Abstract
Background
Evidence for association between asthma and the unfolded protein response (UPR) is emerging. ERp57 is an ER localized redox chaperone involved in folding and secretion of glycoproteins. We have previously demonstrated that ERp57 is up regulated in allergen-challenged human and murine lung epithelial cells. However, the role of ERp57 in asthma pathophysiology is unknown.
Objectives
Here, we sought to examine the contribution of airway epithelial-specific ERp57 in the pathogenesis of allergic asthma.
Methods
We examined the expression of ERp57 in human asthmatic airway epithelium and utilized murine models of allergic asthma to evaluate the relevance of epithelial- specific ERp57.
Results
Lung biopsies from asthmatics and non-asthmatics revealed a predominant increase in ERp57 in asthmatic epithelium. Deletion of ERp57 resulted in a significant decreases in the inflammatory cells and airways resistance in a murine model of allergic asthma. We further observed that disulfide bridges in eotaxin, EGF and periostin were also decreased in the lungs of HDM-challenged ERp57 deleted mice. Fibrotic markers such as collagen and αSMA were also significantly decreased in the lungs of ERp57-deleted mice. Furthermore, adaptive immune responses were dispensable for HDM-induced ER stress and airways fibrosis.
Conclusions
Here we show that ERp57 is increased in the airway epithelium of asthmatics and in mice with allergic airways disease. ERp57 increase is associated with redox modification of pro- inflammatory, apoptotic and fibrotic mediators, and contribute to airways hyperresposiveness (AHR). The strategies to inhibit ERp57 specifically within the airways epithelium may provide an opportunity to alleviate allergic asthma phenotype.
Keywords: UPR, ER Stress, Asthma, HDM, PDI, ERp57, Rag1, Epithelium, AHR
Introduction
Allergic asthma is characterized by airways inflammation, mucus metaplasia and peri-bronchiolar fibrosis which impacts lung structure and function1, 2. Airway epithelial cells (AECs) reside at the intersection of the lung and the external environment3. Recent studies have demonstrated that activation of a number of receptors on the surface of AECs and subsequent secretion of various mediators are required for responses from dendritic cells (DCs) and subsequent immune responses1, 4–6. House Dust Mite (HDM) is one of the most commonly found complex airborne allergens7, inducing an allergic response in approximately 50–85% of asthmatics7, 8. HDM contains numerous antigens, proteases and ligands for Pattern Recognition Receptors (PRRs), resulting in activation of airway epithelial cells and inducing the secretion of growth factors and cytokines that regulate subsequent activation of innate lymphoid cells, T cells, mucus metaplasia, inflammation, airways hyperresponsiveness (AHR), and fibrosis 7, 9, 10.
Complex allergens such as HDM are known to induce the unfolded protein response (UPR)11. Demand for increases in protein synthesis and folding (eg. cytokine or mucus production) can create an imbalance in the endoplasmic reticulum (ER). This leads to an increase in misfolded proteins in the ER, causing ER stress and initiating the UPR 12. In mammalian cells, accumulation of unfolded proteins are sensed by three ER transmembrane proteins: Inositol Requiring Enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK) 13. A prolonged UPR can cause CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP)-induced apoptosis12. Additionally, to cope with excessive protein folding load, the protein disulfide isomerases (PDIs), which construct disulfide bridges (-S-S-) in the ER, are upregulated14. One such PDI, ERp57, mediates misfolded protein-induced apoptosis by oligomerization of pro-apoptotic Bak through the formation of inter-molecular disulfide (-S-S-) bridges and the permeabilization of mitochondria15. Studies thus far from our laboratory and others have shown that ER stress-dependent activation of transcription factors Xbp1 or ATF6α are required during mucus metaplasia and pro-inflammatory responses in ovalbumin or HDM-induced allergic airways disease 16, 17. Our group has previously demonstrated that along with ATF6α, ERp57 is up regulated in both murine and human epithelial cells challenged with HDM11. However, the impact of UPR-mediated induction of ERp57 has not been characterized in the development of allergic asthma. Furthermore, it is not clear whether allergen-induced ERp57 is directly linked to multiple facets of asthma such as inflammation, apoptosis, peri-bronchiolar fibrosis and impairment in respiratory mechanics. The objective of this study was to use clinical specimens and mouse models to gain insight into the function of epithelial-specific up regulation of ERp57 in allergic asthma.
Material and Methods
Human Samples
Physician diagnosed asthma, and non-asthmatic lung tissues were obtained from University of California, San Francisco, Department of Medicine and Cleveland Clinic, Department. Of Pathobiology. The Institutional Review Boards of the University of California, and Cleveland Clinic approved provision of de-identified materials for research at the University of Vermont. All subjects were non-smokers defined as never smoker or former smoker with no smoking for at least 1 year prior to enrollment and total pack-years ≤ 15. All asthmatics were refrained from ICS for 6 weeks prior to enrollment into the study. Six (n=6) non-asthmatics, six (n=6) asthmatics lung biopsies were from UCSF airway tissue bank and three (n=3) non-asthmatics and three (n=3) asthmatics lung biopsies were from Cleveland Clinic (Table S1 A and B).
Animals
For all experiments, age matched male and female mice (C57BL6/J) were used. Bi-transgenic mice carrying the rat club cell secretory protein (CCSP) promoter 5′ to the open reading frame for the reverse tetracycline trans activator (CCSP-rtTA (Line 1, which in adult lung is expressed in bronchiolar and type II epithelial cells) 46, plus seven tetracycline operon 5′ to the open reading frame for Cre recombinase (TetOP-Cre) mice were provided by Dr. Whitsett (Cincinnati Children’s Hospital) 47. CCSP-rtTA+, TetOP-Cre+ mice were bred with mice carrying the ERp57loxp/loxp alleles48. Mice expressing CCSP-rtTA/TetOP-Cre/ERp57loxp/loxp were used to ablate ERp57 from lung epithelial cells (denoted as ΔEpi-ERp57), by feeding doxycycline (Dox) containing chow (6g/kg, Purina Diet Tech, St. Louis, MO) 5 days before exposure to HDM. Mice were maintained on Dox food until the completion of the experiment. Double transgenic littermates either containing CCSP-rtTA/TetOP-Cre or CCSP-rtTA/ERp57loxp/loxp (labeled as Ctr) fed Dox food were used as controls in the experiments. The Rag1−/− mice were maintained in our colony and for the experiments, age matched male and female mice (C57BL6/J) were used as controls (WT).
Statistics
All assays were performed in triplicates. Mice experiments were repeated once (total of 8 to 10 mice in two experiments). Data were analyzed by one-way analysis of variance (ANOVA) and a Tukey’s post-hoc test to adjust for multiple comparisons, or student’s t test where appropriate. Histopathological scores were analyzed using the Kruskal-Wallis and Dunn’s multiple comparison post-hoc tests. Data from multiple experiments were averaged and expressed as mean values ± SEM. Correlations between ERp57 scores and blood eosinophils or bronchodilator response were performed using Spearman’s rank correlation coefficients. The data were analyzed using JMP® Pro 10 (SAS Institute Inc., Cary, NC, USA). P values <0.05 were regarded as statistically significant.
A more detailed information on material and methods is available in the article’s on line data repository (supplementary material and methods section).
Results
ERp57 is increased in humans with asthma and mice with allergic airways disease
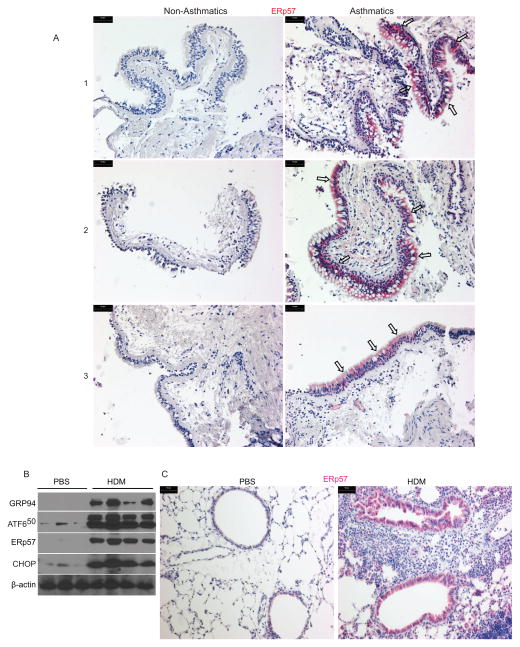
To investigate whether ERp57 expression is altered in the lung during asthma pathogenesis, we stained bronchial biopsy samples from non-asthmatics and asthmatics (refrained from inhaled corticosteroid (ICS) for 6 weeks prior to the study) for ERp57 by immunohistochemistry. Marked increases in ERp57 was observed in asthmatic lung samples (n=9) as compared to non-asthmatics (n=9) (Fig 1 A, supplementary Fig S 1 A & C and supplementary table S1 A & B). Furthermore, these increases were found to be predominantly in the airway epithelium of the asthmatics by semi-quantitative scoring (Figs 1 A, S1 A & C). Increase in ERp57 also showed a positive correlation with increases in levels of eosinophils in blood (n=9/group) and bronchodilator response (% baseline FEV1) in asthma patients (n=8-data not available for 1 of the patients) as compared to non-asthmatic (n=9) subjects (Fig S 1 D & E).
Figure 1.
ERp57 is increased in asthmatics and allergen challenged mouse epithelium. A: Representative images of human lung tissue samples obtained from UCSF airway tissue bank stained for ERp57 (red). B: Western blots of whole lung lysates from PBS or HDM challenged mice probed for various UPR markers, and ERp57. β-actin was used as control. C: Representative images of the lungs of mice challenged with PBS or HDM stained for ERp57 (red). Scale bars represents 50μm.
To explore alterations in ERp57 expression and to investigate the impact on downstream mechanisms of asthma pathogenesis, we used a model of HDM-induced allergic airways inflammation in mice. Evaluation of whole lung homogenates by western blots showed a marked increase in ERp57 as well as other ER stress markers such as GRP94, cleaved 50 kD fragment of ATF6 (ATF650) and CHOP in HDM challenged mice as compared to those challenged with PBS (Fig 1 B). To examine whether ERp57 was increased in specific cell types of the lung as observed in humans, we stained for ERp57 from both PBS and HDM challenged lungs. Immunohistochemistry of the lungs showed that there was a dramatic increase in ERp57 in the epithelium of the HDM treated lungs as compared to PBS treated lungs (Fig 1 C). These results illustrate that both humans with asthma and mice sensitized and challenged with the allergen, HDM show increases in ER stress, associated with an increase in ERp57 predominantly in the lung epithelium.
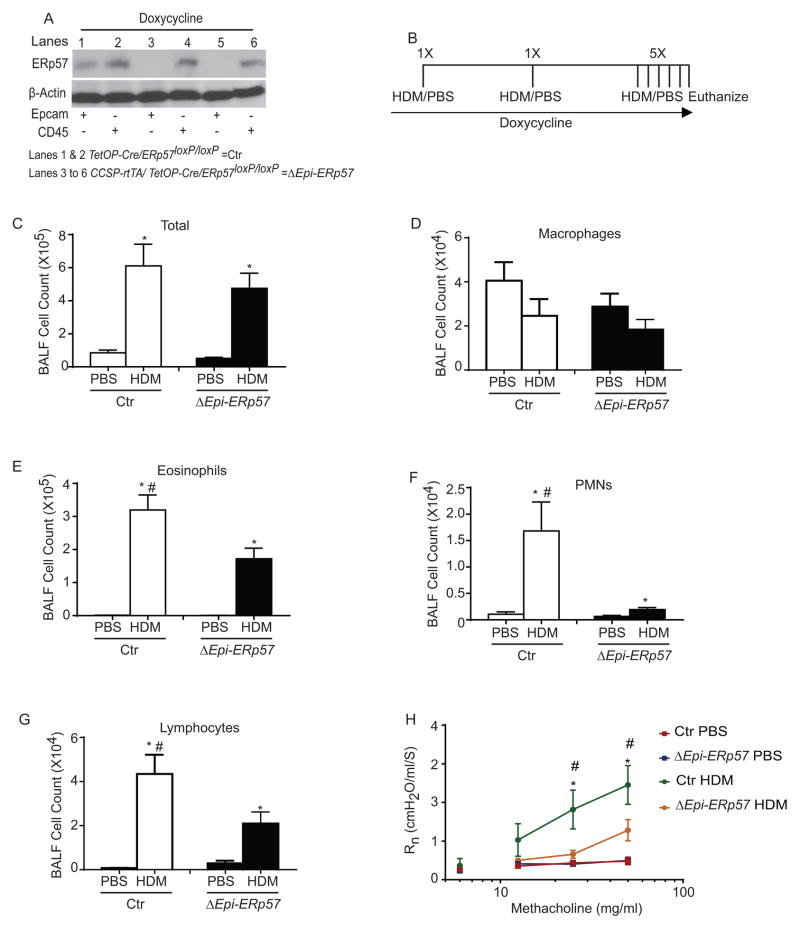
Ablation of ERp57 in lung epithelium attenuates allergen-induced asthma-like responses in mice
To determine whether increased ERp57 in airway epithelial cells may contribute to allergic airways disease in mice, we investigated whether specific deletion of ERp57 in airway epithelial cells attenuated pathophysiology associated with HDM challenge. To answer this question, we generated a doxycyline (Dox) inducible triple transgenic CCSP-rTetA/TetO-Cre/ERp57loxp/loxp (ΔEpi-ERp57) mouse to delete ERp57 specifically in lung epithelial cells. The mice carrying TetO-Cre/ERp57loxp/loxp or CCSP-rTetA/TetO-Cre were used as controls (Ctr). Our analysis showed that there was a clear decrease in ERp57 in the Epcam positive cells of lungs of the Dox treated ΔEpi-ERp57 mice as compared to Ctr mice (Fig. 2 A). For the experiments with allergen challenge, all mice were maintained on Dox for the length of the experiment beginning three days prior to initial sensitization (Fig. 2 B). Analysis of total cells in the BALF showed that there was an increased influx in cells in both HDM treated groups (Ctr and ΔEpi-ERp57) (Fig 2 C). Analysis of specific inflammatory and immune cell types indicated a significant attenuation of eosinophils, neutrophils and lymphocytes in ΔEpi-ERp57 mice challenged with HDM as compared to Ctr mice challenged with HDM. We did not observe any statistically significant changes in macrophage counts in any groups (Fig 2 D–G).
Figure 2.
HDM-induced experimental asthma is attenuated in ERp57 ablated mice. A: Ablation of ERp57 from Epcam+ epithelial cells in mice containing CCSP-rtTA/TetOP-Cre/ERp57loxP/loxP allele. β-actin was used as loading control. B: HDM or PBS instillation regimen. C–G: Analysis of inflammatory and immune cells in the BALF. H: Analysis of methacholine induced AHR in mice. *p<0.05 denotes significant differences compared with PBS groups. # p<0.05 denotes significant differences compared with the HDM groups.
We next determined the consequence of epithelial-specific ablation of ERp57 on airways hyperresponsiveness (AHR) to increasing doses of inhaled methacholine (12.5, 25 and 50mg/ml). These measurements revealed a significant decrease in AHR as measured by changes in central airways resistance (Rn) in ΔEpi-ERp57 mice challenged with HDM as compared to Ctr mice challenged with HDM (Fig 2 H). We did not observe any significant changes within the PBS treated mice from both genotypes (Fig 2 H).
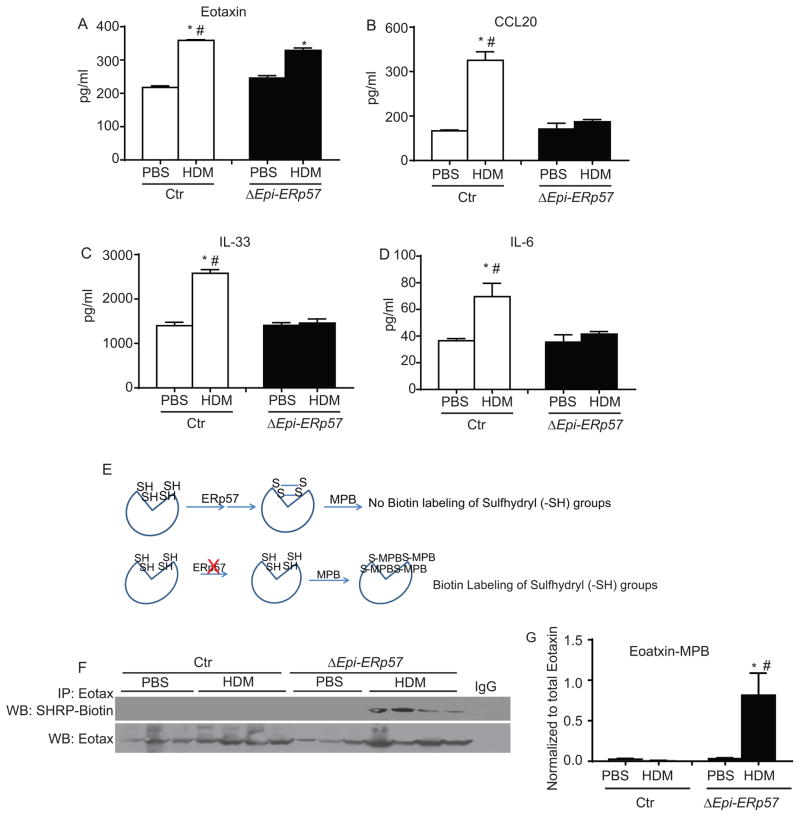
Ablation of ERp57 in airways epithelium attenuates allergen-induced cytokine and chemokine responses in the lung
Deletion of ERp57 in the airways epithelium showed significant decreases in HDM induced eosinophils, neutrophils, lymphocytes and AHR. Therefore we investigated whether these decreases could be explained by decreases in epithelial derived cytokines and chemokines. Our analysis from whole lung tissue lysates from Ctr and ΔEpi-ERp57 mice showed a slight but significant decrease in eotaxin and highly significant decreases in CCL20, IL-33 and IL-6 in HDM challenged ΔEpi-ERp57 mice as compared to Ctr HDM challenged mice (Fig 3 A–D). Although there were decreased neutrophils in the ΔEpi-ERp57 mice, we did not find any significant alterations in production of epithelial derived neutrophil-chemoattractant, G-CSF in PBS or HDM challenged mice in both genetic backgrounds (data not shown).
Figure 3.
Deletion of ERp57 in airway epithelial cells decreases various cytokines and chemokines secreted from epithelial cells. A–D: ELISA for cytokines and chemokines. E: Biotin labeling of free sulfhydryl (-SH) groups by MPB. F: Western blot analysis of sulfhydryl (-SH) content of eotaxin by MPB labeling and immunoprecipitation. G: Densitometry of the eotaxin-MPB in F. *p<0.05 denotes significant differences as compared with PBS groups. # p<0.05 denotes significant differences compared with the HDM groups.
ERp57 is a protein disulfide isomerase that specifically facilitates disulfide (-S-S-) bond formation on glycoproteins being processed and secreted by the ER18. We next determined whether deletion of ERp57 in the airways epithelium had any effect on cytokines such as eotaxin, a glycoprotein containing two disulfide bonds (http://www.uniprot.org/uniprot/P48298). Our analysis using labeling of cysteine sulfhydyl (-SH) groups of (Fig 3 E) eotaxin and subsequent immunoprecipitation revealed considerably less disulfide bonds in eotaxin in the lungs of ΔEpi-ERp57 mice compared to Ctr mice (Fig 3 F & G) challenged with HDM. Collectively these results suggest that ERp57 deletion attenuates HDM-induced cytokine production and may also be affecting the function due to the lack of disulfide bonds.
Epithelial-specific ablation of ERp57 does not alter allergen-induced mucin production
To examine the consequence of ERp57 ablation in airway mucus production we quantified Muc5AC and Gob5 levels in the total lung lysates. Our results show that mRNA for Muc5AC or Gob5 were not decreased in ΔEpi-ERp57 mice challenged with HDM compared to Ctr mice challenged with HDM (Fig. S 2 A & B). Staining for mucus in the airways by Periodic Acid Schiff (PAS) also showed similar mucus production in airway epithelial cells of ΔEpi-ERp57 and Ctr mice challenged with HDM (Fig. S 2 C & D). These results indicate that airway epithelial cell specific deletion of ERp57 has no effect on HDM induced mucus production.
Lung epithelial-specific ablation of ERp57 decreases proapoptotic Bak oligomerization, and caspase-3 activity in mice
ER stress mediated induction of ERp57 leads to interaction with Bak and forms disulfide (-S-S-) mediated Bak oligomerization, promoting intrinsic apoptosis11, 15, 19. Therefore, we next determined whether ERp57 deletion in mice decreases HDM-induced -S-S- mediated oligomerization of Bak and activation of caspase-3 (apoptotic marker). We examined the redox modification of Bak using non-reducing (-DTT) denaturing (+SDS) polyacrylamide gel electrophoresis (PAGE). Our results in figure S3 demonstrate that ER stress mediated increases in ERp57 were associated with the promotion of -S-S- mediated oligomers of Bak as evidenced by DTT mediated decomposition of oligomers in HDM challenged lungs of Ctr mice compared to ΔEpi-ERp57 mice (Fig. S 3 A). As a consequence HDM-induced caspase-3 activity was also attenuated in ERp57 deleted ΔEpi-ERp57 mice as compared to HDM-challenged Ctr mice (Fig S 3 B). Furthermore, staining for both ERp57 and active caspase-3 in serial sections (5μm apart) also revealed a dramatic decreases in active casepase-3 in the same regions of the airway epithelium that corresponds to decreases in ERp57 in ΔEpi-ERp57 mice as compared to HDM-challenged Ctr mice (Fig S 3 C). These results indicate that ablation of ERp57 in lung epithelial cells results in decreased -S-S- mediated oligomerization of Bak and decreased allergen-induced apoptosis in the lung compared to non-ablated (Ctr) mice.
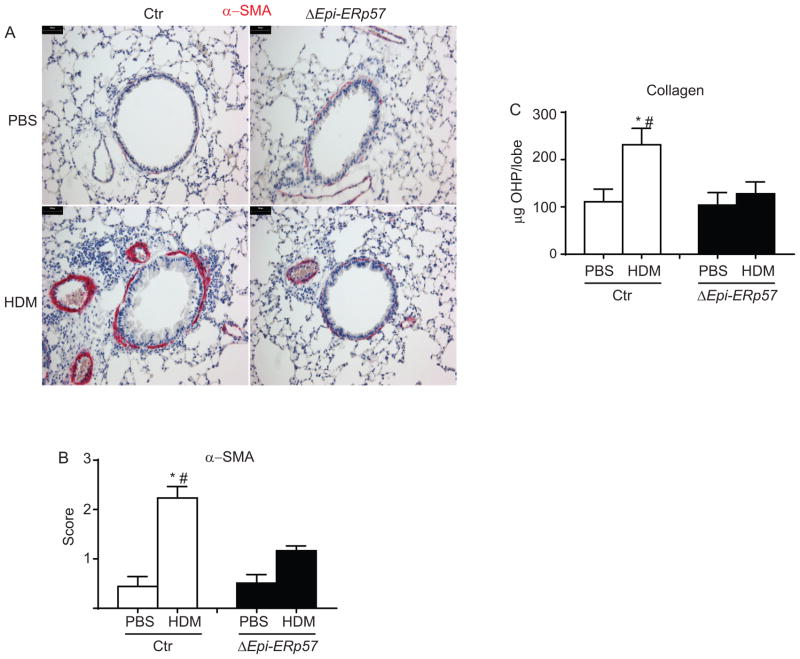
Lung epithelial-specific ablation of ERp57 decreases allergen-induced airways fibrotic alterations
To examine the role of ERp57 in structural airway remodelling, we assessed airways smooth muscle and collagen content following HDM exposure in Ctr and ΔEpi-ERp57 mice. HDM challenge led to increases in alpha-smooth muscle actin (αSMA) in the peri-bronchiolar region of HDM-challenged Ctr mice. Semi-quantitative scoring by three independent scientists blinded to the identity of the samples revealed significant decreases in αSMA staining in HDM challenged ΔEpi-ERp57 mice compared to Ctr mice (Fig. 4 A & B). Similarly, biochemical analysis of collagen deposition showed a reduction in collagen following HDM-challenge in ΔEpi-ERp57 mice compared to Ctr mice (Fig 4 C).
Figure 4.
Ablation of ERp57 in lung epithelial cells decreases smooth muscle hypertrophy and collagen deposition. A: IHC staining for α-SMA in PBS and HDM challenged lungs from Ctr and ΔERp57 mice. B: Histological scores for α-SMA. C: Analysis of collagen deposition. *p<0.05 denotes significant differences as compared with PBS groups. # p<0.05 denotes significant differences as compared with the HDM groups. Scale bars represents 50μm.
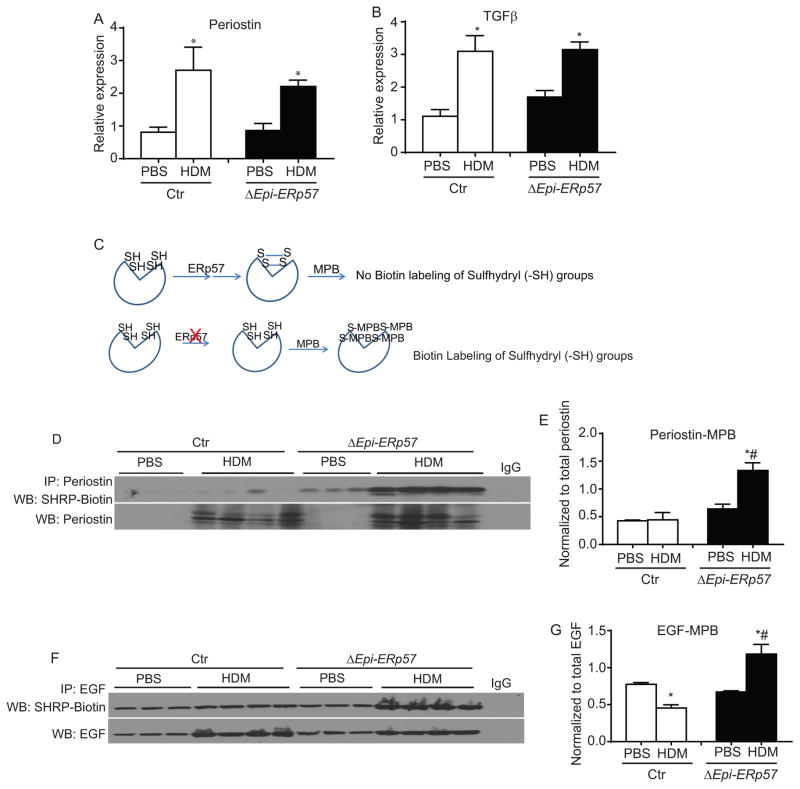
Analysis of pro-fibrotic growth factors, TGFβ and periostin, produced by epithelial cells during injury20, 21 did not show significant differences in expression (Fig. 5 A & B). Glycoproteins such as periostin and EGF (also produced by airway epithelial cells)1 have glycosylated moieties and a number of disulfide bonds in their receptor binding domain (http://www.uniprot.org/uniprot/P01132; http://www.uniprot.org/uniprot/Q62009). Our sulfhydryl labeling experiments indicated that periostin and EGF showed more exposed sulfhydryls (-SH) in the absence of epithelial ERp57. In other words, there were less disulfide bonds (-S-S-) in EGF and periostin from ΔEpi-ERp57 mice as compared to Ctr mice challenged with HDM (Fig 5 D–G). Collectively these results indicate that ER stress mediators, specifically ERp57 controls allergen- induced airways fibrosis in the lung.
Figure 5.
Ablation of ERp57 in lung epithelial cells decreases disulfide bonds (-S-S-) in pro-fibrotic growth factors. A & B: Analysis of mRNA for epithelial derived growth factors. C: Biotin labeling of free sulfhydryl (-SH) by MPB. D: Western blot analysis of sulfhydryl (-SH) content of perisotin. E: Densitometry of the periostin-MPB in D. F: Western blot analysis of sulfhydryl (-SH) content of EGF. G: Densitometry of the EGF-MPB in F. *p<0.05 denotes significant differences as compared with PBS groups. # p<0.05 denotes significant differences compared with the HDM groups.
B and T lymphocytes are dispensable in induction of HDM induced UPR and fibrosis
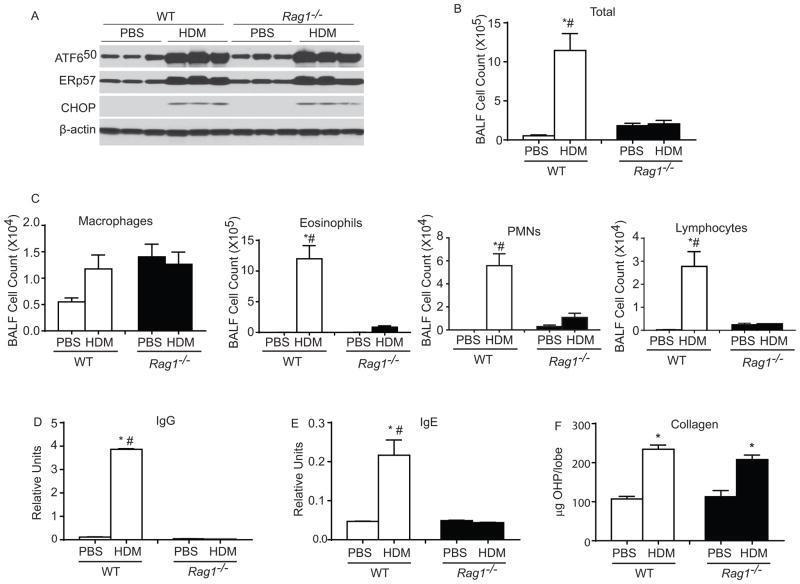
To test whether HDM-induced ER stress and increases in ERp57 were induced as a consequence of the strong adaptive immune responses, we compared the response to HDM challenge in wild type (WT) mice and mice deficient in Recombination-Activating Gene1 (Rag1). Rag1 encodes enzyme involved in the recombination of the immunoglobulin and T cell receptor genes and are essential for the generation of mature B and T lymphocytes22 two essential components of the adaptive immune system. The results (Fig 6) demonstrate that although HDM increased ER stress markers such as ATF650 and CHOP in both WT and Rag1−/− mice, there was no difference in up-regulation of ER stress markers between the groups. Furthermore, we also observed similar increases in ERp57 in HDM challenged WT and Rag1−/− mice (Fig 6 A). As expected Rag1−/− mice exhibited dramatic decreases in Total BALF cell counts, eosinophils, PMNs, lymphocytes and immunoglobulin production (IgG & IgE) (Fig 6 BE). Interestingly, we did not see any significant alterations in collagen content in HDM-challenged Rag1−/− mice as compared to WT mice (Fig. 6 F). These results suggested that HDM induced T helper cell and B cell responses are not required for HDM-induced UPR and fibrosis in the lungs of HDM-challenged mice.
Figure 6.
T or B cells are not required for HDM induced ER stress activation and collagen deposition. A: Western blot analysis for various ER stress markers and ERp57 in WT and Rag1−/− mice, β-actin was used as loading control. B & C: Analysis of inflammatory and immune cells in the BALF. D & E: Assay for production of IgG and IgE. F: Hydroxyproline assay. p<0.05 denotes significant differences as compared with PBS groups. # p<0.05 denotes significant differences compared with the HDM groups.
Discussion
Our results show that asthmatics exhibit a marked increases in ERp57 as compared to non-asthmatics, predominantly in the lung epithelium. Using a murine model of allergic asthma, we demonstrated that epithelial specific up-regulation of protein disulfide isomerase-ERp57 modulates allergen induced inflammation, AHR and airways fibrosis in the lung. Furthermore we also report that adaptive immune responses are dispensable for the development of allergen induced UPR, up-regulation of ERp57 and airways fibrosis.
Perturbations in ER homeostasis can cause unfolded protein response (UPR), leading to inflammation and, when unresolved, cell death 23. Recent reports suggest that UPR mediated activation of transcription factor X-box binding protein 1 (XBP-1) is required to induce mucus metaplasia in the lungs of mice challenged with ovalbumin16, 17. These reports did not address the implications of UPR in other facets of allergic asthma, such as inflammation, epithelial apoptosis, AHR and peri-bronchiolar fibrosis. In our earlier work, we demonstrated that in vivo, a complex allergen, HDM induces higher levels of UPR markers and upregulation of ERp57 as compared to murine models of LPS or Ovalbumin+LPS challenge11. Additionally, in contrast to the recent reports16, 17, our studies with human epithelial cells (in vitro) demonstrated the activation of ATF6α and up regulation of ERp5711. We believe that the differences in the activation of specific UPR transducers may be due to the complex signaling pathways activated (by multiple PRR agonists, Uric acid and proteases) in the epithelium by HDM, compared to the simple antigen ovalbumin or TLR4 agonist, LPS 7, 9. Thus, our results indicate a multifaceted mechanism of allergen-specific activation of ER stress mediators in mouse and human airway epithelial cells.
Interestingly ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) a protein linked to asthma susceptibility, contributes to alterations in ceramide and calcium homeostasis24–26 and can induce ATF6α activation in epithelial cells26. How ATF6α activation contributes to asthma pathophysiology remains to be determined. Previous work performed by our laboratory demonstrated that ATF6α knockdown in human bronchiolar epithelial cells decreased HDM-induced up-regulation of ERp57 and apoptosis11. However, these studies did not address the role of ERp57 as a regulator of HDM-induced pro-inflammatory/pro-fibrotic response from epithelial cells in vivo. Our experiments here demonstrate that lung epithelial-specific deletion of ERp57 significantly decreased HDM-induced influx of neutrophils and, lymphocytes, and modestly decreased eosinophilic infiltration into the lung. To elucidate the mechanism by which ERp57 acts to regulate immune responses, we analyzed epithelial derived cytokine and chemokine production1, 27. Our results show that deletion of ERp57 in lung epithelial cells dramatically decreased IL-33, IL-6, and CCL20 production, and slightly, but significantly decreased eotaxin production following HDM challenge in ERp57 deleted mice. These results indicate that deletion of ERp57 in epithelial cells could directly affect the production of the above epithelial-derived, innate lymphoid cell, T cell and dendritic cell activators1 that are involved in innate and adaptive immune responses following HDM exposure.
We hypothesized that being a protein disulfide isomerase with specificity towards glycoproteins (eg. cytokines and growth factors), ERp57 could be involved in formation of disulfide bonds (-S-S-) in specific cytokines. Furthermore, ablation of ERp57 would likely both decrease the secretion of cytokines and also affect their function. We tested this hypothesis on eotaxin since its levels were not dramatically altered yet we observed significant attenuation of eosinophils in ERp57 deleted mice. Our results showed that deleting ERp57 in epithelial cells exposed more –SH groups in eotaxin compared to Ctr mice challenged with HDM. Based on our results we speculate that although eotaxin is produced in copious amounts, deficiency in disulfides may be affecting the function of eotaxin in ERp57 deleted mice. ERp57 is also known to modulate the activity of a redox active transcription factor Ref-128. At this juncture we can only speculate that the decrease in cytokines such as IL-33, IL-6 and chemokine CCL20 could be due to inactivity of Ref-1 in ERp57 deleted airway epithelial cells. However, additional targeted experiments are required to substantiate the role of the ERp57 in oxidative folding of eotaxin and Ref-1 regulation in HDM-induced allergic airways disease.
Mucins are large glycosylated proteins encompassing multiple disulfide bonds29. However, it is known that mucins are folded and secreted by epithelial cells through PDI-AGR216, 29, 30, and not ERp57. Therefore, there was no significant decreases in mucin production in HDM challenged ΔEpi-ERp57 mice compared to Ctr mice.
Allergen-induced structural remodeling of the lung was thought to be the consequence of damage to the airways epithelium and subsequent cross talk between airways epithelial cells (AECs), mesenchymal and immune cells31, 32. Recent studies on ER stress-mediated apoptosis have also shown involvement of ERp57 in disulfide-mediated oligomerization of proapoptotic Bak on the ER and mitochondria associated membranes11, 15, 19, 33. Based on the results presented herein indicating that ERp57 deleted mice showed decreased oligomerization of Bak and apoptosis marker active caspase-3, it is reasonable to speculate that ERp57 could be regulating apoptosis of epithelial cells during HDM challenge.
Growth factors such as EGF, TGFβ and periostin, released by injured airway epithelium are thought to be the potential pro-fibrotic growth factors involved in airways structural remodeling in asthma1, 34. The allergen dependent chronic activation of ER stress and apoptosis can cause repeated injury to the airway epithelium. In fact, injured epithelium in human asthmatics as well as in mouse models up regulate pro fibrotic growth factors, stimulating proliferation of the underlying smooth muscle cells, and subsequently leading to the deposition of collagen 34. Measurement of profibrotic-growth factor mRNAs in our study did not yield any significant differences between the two genotypes challenged with HDM. This suggests that ERp57 deficiency in the epithelial cells does not alter the transcription of these mRNAs. However, our analysis of sulfhydryl groups in EGF and periostin (both are primarily secreted by epithelial cells in response to damage and repair)20, 35–37 revealed more sulhydryl groups exposed in ERp57 deleted HDM challenged mice as compared to Ctr mice challenged with HDM. These results suggest that ERp57 may also be controlling the oxidative disulfide mediated (-S-S-) folding of EGF and periostin (http://www.uniprot.org/uniprot/P01132; http://www.uniprot.org/uniprot/Q62009) (cysteine rich-glycosylated growth factors). Therefore deletion of ERp57 in epithelial cells may not affect the production but rather inhibits their function due to lack of disulfide bonds which are required for tertiary structure and of the functional ligands.
ER stress transducers, such as ATF6 and CHOP, play a prominent role in apoptosis of alveolar type II epithelial cells in fibrotic interstitial lung diseases38, 39. Recent studies have suggested that asthmatics and HDM based mouse models of asthma develop sub-epithelial thickening marked by αSMA (smooth muscle hyperplasia) and increased collagen deposition40–42, resulting in peri-bronchiolar fibrosis. Accordingly results presented here show that HDM induces severe ER stress, leading to apoptosis of airway epithelial cells and subsequent fibrosis.
Deletion of ERp57 in airways epithelial cells also resulted in a significant decrease in HDM-induced central airway resistance (Rn). We did not observe statistically significant differences in tissue resistance (G) and tissue stiffness (H) in HDM-challenged, Ctr mice as compared to HDM-challenged, ERp57 deleted mice (data not shown). This suggests that the effect of ERp57 on AHR is mediated through effects on central but not peripheral, airway function. Indeed it is difficult to compare functional measures of respiratory impedance with exact anatomical locations. Nonetheless, we believe that an effect on only central airway function is consistent with our findings of an effect of ERp57 on airway smooth muscle content (α-SMA staining) but not on mucus production (PAS staining, MUC5AC mRNA, Gob5 mRNA). This is consistent with the increase in airway smooth muscle, which would be expected to predominantly increase central airway narrowing in response to methacholine. In contrast, we have previously shown that increased peripheral airway responses to methacholine are predominantly due to increased airway closure, which is likely due to mucus metaplasia 43. Furthermore, enhanced apoptosis of epithelial cells likely decreased the protective barrier layer of the airways. Increased permeability of larger airways could perhaps allow increased access of methacholine to smooth muscle cells 44, 45, resulting in increased central airway narrowing. However, the mechanisms by which ERp57 increase AHR of central, but not peripheral airways are yet to be determined.
Conclusion
Collectively, our work illuminates a previously unexplored mechanism of HDM-induced UPR and epithelial ERp57. We demonstrate that ERp57 up-regulation in the epithelial cells play a regulatory role in airway inflammation, peri-bronchiolar fibrosis and AHR. Based on our results we believe that strategies to inhibit ERp57 in airway epithelial cells may offer beneficial effects in treating allergic asthma.
Supplementary Material
Key Messages.
Asthmatics exhibit induction of ERp57 predominantly in airways epithelium.
Allergen exposure also induces ERp57 in the airways epithelium of mice.
ERp57 increase is associated with redox modification of key mediators of asthma phenotype.
Strategies to inhibit ERp57 specifically within the airways epithelium may provide an opportunity to alleviate allergic inflammation and airways fibrosis associated with asthma.
Acknowledgments
This work is supported by NIHR01HL122383, Parker B. Francis Fellowship, ATS unrestricted grant, AAFA-Sheldon C. Siegel award, UVM-College of medicine internal grant Program to VA and NIHR01HL079331 to YJH. DGC is a recipient of a CJ Martin Fellowship from the national Health and Medical Research Council of Australia (1053790). SE and SAC are supported by NIH grants HL103453 and HL081064. Authors also thank UCSF Airway Tissue Bank, college of medicine microscopy core facility, flow cytometry facility (P20GM103496) and Vermont lung center phenotyping core (P30GM103532). We thank Drs. Natalio Garbi and Gunther Hammerling (Heidelberg University Medical School, Germany) for providing ERp57loxp/loxp mice. We thank Dr. Jeffrey Whitsett (Division of Pulmonary Biology, Cincinnati Children’s Hospital, Cincinnati) for permission to use CCSP-rTetA mice.
Abbreviations
- AECs
Airways Epithelial Cells
- AHR
Airways Hyperresponsiveness
- ATF6
Activating Transcription Factor 6
- BAL
Bronchoalveolar lavage
- CHOP
C/EBP homologous protein
- CCSP
Club cell secretory protein promoter
- ER
Endoplasmic Reticulum
- ERp57
Endoplasmic Reticulum protein 57
- GRP78
Glucose Regulated Protein 78
- GRP94
Glucose Regulated Protein 94
- HDM
House Dust Mite
- IRE
Inositol requiring enzyme
- LPS
Lipopolysaccharide
- LoxP
Locus of cross-over in P1 sites
- OVA
Ovalbumin
- PERK
PKR-like ER kinase
- PDI
Protein Disulfide Isomerase
- Rn
Central airway resistance
- rTetA
Reverse tetracycline trans activator
- Teto
Tetracycline operon promoter
- UPR
Unfolded Protein Response
- XBP-1
X-box binding protein
Footnotes
Author Contributions: VA, MP, SH and DC designed the study. VA, SH, DC, KL, JM, GR, ND, MA, and MP performed the study. KF provided Rag1−/− mice and littermate controls. SE, SC, PW, NB provided human samples, patient characteristics and help with data interpretation. AD, CI and YJ-H provided valuable suggestions in data interpretation and reagents to complete the study. VA, MP, SH and DC analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–19. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 3.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14:686–98. doi: 10.1038/nri3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rate A, Upham JW, Bosco A, McKenna KL, Holt PG. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J Immunol. 2009;182:72–83. doi: 10.4049/jimmunol.182.1.72. [DOI] [PubMed] [Google Scholar]
- 6.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–8. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–11. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson RP, Jr, DiNicolo R, Fernandez-Caldas E, Seleznick MJ, Lockey RF, Good RA. Allergen-specific IgE levels and mite allergen exposure in children with acute asthma first seen in an emergency department and in nonasthmatic control subjects. J Allergy Clin Immunol. 1996;98:258–63. doi: 10.1016/s0091-6749(96)70148-3. [DOI] [PubMed] [Google Scholar]
- 9.Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–61. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Lan RS, Stewart GA, Henry PJ. Role of protease-activated receptors in airway function: a target for therapeutic intervention? Pharmacol Ther. 2002;95:239–57. doi: 10.1016/s0163-7258(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, et al. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffstrom BG, Kaplan A, Letso R, Schmid RS, Turmel GJ, Lo DC, et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat Chem Biol. 2010;6:900–6. doi: 10.1038/nchembio.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, et al. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–85. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, et al. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–54. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turano C, Gaucci E, Grillo C, Chichiarelli S. ERp57/GRP58: a protein with multiple functions. Cell Mol Biol Lett. 2011;16:539–63. doi: 10.2478/s11658-011-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao G, Lu H, Li C. Proapoptotic Activities of Protein Disulfide Isomerase (PDI) and PDIA3 Protein, a Role of the Bcl-2 Protein Bak. J Biol Chem. 2015;290:8949–63. doi: 10.1074/jbc.M114.619353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, et al. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014;134:1433–42. doi: 10.1016/j.jaci.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holgate ST. Epithelial damage and response. Clin Exp Allergy. 2000;30 (Suppl 1):37–41. doi: 10.1046/j.1365-2222.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 22.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–63. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–67. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet. 2010;19:111–21. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- 26.Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, et al. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci U S A. 2012;109:16648–53. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123:612–8. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grillo C, D’Ambrosio C, Scaloni A, Maceroni M, Merluzzi S, Turano C, et al. Cooperative activity of Ref-1/APE and ERp57 in reductive activation of transcription factors. Free Radic Biol Med. 2006;41:1113–23. doi: 10.1016/j.freeradbiomed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–5. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–24. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009;6:678–82. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 33.Ono Y, Tanaka H, Tsuruma K, Shimazawa M, Hara H. A Sigma-1 Receptor Antagonist (NE-100) Prevents Tunicamycin-Induced Cell Death via GRP78 Induction in Hippocampal Cells. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–9. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 35.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–21. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–46. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108:10562–7. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–85. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 41.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–64. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 42.Noble PB, Ansell TK, James AL, McFawn PK, Mitchell HW. Airway Smooth Muscle Dynamics and Hyperresponsiveness: In and outside the Clinic. J Allergy (Cairo) 2012;2012:157047. doi: 10.1155/2012/157047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–74. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates JH, Cojocaru A, Haverkamp HC, Rinaldi LM, Irvin CG. The synergistic interactions of allergic lung inflammation and intratracheal cationic protein. Am J Respir Crit Care Med. 2008;177:261–8. doi: 10.1164/rccm.200706-832OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, et al. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol. 2004;173:7003–9. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol. 2009;40:1–3. doi: 10.1165/rcmb.2008-0011ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol. 2005;33:455–62. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–95. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.