Summary
Primary gustatory cortex (GC) is connected (both mono- and poly-synaptically) to primary olfactory (piriform) cortex (PC)—connections that might be hypothesized to underlie the construction of a “flavor” percept when both gustatory and olfactory stimuli are present. Here, we use multi-site electrophysiology and optical inhibition of GC neurons (GCx, produced via infection with ArchT) to demonstrate that, indeed, during gustatory stimulation, taste-selective information is transmitted from GC to PC. We go on to show that these connections impact olfactory processing even in the absence of gustatory stimulation: GCx alters PC responses to olfactory stimuli presented alone, enhancing some and eliminating others, despite leaving the path from nasal epithelium to PC intact. Finally, we show the functional importance of this latter phenomenon, demonstrating that GCx renders rats unable to properly recognize odor stimuli. This sequence of findings suggests that sensory processing may be more intrinsically integrative than previously thought.
Introduction
The brain is divided into multiple sensory systems, each classically described as processing its own unique aspects of the outside world. In their natural environments, however, animals typically treat multiple sources of sensory information as unified events [1-7]. Recent work performed in recognition of this fact has challenged the idea that sensory systems function in isolation, demonstrating anatomical as well as functional connectivity between putatively unimodal sensory cortices.
One known consequence of such crossmodal connectivity is the fact that sensory input to one modality (e.g., an auditory stimulus) can cause or modulate responses in heteromodal cortex (e.g., visual cortex) [8-11]. Less well studied is the influence that “spontaneous” activity in one primary sensory cortex might have upon activity in another to which it is connected—that is, how the very presence of projections from one system might affect local processing performed on unimodal stimuli in another. Imaging studies have shown that functional connections between nodes of a network are present even in the absence of external input [12-14]. It is therefore reasonable to hypothesize that sensory processing in one system might be dependent on the mere presence of a second to which it is connected—that even spontaneous neural activity in one sensory system will impact unimodal processing by another.
Here we have tested this hypothesis, using simultaneous electrophysiological recordings and optical inhibition. Specifically, we tested the specific prediction that gustatory cortex (GC) is required for normal olfactory (piriform) cortical (PC) processing of odors. We first established the functionality of connections between PC and GC of the rat, using a combination of sensory stimulation and multi-site electrophysiology. We then evaluated the impact of optically inhibiting GC (GCx) on spiking activity in PC. This manipulation had the predictable impact of eliminating taste responses in PC. More surprising was the fact that GCx modulated olfactory processing in PC. Subsequent behavioral testing confirmed the functional importance of this phenomenon, showing that olfactory perception (measured in a unisensory odor preference task) is altered by GCx. These data demonstrate that GC plays a role in olfactory processing, even when taste stimuli are not present.
Results
Olfactory cortex responds to taste stimuli
We first recorded the spiking activity of neurons in posterior piriform cortex (PC) of awake rats in response to basic taste stimuli—sweet (sucrose), salty (sodium chloride), sour (citric acid) and bitter (quinine) tastes, delivered directly onto the tongue through intra-oral cannulae (see Experimental Procedures). As shown in an earlier paper from our lab [11], taste-evoked PC responses evolve over several seconds following delivery to the tongue, similar to the protracted dynamics that are typically observed along the gustatory pathway in response to intra-oral delivery of taste stimuli [15-18] (see Figure 1B for examples of PC and GC taste responses). Taste selectivity in these responses was assessed using two-way ANOVA on average firing rates with factors Taste (sweet, salty, sour and bitter) and Time (3 consecutive 1 s bins following stimulus onset) [11]. Out of a total of 71 PC neurons recorded for this study, 31 (44%) responded in a taste-selective manner, as measured by a significant main effect of Taste; four neurons (6%) showed non-specific responses, as measured by a main effect of Time, and one neuron (1%) showed a Taste × Time interaction. For comparison, analogous analysis almost never yielded evidence for stimulus-specificity in pre-stimulus firing rates (n=3/71 [4%], effectively chance results), thus confirming the stability of spontaneous firing rates (identical results were observed in relation to each effect described below, and will not be discussed further).
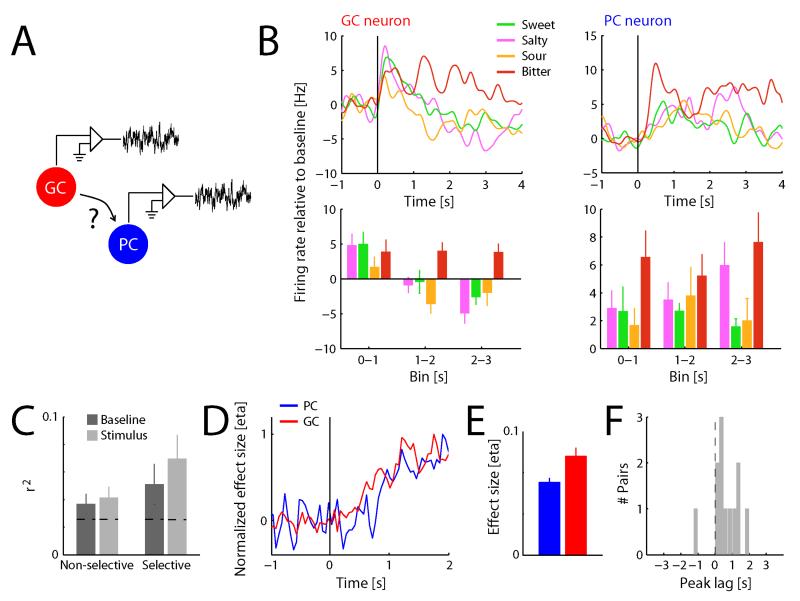
Figure 1. PC forms functional interactions with GC.
A. Simultaneous recordings from PC and GC allows for assessment of functional connectivity between the two regions. B. Example of a taste-selective pair of neurons recorded from GC and PC. Upper panels show spike density functions of the responses to basic taste stimuli presented directly to the tongue (baseline subtracted); lower panels show average spiking activity (baseline subtracted) in three consecutive 1 s bins following stimulus onset. . Both neurons show significant effects of Taste (n=10 trials/condition; two-way ANOVA, p<0.05) C. Trial-by-trial correlation in baseline and stimulus-evoked firing rate, averaged over taste-selective (n=12) and non-selective (n=45) PC-GC neuron pairs. Measured correlations are significantly higher than trial-shuffled control data (horizontal dashed lines; Kolmogorov-Smirnoff test, p<0.01); correlations between taste-selective neuron pairs are significantly higher than correlations between non-selective neuron pairs (Kolmogorov-Smirnoff test, p<0.05). D. Normalized taste selectivity as a function of time, averaged over the population of PC (n=71) and GC (n=27) neurons. E. Taste selectivity (averaged over 3 s following stimulus onset) in GC is significantly higher than in PC (t-test, p<0.01). F. Histogram of peak lag times obtained from cross-correlating the time course of taste selectivity (over 3 s following stimulus onset) between simultaneously recorded taste-selective PC-GC neuron pairs. Peaks are significantly skewed towards positive values (t-test, p<0.05), indicating PC lagging GC.
Our previous report already demonstrated that such PC responses to taste stimuli are eliminated by inactivation of the lingual epithelium and unaffected by nasal epithelial decilliation [11], confirming their origin from the oral cavity. Thus, taste responses in PC are robust, selective and significant, if not necessarily as visually compelling as classic visual, somatosensory or auditory cortical responses.
Olfactory cortical taste responses are functionally correlated with, and lag behind, gustatory cortical taste responses
It is reasonable to hypothesize that the neural pathway responsible for the appearance of taste-related firing in PC arrives via primary gustatory cortex (GC, which is a part of insular cortex [19, 20]). Anatomical studies have indicated that one of several sources of input to posterior PC is insular cortex [21-23], thus making GC a likely candidate for relaying taste-specific input to PC.
We performed multiple tests of this hypothesis. First, we compared neural response patterns in simultaneously-recorded PC and GC neurons (Figure 1A; see Figure 1B for an example pair of simultaneously recorded PC and GC neurons). These paired recordings were used to test whether the two structures are functionally connected in the context of tasting. We first calculated spontaneous activity (average firing rate during 1 s preceding stimulus delivery) and stimulus-evoked activity (average firing rate during the 3 s following stimulus delivery, baseline subtracted) for each trial. We then computed, for each simultaneously recorded PC and GC neuron pair (n=57 pairs), correlations in trial-by-trial variation of both spontaneous and stimulus-evoked activity patterns [24].
The average variance accounted for by these correlations (Figure 1C) proved to be significantly higher than that obtained from trial-shuffled control data (horizontal dashed line; Kolmogorov-Smirnov test: KS=1.0, p<0.01). Correlations in spontaneous activity patterns did not differ from those in stimulus-evoked activity patterns (KS=0.1, p=0.60), but correlations between pairs of taste-selective neurons (i.e., pairs of neurons in which both the PC and the GC neuron showed a taste-selective response, n=12) were significantly higher than those between non-selective pairs of neurons (n=45; KS=0.3, p=0.03). Correlations were therefore not the result of global fluctuations in modulatory activity, but instead reflected functional connectivity specifically shared by taste-selective GC and PC neurons. Moreover, further analyses and experiments (described below) demonstrated that correlations between GC and PC are not driven by a third area.
Further analysis suggested that taste selectivity in PC, averaged over all recorded neurons (n=71), evolved with similar temporal dynamics as in GC (n=27). Figure 1D illustrates the temporal dynamics of taste selectivity in the population of GC and PC neurons. Note that taste selectivity in Figure 1D is normalized to peak in order to highlight the similarity in time courses. Direct comparison of the magnitude of taste selectivity in the two regions revealed that taste selectivity in PC is smaller than that observed in GC (Figure 1E; t96=3.1, p<0.01), consistent with GC being a primary taste region (receiving direct thalamic taste input), and PC being a region that is indirectly modulated by taste input.
The striking similarity of the evolution of taste selectivity in PC and GC shown in Figure 1D, and the fact that the PC function seems to slightly lag behind the GC function, suggests (but does not test) the possibility that taste information may be passed from the latter to the former. To more rigorously examine this possibility, we calculated the cross-correlation between the time course of taste selectivity in each individual simultaneously-recorded PC and GC neuron pairs, reasoning that if PC taste selectivity originates in GC, taste selectivity should appear in the GC response before it appears in the PC response. The results of this analysis—a histogram of the timing of peaks in cross-correlation for all pairs of taste-selective neurons—are shown in Figure 1F. The distribution of peaks is significantly skewed towards positive values (t11=2.5, p=0.03)—in fact, we found only one example (out of 12 simultaneously-recorded GC-PC neuron pairs both showing taste selectivity) that failed to accord to this pattern.
Olfactory cortical taste responses are reduced by optical inhibition of gustatory cortex
While these data suggest that taste-selective information reaches PC via GC, this evidence is explicitly correlational. In order to more directly test the causal influence of GC input on gustatory processing in PC, we expressed the light-sensitive inhibitory channel ArchT [25, 26] in GC neurons (see Experimental Procedures). Illuminating GC neurons via optic fibers implanted in GC resulted in overall inhibition of GC spiking activity (GCx), effectively removing excitatory input from GC to PC (Figure 2A; see also below and Supplementary Figure S1). The high temporal resolution of optogenetic inhibition allowed within-session comparison of PC activity in the presence and absence of GC input.
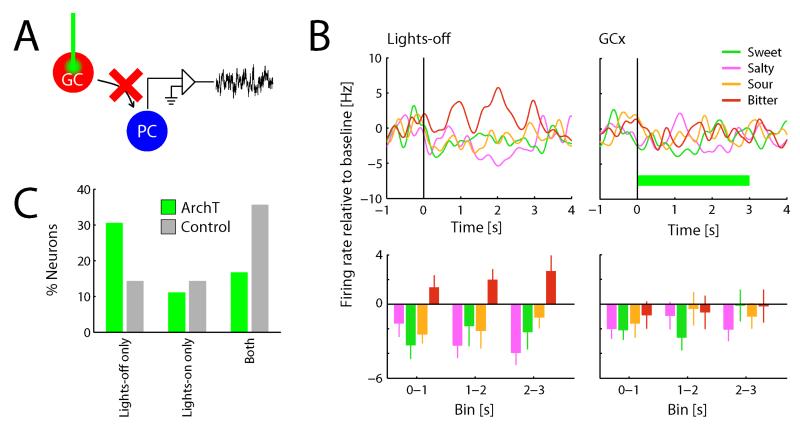
Figure 2. GC is the source of taste-selective input to PC.
A. Simultaneous GCx and recording from PC allows assessment of causal influences of GC on neural processing in PC. B. Example of a PC neuron that exhibits a taste-selective response during lights-off trials, but not during GCx trials (n=10 trials/condition; same conventions as in Figure 1B; horizontal green bar indicates lights-on period). C. Percentage of taste-selective responses observed during lights-on and lights-off trials across the complete sample of neurons obtained from subjects expressing ArchT (n=36) as well as control subjects (n=14). The proportion of taste-selective neurons during lights-off trials is significantly higher than during lights-on trials for ArchT subjects (Χ2 test, p<0.05), and this distribution is significantly different than for control subjects (Χ2 test, p<0.01).
We once again recorded the spiking responses of PC neurons to intra-oral delivery of taste solutions, but on a randomly-selected half of the trials, GC was illuminated at the time of stimulus presentation; on the other half of the trials, no illumination was applied. If GC indeed provides taste-selective input to PC, taste selectivity in PC should be decreased on GCx trials, relative to lights-off trials.
Figures 2B and 2C show the result of this experiment. Figure 2B shows the response of an example PC neuron to taste stimuli during GCx and lights-off trials. In the latter condition, this neuron exhibited a robustly taste-selective response, most notably displayed as a distinctive, selective, and significant increase of firing rate in response to quinine; during GCx trials, this taste-selective response was absent. This result was representative of those observed across the population of PC neurons recorded in this experiment (n=36; Figure 2C): almost 3 times as many PC neurons lost responses during GCx as showed the opposite pattern (taste selectivity during lights-on [GCx] only). Overall, taste selectivity was almost twice as robust during lights-off trials as GCx trials (Χ2=5.1, p=0.02), indicating that GCx reduces taste selectivity of PC neurons. We did not observe any specificity in which tastes were more likely affected by GCx than others.
Note that taste selectivity was not completely eliminated by GCx, but merely reduced. This likely reflects the fact that infection is not complete (i.e., not every cell expresses ArchT, and illumination suppressed but did not eliminate spiking, see Supplementary Figure S1). As a control, therefore, we subjected a separate group of subjects to the exact same stimulation protocol (both taste and optical), but without first infecting those subjects with ArchT. Light stimulation alone did not affect the likelihood of observing taste selectivity in PC (Figure 2C, grey bars; lights-on compared to lights-off for control group; Χ2=0.0, p=1; ArchT versus control: Χ22=11.4; p<0.01), although a low level of change was observed (see below).
We also performed tests of the possibility that suppression of taste responses in PC by GCx is the result of altered orofacial behavior in response to taste stimuli (as opposed to altered taste responses themselves). These experiments demonstrated that the palatability-relatedness of taste behavior is maintained during GCx, as are the metrics of mouth movements themselves (see Supplementary Figure S1). Thus, the findings shown in Figure 3 cannot be explained in terms of the impact of GCx on oromotor responses to tastes.
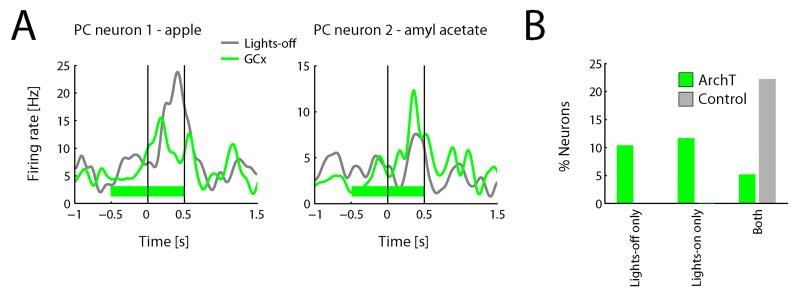
Figure 3. Spontaneous activity in GC modulates olfactory processing in PC.
A. Examples of odor-selective responses recorded from PC that are modulated by GCx. Traces show average responses during GCx and lights-off trials. Solid vertical lined indicate odor on- and offset, horizontal green bar indicates lights-on period. Both neurons show significant effects of Odor and Stimulation (n=10 trials/condition; two-way ANOVA, p<0.05). B. Percentage of odor-selective responses during GCx and lights-off trials across the population on a neuron-by-neuron basis (n=77).
These data demonstrate that the removal of GC input decreases taste selectivity in PC, thus providing causal evidence that taste selectivity reaches PC via mono- or poly-synaptic connections with GC.
Optogenetic inhibition of gustatory cortex modulates odor responses in olfactory cortex neurons
The results presented thus far indicate that multisensory connectivity relays taste-selective information from gustatory to olfactory cortex, and suggest a substrate whereby even spontaneous neural activity in GC (i.e., activity in the absence of taste stimuli) impacts PC firing [12-14] (see Figure 1C). That is, if spontaneous firing in GC courses to PC as taste-related firing does, it is reasonable to hypothesize that GCx might not only eliminate gustatory processing but also change olfactory processing in PC. Note that we are specifically hypothesizing a change in processing, not the elimination of odor processing, under the assumption that olfactory information will reach PC (via the olfactory bulb) regardless.
To test this hypothesis, we recorded spiking activity of PC neurons as subjects sampled odorants presented in an air stream (via an olfactometer). On a random half of the trials, GC neurons were optogenetically inhibited just before and during stimulus presentation; on the other half of the trials, no illumination was applied. That is, this experiment was essentially identical in form to the previous experiment on taste responses, with one notable distinction: because any set of olfactory stimuli comprise only a tiny portion of the potential stimulus space (unlike the small battery of taste stimuli, which spans almost the entire space of basic taste qualities), we could not determine the overall odor-responsiveness of the neurons on the basis of their responses to a small set of odors; our initial analyses of olfactory responses therefore proceeded on an individual odor basis [27, 28] (see below for a neuron-based analysis similar to the analysis performed on taste responses).
Across the sample of 142 neuron-odor pairs, 18 (13%) were modulated by GCx (in a two-way ANOVA with factors Odor [odor versus clean air] and Stimulation [lights-on versus lights-off]) in the absence of gustatory stimulation. Restricting our analysis to those neuron-odor pairs that exhibited an odor-selective response (n=42), 21% were significantly modulated by GCx.
A closer look revealed a basic difference between the impact of GCx on odor responses and the impact of GCx on taste responses. Figure 3A, which shows the odor-evoked firing rate of two example odor-responsive PC neuron-odor pairs in GCx and lights-off conditions, reveals two distinct types of significant effects of Stimulation: the example on the left shows a suppressed odor response on GCx trials; the example on the right an enhanced odor response on GCx trials. This diversity of effects characterized the entire sample: across the 18 neuron-odor pairs that showed a significant effect of stimulation, increases (n=11) and decreases (n=7) in firing rate occurred similarly often.
Again, this bi-directionality distinguishes the impact of GCx on odor processing from its impact on gustatory processing (recall that the vast majority of changes in taste responses were decreases). Figure 3B permits a more direct comparison between these two effects, plotting odor selectivity (one-way ANOVA including responses to all odors, on a neuron-by-neuron basis) in the different stimulation conditions: odor selectivity was equally likely observed during GCx and lights-off trials (Χ2=0.1, p=0.75)—a pattern that was significantly different from the one observed for taste selectivity (Χ22=7.7, p<0.05).
The fact that GCx had distinct effects on PC responses to tastes and odors renders it unlikely that our results reflect artifactual impact of illumination itself on OC activity. Nonetheless, we went on to directly test this possible influence by subjecting a separate group of animals not infected with ArchT to the identical stimulation paradigm as described above. These experiments did not yield any significant effects of Stimulation on odor responses (Figure 3B; n=0 out of 18 neuron-odor pairs). To further address the possibility that mere light stimulation affects neural activity in PC neurons, we assessed the effect of light stimulation in uninfected animals on spontaneous firing rate in PC neurons. In this condition, only one neuron out of a total of 24 (4%, i.e., chance level) showed a significant modulation by light stimulation.
These data confirm that the effects observed on odor responses in the GCx condition are not due to sensory stimulation associated with the laser turning on, nor to a direct influence of light on neural tissue in GC, but instead are due to ArchT mediated inactivation of GC neurons. Rather, GC continuously modulates neural activity in PC, such that when input from GC is removed, some odor-evoked responses appear while others vanish—effectively changing the ensemble activated by a given odor.
Optogenetic inhibition of gustatory cortex affects unimodal odor perception
The findings presented above suggest an integral role for GC in olfactory coding, but stop short of demonstrating that this role has functional relevance. If the changes in PC activity observed during GCx actually reflect changed odor representations, then it should be possible to show GCx changing how odor stimuli are perceived by the subject.
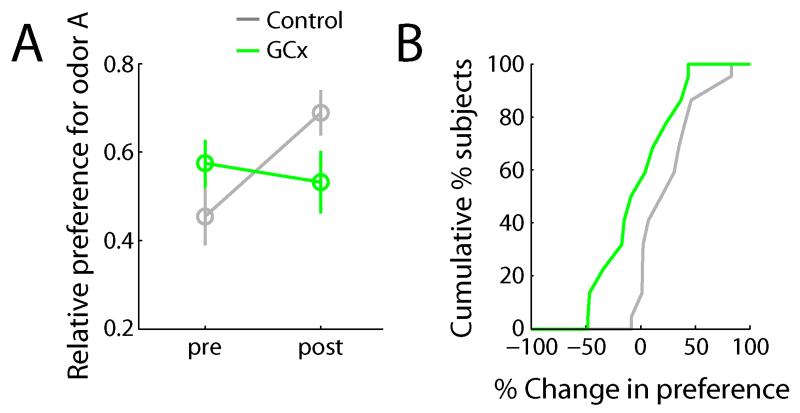
In order to probe this possible functional consequence of GCx, we tested subjects’ ability to express a previously learnt odor preference during GCx. Relative preferences for two odors (measured in terms of relative consumption of odorants A and B in water) were assessed before and after training sessions in which the subjects learned to associate odor A with saccharin, a non-caloric sweet taste reward [29]. Figure 4A shows relative preference for odor A, before and after training sessions. Control subjects (n=11), as a group, reliably expressed a preference for odor A after training, as evidenced by an increase in relative consumption of odor A versus odor B (Figure 4B, t10=2.9, p=0.02). Individual animals’ results paralleled the group average: 10 out of 11 (91%) subjects showed an increase in preference.
Figure 4. GC influences odor perception.
A. Average relative preference for odor A before and after training sessions for GCx (n=11) and control (n=11) groups. Preference after training sessions is significantly higher than before for control subjects (t-test, p<0.05). B. Cumulative histogram of change in preference (post – pre) for GCx and control subjects. Change in preference is significantly higher for control subjects compared to GCx subjects (t-test, p<0.05).
When we optogenetically inhibited GC during odor sampling during the testing session (i.e., after preference learning had taken place), in contrast, this group of subjects (n=11) did not express an increase in preference for odor A (t10=0.5, p=0.63; change in preference compared to control: t20=2.2, p=0.04). Again, individual animals’ consumption parallel the group average: 5 out of 11 (45%) subjects increased (by any amount) their consumption of odor A; 6 (55%) subjects decreased their consumption (by any amount) of odor A. Note that pre-training preferences did not differ between groups (t=1.8, p=0.09).
The magnitude of the behavioral effect (i.e., no evidence of preference for odor A, as opposed to reduced preference for odor A during GCx), despite a limited effect on neural responses—only 21% of odor responses appear to be affected by GCx—can be explained by the possibility that from a behavioral perspective, there is no continuous assessment of similarity in the two conditions: the animal either recognizes the odor or it does not. In our experiment, most animals did not recognize the odor, leading to a failure to perform the task altogether.
With these data, we conclude that removal of gustatory cortex perturbs putatively unimodal olfactory coding; that is, activity in gustatory cortex modulates odor perception, even in the absence of taste stimulus presentation.
Discussion
Numerous studies have shown that sensory systems influence each other. By probing sensory systems with multisensory input, these studies have provided ample evidence that sensory processing is influenced, even at the level of primary sensory cortex, by heteromodal stimuli (vision [8, 30-32]; audition [9, 10, 33]; gustation [15, 34]; and olfaction [11, 35, 36]). The work presented here provides novel insight into the nature of functional interactions between sensory systems, and into the impact of such interactions on sensory processing. Specifically, we identified a functional connection between the taste and olfactory systems, such that projections from taste to olfactory cortex, besides providing information about taste stimuli, modulate native functionality of the olfactory system, even in the absence of a taste stimulus. That is, we show here that gustatory cortex is involved in olfactory perception.
These results reveal that crossmodal circuitry plays an even more integral role in sensory processing than previously thought. Early accounts [37] acknowledged the existence of multisensory convergence sites at the borders between sensory cortices (i.e., beyond classic multisensory zones such as the superior colliculus [3] and prefrontal cortex [38]). Subsequently this idea was extended to include core regions of sensory cortex as receiving multisensory input [39]. The present findings further extend the view of sensory processing as inherently multisensory by demonstrating that, in the case of olfactory cortex, unisensory odor stimuli are processed by a multisensory network involving taste cortex.
Contrary to the common intuition that smell influences taste, which is based on the interchangeable use of the words “taste” and “flavor” in everyday language, the influence of taste stimuli on smell is at the heart of several basic food-related behaviors, such as the acquisition and expression of food preferences [29, 40] and aversions [41-43]. Our results implicate PC as an important node in the network mediating such flavor-driven behaviors. The functional significance of GC influencing PC in the absence of taste stimulation remains unclear, however. Previous work has shown that pharmacological inactivation of GC influences performance on an ethological olfactory learning task in a state-dependent manner: olfactory preferences that normally form during a “training” interaction between a subject rat and a recently-fed conspecific were not expressed with GC firing inhibited during either training or testing sessions, but were rescued when GC was inactivated during both training and testing. This state-dependency of olfactory perception suggested that GCx does not impair, but rather changes olfactory perception [40] (see also [44]).
Our finding that GCx results in increases as well as decreases in odor responses is consistent with this suggestion. Moreover, that fact that our behavioral results revealed a complete inability of the animals to express learnt odor preferences during GCx further supports the notion that GCx modulates odor representations: by changing the nature of responsive PC ensembles, GCx does not diminish the amount of olfactory information, but essentially changes the olfactory code, and as a consequence affects how an odor is perceived by the animal. Given that the nature of this effect on olfactory representations differed from the effect of GCx on taste representations, non-specific effects of GCx cannot explain our results. Which specific aspect of the odor representation is affected by GCx is thus far unknown, however. The current behavioral findings, in conjunction with our earlier papers on GC taste responses [15, 17, 45], make it reasonable to propose that GC may provide input relating to the visceral/hedonic aspect of an odor stimulus to PC.
The idea that GC might influence a specific aspect of the olfactory code may seem to argue against a mere “modulatory” influence of GC on PC. Previous work at the circuit level has demonstrated somatosensory modulation of auditory cortex [9] and auditory modulation of visual cortex [8], and is characterized by a non-specific influence of the heteromodal stimulus on native sensory cortex, affecting the probability that native sensory input evokes a response, not the nature of the response. GC may provide a similar influence on PC, affecting the probability that an odor stimulus evokes a response in a given neuron. However, the proposed distributed nature of the olfactory code [46] in PC would allow for specific aspects of odor information to be altered via this mechanism. Thus, modulation of responses in individual neurons could lead to qualitative differences in odor coding. Finally, the present analyses only considered effects of GCx on overall firing rate in response to odor stimuli. It is entirely possible that input from GC changes temporal aspects of the olfactory response as well (e.g., see [47]for an example of temporal modulation by heteromodal input to the auditory system). Indeed, recent work has suggested that temporal aspects of olfactory responses are relevant for encoding odor valence [48, 49].
Our data identify a novel systems-level phenomenon—the effect of multisensory network connectivity on unisensory processing. Identification of the exact circuit underlying this intriguing and novel effect, a task that is above and beyond the hypotheses tested here, will be the topic of future work for which the current project sets the stage (similarly beyond the scope of this first project are questions regarding whether the novel phenomenon depends on the action of particular cell types). It is possible that axons directly connecting GC and PC [21-23] provide the substrate for the observed effects. It is also possible, however, that GC influences PC only indirectly, via multisynaptic connections. Indeed, our finding of relatively long lags (on the order of hundreds of milliseconds) separating taste selectivity of GC and PC argues against monosynaptic input from GC to PC. By one possible alternative route, GC may project to orbitofrontal cortex, which then feeds back to PC. Multisensory modulation of primary sensory cortex via a higher order multisensory region has been suggested to underlie modulation of primary auditory cortex by visual stimuli [10, 50, 51].
It is also conceivable that even olfactory information reaches PC via GC—previous work has suggested that GC responds to odor stimuli [52, 53]. Although we cannot definitively rule out this possibility, it is unlikely to explain our findings, because GCx would then be expected to diminish olfactory responses, just as it does taste responses. We instead found both suppressed and enhanced olfactory responses in equal numbers. The total size of the olfactory response (i.e., the number of responsive neurons) in PC was similar before and during GCx, suggesting that our manipulation did not cut the route whereby olfactory information reached PC. The likely source of that information is of course the olfactory bulb. The most far-reaching aspect of our results, therefore, is that patency of the bulbar-PC connection is necessary but insufficient to assure reliable PC odor responses.
Well-known functional and adaptive interactions between taste and smell, as well as anatomical connections between PC and OC, formed the basis for the hypotheses tested in the present paper. However, the specificity of the observed effects remains unknown. It is possible that the observed effects of GCx reflect a general network phenomenon. That is, inhibition of spontaneous activity in any sensory system (or more general, any brain region) projecting to PC affects olfactory responses. For example, previous work has identified functional auditory inputs to the olfactory tubercle [36]. While it is likely that inhibiting spontaneous activity in these auditory projections has some effect on neural activity in the olfactory system, the auditory responses observed by Wesson et al. [36] were much less prevalent and much less pronounced than the taste responses in piriform cortex described here. Moreover, auditory inputs likely play a different functional role in olfaction than taste inputs do. Auditory inputs are therefore unlikely to affect olfactory coding in the same way and to the same degree as taste inputs do.
Another interesting question for future research is the generalizability of the present findings to other sensory interactions. For example, previous work has identified strong functional interactions between auditory and visual cortex [33, 51] that are thought to play a key role in various adaptive behaviors. The effects of inhibiting spontaneous activity in visual cortex on auditory processing is to date unknown, but we predict that any changes reflect the behavioral relevance of visual input to the auditory system.
Experimental Procedures
Subjects
Naïve adult female Long-Evans rats (www.criver.com), weighing between 250 and 325 g at the time of surgery served as subjects. All subjects were individually housed and kept on a 12/12 hour light/dark cycle. Experiments were conducted during the light cycle, and complied with the Brandeis University Institutional Animal Care and Use Committee guidelines.
Surgery
Stereotaxic surgery was performed under ketamine/xylazine anesthesia. Multi-electrode assemblies (16 wires/assembly [15]) were implanted into left posterior piriform olfactory cortex (PC, 1.4 mm posterior to bregma, 5.5 mm lateral to the midline, 7 mm ventral from the surface of the brain) and left insular gustatory cortex (GC, 1.4 mm anterior to bregma, 5 mm lateral from the midline, 4.7 mm ventral from the surface of the brain). Optic fibers were implanted into GC bilaterally. Intra-oral cannulae (IOC) were implanted into the oral cavity [54].
Adeno-associated virus (serotype 9) coding for ArchT (AAV-CAG-ArchT-GFP; www.genetherapy.unc.edu) was injected into GC bilaterally (5 μl/hemisphere), three weeks before implantation of electrodes, optic fibers and IOCs. AAV serotype 9 is known to spread well across the tissue and infect all cell types [55].
For purposes of both electrode and optic fiber implantation, GC was defined as the region of insular cortex where we [15-17, 45] and others [20, 53, 56] have repeatedly found a high density of taste responses. This region corresponds with the region previously identified as receiving projections from the gustatory thalamus [19].
Electrophysiological recording and data analysis
Spike waveforms from the extracellular signal recorded with each electrode were amplified, filtered and clustered into single unit records (www.plexon.com). Spike time stamps were aligned to stimulus onset, binned in 1 ms bins and averaged over trials. Average baseline firing rate (1 s immediately preceding stimulus onset) was subtracted from the average responses to tastes (3 s immediately following stimulus onset) before any further analysis. Spike-density functions were computed for display purpose only by convolving spike times with a Gaussian. Transient noise artifacts resulting from opening and closing of the valves controlling taste delivery, which were excluded from all analyses, but caused seeming baseline variability in displays of spike density functions, were removed manually before plotting example figures.
Stimuli
Taste stimuli consisted of aliquots (30 μl) of basic tastes spanning the entire taste quality space: sucrose (100 mM), sodium chloride (100 mM), citric acid (100 mM) and Quinine-HCl (1 mM) solutions. All taste solutions were presented at room temperature, in aqueous solution of the same volume, viscosity, and lubricity, using the same delivery method, ensuring identical mouthfeel. Moreover, the concentrations used are far below those known to activate the trigeminal system.
Odor quality space is virtually infinite (due to the synthetic nature of olfaction), making it impossible to even approximate complete sampling of this space. Instead, we chose exemplars of monomolecular odorants: amyl acetate and methyl valerate, as well as complex odorant mixtures: apple and strawberry aroma. All odor stimuli consisted of saturated vapor (in N2, on medical grade air). Medical grade air alone was used as a control stimulus.
Optical stimulation
GC was illuminated bilaterally with 532 nm light from a laser (30-40 mW) through multimode optic fibers (200 μm diameter) connected via a ferrule (www.thorlabs.com) [57]. Optic fibers were implanted just above GC, identified (see above) as the thalamic-recipient region within which we have repeatedly observed large numbers of taste responses [15-17, 20, 45, 53, 56]. Light strength was chosen to allow sufficient power to cause inactivation of cells at a depth of up to 1 mm from the tip of the fiber [58], thus covering a large portion of identified taste cortex while leaving unaffected cells outside of this region (See supplementary Figure S1)—thus we have high confidence that all of the impact of illumination was confined to the region of taste cortex.
Illumination was applied at the onset of stimulus presentation and lasted for the duration of the stimulus (taste: 0-3 s; odor: 0-0.5 or 0-1 s after stimulus onset). In a subset of sessions, illumination was applied starting 0.5 s prior to odor onset and lasted for the duration of the stimulus (−0.5-0.5 s around stimulus onset). Lights-on and lights-off trials were randomly interleaved.
Note that we specifically avoided, as much as possible, complex network effects that arise when only one particular cell type is manipulated (e.g., the fact that inactivation of interneurons necessarily disinhibits the firing of other cortical neurons), by inactivating GC neurons in a cell type general manner (similar to pharmacological inactivation, but with vital temporal control).
Behavior
Sensory stimulation task
Taste stimuli were delivered passively through IOC directly into the oral cavity (10-20 repetitions per stimulus, ITI: 15-30 s). Odor stimuli were delivered via an olfactometer, immediately upon the subject triggering an infra-red beam in a nose poke (10-20 repetitions per stimulus, ITI: >10 s). Subjects were trained to keep their nose in the odor port for the duration of the stimulus (0.5 s or 1 s) and were rewarded for successful trials (30 μl water, presented through IOC, 1 s after odor offset). Stimuli were presented randomly within taste and odor stimulation blocks.
Odor preference learning task
After habituation to the experimental setup, each subject was subjected to the following experimental protocol:
Day 1 – Preference testing: odor A in water versus odor B in water.
Day 2-5 – Training: odor A in 0.2% saccharin (2 sessions) and odor B in water (2 sessions).
Day 6 – Preference testing: odor A in water versus odor B in water.
During preference testing sessions (30 minutes duration), subjects were free to approach a pair of nose pokes giving access to two different odorized solutions, delivered via IOC immediately upon the subject triggering the infra-red beam in the nose pokes (30 μl; ITI: 3 s). Preference was calculated as relative consumption: odor A / (odor A + odor B). During training sessions, subjects were free to approach a single nose poke giving access to one odorized solution (odors A and B were presented on alternating days). Odor stimuli were 0.025% amyl acetate and 0.025% methyl valerate (in water), and the identity of odor A was counterbalanced across subjects. Illumination was applied for 2.5 s immediately upon the subject triggering the infra-red beam, during the final preference testing session only (day 6).
Statistics
Significance of effects was assessed using standard tests (defined in the results section). Distributions were visually inspected to make sure that comparisons were made between groups with similar variance, and that the data meet the assumptions of the tests used. Sample size, both in terms of number of trials and number of neurons are typical for sensory electrophysiology studies. Effect size was calculated as eta-squared, a standard measure of effect size, that describes the ratio of variance explained in a dependent variable (e.g., Taste) by a predictor relative to other predictors, and is obtained by dividing the sum of squares for that factor by the total sum of squares: eta=SS(factor)/SS(total).
Supplementary Material
Acknowledgments
Many thanks to Daniel Ley for technical assistance. This work was supported by R03 DC14017 (to JXM) and R01 DC7702 and DC6666 (to DBK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Experimental design (JXM, MLB, JXL, DBK), data collection (JXM, MLB), data analysis (JXM, JXL), manuscript preparation (JXM, DBK).
References
- 1.Rowe C. Receiver psychology and the evolution of multicomponent signals. Animal behaviour. 1999;58:921–931. doi: 10.1006/anbe.1999.1242. [DOI] [PubMed] [Google Scholar]
- 2.Small DM, Green BG. A Proposed Model of a Flavor Modality. In: Murray MM, Wallace MT, editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): 2012. [Google Scholar]
- 3.Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge, Mass.: 1993. [Google Scholar]
- 4.McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa N, Ichihara S. Hearing visual motion in depth. Nature. 2002;416:172–174. doi: 10.1038/416172a. [DOI] [PubMed] [Google Scholar]
- 6.Shams L, Kamitani Y, Shimojo S. Illusions. What you see is what you hear. Nature. 2000;408:788. doi: 10.1038/35048669. [DOI] [PubMed] [Google Scholar]
- 7.Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- 8.Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73:814–828. doi: 10.1016/j.neuron.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier JX, Wachowiak M, Katz DB. Chemosensory convergence on primary olfactory cortex. Journal of Neuroscience. 2012;32:17037–17047. doi: 10.1523/JNEUROSCI.3540-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 15.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontanini A, Katz D. State-dependent modulation of time-varying gustatory responses. J Neurophysiol. 2006;96:3183–3193. doi: 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sadacca BF, Rothwax JT, Katz DB. Sodium concentration coding gives way to evaluative coding in cortex and amygdala. Journal of Neuroscience. 2012;32:9999–10011. doi: 10.1523/JNEUROSCI.6059-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. Journal of Neuroscience. 2012;32:9981–9991. doi: 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain research. 1986;379:342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- 20.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain research. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 21.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. Journal of Comparative Neurology. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 22.Shi C, Cassell M. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Datiche F, Litaudon P, Cattarelli M. Intrinsic association fiber system of the piriform cortex: A quantitative study based on a cholera toxin B subunit tracing in the rat. J Comp Neurol. 1996;376:265–277. doi: 10.1002/(SICI)1096-9861(19961209)376:2<265::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nature neuroscience. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nature neuroscience. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- 29.Holman E. Immediate and delayed reinforcers for flavor preferences in rats. Learn. Motiv. 1975;6:91–100. [Google Scholar]
- 30.Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- 31.Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 33.Kayser C, Petkov CI, Logothetis NK. Visual modulation of neurons in auditory cortex. Cerebral Cortex. 2008;18:1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- 34.Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiology & behavior. 1999;67:489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 35.Carlson KS, Xia CZ, Wesson DW. Encoding and representation of intranasal CO2 in the mouse olfactory cortex. J Neurosci. 2013;33:13873–13881. doi: 10.1523/JNEUROSCI.0422-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wesson DW, Wilson DA. Smelling sounds: olfactory-auditory sensory convergence in the olfactory tubercle. Journal of Neuroscience. 2010;30:3013–3021. doi: 10.1523/JNEUROSCI.6003-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- 39.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends in cognitive sciences. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, Katz DB. State dependence of olfactory perception as a function of taste cortical inactivation. Nature neuroscience. 2010;13:158–159. doi: 10.1038/nn.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmerino CC, Rusiniak KW, Garcia J. Flavor-illness aversions: the peculiar roles of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- 42.Rusiniak KW, Hankins WG, Garcia J, Brett LP. Flavor-illness aversions - potentiation of odor by taste in rats. Behav. Neural Biol. 1979;25:1–17. doi: 10.1016/s0163-1047(79)90688-5. [DOI] [PubMed] [Google Scholar]
- 43.Slotnick BM, Westbrook F, Darling FMC. What the rat’s nose tells the rat’s mouth: Long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim Learn Behav. 1997;25:357–369. [Google Scholar]
- 44.Mak YE, Simmons KB, Gitelman DR, Small DM. Taste and olfactory intensity perception changes following left insular stroke. Behavioral Neuroscience. 2005;119:1693–1700. doi: 10.1037/0735-7044.119.6.1693. [DOI] [PubMed] [Google Scholar]
- 45.Maier JX, Katz DB. Neural dynamics in response to binary taste mixtures. J Neurophysiol. 2013;109:2108–2117. doi: 10.1152/jn.00917.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chemical senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 47.Chandrasekaran C, Lemus L, Ghazanfar AA. Dynamic faces speed up the onset of auditory cortical spiking responses during vocal detection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4668–4677. doi: 10.1073/pnas.1312518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69:1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gire DH, Whitesell JD, Doucette W, Restrepo D. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nature neuroscience. 2013;16:991–993. doi: 10.1038/nn.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder CE, Foxe JJ. The timing and laminar profile of converging inputs to multisensory areas of the macaque neocortex. Brain Research. Cognitive Brain Research. 2002;14:187–198. doi: 10.1016/s0926-6410(02)00073-3. [DOI] [PubMed] [Google Scholar]
- 52.Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM. The insular taste cortex contributes to odor quality coding. Front Hum Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste Responses of Cortical-Neurons in Freely Ingesting Rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- 54.Phillips MI, Norgren RE. A Rapid Method for Permanent Implantation of an Intraoral Fistula in Rats. Behav Res Meth Instr. 1970;2:124–&. [Google Scholar]
- 55.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PloS one. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012;74:410–422. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature protocols. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.