery
In this chapter, we examine the clinical evidence suggesting that regional anesthesia has meaningful benefits for our patients and society beyond the immediate perioperative period. While there are several other outcomes of interest, such as morbidity and mortality or cancer recurrence, we focus on three long-term outcomes after elective surgery based on their particular importance:
Persistent pain
Joint function
Cognitive outcomes
We also selected these outcomes because their impact and significance are easy to convey to any interlocutor—surgical colleague, lay person, hospital administrator, or politician—regardless of their prior training or experience.
FOCUS 1: REGIONAL ANESTHESIA FOR THE PREVENTION OF PERSISTENT PAIN AFTER SURGERY
Case in Point
Your patient, an otherwise healthy 58-year-old woman, is about to undergo a right mastectomy for cancer. She is asking you what are the respective benefits of general and regional anesthesia. She is very adamant about being asleep and not knowing what happens during surgery, but seems open to the idea of an epidural or a paravertebral block to decrease the pain after surgery. What do you tell her?
Impact
Persistent pain (beyond six months after surgery) is a neglected, yet often severe and surprisingly frequent condition, as shown in Table 1, which details the risk after several types of surgical intervention. There are few effective treatment options to date.8 Prevention is therefore paramount (Supplemental Digital Content 1, http://links.lww.com/ASA/A567). Mild chronic pain can significantly diminish quality of life and impair daily functioning10; intractable chronic pain can be devastating to both patient and family. Between 25 and 40% of patients undergoing thoracotomy, amputation, or breast surgery continues to suffer from persistent pain for months afterward.11 Even for procedures with a lower risk of chronic postsurgical pain, such as hernia repair or cesarean section, prevention becomes important in light of their increasing frequency.12 Some 5% of patients suffer from persistent pain after minor surgery, and around 40% of patients after limb amputation or thoracotomy.13 About 10% of patients develop persistent pain after a cesarean section.12
Table 1.
Risk for Persistent Postoperative Pain (PPP) after Various Types of Surgery with Estimated Surgical Volumes
| Type of Surgery | Risk of PPP* | Risk of Severe PPP* | Surgical Volume† (cases/year in the US in 2003) |
|---|---|---|---|
| Amputation | 40% | 10% | ~130,000 |
| Breast surgery | 25% | 10% | ~110,000 |
| Thoracotomy | 40% | 10% | ~70,000 |
| Hernia repair | 10% | 4% | ~250,000 |
| Cesarean section | 10% | 4% | ~1,200,000 |
Even though the risk may seem low for some interventions, the sheer volume (roughly estimated for the United States) still leads to a significant number of previously healthy patients ending up with severe, disabling pain after surgery.
Risk values adapted from Kehlet et al.
Number of surgical cases adapted from Merrill and Elixhauser.9
How This Intervention Might Work
Figure 1 explains how regional anesthesia may prevent chronic pain from developing after surgery by interrupting the development of central sensitization. Panel A shows the physiological transmission of pain from the primary nociceptor to the synapse in the dorsal horn of the spinal cord to the secondary neuron, which transmits the signal to the brain. During surgery, the barrage of nociceptive input from the surgical site induces central sensitization—a permanent increase in synaptic strength. These pathological changes in synaptic transmission from the primary to the secondary neuron lead to perception of pain out of proportion to the stimulus (Panel B); this is the physiological basis for the development of hyperalgesia (exaggerated perception of painful stimuli) and allodynia (painful perception of nonpainful stimuli, such as touch) and persistent pain after surgery.14 An effective nociceptive block of regional anesthesia prevents pain impulses from being conducted from the surgical site to the central nervous system with subsequent sensitization, thus preventing the development of chronic pain (Panel C).
Figure 1.
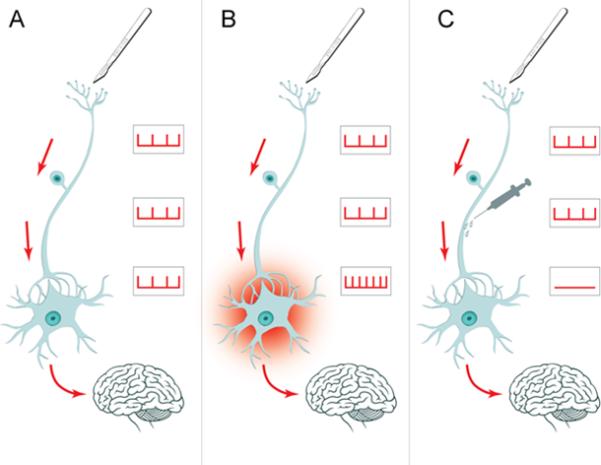
Normal perception: Panel A shows the normal pain pathways; the peripheral primary nociceptor transmits a pain signal to the secondary neuron in the spinal dorsal horn. From there, the pain is transmitted to the brain. Central sensitization: In Panel B, the intense barrage of pain signals from a surgical incision leads to permanent signal amplification in the spinal horn. This is the pathophysiological basis for hyperalgesia and persistent pain after surgery. Prevention of chronic pain: In Panel C, regional anesthesia prevents the development of chronic pain after surgery by blocking the pain signal. No central sensitization occurs.
Effects of the Intervention and Evidence Synthesis
A systematic review and meta-analysis for the Cochrane Collaboration found 23 randomized controlled trials (RCTs) investigating regional anesthesia or local anesthetics for the prevention of chronic pain after surgery.15,16 Pooled data from 250 patients in four RCTs with dichotomous outcomes at six months after thoracotomy strongly favored epidural anesthesia with an odds ratio (OR) of 0.34 and 95% confidence interval (CI) of 0.19–0.60. Data from 89 participants in two RCTs with outcomes at five or six months favored paravertebral block after breast cancer surgery with an OR of 0.37 and 95% CI of 0.14–0.94. Pooled results are shown in Figure 2 as forest plots. Including studies after cosmetic breast surgery and a study with multimodal topical analgesia increased the strength of the evidence. However, the conclusions were weakened by the intermediate methodological quality of the included studies.
Figure 2.
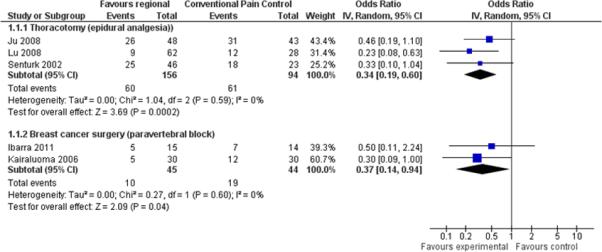
Forest plot comparison of regional analgesia vs. conventional analgesia for dichotomous pain outcomes at six months for thoracotomy and breast cancer surgery. (From Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No.: CD007105. DOI: 10.1002/14651858.CD007105.pub2. Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.)
Discussion of Included Studies and Their Limitations
We will discuss the individual studies and their strengths and weaknesses, stratifying them by surgical intervention. Studies and their designs are summarized in Table 2.
Table 2.
Randomized Controlled Trials Investigating the Effect of Regional Anesthesia/Analgesia versus General Anesthesia and/or Conventional Analgesia for Persistent Postoperative Pain after Breast Surgery and after Thoracotomy
| Study | Intervention vs. Control | Adjuvant | Outcomes | Follow-up (months) |
|---|---|---|---|---|
| Breast Surgery | ||||
| Baudry17 | Single-shot postoperative local infiltration vs. none | None | Pain/no pain Allodynia/hyperalgesia |
12 |
| Bell18 | Single-shot preincision local infiltration vs. none | None | Pain/no pain | 6 |
| Ibarra19 | Single-shot preincision paravertebral block vs. none | None | Myofascial, phantom, or neuropathic pain | 4-5 |
| Kairaluoma20 | Single-shot preincision paravertebral block vs. none | None | NRS >3 Analgesic consumption |
12 |
| Fassoulaki21 | Single-shot blocks + continuous postoperative topical application vs. none | Gabapentin vs. none | Pain/no pain Analgesic consumption |
6 |
| Thoracotomy | ||||
| Ju22 | Continuous preincision and postoperative epidural analgesia vs. none | None | Pain/no pain Allodynia |
12 |
| Senturk23 | Continuous preincision and postoperative epidural analgesia vs. none | None | Pain/no pain NRS Pain affecting daily living |
6 |
| Lu24 | Continuous preincision and postoperative epidural analgesia vs. none | None | Pain/no pain | 6 |
| Katz25 | Single-shot preincision intercostal blocks vs. none | Morphine, perphenazine, and indomethacin vs. midazolam and placebo | Pain/no pain VRS Analgesic consumption |
18 |
NRS, numeric rating scale; VRS, verbal rating scale.
Adapted with permission from Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 2012 Oct 17;10:CD007105.15
Breast Surgery
(Supplemental Digital Content 2, http://links.lww.com/ASA/A568). Baudry et al. studied 81 patients undergoing breast cancer surgery and randomly assigned them to receive an infiltration with 40 mL of 4.75 mg/mL ropivacaine or 40 mL of saline at the end of the operation.17 Fifty-three patients were interviewed by telephone one year postoperatively and no difference was found in the rates of chronic pain evaluated using a French version of the McGill questionnaire (16 patients in the ropivacaine group vs. 8 in the control group, p = 0.19). Limitations included the fact that patients undergoing both lumpectomy and mastectomy were included (although the proportions were similar in both groups); the intervention was a superficial wound infiltration rather than a nerve block or an epidural technique; it was performed at the end of the operation, not blocking painful stimuli from the surgical procedure; and the assessment was made by telephone rather than in a face-to-face interview.
Bell et al. recruited 8 patients undergoing bilateral reduction mammoplasty.18 Patients were randomly assigned to have one breast infiltrated preoperatively with 100 mL of 5 mg/mL lidocaine and epinephrine, while the other was infiltrated with saline and epinephrine. Six months after surgery, patients were reassessed for spontaneous pain. Three patients reported ongoing periodic pain. For 2, the pain was bilateral; however, 1 patient had undergone bilateral scar revision under local anesthesia shortly before the assessment. One patient reported pain localized “deep” in the lidocaine-treated breast. Issues include the small number of patients and the use of a short-acting local anesthetic.
Ibarra et al. randomly assigned 40 patients scheduled for radical mastectomy to general anesthesia with or without a paravertebral block, then assessed 29 patients by telephone four to five months later.19 Only 5 patients (33%) in the paravertebral block group reported chronic neuropathic pain, and none had phantom breast pain. In the group that received general anesthesia alone, 1 patient reported phantom breast pain and 6 had neuropathic pain, in 2 cases associated with phantom breast pain. The main limitations of this study were that breast-conserving operations were not included, despite evidence that those procedures lead to a higher rate of chronic pain,26 and that a single-injection paravertebral block was used rather than injections at multiple levels.
Kairaluoma et al.20 followed up 59 patients out of 60 who had been randomly assigned to receive a preincisional paravertebral block using 1.5 mg/kg of 5 mg/mL bupivacaine at the third thoracic vertebra or a sham block using subcutaneous saline. After one year, 5 patients (16.7%) in the paravertebral block group still had pain, while 12 (40%) of those in the sham group had pain (p = 0.008). The difference was still present when patients who had undergone an axillary dissection and those who had not were analyzed separately.
Fassoulaki et al.21 randomly assigned 50 patients undergoing breast cancer surgery (lumpectomy or mastectomy) to receive a combination of gabapentin 400 mg every 6 hours for 8 days, 20 g of eutectic mixture of local anesthetic (EMLA) cream applied daily from the day of surgery until the third postoperative day, and an axillary brachial plexus block with 10 mL of 7.5 mg/mL ropivacaine as well as intercostal blocks with 3 mL of 7.5 mg/mL ropivacaine at the third, fourth, and fifth intercostal spaces, or a triple placebo. At three months, significantly fewer patients in the treatment group (10 of 22, 45%) than in the control group (18 of 22, 77%) had chronic pain. The difference (6 of 20 vs. 12 of 21) was not statistically significant at six months, however the study was probably underpowered. One obvious confounder is that patients in the treatment group received gabapentin as well as regional analgesia, and thus the respective effects are impossible to tease out.
Thoracotomy
Ju et al.22 compared epidural analgesia with intercostal nerve cryoanalgesia in 107 patients undergoing thoracotomy. After one year, 16 of 38 patients (42.1%) in the epidural group had chronic pain vs. 22 of 39 (56.4%) in the cryoanalgesia group (p = 0.209), but 3 of 38 (7.9%) in the epidural group had pain that interfered with daily life vs. 13 of 19 (33.3%) in the cryoanalgesia group (p = 0.014). Limitations included the use of cryoanalgesia rather than placebo, as cryoanalgesia might worsen neuropathic pain. Rather than performing an intent-to-treat analysis, the authors withdrew 7 patients from the study who had been assigned to the epidural arm but whose catheter was dislodged during the first two postoperative days. Also, the rate of chronic pain was not significantly different between groups; only the number of patients whose pain interfered with daily life activities reached significance.
Sentürk et al.23 compared three analgesic regimens after thoracotomy: bupivacaine and morphine epidural analgesia initiated before incision, bupivacaine and morphine epidural analgesia initiated postoperatively, and intravenous morphine patient-controlled analgesia (IV PCA). After six months, 18 out of 23 (78%) IV PCA patients reported chronic pain vs. 10 out of 22 (46%) in the preoperative epidural group (p = 0.023). No significant difference was found when the postoperative epidural group was compared with the other two groups. Of note, no patient reported that pain interfered with daily life activities.
Lu et al.24 performed a similar study, randomly assigning 105 patients undergoing thoracotomy to one of three groups: ropivacaine and morphine epidurally, initiated either preoperatively or postoperatively, or intravenous fentanyl. After 6 months, significantly fewer patients had pain in the epidural groups than in the IV fentanyl group (p = 0.010 and 0.003 for the groups receiving epidural analgesia initiated preoperatively and postoperatively, respectively). In this study, there did not seem to be a difference with epidural analgesia initiated before or after surgery.
Katz et al.25 contacted patients from a prior study by telephone 18 months after their surgery. In that previous study,27 they had randomly assigned 30 patients undergoing thoracotomy to receive either intercostal nerves with 3 mL of bupivacaine 5 mg/mL with epinephrine 1:200,000 at the level of the incision as well as two levels above and below, or saline placebo. The treatment group also received preoperative morphine, perphenazine (an antipsychotic), and indomethacin, while the control group received midazolam and a placebo. In the initial study, the treatment group used less morphine in the first 6 hours, but more on postoperative days 2 and 3. There was no difference in pain scores. At the latest follow-up, 7 of 13 (53.8%) in the treatment group and 5 of 10 (50%) in the control group had evidence of chronic pain (p = 1.000; data obtained from the authors and not included in the original publication). Issues with this study included the small number of patients with large attrition by the last follow-up; the fact that the groups received a different pharmacological regimen besides the blocks with bupivacaine or saline; that blocks were performed under general anesthesia and no effort was made to determine whether blockade was or was not successful; and that there was no difference in pain scores initially (despite the differences in morphine use), and thus the discussed mechanism for the reduction of chronic pain could not have intervened. With a follow-up at 18 months—outside the predefined end point at 6 and 12 months—this study could not be pooled with the other three RCTs.
Summary
Evidence synthesis supports the use of epidural anesthesia for patients undergoing open thoracotomy, and paravertebral blocks for women undergoing breast cancer surgery, to reduce the risk of developing persistent pain six months after surgery. Persistent postsurgical pain may be devastating and resistant to treatment, but may be preventable in one patient out of every four by an effective perioperative nociceptive blockade. It is remarkable how homogeneous and consistent the different RCTs, conducted in diverse settings, were in their estimates of the long-term effect. On a cautionary note, effects with specific regional blocks in one specific surgical intervention may not translate to other surgical procedures or regional anesthesia techniques (Supplemental Digital Content 3, http://links.lww.com/ASA/A569).
You can tell your patient that perioperative regional anesthesia and analgesia likely reduces the risk of developing chronic pain after surgery. Outside the scope of this chapter, there is also evidence suggesting that avoiding general anesthesia and opioids, and using regional anesthesia and analgesia, can reduce the risk of cancer recurrence.28 These are compelling arguments in favor of regional anesthesia in this case.
FOCUS 2: REGIONAL ANESTHESIA TO IMPROVE LONG-TERM FUNCTION AFTER MAJOR JOINT SURGERY
Case in Point
The chair of orthopedics at your hospital is asking your group to justify performing nerve blocks on his joint replacement patients. He wonders if it is worth the time and effort. He has the impression that his patients walk just as well after knee or hip replacement three months postoperatively regardless of whether or not they received a block. What do you answer?
Impact
With an aging population, severe pain or dysfunction of the major joints has become much more frequent, leading to higher numbers of total joint replacements (shoulder, hip, knee). Sustained improved flexibility and function, rather than immediate postoperative results, are the sought-after outcomes of these interventions; function and mobility define the activity and independence of our aging population and drive secondary comorbidities such as diabetes mellitus, obesity, and possibly even cognitive performance.
How This Intervention Might Work
Severe postoperative pain can hinder effective rehabilitation. Pain may lead to reflex inhibition of muscle fibers, limiting strength and muscle building. Optimal pain control enables patients to participate more actively in more forceful motion exercise. Single injection, continuous delivery through catheters, or sustained-release preparations of local anesthetics block the conduction of pain impulses from the operated joint to the central nervous system and may offer analgesia superior to that achieved with a combination of nonsteroidal antiinflammatory drugs (NSAIDs), gabapentinoids, and opioids. Better pain control should allow more aggressive rehabilitation.29 Early mobilization and range of motion exercises are probably the key factors in optimal long-term joint function—the outcome of interest. Early activity is associated with decreased adverse effects of immobilization on muscles and joints.30 Range of motion exercises facilitated by regional analgesia can increase passive knee flexion; these gains may be sustained for at least a few months after surgery31 (Supplemental Digital Content 4, http://links.lww.com/ASA/A570).
Effects of the Intervention and Evidence Synthesis
We report and discuss the preliminary findings of our systematic review and meta-analysis for the Cochrane Collaboration, which is still unpublished and in the editorial phase.32 In Table 3, we list 8 RCTs reporting functional outcomes at or after 3 months following major joint replacement. Preliminary results, pooling data from 140 participants from three studies, suggest no significant improvement in range of motion (improvement of 4 degrees with 95% CI ranging from −2.23 to 10.21) (Supplemental Digital Content 5, http://links.lww.com/ASA/A571).
Table 3.
Randomized Controlled Trials Investigating the Effect of Regional Anesthesia/Analgesia versus General Anesthesia and/or Conventional Analgesia for Long-term Functional Outcomes after Major Joint Replacement
| Study Blinding | Intervention | Control | Outcomes |
|---|---|---|---|
| Primary Total Hip Replacement | |||
| Ilfeld33 Triple | CLPB until POD 4 | CLPB until POD 1 | WOMAC at 1, 2, 3, 6, and 12 months |
| Primary Total Knee Replacement | |||
| Ilfeld34 Triple | CFNB until POD 4 | CFNB until POD 1 | WOMAC at 1, 2, 3, 6, and 12 months |
| Kadic35 Single | CFNB | No block | Knee function after 3 months (knee range of motion, KSS), WOMAC |
| Nader36 Unblinded | CFNB Epidural infusion until POD 1 |
No block Epidural infusion until morning of POD 1 |
Knee flexion and functional outcomes at 1, 6, and 12 months |
| Singelyn29 Unblinded | -CFNB -Epidural analgesia |
No block | Knee flexion at 6 weeks and 3 months postoperatively |
| Tammachote37 Single | Periarticular infiltration | Intrathecal morphine | Knee flexion and Thai version of the WOMAC at 6 and 12 weeks |
| Wu38 Unblinded | CFNB until POD 3 | No block | KSS (difference with preoperative value) at discharge, 6 weeks, 3 months, and 6 months postoperatively |
| Zhang39 Double | Periarticular infiltration then ropivacaine/ketorolac infusion | Saline intraarticular infusion | Maximum knee flexion at 90 days |
CLPB, continuous lumbar plexus block; CFNB, continuous femoral nerve block; POD, postoperative day; KSS, Knee Society Score; WOMAC, Western Ontario and McMaster Osteoarthritis Index.
Discussion of Included Studies and Their Strengths and Weaknesses
Extended Regional Blocks Fail to Show Improved Long-term Function
Ilfeld et al. examined the potential benefit of extending the duration of regional analgesia to several days postoperatively in both hip and knee replacement patients. An initial study was performed, enrolling patients undergoing total hip replacement (THR)40 or total knee replacement (TKR).41 A continuous nerve block, lumbar plexus for hip patients and femoral for knee patients, was initiated preoperatively, and was continued for 24 hours with a subsequent saline infusion for patients in the control group or for four days for patients in the treatment group. The initial studies evaluated discharge readiness. Follow-up studies33,34 were performed one year after surgery to assess health-related quality of life using the WOMAC score (Western Ontario and McMaster Osteoarthritis Index)42 and found no difference between groups. Significant attrition might have watered down any effect of regional anesthesia (THR: 47 patients enrolled and assessed at one year; TKR: 77 patients enrolled, 53 assessed at one year).
Kadic et al. examined 53 patients scheduled for TKR to see whether a continuous femoral nerve block improved function, assessing outcomes with the WOMAC score as well as the Knee Society Score.35 While initially patients in the femoral nerve block group achieved better flexion 6 days after surgery, there was no difference in functional scores between groups at 3 months. One can argue that, strictly speaking, these studies do not compare regional analgesia vs. conventional analgesia, but rather, two regimens of regional analgesia of different duration.
Regional Analgesia versus Conventional Pain Control Fails to Improve Outcomes at Six Months
Nader et al. studied 62 patients undergoing TKR and receiving epidural analgesia until the first postoperative day.36 Upon removal of the epidural catheter, patients were randomly assigned to continuous femoral nerve blockade for 24 hours or oral analgesics. There was no difference in knee flexion after 6 or 12 months. WOMAC scores were not significantly different at 6 months and were significantly higher (worse outcome) in the continuous femoral nerve block group at 12 months. The authors attributed this result to the fact that this was a global function score and that more patients in the experimental group underwent further joint replacement procedures in the intervening year. As in Ilfeld's studies, all patients received regional analgesia for the first day and night.
Singelyn et al.29 performed a three-arm study, randomly assigning 45 patients undergoing TKR under general anesthesia to receive, during the first 48 postoperative hours, either IV PCA with morphine, epidural analgesia, or continuous femoral nerve blockade (infusion of 1.25 mg/mL ropivacaine with sufentanil 0.1 μg/mL and clonidine 1 μg/mL in both continuous regional analgesia groups). Postoperative pain scores were lower in the regional anesthesia groups than in the IV PCA group, and patients in the IV PCA group took significantly more days to achieve 90-degree flexion than those in the other groups. However, no difference was noted between groups after three months.
Tammachote et al.37 randomly assigned 57 patients undergoing TKR under spinal anesthesia to one of two groups: intrathecal morphine (0.2 mg) or periarticular infiltration (100 mL containing 100 mg bupivacaine, 5 mg morphine, and 30 mg ketorolac). Patients then received a ketorolac IV PCA. There was no difference in WOMAC score or knee flexion after 3 months.
Wu and Wong38 randomly assigned 60 patients undergoing TKR under spinal anesthesia to receive a continuous femoral nerve block (infusion continued until the third postoperative day) or no block. Patients received acetaminophen and diclofenac. Block patients received oral opioids as needed, while a morphine IV PCA was used in the other group. Knee Society scores did not differ between the groups postoperatively or at 6 weeks, 3 months, or 6 months postoperatively. This study had a high rate of selection bias in that 79 patients were effectively enrolled, but 19 were excluded after entering the study because of technical problems or medical complications.
Zhang et al. performed a three-arm study, randomly assigning 80 patients undergoing TKR under general anesthesia to receive, during the first 48 postoperative hours, either single-injection periarticular infiltration, continuous periarticular infiltration, or placebo.39 Knee flexion at 90 days was significantly higher in the infiltration groups than in the control group, and in the continuous infiltration group compared with the single-injection group.
Summary
While well-documented short-term improvements in function were not sustained in the long run (at least in the preliminary results from this meta-analysis), we should point out important limitations in the quality of evidence. The diversity of patients, interventions, and agents used renders evidence synthesis challenging and may lead to missing an effect if it is really there. Indeed, the studies might have been underpowered to detect a small but meaningful effect. Generally, the considerable heterogeneity in interventions used and the outcomes reported hindered evidence synthesis. No study detailed the rehabilitation protocol used, and one might wonder how these could be improved to maximize the benefit of regional blockade. There were no studies of long-term shoulder function and only one study of patients following hip replacement.
Thus, the jury is still out with regard to regional anesthesia for improved long-term function after total knee surgery. More research is needed, for example, on newer regional analgesia interventions such as the adductor canal block, and taking full advantage of the aggressive rehabilitation possible with the exquisite pain control only attainable with regional anesthesia.
At this time, beyond the humane concept that even short-lived severe suffering is worth alleviating and that acute pain is an important determinant of Press Ganey scores for the orthopedic department (and hence the public perception of its surgical performance), there is insufficient evidence to convince the chair of orthopedics that nerve blocks are leading to sustainable, demonstrable functional improvement for joint replacement patients beyond the immediate perioperative period. A caveat: this might be a case of absence of evidence rather than evidence of absence; the small number of studies and the subject attrition underline this argument. However, many orthopedic surgeons have been using periarticular infiltration, often with extended-release local anesthetics—thus in effect providing regional analgesia to their patients—without any convincing Level 1 evidence of long-term benefits, to our knowledge.
FOCUS 3: REGIONAL ANESTHESIA FOR IMPROVED COGNITIVE OUTCOMES AFTER NONCARDIAC SURGERY
Case in Point
An 80-year-old patient presents for total hip replacement. Airway concerns discourage you from using a spinal anesthetic with sedation and the patient in the lateral position. You suggest to the patient that general anesthesia might be a safer option. He tells you that his brother-in-law had general anesthesia recently and “has not been the same since then.” He is worried about how general anesthesia might impact his cognitive function, and asks whether regional anesthesia would be safer from that point of view. What do you tell him?
Impact
Long-term cognitive outcomes are very important to an increasingly elderly surgical population; postoperative cognitive dysfunction (POCD) impacts quality of life and independence of the elderly after surgery while often negating the success of the surgical intervention.43 Three months after surgery, POCD44 may be present in one out of ten high-risk (elderly) patients.45 POCD and delirium independently predict other long-term outcomes such as increased morbidity and mortality, higher associated healthcare costs, long-term cognitive impairment, and further decline beyond 12 months.46
How This Intervention Might Work
The etiology of POCD after surgery is unclear.43 Perioperative imbalances of neurotransmitter and inflammatory mediators have been implicated. Microemboli are a concern in orthopedic and cardiac surgery. Regional anesthesia may improve cognitive outcomes after surgery by attenuating the inflammatory response. The administration of benzodiazepines, opioids, and other psychotropic medications is associated with POCD and may trigger a vicious cycle of POCD leading to more medication leading to more POCD. Regional anesthesia, by controlling pain and obviating the need for opioids, may improve cognitive outcomes, but it is unclear whether these mechanisms lead to sustained improvement over conventional anesthetic approaches.
Effects of the Intervention and Evidence Synthesis
While some systematic reviews of short-term cognitive outcomes suggest a clear benefit of regional anesthesia in the elderly,47 others do not.48,49 Individual studies, summarized in Table 4, do not support the hypothesis that regional anesthesia versus general anesthesia or regional analgesia versus conventional analgesia improves long-term cognitive function after surgery. A meta-analysis56 of 26 RCTs with a total of more than 1,100 patients in each group (combining both long-term and short-term studies) failed to support the concern that general anesthesia contributed to long-term POCD in adults (standardized difference in means −0.08; 95% CI −0.17–0.01; P = 0.094) (Supplemental Digital Content 6, http://links.lww.com/ASA/A572). Some might question whether pooling the plethora of cognitive outcome measurements and patient population is meaningful.
Table 4.
RCTs Comparing Regional vs. General Anesthesia with Cognitive Outcome beyond 90 Days
| Study | Surgery | Intervention vs. Control | Outcomes evaluated |
|---|---|---|---|
| Jones50 | Total hip or knee replacement | Spinal anesthesia vs. general anesthesia | National Adult Reading Test Choice Reaction Time Critical flicker fusion threshold Objective learning test Digit copying test Functional life scale Cognitive difficulties test |
| Nielson51 | Total knee replacement | Spinal anesthesia vs. general anesthesia | Wechsler Adult Intelligence Scale–Revised Trail Making Test Controlled Oral Word Association Test Finger Oscillation Test Two-point discrimination Hand preference questionnaire Sickness impact profile |
| Bigler52 | Hip fracture | Spinal anesthesia vs. general anesthesia | Abbreviated Mental Test |
| Williams-Russo53 | Total knee replacement | Epidural anesthesia (followed in more than 95% of the cases by epidural analgesia) vs. general anesthesia (followed by IV PCA) | Boston Naming Test Controlled word association Digit symbol Trail Making Tests A Trail Making Tests B Digit span test Benton Visual Retention Test Benton Visual Recognition Test Mattis-Kovner Verbal Recall Mattis-Kovner Verbal Recognition |
| Rasmussen54 | Major noncardiac surgery | Regional (spinal or epidural) anesthesia vs. general anesthesia | Part of the Visual Verbal Learning Test Part of the Concept Shifting Test Part of the Stroop Color Word Interference Test Part of the Letter Digit Coding Test Geriatric Depression Scale Subjective cognitive functioning questionnaire (patients and relatives) Instrument for activities of daily living (patients and relatives) |
| Campbell55 | Cataract | Regional (topical + retrobulbar or peribulbar ) anesthesia vs. general anesthesia | Part of the Rivermead Behavioural Memory Test 2 parts of the Fuld Object Memory Test Part of the Wechsler Adult Intelligence Scale–Revised Digit copying test Felix post unit questionnaire Holbrook activity index National Adult Reading Test |
Discussion of the Few Randomized Trials with Long-term Outcomes
We will discuss the few randomized controlled clinical trials with long-term cognitive outcomes comparing a regional anesthesia intervention versus a conventional general anesthesia approach; these RCTs are tabulated in Table 4.
Jones et al.50 randomly assigned 146 patients over 60 years of age and scheduled to undergo TKR or THR to general or spinal anesthesia and followed them for 90 days. Cognitive and functional competence in these elderly patients was not detectably impaired after either general or regional anesthesia when attention was paid to the known perioperative influences on mental function.
Nielson et al.51 included 98 individuals aged between 60 and 86 undergoing TKR and randomly assigned them to general or spinal anesthesia. There were no cognitive or psychosocial effects of general or regional anesthesia after 3 months.
Bigler et al.52 studied 40 patients above 60 years of age about to undergo surgery for hip fracture and randomly assigned them to receive either general or spinal anesthesia. There were no significant cognitive differences between the two groups at any time up to the last follow-up at 90 days.
Williams-Russo et al.53 enrolled 262 patients older than 40 (median age, 69 years) undergoing TKR and randomly assigned them to receive epidural anesthesia (followed in more than 95% of the cases by epidural analgesia) or general anesthesia (followed by IV PCA). There was a generalized pattern of decline at 1 week, followed at 6 months by either a return to baseline levels or improvement, but there were no significant differences between the groups in within-subject change from baseline on any of the 10 cognitive test results at either 1 week or 6 months.
Rasmussen et al.,54 in a multicenter trial involving twelve hospitals in seven countries, randomly allocated 428 patients aged over 60 years and undergoing major noncardiac surgery to regional anesthesia (spinal or epidural anesthesia; postoperative epidural analgesia was encouraged) or general anesthesia. No significant difference was found in the incidence of cognitive dysfunction after 3 months.
Campbell et al.55 randomized 169 patients aged 65 to 98 years and undergoing cataract surgery to receive general or regional anesthesia. No evidence of long-term postoperative cognitive dysfunction was detected and there was no significant difference in performance between the two groups up to the longest follow-up of 18 months.
Summary
We caution that our conclusions are based mostly on older small studies (in Guay's review,56 18 out of 26 studies date to 1995 or earlier) with significant attrition, while most recent RCTs did not follow patients long enough to assess long-term cognitive outcomes. Patients with worse POCD may have selectively dropped out to avoid the embarrassment of failure in cognitive tests, biasing the studies toward the null hypothesis. Many patients in the regional anesthesia groups received midazolam, which may offset the potential benefit of regional anesthesia.57 Most importantly, the focus of these studies was on intraoperative management, not on extended postoperative regional analgesia, where opioid sparing might have the strongest effect in preventing cognitive dysfunction.
Thus, while there is evidence that short-term cognitive impairment might be more likely after general rather than regional anesthesia, you can reassure your patient that long-term differential effects have not been demonstrated. Especially in elderly patients preferring regional anesthesia when the procedure is amenable to it and there are no medical contraindications, a regional technique is reasonable, and an excellent perioperative nociceptive regional anesthetic may avoid pain and the confusion sometimes induced by opioids in the elderly.
CONCLUSION
In our changing healthcare system, we need to convince patients, payers, providers, and the public that regional anesthesia is worth the resources invested. Indicating its obvious immediate benefits right after surgery may not be sufficient. We need to validate its sustained effects on long-term outcomes, among them for chronic pain after surgery, long-term function after joint replacement, and sustained cognitive outcomes in the elderly.
The effects of regional anesthesia for the prevention of persistent postsurgical pain are robust. They are supported by many RCTs and a Cochrane review, and are based on a well-described molecular mechanism. At this time, there is insufficient evidence for improvement of long-term function after major joint repair, but because the rationale seems compelling, further studies are warranted. The benefit of regional anesthesia for long-term cognitive outcomes is unclear at present, and no molecular or animal model offers a clear mechanism easily amenable to a regional anesthesia intervention.
To ensure that the benefits of regional anesthesia will remain available to our patients, the regional anesthesia community should focus research and development on sustained benefits of patient-centered extended perioperative nociceptive blockade and engage itself deeply in the rehabilitation effort.
Supplementary Material
Learning Objectives.
As a result of completing this activity, the participant will be able to
Describe the advantages of regional anesthesia and analgesia for long-term rather than short-term outcomes
Summarize the evidence for regional anesthesia and analgesia to prevent chronic pain after surgery
Discuss the usefulness of regional anesthesia and analgesia to improve functional outcomes after total joint replacement
Explain the usefulness of regional anesthesia and analgesia to decrease postoperative cognitive dysfunction, especially in elderly patients
Acknowledgments
This publication was supported in part by CTSA Grant 1 UL1 TR001073-01, 1 TL1 TR001072-01, 1 KL2 TR001071-01 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
This chapter is based in part on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) 2012, Issue 10, DOI: 10.1002/14651858. CD007105.pub2 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.
We wish to acknowledge the contribution of our Cochrane review coauthors (Drs. G. Schwartz, C.B. Hall, C.M. Lajam, and D.A. Andreae) and the support of the Cochrane Anesthesia Review Group in publishing the Cochrane Review.
Footnotes
Author Disclosure Information:
Dr. Atchabahian has disclosed that he receives consulting fees from Pacira. Dr. Andreae has disclosed that he has no financial interests in or significant relationship with any commercial companies pertaining to this educational activity.
Contributor Information
Arthur Atchabahian, Department of Anesthesiology, New York University School of Medicine, New York, New York.
Michael Andreae, Department of Anesthesiology, Albert Einstein College of Medicine, Bronx, New York.
REFERENCES
- 1.Vetter TR, Boudreaux AM, Jones KA, Hunter JM, Jr, Pittet JF. The perioperative surgical home: how anesthesiology can collaboratively achieve and leverage the triple aim in health care. Anesth Analg. 2014 May;118(5):1131–6. doi: 10.1213/ANE.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 2.Methodology Committee of the Patient-Centered Outcomes Research Institute (PCORI) Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA. 2012 Apr 18;307(15):1636–40. doi: 10.1001/jama.2012.466. [DOI] [PubMed] [Google Scholar]
- 3.Szokol JW, Stead S. The changing anesthesia economic landscape: emergence of large multispecialty practices and Accountable Care Organizations. Curr Opin Anaesthesiol. 2014 Apr;27(2):183–9. doi: 10.1097/ACO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 4.Mariano ER. Making it work: setting up a regional anesthesia program that provides value. Anesthesiol Clin. 2008 Dec;26(4):681–92. doi: 10.1016/j.anclin.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008 Aug;248(2):189–98. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 6.Liu SS, Wu CL. The effect of analgesic technique on postoperative patient-reported outcomes including analgesia: a systematic review. Anesth Analg. 2007 Sep;105(3):789–808. doi: 10.1213/01.ane.0000278089.16848.1e. [DOI] [PubMed] [Google Scholar]
- 7.Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009 Jul 9;361(2):109–12. doi: 10.1056/NEJMp0904131. [DOI] [PubMed] [Google Scholar]
- 8.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006 May 13;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. Table. [DOI] [PubMed] [Google Scholar]
- 9.Merrill C, Elixhauser A. Procedures in U.S. Hospitals, 2003. Rockville, MD: Agency for Healthcare Research and Quality. 2005. HCUP Fact Book No.7. AHRQ Publication No. 06-0039. [Google Scholar]
- 10.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006 Mar;104(3):594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008 Jul;101(1):77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 12.Sng BL, Sia AT, Quek K, Woo D, Lim Y. Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesth Intensive Care. 2009 Sep;37(5):748–52. doi: 10.1177/0310057X0903700513. [DOI] [PubMed] [Google Scholar]
- 13.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003 Jul;104(1-2):1–13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 14.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000 Jun 9;288(5472):1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 15.Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 2012 Oct 17;10:CD007105. doi: 10.1002/14651858.CD007105.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013 Nov;111(5):711–20. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baudry G, Steghens A, Laplaza D, Koeberle P, Bachour K, Bettinger G, Combier F, Samain E. Infiltration de ropivacaïne en chirurgie carcinologique du sein: effet sur la douleur postopératoire aiguë et chronique [Ropivacaine infiltration during breast cancer surgery: postoperative acute and chronic pain effect]. Ann Fr Anesth Reanim. 2008 Dec;27(12):979–86. doi: 10.1016/j.annfar.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Bell RF, Sivertsen A, Mowinkel P, Vindenes H. A bilateral clinical model for the study of acute and chronic pain after breast-reduction surgery. Acta Anaesthesiol Scand. 2001 May;45(5):576–82. doi: 10.1034/j.1399-6576.2001.045005576.x. [DOI] [PubMed] [Google Scholar]
- 19.Ibarra MM, S-Carralero GC, Vicente GU, Cuartero del Pozo A, López Rincón R, Fajardo del Castillo MJ. [Chronic postoperative pain after general anesthesia with or without a single-dose preincisional paravertebral nerve block in radical breast cancer surgery]. Rev Esp Anestesiol Reanim. 2011 May;58(5):290–4. doi: 10.1016/s0034-9356(11)70064-0. [DOI] [PubMed] [Google Scholar]
- 20.Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006 Sep;103(3):703–8. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 21.Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005 Nov;101(5):1427–32. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 22.Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain. 2008 Apr;12(3):378–84. doi: 10.1016/j.ejpain.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Sentürk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalçin S, Dilege S, Pembeci K. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002 Jan;94(1):11–5. doi: 10.1213/00000539-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Lu YL, Wang XD, Lai RC. [Correlation of acute pain treatment to occurrence of chronic pain in tumor patients after thoracotomy]. Ai Zheng. 2008 Feb;27(2):206–9. [PubMed] [Google Scholar]
- 25.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996 Mar;12(1):50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995 May;6(5):453–9. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh BP, Katz J, Sandler AN, Nierenberg H, Roger S, Boylan JF, Laws AK. Multimodal analgesia before thoracic surgery does not reduce postoperative pain. Br J Anaesth. 1994 Aug;73(2):184–9. doi: 10.1093/bja/73.2.184. [DOI] [PubMed] [Google Scholar]
- 28.Vaghari BA, Ahmed OI, Wu CL. Regional Anesthesia-Analgesia: Relationship to Cancer Recurrence and Infection. Anesthesiol Clin. 2014 Dec;32(4):841–851. doi: 10.1016/j.anclin.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998 Jul;87(1):88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop Relat Res. 1987 Jun;(219):28–37. [PubMed] [Google Scholar]
- 31.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999 Jul;91(1):8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Atchabahian Cochrane Database Syst Rev. 2012 DOI: 10.1002/14651858.CD010278. [Google Scholar]
- 33.Ilfeld BM, Ball ST, Gearen PF, Mariano ER, Le LT, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Maldonado RC, Meyer RS. Health-related quality of life after hip arthroplasty with and without an extended-duration continuous posterior lumbar plexus nerve block: a prospective, 1-year follow-up of a randomized, triple-masked, placebo-controlled study. Anesth Analg. 2009 Aug;109(2):586–91. doi: 10.1213/ane.0b013e3181a9db5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilfeld BM, Shuster JJ, Theriaque DW, Mariano ER, Girard PJ, Loland VJ, Meyer S, Donovan JF, Pugh GA, Le LT, Sessler DI, Ball ST. Long-term pain, stiffness, and functional disability after total knee arthroplasty with and without an extended ambulatory continuous femoral nerve block: a prospective, 1-year follow-up of a multicenter, randomized, triple-masked, placebo-controlled trial. Reg Anesth Pain Med. 2011 Mar-Apr;36(2):116–20. doi: 10.1097/aap.0b013e3182052505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadic L, Boonstra MC, De Waal Malefijt MC, Lako SJ, Van Egmond J, Driessen JJ. Continuous femoral nerve block after total knee arthroplasty? Acta Anaesthesiol Scand. 2009;53(7):914–20. doi: 10.1111/j.1399-6576.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 36.Nader A, Kendall MC, Wixson RL, Chung B, Polakow LM, McCarthy RJ. A randomized trial of epidural analgesia followed by continuous femoral analgesia compared with oral opioid analgesia on short- and long-term functional recovery after total knee replacement. Pain Med. 2012;13(7):937–47. doi: 10.1111/j.1526-4637.2012.01409.x. [DOI] [PubMed] [Google Scholar]
- 37.Tammachote N, Kanitnate S, Manuwong S, Yakumpor T, Panichkul P. Is pain after TKA better with periarticular injection or intrathecal morphine? Clin Orthop Rel Res. 2013;471(6):1992–9. doi: 10.1007/s11999-013-2826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu JW, Wong YC. Elective unilateral total knee replacement using continuous femoral nerve blockade versus conventional patient-controlled analgesia: perioperative patient management based on a multidisciplinary pathway. Hong Kong Med J. 2014;20(1):45–51. doi: 10.12809/hkmj133899. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Wang F, Lu ZD, Zhang L, Jin QH. Effect of single-injection versus continuous local infiltration analgesia after total knee arthroplasty: A randomized, double-blind, placebo-controlled study. J Int Med Res. 2011;39(4):1369–80. doi: 10.1177/147323001103900423. [DOI] [PubMed] [Google Scholar]
- 40.Ilfeld BM, Ball ST, Gearen PF, Le LT, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Meyer RS. Ambulatory continuous posterior lumbar plexus nerve blocks after hip arthroplasty: a dual-center, randomized, triple-masked, placebo-controlled trial. Anesthesiology. 2008 Sep;109(3):491–501. doi: 10.1097/ALN.0b013e318182a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010 Sep;150(3):477–84. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 43.Wu CL, Hsu W, Richman JM, Raja SN. Postoperative cognitive function as an outcome of regional anesthesia and analgesia. Reg Anesth Pain Med. 2004 May-Jun;29(3):257–68. doi: 10.1016/j.rapm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. 2011 Jul 20;343:d4331. doi: 10.1136/bmj.d4331. [DOI] [PubMed] [Google Scholar]
- 45.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998 Mar 21;351(9106):857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 46.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000 Jun;48(6):618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 47.Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004 Oct;18(4):CD000521. doi: 10.1002/14651858.CD000521.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Barbosa FT, Jucá MJ, Castro AA, Cavalcante JC. Neuraxial anaesthesia for lower-limb revascularization. Cochrane Database Syst Rev. 2013 Jul 29;7:CD007083. doi: 10.1002/14651858.CD007083.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22(Suppl 3):67–79. doi: 10.3233/JAD-2010-101086. [DOI] [PubMed] [Google Scholar]
- 50.Jones MJ, Piggott SE, Vaughan RS, Bayer AJ, Newcombe RG, Twining TC, Pathy J, Rosen M. Cognitive and functional competence after anaesthesia in patients aged over 60: controlled trial of general and regional anaesthesia for elective hip or knee replacement. BMJ. 1990 Jun 30;300(6741):1683–7. doi: 10.1136/bmj.300.6741.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielson WR, Gelb AW, Casey JE, Penny FJ, Merchant RN, Manninen PH. Long-term cognitive and social sequelae of general versus regional anesthesia during arthroplasty in the elderly. Anesthesiology. 1990 Dec;73(6):1103–9. doi: 10.1097/00000542-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Bigler D, Adelhøj B, Petring OU, Pederson NO, Busch P, Kalhke P. Mental function and morbidity after acute hip surgery during spinal and general anaesthesia. Anaesthesia. 1985 Jul;40(7):672–6. doi: 10.1111/j.1365-2044.1985.tb10949.x. [DOI] [PubMed] [Google Scholar]
- 53.Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA. 1995 Jul 5;274(1):44–50. [PubMed] [Google Scholar]
- 54.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT, ISPOCD2(International Study of Postoperative Cognitive Dysfunction) Investigators Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003 Mar;47(3):260–6. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 55.Campbell DN, Lim M, Muir MK, O'Sullivan G, Falcon M, Fison P, Woods R. A prospective randomised study of local versus general anaesthesia for cataract surgery. Anaesthesia. 1993 May;48(5):422–8. doi: 10.1111/j.1365-2044.1993.tb07019.x. [DOI] [PubMed] [Google Scholar]
- 56.Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: A meta-analysis. Indian J Anaesth. 2011 Jul;55(4):358–63. doi: 10.4103/0019-5049.84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010 Jan;85(1):18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


