SUMMARY
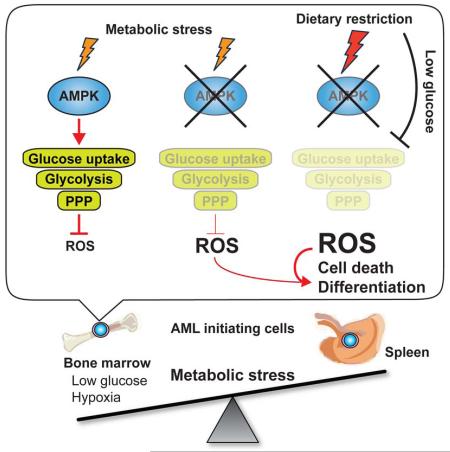
How cancer cells adapt to metabolically adverse conditions in patients and strive to proliferate is a fundamental question in cancer biology. Here we show that AMP-activated protein kinase (AMPK), a metabolic checkpoint kinase, confers metabolic stress resistance to leukemia-initiating cells (LICs) and promotes leukemogenesis. Upon dietary restriction, MLL-AF9-induced murine AML activated AMPK and maintained leukemogenic potential. AMPK deletion significantly delayed leukemogenesis and depleted LICs by reducing the expression of glucose transporter 1 (Glut1), compromising glucose flux, and increasing oxidative stress and DNA damage. LICs were particularly dependent on AMPK to suppress oxidative stress in the hypoglycemic bone marrow environment. Strikingly, AMPK inhibition synergized with physiological metabolic stress caused by dietary restriction and profoundly suppressed leukemogenesis. Our results indicate that AMPK protects LICs from metabolic stress, and that combining AMPK inhibition with physiological metabolic stress potently suppresses AML by inducing oxidative stress and DNA damage.
Keywords: AMPK, AML, leukemia-initiating cells, metabolic stress, dietary restriction, glycolysis, ROS, DNA damage
INTRODUCTION
A fundamental question in cancer biology concerns how cancer cells residing in metabolically adverse conditions with low oxygen, glucose, and nutrient levels strive to proliferate, and whether this feature of cancer cells can be turned into vulnerability by interventions (Cairns et al., 2011; Schulze and Harris, 2012). Dietary restriction (DR) causes systemic changes in metabolic parameters such as reduced insulin, insulin-like growth factor 1 (IGF1), and glucose levels (Masoro, 2005), and delays the incidence and growth of various tumor models (Colman et al., 2009; Curry et al., 2013; Kalaany and Sabatini, 2009; Longo and Fontana, 2010; Mattison et al., 2012; Mihaylova et al., 2014), demonstrating that cancer cells can be targeted by modulating systemic metabolic parameters. Nonetheless, some cancer cells acquire adaptive mutations, such as activating mutations in the phosphoinositide 3-kinase (PI3K) pathway, and become insensitive to insulin/IGF1 lowering effects of DR (Curry et al., 2013; Kalaany and Sabatini, 2009). It is, however, still unknown whether the PI3K pathway mutations are the sole determinant of DR sensitivity, or whether cancer cells are equipped with other protective mechanisms independent of their mutation status. Moreover, how DR affects expansion of cancer cells by regulating cancer-initiating cells is unexplored.
AMP-activated protein kinase (AMPK) orchestrates cellular metabolic state with proliferation by promoting catabolism and inhibiting anabolism (Hardie et al., 2012). AMPK is a heterotrimeric complex consisted of a catalytic α subunit and two regulatory subunits (β and γ). Energetic stress increases AMP:ATP ratio, upon which AMPK is activated in a manner dependent on the phosphorylation by the tumor suppressor kinase Lkb1 (Shackelford and Shaw, 2009). Activated AMPK promotes multiple catabolic processes to maintain metabolic homeostasis, such glucose uptake via glucose transporters Glut1 and Glut4 (Barnes et al., 2002; Kurth-Kraczek et al., 1999), glycolysis (Almeida et al., 2004), fatty acid uptake and oxidation (Hardie et al., 2012), and mitochondrial biogenesis (Jager et al., 2007; Zong et al., 2002). Furthermore, AMPK activation suppresses anabolic processes such as protein and lipid synthesis. Of note, AMPK inhibits the mammalian target of rapamycin (mTOR) pathway, which promotes protein translation and cell growth, and is thus frequently over-activated in many cancers (Laplante and Sabatini, 2012). Consistent with the tumor suppressive role of the Lkb1-AMPK pathway, AMPK activating drugs suppress cancer cell proliferation and tumor formation (Shackelford and Shaw, 2009), while disruption of AMPK promotes some tumors (Faubert et al., 2013). On the contrary, AMPK can promote cancer cell maintenance in vitro and in xenograft models by regulating redox homeostasis (Jeon et al., 2012). Thus, it remains elusive how AMPK function affects cancer in a physiological setting (Faubert et al., 2015; Liang and Mills, 2013; Saito and Nakada, 2014).
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, and it appears increasingly with age with devastating prognosis for elder patients (Ferrara and Schiffer, 2013). AML is a heterogeneous disease caused by various genetic lesions, among which a translocation between the mixed lineage leukemia (MLL) and AF9 genes (producing MLL-AF9) is often found and have poor prognosis (Krivtsov and Armstrong, 2007; Muntean and Hess, 2012). Leukemia-initiating cells (LICs), a cell population capable of initiating leukemias, have been functionally identified in murine AML models as well as in human AML specimens through transplantation assays, and have been postulated to be involved in disease initiation, progression, and relapse (Bonnet and Dick, 1997; Huntly and Gilliland, 2005; Kreso and Dick, 2014; Lapidot et al., 1994). Similar to normal hematopoietic progenitors, LICs demand tightly regulated metabolism, since disruption of either glycolysis or mitochondrial respiration impairs leukemogenesis (Lagadinou et al., 2013; Wang et al., 2014b). LICs of human AML maintain low oxidative stress compared to the bulk of the leukemia and utilize mitochondrial respiration to support metabolic homeostasis (Lagadinou et al., 2013). Since both LICs of AML and normal hematopoietic stem cells (HSCs) reside in the hypoxic bone marrow environment (Ishikawa et al., 2007; Morrison and Scadden, 2014; Nombela-Arrieta et al., 2013; Spencer et al., 2014; Suda et al., 2011), this raises a question of whether LICs share metabolic regulation with HSCs to meet the bioenergetic demands in the hypoxic environment, or whether maintenance of LICs in hypoxia is regulated by leukemia specific mechanisms, potentially providing a therapeutic target.
Here, we demonstrate that LICs activate AMPK upon systemic metabolic stress caused by DR, and that AMPK deletion profoundly depletes LICs residing in the hypoxic bone marrow environment by attenuating glucose metabolism. Interrupting the AMPK pathway rendered LICs sensitive to physiological metabolic stress caused by DR, and this combination profoundly suppressed AML. Since AMPK deletion does not impair normal HSC function (Nakada et al., 2010), our results indicate that LIC metabolism can be targeted to make them vulnerable to metabolic stress in the bone marrow, without affecting normal HSCs.
RESULTS
Dietary restriction activates AMPK in MLL-AF9-induced AML
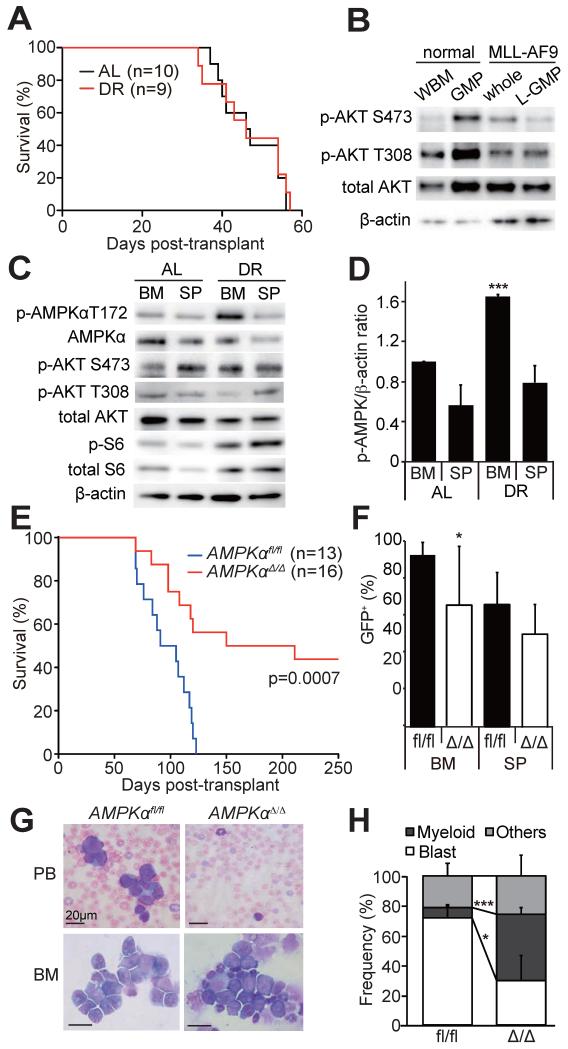
To examine how metabolic stress caused by diet affects LICs, we used a murine AML model driven by MLL-AF9 oncogene (Krivtsov et al., 2006; Somervaille and Cleary, 2006). In this model, LICs have been immunophenotypically identified as lineagelowSca-1−c-kit+CD16/32+CD34+ cells, which shares the same immunophenotype as granulocyte-macrophage progenitors (GMPs) and are thus termed GMP-like leukemic cells (L-GMPs) (Krivtsov et al., 2006). Murine hematopoietic progenitor cells were transduced with retrovirus encoding both MLL-AF9 and GFP, and transplanted into irradiated syngeneic mice. Upon development of AML, AML cells were transplanted into secondary recipients, which were either fed ad libitum (AL) or subjected to dietary restriction (DR, 70% caloric uptake (Ertl et al., 2008)). Blood glucose levels dropped from 148±17 mg/dl (AL) to 104±14 mg/dl (DR, p<0.01, Figure S1A) within 14 days of DR. However, all recipient mice died within 60 days regardless of the dietary manipulation, indicating that MLL-AF9-induced AML are not significantly affected by this DR regimen (Figure 1A). Consistent with a previous report (Sykes et al., 2011), L-GMPs had low levels of phosphorylated Akt (Figure 1B), unlike other tumors in which hyperactivated PI3K-Akt pathway rendered tumors DR resistant (Curry et al., 2013; Kalaany and Sabatini, 2009).
Figure 1. AMPK is activated in AML cells upon DR and promotes leukemogenesis.
(A) Secondary recipients of 1,000 MLL-AF9-induced AML cells were either fed ad libitum (AL, n=10) or subjected to dietary restriction (DR, n=9). DR did not extend the survival of AML recipient mice. (B) Immunoblotting was performed on freshly isolated whole bone marrow cells (WBM) and GMPs from non-leukemic mice, and whole AML cells (sorted GFP+ cells) and L-GMPs from leukemic mice, all fed AL. PI3K pathway, as determined by phosphorylation of Akt, was not activated in AML cells compared to normal progenitors. (C) Immunoblotting of freshly isolated AML cells from AL or DR mice revealed that AMPK was highly activated in the bone marrow (BM), but not the spleens (SP), of DR mice. (D) Quantification of the results shown in (C). p-AMPK signals were normalized to the signals from β-actin. (E) Survival of mice after transplanting MLL-AF9 transduced AMPKαfl/fl (n=13) or AMPKαΔ/Δ cells (n=16, three independent experiments). (F) Frequencies of GFP+ AML cells in the bone marrow or the spleens of leukemic mice shown in (E). (G) Wright-giemsa staining of peripheral blood (PB) or the bone marrow (BM) samples from AML mice revealed fewer blasts in the recipients of AMPKαΔ/Δ AML. (H) Quantification of the cell types in the bone marrow. In all figures, data represent mean±standard deviation; *, p<0.05; **, p<0.005; and ***, p<0.0005 by Student’s t-test, except for comparison of the survival curves in which the significance was accessed by a log-rank test. See also Figure S1.
To examine how MLL-AF9-induced AML responds to DR, we isolated AML cells from the bone marrow and spleens of AL and DR mice, and examined the activation status of several metabolic pathways. Interestingly, we found that AMPK is highly activated in AML cells from the bone marrow of DR mice compared to AL mice, whereas cells from the spleens showed little change (Figure 1C, D). Activation of the PI3K/Akt/mTOR pathway did not change upon DR (Figure 1C and Figure S1B). These results raised a possibility that AMPK activation is an adaptive mechanism that protects AML cells from metabolic stress caused by diet, and that the levels of stress DR causes is much higher in the bone marrow compared to that in the spleen.
AMPK is required for the maintenance of LICs in myeloid leukemias
We next tested whether AMPK regulates AML. We treated Mx1-Cre; Prkaa1fl/fl; Prkaa2fl/fl and control Prkaa1fl/fl; Prkaa2fl/fl mice (Nakada et al., 2010) with polyinosinic:polycytidylic acid (poly(I:C)) to delete Prkaa1 and Prkaa2, two genes encoding the catalytic α subunits of AMPK, from hematopoietic cells. Prkaa1fl/fl; Prkaa2fl/fl and Mx1-Cre; Prkaa1fl/fl; Prkaa2fl/fl mice treated with poly(I:C) are hereafter described as AMPKαfl/fl and AMPKαΔ/Δ mice, respectively. All mice transplanted with MLL-AF9 transduced AMPKαfl/fl cells developed AML and succumbed within 130 days post transplantation (Figure 1E). In contrast, recipients of MLL-AF9 transduced AMPKαΔ/Δ cells had significantly extended survival (Figure 1E). Moreover, AMPKαΔ/Δ AML cells proliferated less extensively than AMPKαfl/fl cells in vitro and exhibited differentiated morphologies (Figure S2A and S2B). AMPKαΔ/Δ MLL-AF9 transduced cells caused AML in some of the recipients, exhibiting increased GFP+ AML cells in the bone marrow and the spleens (Figure 1F) and increased blast cells in peripheral blood and in the bone marrow (Figure 1G, H), although the frequencies of AML cells and blasts were lower than that in the recipients of AMPKαfl/fl cells. We also used two additional murine leukemia models to examine the role of AMPK in other leukemias. Hematopoietic progenitor cells infected with MOZ-TIF2 (Deguchi et al., 2003) or BCR-ABL (Pear et al., 1998) expressing retrovirus were transplanted into irradiated recipient mice. Although MOZ-TIF2 expression in AMPKαfl/fl cells induced AML in most of the recipient mice as reported before (Deguchi et al., 2003), MOZ-TIF2 expressing AMPKαΔ/Δ cells developed AML in only one out of 9 recipients (Figure S1C). Expression of BCR-ABL caused fully penetrant chronic myelogenous leukemia (CML)-like disease as reported before (Figure S1D) (Pear et al., 1998). AMPK deletion significantly delayed the onset of the disease (Figure S1D). These results indicate that AMPK, which is phosphorylated at a basal level in steady state (Figure 1C and S2F), is required to maintain the full leukemogenic potential of multiple leukemia models, including those induced by MLL-AF9, MOZ-TIF2, and BCR-ABL.
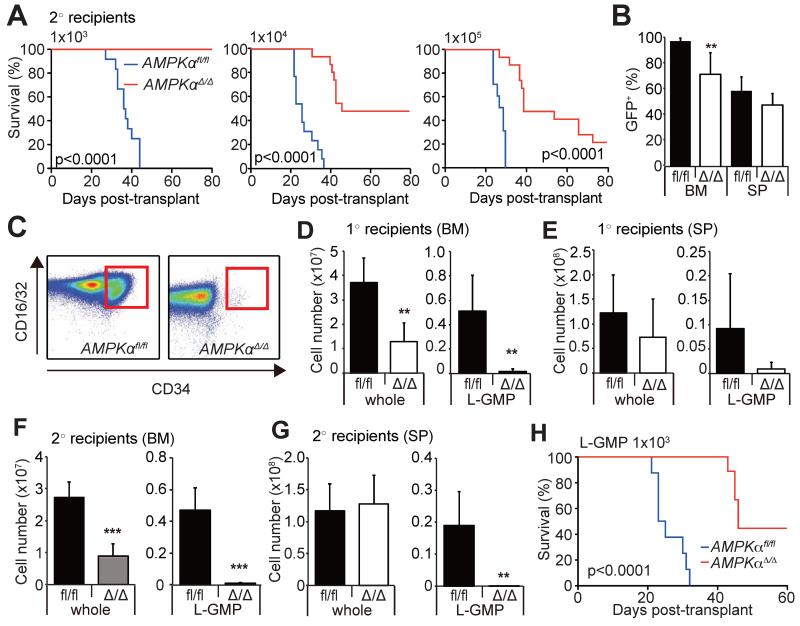
To examine the function of AMPK in LICs in the MLL-AF9 model, we performed secondary transplantation with 103 to 105 AML cells isolated from primary recipient mice. AMPKαfl/fl cells, regardless of the cell dose infused, caused AML in secondary recipients within 60 days of transplantation, whereas AMPKαΔ/Δ cells were severely compromised in their ability to develop AML, suggesting that the LIC fraction is negatively affected by AMPK deletion (Figure 2A). The expansion of AMPKαΔ/Δ AML cells in the bone marrow, but not in the spleen, was significantly lower than that of AMPKαfl/fl AML cells (Figure 2B). Tertiary transplantation of bone marrow cells from moribund secondary recipients of AMPKαΔ/Δ cells caused AML, but again with delayed onset compared to AMPKαfl/fl cells (Figure S2C). Consistent with the previous report (Krivtsov et al., 2006), we confirmed that clonogenic cells reside in the L-GMP fraction, and that L-GMPs are highly enriched for LIC activity (Figure S2D, E). Flow cytometry revealed that L-GMP fraction was markedly depleted in the bone marrow of AMPKαΔ/Δ primary recipients compared to AMPKαfl/fl recipients (Figure 2C-E), as well as in the secondary recipients of AMPKαΔ/Δ AML cells (Figure 2F-G). The defective leukemogenic potential of AMPKαΔ/Δ cells (Figure 2A) were not solely due to the depletion of LICs, since transplantation of 103 purified AMPKαΔ/Δ L-GMPs had significantly delayed onset of AML compared to AMPKαfl/fl L-GMPs (Figure 2H). Western blotting with purified L-GMPs and whole AML cells (sorted GFP+ cells) confirmed efficient deletion of AMPK accompanied by reduced phosphorylation of the AMPK target ACC, without any changes in the expression of MLL-AF9 (Figure S2F). Gene expression profiling of AMPKαfl/fl and AMPKαΔ/Δ L-GMPs revealed that AMPKαΔ/Δ L-GMPs have increased expression of genes regulating myeloid differentiation (Figure S2G-H). Freshly isolated AMPKαΔ/Δ L-GMPs also displayed differentiated morphologies compared to AMPKαfl/fl L-GMPs, suggesting that AMPK suppresses differentiation of L-GMPs (Figure S2I). These data indicate that AMPK promotes leukemogenesis by both maintaining the LIC pool size and the function in the MLL-AF9 model.
Figure 2. AMPK deletion suppresses leukemogenesis and depletes LICs.
(A) Secondary transplantation of 103, 104, 105 GFP+ AML cells revealed significantly delayed onset of leukemogenesis by AMPKαΔ/Δ cells. (B) Frequencies of GFP+ AML cells in the bone marrow or the spleens of leukemic mice shown in (A). (C) Representative FACS profiles gated on lineage− Sca-1−c-kit+cells show reduced CD34+CD16/32+ L-GMPs (red box) after AMPK deletion. (D and E) Total numbers of L-GMPs and whole AML cells in the bone marrow and spleens of primary recipients. (F and G) Secondary recipients of AMPKαΔ/Δ AML cells had significantly reduced total numbers of whole AML cells and L-GMPs in the bone marrow (F), and L-GMPs in the spleens (G). (H) 103 L-GMPs isolated from primary recipients of AMPKαfl/fl and AMPKαΔ/Δ AML were transplanted into secondary recipient mice. AMPKαΔ/Δ L-GMPs had delayed onset of leukemogenesis compared to AMPKαfl/fl L-GMPs, indicating that AMPK deletion impairs L-GMP function. See also Figure S2.
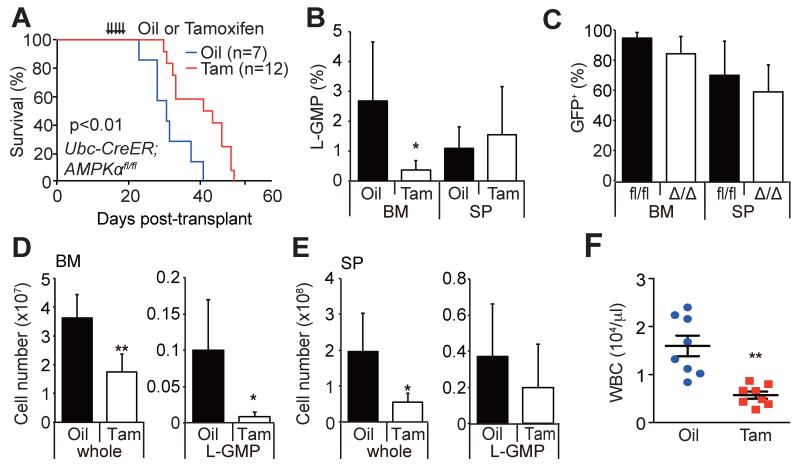
To determine whether AMPK is continuously required for the maintenance of leukemia after its development, we conditionally deleted AMPK from established AML using a tamoxifen inducible Ubc-Cre-ERT2 model. After establishing primary MLL-AF9-induced AML with Ubc-Cre-ERT2; AMPKαfl/fl cells, we performed serial transplantation, and secondary recipients were treated with control oil or tamoxifen 14 days after transplantation to delete AMPK. Efficient deletion of AMPKα by tamoxifen treatment was confirmed by PCR with sorted AML cells (Figure S3A). Acute deletion of AMPK from fully established AML significantly extended the survival of recipient mice, although it did not completely block leukemia progression (Figure 3A, Figure S3B). Interestingly, this acute deletion of AMPK led to rapid reduction in the frequencies of L-GMPs residing in the bone marrow but not in the spleens (Figure 3B), without significantly affecting the frequencies of GFP+ AML cells in either tissue (Figure 3C). Acute AMPK deletion also led to rapid reduction in the total numbers of L-GMPs in the bone marrow (Figure 3D and 3E) and reduced the white blood cell (WBC) counts that were increased by AML progression (Figure 3F). The depletion of L-GMPs in the bone marrow paralleled the reduction of whole AML cell numbers in the bone marrow and spleens (Figure 3D and 3E). These results indicate that AMPK is required within established leukemias to maintain L-GMPs, particularly those that reside in the bone marrow.
Figure 3. AMPK is required within established leukemias to maintain LICs.
(A and B) Conditional deletion of AMPK from established AMLs by treating mice carrying MLL-AF9-transduced Ubc-Cre-ERT2; AMPKαfl/fl AML cells with tamoxifen led to significant extension of survival (A) and reduced frequency of L-GMP in the bone marrow but not in the spleens (B). (C) Acute AMPK deletion did not affect the frequencies of GFP+ AML cells in the bone marrow or in the spleens. (D and E) Acute AMPK deletion significantly reduced the numbers of L-GMPs in the bone marrow but not in the spleens, and reduced the numbers of whole AML cells in these two tissues (n=7). (F) AMPK deletion by tamoxifen treatment reduced WBC in the peripheral blood compared to oil-treated mice (n=8). See also Figure S3.
The LIC population of the BCR-ABL-induced CML-like disease has been identified as lineage−Sca-1+c-kit+ (LSK) cells (Hu et al., 2006; Naka et al., 2010; Neering et al., 2007). We found that the leukemic LSK cells were reduced by AMPK deletion in the bone marrow but not in the spleen (Figure S1F and G). These results further support our notion that LICs residing in the bone marrow depend more upon AMPK than those in the spleen.
AMPK suppresses ROS and DNA damage in AML
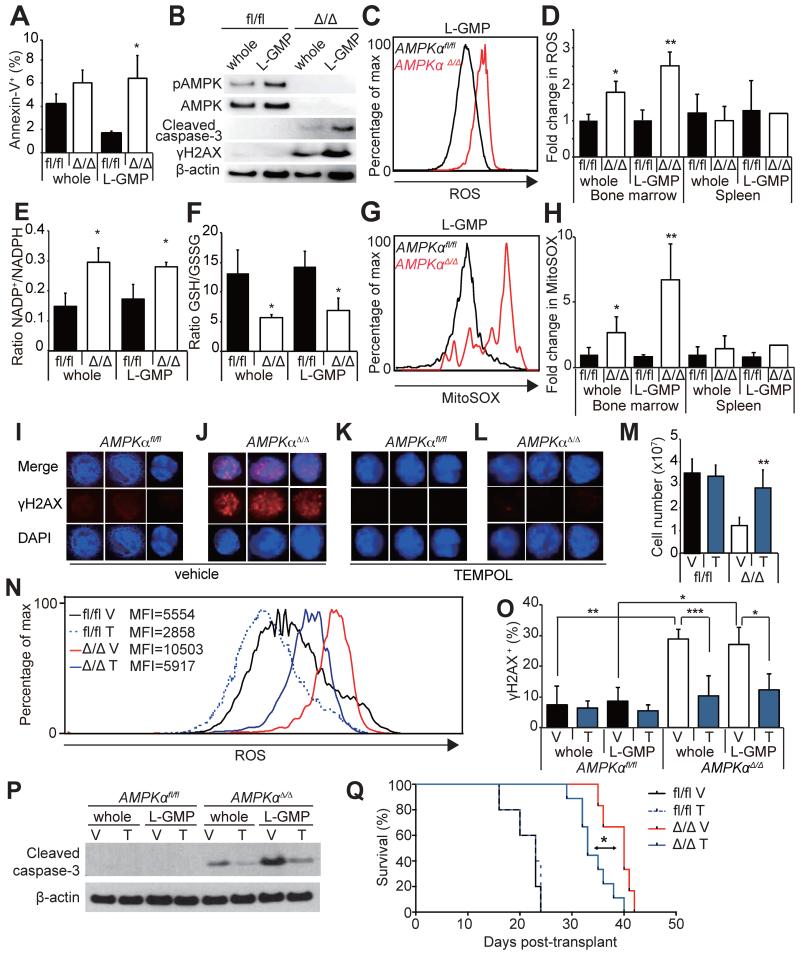
To determine how AMPK regulates leukemogenesis, we examined cell death in L-GMPs. Mx1-Cre; AMPKαΔ/Δ L-GMPs in the bone marrow had significantly increased frequencies of Annexin-V+ cells and increased level of cleaved caspase-3, indicating that AMPK suppresses cell death of L-GMPs (Figure 4A and 4B). Increased levels of reactive oxygen species (ROS) in hematopoietic progenitors and leukemia cells are associated with myeloid differentiation, and suppression of ROS is crucial to maintain LICs (Lagadinou et al., 2013; Tothova et al., 2007). We found that AMPK deletion significantly increased the level of ROS (Figure 4C and 4D) and mitochondrial superoxide (Figure 4G and 4H) in whole AML cells and L-GMPs in the bone marrow. Interestingly, induction of ROS or superoxide was not observed in the splenic GFP+ AML cells or L-GMPs after AMPK deletion (Figure 4D and 4H), suggesting that AML cells are more dependent upon AMPK to suppress oxidative stress in the bone marrow environment. Consistent with the increased oxidative stress, reducing agents in AMPKαΔ/Δ AML cells and L-GMPs in the bone marrow were depleted as indicated by the increased NADP+/NADPH and reduced GSH/GSSG ratios (Figure 4E and 4F). The induction of ROS in the bone marrow AML cells correlated with increased DNA damage, shown by γH2AX immunoblotting and immunofluorescence of freshly isolated bone marrow L-GMPs and whole AML cells (Figure 4B, 4I and 4J).
Figure 4. AMPK deletion induces oxidative stress and DNA damage in LICs.
(A) AMPK deletion significantly increased the frequencies of Annexin-V+ L-GMPs in the bone marrow (n=3). (B) Western blotting of AMPKαfl/fl and AMPKαΔ/Δ AML cells and L-GMPs from the bone marrow revealed increased cleaved caspase-3 consistent with the increased cell death, as well as increased γH2AX indicating increased DNA damage. (C and D) AMPK deletion increased the levels of ROS in whole AML cells and L-GMPs in the bone marrow but not in the spleens (n=5). (E and F) Deletion of AMPK from L-GMPs and whole AML cells from the bone marrow increased the NADP+/NADPH ratio and reduced the GSH/GSSG ratio consistent with the increased oxidative stress (n=3). (G and H) AMPK deletion also increased the levels of mitochondrial superoxide (as indicated by the MitoSOX staining) in whole AML cells and L-GMPs in the bone marrow but not in the spleens (n=5). (I-L) Immunofluorescence staining of freshly isolated bone marrow L-GMPs with anti-γH2AX antibodies revealed that AMPK deletion (J) increased DNA damage, which was significantly suppressed by treating the mice with an antioxidant TEMPOL (L). Scale bars indicate 5 μm. Quantification of I-L is shown in O (V: vehicle, T: TEMPOL, n=5). (M) TEMPOL treatment increased the numbers of AMPKαΔ/Δ AML cells in the bone marrow, and reduced ROS levels in bone marrow L-GMPs (N). MFI indicates the mean fluorescence intensity of each sample. (P) Western blotting of AMPKαfl/fl and AMPKαΔ/Δ AML cells and L-GMPs isolated from the bone marrow of mice subjected to TEMPOL or vehicle treatment revealed that the levels of cleaved caspase-3 in AMPKαΔ/Δ AML cells and L-GMPs were partially reduced by TEMPOL treatment. (Q) 104 GFP+ AML cells from secondary recipients were transplanted and recipients treated with either control vehicle or TEMPOL. TEMPOL treatment slightly but significantly accelerated the onset of leukemogenesis by AMPKαΔ/Δ AML cells.
To test whether the oxidative stress caused by AMPK deletion induced cell death of AMPKαΔ/Δ AML cells, AML recipient mice were given a superoxide scavenger TEMPOL (Wang et al., 2010) in their drinking water. TEMPOL treatment of AMPKαΔ/Δ AML recipient mice significantly increased the total numbers of AML cells in the bone marrow (Figure 4M) and partially reduced the levels of ROS, DNA damage, and apoptosis of AML cells and L-GMPs in the bone marrow (Figure 4I-4L, 4N-P). TEMPOL treatment also slightly accelerated the onset of leukemogenesis by AMPKαΔ/Δ AML cells (Figure 4Q), consistent with the partial suppression of ROS, DNA damage, and apoptosis by TEMPOL treatment. These results indicate that loss of AMPK compromises leukemogenesis, at least partially, by inducing oxidative stress, DNA damage, and cell death.
AMPK promotes glucose uptake and metabolism in AML
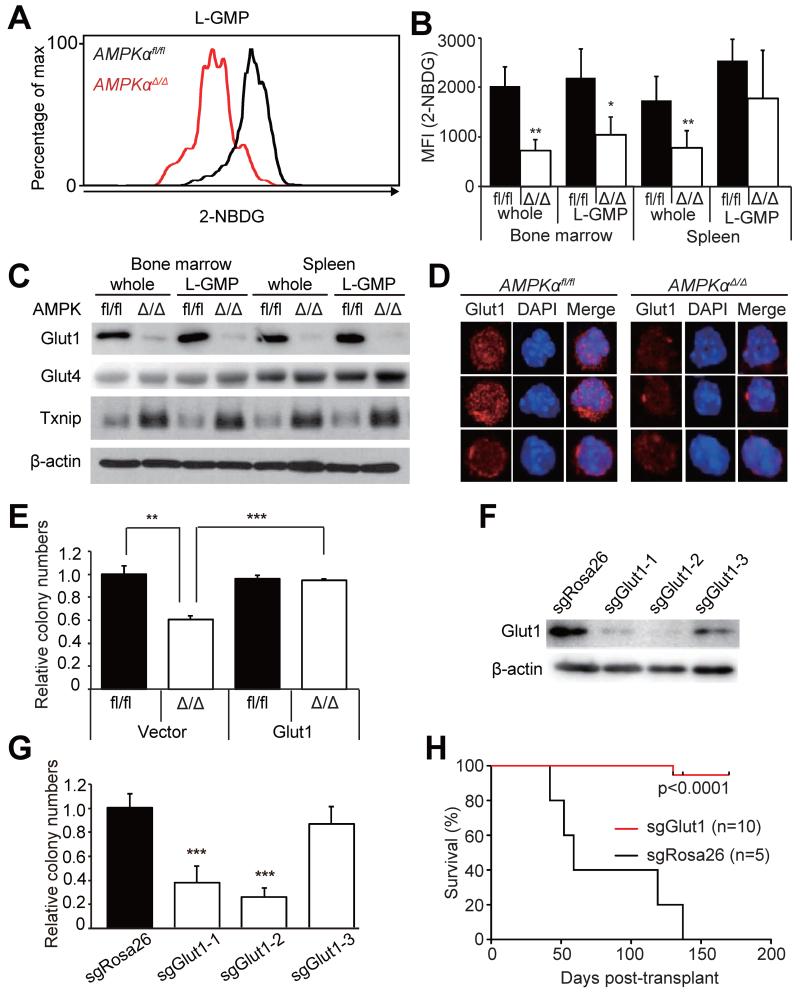
We next examined whether AMPK regulates energy metabolism in AML cells. A metabolic phenotyping microarray analysis (Bochner et al., 2011) was performed to identify metabolites whose consumption is regulated by AMPK. We found that immature c-kit+ AML cells metabolized glucose at an extremely high level compared to normal GMPs consistent with the Warburg effect (Cairns et al., 2011; Warburg, 1956), and that AMPK deletion attenuated glucose metabolism of AML cells (Figure S4). We then tested whether AMPK regulates glucose uptake in vivo. Leukemic mice were injected with a fluorescent glucose analog 2-NBDG, and 2-NBDG uptake was analyzed by flow cytometry as previously described (Warr et al., 2013). AMPKαΔ/Δ whole AML cells and L-GMPs in the bone marrow, but not L-GMPs in the spleen, had significantly reduced 2-NBDG uptake compared to AMPKαfl/fl cells (Figure 5A and 5B). Furthermore, we found that the mRNA and protein levels of a glucose transporter Glut1, but not Glut4, were significantly reduced in whole AML cells and L-GMPs upon AMPK deletion (Figure 5C and 5D, Figure S5B). AMPK phosphorylates and destabilizes Txnip1, which suppresses Glut1 expression transcriptionally and by preventing plasma membrane translocation of Glut1 protein (Wu et al., 2013). Consistent with this finding, AMPKαΔ/Δ whole AML cells and L-GMPs from the bone marrow had increased protein levels of Txnip1 (Figure 5C), suggesting that AMPK may regulate Glut1 levels through Txnip1. We then tested whether overexpression of Glut1 can rescue the reduced proliferative potential of AMPKαΔ/Δ AML cells. Overexpression of Glut1 in AMPKαΔ/Δ AML cells substantially rescued the defective clonogenic potential, indicating that the defective Glut1-mediated glucose uptake is at least partly responsible for the impaired AML cell proliferation in vitro (Figure 5E). We also tested whether Glut1 is required for the proliferative potential of AML cells, by deleting Glut1 from AML cells. We derived AML clones that express doxycycline-inducible Cas9 endonuclease, and introduced short guide RNAs (sgRNAs) against Rosa26 or Glut1. We found two sgRNA clones against Glut1 (clones 1 and 2) that reduced Glut1 protein levels and reduced clonogenicity of AML cells (Figure 5F and 5G). Transplantation of these Glut1-deleted AML cell revealed that Glut1 deletion significantly suppressed AML, with only one out of ten recipients developing AML (Figure 5H). Collectively, these results indicate that AMPK maintains glucose metabolism and leukemogenic potential of AML cells at least partly through Glut1.
Figure 5. AMPK regulates glucose uptake through Glut1.
(A) A representative histogram showing reduced 2-NBDG uptake by AMPKαΔ/Δ bone marrow L-GMPs in vivo. (B) AMPK deletion significantly reduced glucose uptake of whole AML cells in the bone marrow and spleens, and L-GMPs in the bone marrow but not in the spleens (n=5). Immunoblotting (C) and immunostaining (D) assays revealed that AMPK deletion reduced the protein levels of Glut1, but not Glut4, in whole AML cells and L-GMPs, concomitant with increased Txnip1 levels. Scale bars indicate 5 μm. (E) Overexpression of Glut1 substantially rescued the defective clonogenicity of AMPKαΔ/Δ AML cells (n=4). (F) Immunoblotting against Glut1 using gene-edited AML cells revealed that Glut1 sgRNA clones 1 and 2 reduced Glut1 protein levels, whereas clone 3 had little effects. (G) Clonogenecity of the gene-edited cells as in (F). Glut1 sgRNA clones 1 and 2 significantly reduced clonogenicity, whereas clone 3 did not (n=4). (H) AML cells expressing sgRosa26 (n=5) or sgGlut1 (clone 1, n=5; clone 2, n=5) were transplanted. Deletion of Glut1 by sgRNAs significantly attenuated leukemogenesis. See also Figure S4.
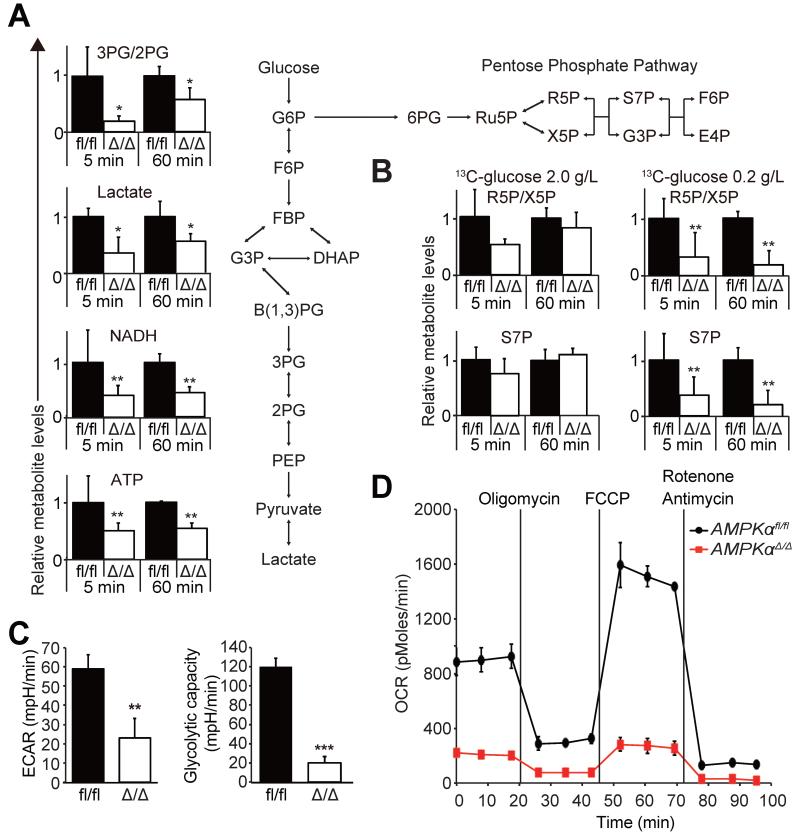
To better understand how AMPK regulates glucose metabolism in AML, we performed metabolic flux analysis using 13C-glucose to trace cellular carbon flux. MLL-AF9-induced AML cells were isolated from recipient mice, briefly exposed to 13C-labeled glucose tracer, and 13C incorporation into metabolites was analyzed by mass spectrometry. We found that AMPKαΔ/Δ AML cells have significantly reduced 13C incorporation into glycolytic intermediates 3-phosphoglycerate/2-phosphoglycerate and lactate, as well as ATP and NADH (Figure 6A). To directly access glycolysis, we analyzed extracellular acidification rates (ECAR) of freshly isolated AML cells from the bone marrow. AMPKαΔ/Δ AML cells had significantly reduced basal ECAR and the glycolytic capacity, as determined by the ECAR after oligomycin treatment (Figure 6C), indicating that AMPK is required for glycolysis of AML cells. Consistently, GSEA and qPCR analyses revealed decreased expression of genes involved in glycolysis in AMPKαΔ/Δ L-GMPs (Figure S5A-B). AMPKαΔ/Δ AML cells also exhibited significantly reduced oxygen consumption rates (OCR), indicating that mitochondrial respiration is compromised by AMPK deletion (Figure 6D). The reduced OCR of AMPKαΔ/Δ AML cells is reminiscent of Lkb1-deficient hematopoietic cells (Gurumurthy et al., 2010), and suggests that the increased ROS of AMPKαΔ/Δ AML cells is unlikely to be caused by increased oxidative phosphorylation. Collectively, these data indicate that AMPK promotes glucose uptake and subsequent glucose metabolism.
Figure 6. AMPK regulates glucose metabolism.
(A) 13C-glucose flux analysis performed on freshly isolated AMPKαfl/fl and AMPKαΔ/Δ AML cells exposed to 2.0 g/L 13C-glucose for the indicated time revealed significantly reduced 13C incorporation into glycolysis intermediates 3PG/2PG and lactate, in addition to NADH and ATP. The amounts of 13C-labeled metabolites were normalized to the values of AMPKαfl/fl cells (n=3). Schematic of glycolysis and the pentose phosphate pathway (PPP) is shown on right. (B) 13C-glucose flux analysis was performed as in (A) with either 2.0 g/L (left panels) or 0.2 g/L glucose (right panels) to access the glucose flux through PPP (n=3). (C) Basal ECAR and ECAR after oligomycin treatment were significantly reduced in freshly isolated AMPKαΔ/Δ AML cells compared to AMPKαfl/fl AML cells (n=3). (D) Extracellular Flux analysis revealed that AMPKαfl/fl AML cells have significantly reduced OCR (n=3). See also Figure S5.
Since AMPK deletion significantly increased ROS (Figure 4C, D, G, H) and reduced the amount of antioxidants (Figure 4E, F), we tested whether AMPK regulates the pentose phosphate pathway (PPP), a metabolic pathway that branches from glycolysis and produces antioxidants (Patra and Hay, 2014). 13C-glucose metabolic flux analysis using 2.0 g/L glucose only revealed a trend towards reduced glucose flux through PPP by AMPK deletion (Figure 6B, left). Interestingly, a similar flux analysis in the presence of low glucose (0.2 g/L) revealed significantly reduced flux into the PPP by AMPK deletion, as shown by reduced 13C incorporation into ribose-5-phosphate (R5P)/xylulose-5-phosphate (X5P) and sedoheptulose-7-phosphate (S7P, Figure 6B, right). These results indicate that AMPK is important for carbon flux through the PPP in AML cells, particularly under low glucose conditions.
Dietary restriction suppresses AMPK-deficient AML
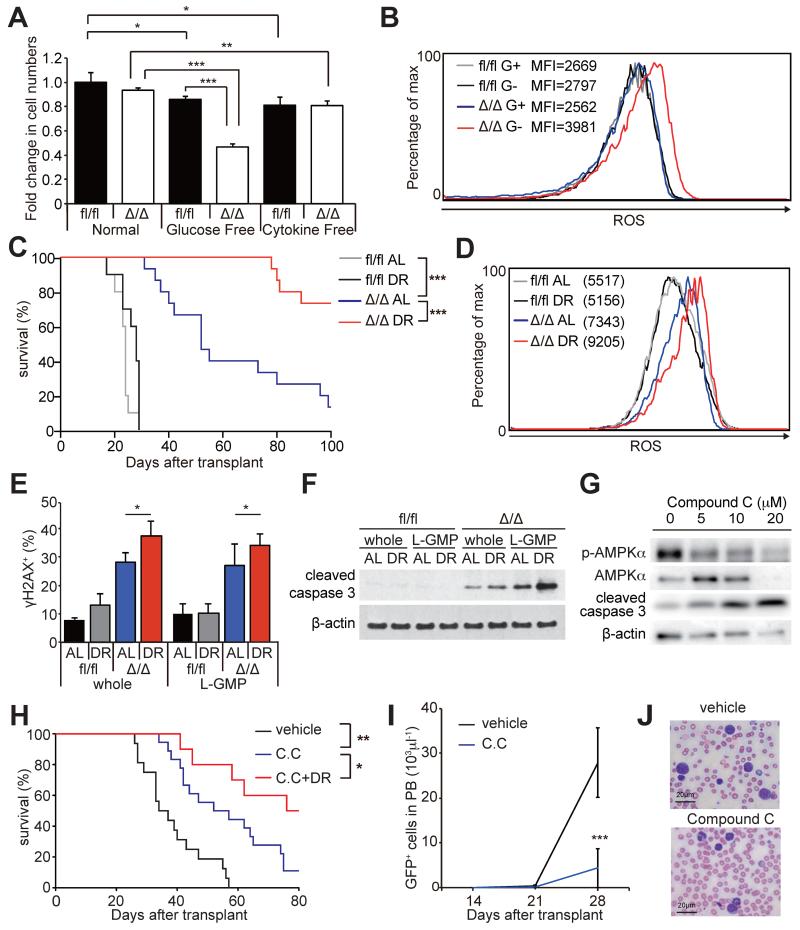
AMPK was hyper-activated in the bone marrow but not spleens of DR mice (Figure 1C), and L-GMPs and AML cells residing in the bone marrow were more dependent upon AMPK than those in the spleens for their maintenance and to suppress ROS (Figure 3B, 4D, and 4H). We found that the glucose levels in the bone marrow were much lower than that in the spleens or peripheral blood, and that DR further reduced the glucose levels in the bone marrow (Figure S1A). This raised a possibility that AML cells become acutely depleted in the bone marrow upon AMPK deletion (Figure 3B) due to the lower glucose levels in the bone marrow that in the spleens. Consistently, exposure of AMPKαΔ/Δ AML cells in vitro with no or low glucose conditions, but not cytokine deprivation, was sufficient to reduce cell survival (Figure 7A and S6A). Interestingly, upon glucose deprivation, AMPKαΔ/Δ AML cells upregulated ROS (Figure 7B), consistent with our observation that the PPP flux is reduced in AMPKαΔ/Δ AML cells under low glucose condition (Figure 6B). These results suggest that AMPK is particularly important to suppress ROS in a hypoglycemic condition such as in the bone marrow.
Figure 7. AMPK inhibition sensitized AML to dietary restriction.
(A) In vitro glucose deprivation, but not cytokine deprivation, significantly reduced survival of AMPKαΔ/Δ AML cells during a 24-hour culture (n=4). (B) Representative histograms showing increased ROS levels in AMPKαΔ/Δ AML cells after glucose deprivation in vitro. G+ and G− indicates cultured with or without glucose, respectively. (C) Secondary recipients of 104 AMPKαfl/fl (n=10 per group) and AMPKαΔ/Δ AML cells (n=15 per group) were either fed AL or subjected to DR and their survival monitored. DR did not extend the survival of AMPKαfl/fl AML recipients, but did significantly extend the survival of AMPKαΔ/Δ AML recipients. (D) Representative histograms showing that DR further increased the higher ROS levels of AMPKαΔ/Δ L-GMPs (n=3). (E) The frequencies of whole AML cells and L-GMPs positive for γH2AX examined by immunofluorescence assay. (F) Immunoblotting assay using AMPKαfl/fl and AMPKαΔ/Δ whole AML cells and L-GMPs isolated from the bone marrow of the recipient mice subjected to AL or DR. (G) Immunoblotting assay of primary AML cells treated with compound C. (H) Secondary recipient mice of 103 MLL-AF9 AML cells were fed AL or subjected to DR after transplantation. 7 days after transplantation, mice were treated with 4 mg/kg of compound C (C.C) or control vehicle. Compound C treatment significantly extended the survival, and this was further extended when combined with DR (vehicle; n=16, C.C; n=18, C.C+DR; n=10). (I and J) Compound C treatment significantly reduced the total numbers of GFP+ AML cells (I) and the frequencies of blasts (J) in peripheral blood (vehicle; n=5, C.C; n=8). Scale bars indicate 20 μm. See also Figure S6.
We then tested whether AMPK inhibition sensitizes AML cells to metabolic stress caused by DR by subjecting secondary recipient mice of AMPKαfl/fl and AMPKαΔ/Δ AML cells to AL or DR. Strikingly, although DR did not extend the survival of mice transplanted with AMPKαfl/fl AML cells (as in Figure 1A), it significantly extended the survival of mice transplanted with AMPKαΔ/Δ AML cells (Figure 7C). Additionally, AMPKαΔ/Δ L-GMPs from mice subjected to DR had significantly increased levels of ROS, DNA damage, and apoptosis compared to mice subjected to AL, consistent with our findings that glucose deprivation increases oxidative stress in AMPKαΔ/Δ AML cells (Figure 7D-F). DR did not affect the frequencies of normal hematopoietic progenitors including HSCs, multipotent progenitors (MPPs), and GMPs of AMPK-deficient mice (Nakada et al., 2010) even though DR activated the AMPK pathway in bone marrow cells, indicating that normal hematopoietic progenitors do not depend upon AMPK during DR (Figure S6B-D). AMPK-deficient hematopoietic progenitor cells including HSCs, MPPs, MEPs, CMPs, and GMPs were not sensitive to metabolic stress caused by metformin, even though metformin treatment activated AMPK in bone marrow cells (Figure S6E-F). These results indicate that although both normal hematopoietic cells and AML cells activate AMPK upon metabolic stress, AML cells are particularly dependent on AMPK to suppress oxidative stress, DNA damage, and apoptosis upon metabolic stress.
Finally, we tested whether pharmacological inhibition of AMPK by an AMPK inhibitor compound C suppresses AML. Treatment of MLL-AF9 transduced AML cells with compound C in vitro reduced AMPK activation and induced apoptosis (Figure 7G). Moreover, L-GMPs were more sensitive to compound C than normal GMPs (Figure S6H). Next, we examined whether compound C inhibits murine AML in vivo. We treated secondary recipients of MLL-AF9 AML cells with compound C (4mg/kg/day) or control vehicle starting from 7 days after transplantation. Compound C treatment significantly extended the survival of recipient mice (Figure 7H) and reduced the number of circulating GFP+ AML cells in peripheral blood (Figure 7I and J). Interestingly, the combination of compound C with DR markedly prolonged the survival compared to compound C treatment alone (Figure 7H) similar to the results obtained by genetically deleting AMPK, suggesting that AMPK can be manipulated pharmacologically to sensitize AML to physiological metabolic stress. To test whether human AML cells can be targeted by this combination therapy we transplanted MOLM13 cells, which carry MLL-AF9 rearrangements, into NOD-SCID mice and treated them with compound C and DR. The survival of recipient mice was significantly extended by both compound C and DR regimen, and further extended by the combination of compound C and DR (Figure S6I). Together with the genetic data, these results suggest that AMPK inhibition renders both murine and human AML cells sensitive to physiological metabolic stress, which can be exacerbated by dietary manipulation.
DISCUSSION
Maintaining metabolic homeostasis under stressful conditions is essential for cancer cells that propagate in metabolically adverse environment, such as in the bone marrow where AML cells expand. Our study shows that AMPK is critical for the maintenance of leukemic cells within the bone marrow, and that deletion of AMPK renders leukemias susceptible to physiological metabolic stress caused by dietary restriction. AMPK has been shown to have both tumor suppressor roles and tumor supportive roles. Supporting the tumor suppressive roles of AMPK, AMPK activator such as metformin has been shown to suppress tumor cell proliferation in vitro and in vivo (Faubert et al., 2015; Jeon and Hay, 2015; Liang and Mills, 2013; Saito and Nakada, 2014). Deletion of the α1 catalytic subunit of AMPK accelerated Eμ-Myc-induced B-cell lymphomagenesis by promoting aerobic glycolysis (Faubert et al., 2013). The difference between our results showing that multiple AML and CML models depend upon AMPK and the results from Faubert et al. showing that B-cell lymphomas are suppressed by AMPK might be due to the different cell types in which the cancer was initiated. The metabolism of myeloid cells and lymphoid cells are known to be different (Fox et al., 2005; O’Neill and Hardie, 2013). AMPK activation in monocytes promoted glycolysis (Marsin et al., 2002), whereas in T-lymphocytes AMPK suppressed glycolysis (MacIver et al., 2011). The functions of FoxO transcription factors are also different between AML and B-lymphomas. MLL-AF9 driven AML maintain low Akt signaling in order to allow FoxO transcription factors to suppress differentiation (Sykes et al., 2011), whereas Eμ-Myc-induced B-cell lymphomagenesis was promoted by ablation of FoxO (Bouchard et al., 2007). Regardless of whether different tumors use AMPK to promote or suppress glycolysis, our results are overall consistent with the idea that AMPK inhibition sensitizes cancer cells to metabolic stress by increasing oxidative stress (Faubert et al., 2013; Jeon et al., 2012). Our results are also consistent with the findings from non-small cell lung cancer in which Lkb1-deficient tumors with attenuated AMPK activity were sensitive to metabolic stress caused by phenformin and exhibited increased apoptosis and ROS (Shackelford et al., 2013). Our results further demonstrate that AMPK inhibition can make LICs residing in the hypoxic bone marrow environment vulnerable to physiological metabolic stress caused by changes in diet, pointing to differential vulnerability of cancer cells on AMPK inhibition depending upon their tumor microenvironment.
AMPK was required to maintain the expression of Glut1, the predominant glucose transporter in hematopoietic cells (Rathmell et al., 2003), and to uptake glucose. AMPK deletion led to impaired glycolytic flux, reduced synthesis of ATP and reducing agents NADPH and GSH. Consistent with our results, AMPK deletion in cancer cell lines and mouse embryonic fibroblasts has been shown to decrease the levels of NADPH and GSH and increase ROS upon glucose deprivation (Jeon et al., 2012). Other regulators of glycolysis such as PKM2 and LDHA have also been shown to be critical for LIC maintenance by promoting glycolysis (Wang et al., 2014b). Additionally, Glut1 has been identified in a screen as important for cancer cell proliferation in low glucose culture condition (Birsoy et al., 2014). Consistent with a previous report (Wu et al., 2013), we found that AMPK deletion increases the protein levels of Txnip1, which reduces Glut1 expression. AMPK has been shown to phosphorylate and destabilize Txnip1, thereby increasing Glut1 expression. Our results therefore suggest that the previously documented AMPK-Txnip1-Glut1 module is functionally important for LIC maintenance. We note, however, that it is still unclear whether AMPK phosphorylates and destabilizes Txnip1 in AML cells, and whether increased Txnip1 is the cause of Glut1 depletion upon AMPK deletion in AML cells. Future studies will be needed to fully elucidate the role of Txnip1 in AMPK-dependent glucose metabolism of AML cells.
We found that LICs residing in the bone marrow were more dependent on AMPK to suppress oxidative stress than those residing in the spleen, indicating that the cancer microenvironment determines the extent to which cancer cells depends upon AMPK. Little is know about the difference in the metabolic milieu of the bone marrow and the spleens. We found that the glucose levels in the bone marrow were significantly lower that those in the spleen and in the peripheral blood (Figure S1A). Consistent with the idea that the low glucose concentration in the bone marrow renders LICs in the bone marrow particularly dependent upon AMPK, AMPK-deficient AML cells cultured briefly in low or glucose free medium had reduced glucose flux through PPP, a metabolic pathway that branches from glycolysis and produces NADPH, leading to increased ROS and induced cell death. Thus, low glucose levels in the bone marrow at least partially contribute to the higher dependence of LICs upon AMPK. In addition to the lower glucose level in the bone marrow, other factors such as the oxygen tension (Nombela-Arrieta et al., 2013; Spencer et al., 2014) or IGF-1 (Kalaany and Sabatini, 2009; Longo and Fontana, 2010) may also contribute to increased dependence on AMPK in the bone marrow.
In contrast to myeloid leukemias that were dependent on AMPK, normal HSCs were not dependent upon AMPK during homeostasis, upon transplantation (Nakada et al., 2010), and even under metabolic stress by DR or metformin treatment (Figure S6). This raises a possibility that AMPK inhibition provides a therapeutic window to treat leukemias without harming normal hematopoiesis. Consistent with this hypothesis, we found that L-GMPs were more sensitive to an AMPK inhibitor, compound C, than normal GMPs. In both murine and human AML transplantation models, compound C single treatment delayed the onset of leukemogenesis and the combination of compound C with DR further delayed leukemogenesis. Although compound C may have AMPK-independent targets (Hao et al., 2010; Yu et al., 2008), our genetic analyses using three different myeloid leukemia models provide strong support for the idea that AMPK inhibition may suppress multiple myeloid malignancies.
In conclusion, our results demonstrate that targeting AMPK suppresses myeloid leukemias by interrupting glucose metabolism, and reveal different metabolic requirements of leukemias residing in the bone marrow and in the spleen. AMPK inhibition combined with dietary manipulation or its mimetics may also suppress other tumors that reside in hypoglycemic, hypoxic, nutrient poor conditions that are difficult to target with conventional therapies (Wilson and Hay, 2011).
EXPERIMENTAL PROCEDURES
Mice and treatments
Prkaa1fl and Prkaa2fl mice were reported before (Nakada et al., 2010). C57BL/Ka-Thy-1.2 (CD45.1), C57BL/Ka-Thy-1.1 (CD45.2), and NOD-SCID mice (8-12 weeks of age) were used for AML transplantation assays. Poly(I:C) (Amersham, Piscataway, NJ) was injected intraperitoneally with 0.5 μg/gram of body mass every other days for 6 days. 5 mg of tamoxifen (Sigma, St. Louis, MO) was administered daily by oral gavage for 5 days. 1 mM Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy) (Enzo Life Science, Farmingdale, NY) was given to mice in the drinking water. 4 mg/kg of compound C (Cayman Chemical, Ann Arbor, MI) diluted in DMSO was intraperitoneally injected for 4 weeks. Metformin (1.5 mg/ml) was given to mice in the drinking water. All mice were fed Harlan Rodent Diet 2920X, either ad libitum (AL) or with DR regimen (70% of the normal food intake). Mice were housed in AAALAC-accredited, specific-pathogen-free animal care facilities at Baylor College of Medicine (BCM). All procedures were approved by BCM Institutional Animal Care and Use Committees.
Retroviral bone marrow transduction assays
5-FU primed bone marrow cells were incubated in X-Vivo 15 (Lonza, Allendale, NJ) supplemented with 50 ng/ml SCF, 50 ng/ml TPO, 10 ng/ml IL-3 and 10 ng/ml IL-6 (all from Peprotech, Rocky Hill, NJ) for 24 hours. After incubation, cells were spin-infected with retroviral supernatant supplemented.
CRISPR/Cas9-mediated gene editing in AML cells
AML cells were infected with doxycycline-inducible Cas9 expressing lentivirus (pCW-Cas9: Addgene 50661) (Wang et al., 2014a) and sgRNA expressing lentivirus (pKLV-U6gRNA(BbsI)-PGKpuro2ABFP: Addgene 50946) (Koike-Yusa et al., 2014). Infected clones were selected with puromycin (0.5 μg/ml, Life Technologies) for 5 days, and BFP+ cells were sorted for further analysis.
Flow cytometry
ROS was measured using CellROX Deep Red Reagent (Life Technologies, Carlsbad, CA) or MitoSOX Red Mitochondrial Superoxide Indicator (Life Technologies). To analyze glucose uptake in vivo, 100 μl of 2mM 2-(n-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (Life Technologies) was injected intravenously into mice transplanted with pMIR-MLL-AF9. One hour later, mice were sacrificed and bone marrow cells were collected and stained with antibodies to identify L-GMPs.
13C-glucose tracing experiments
Freshly isolated cells from mice were incubated with RPMI-1640 containing 2% dialyzed FBS (Thermo Scientific, Waltham, MA), 2.0 g/L glucose and cytokines for 16 hours, then replaced in pre-warmed labeling medium containing 2.0 g/L or 0.2 g/L 1,2-13C2-glucose (Cambridge Isotope Laboratories, Andover, MA) with cytokines and incubated for 5 or 60 min at 37°C. Metabolites were extracted with an organic solvent (ice cold 90% 9:1 MeOH: CHCl3) and separated on a 1mm ×150mm HILIC column in a 35 min cycle. All analytes and internal standards were measured by ESI− ionization on an Agilent 6520 QTOF mass spectrometer equipped with a 1260 LC unit (Agilent, Santa Clara, CA).
Measurement of extracellular acidification rates (ECAR) and oxygen consumption rates (OCR)
ECAR and OCR were analyzed with a XF24 extracellular flux analyzer (Seahorse Bioscience, Chicopee, MA) following the manufacturer’s protocol.
Supplementary Material
HIGHLIGHTS.
AMPK is activated upon metabolic stress and promotes survival of AML
AMPK deletion disrupts glucose metabolism of AML cells and increases ROS
AMPK is more important for LICs in the bone marrow compared to those in the spleen
AMPK deletion sensitizes AML to metabolic stress caused by dietary restriction
ACKNOWLEDGEMENTS
This work was supported by the Cancer Prevention and Research Institute of Texas, the Gabrielle’s Angel Foundation for Cancer Research, and the National Institutes of Health R01CA193235 (D.N.). Flow-cytometry was partially supported by the NIH (NCRR grant S10RR024574, NIAID AI036211 and NCI P30CA125123) for the BCM Cytometry and Cell Sorting Core. Metabolomics Core Services was supported by grant U24 DK097153 of NIH Common Funds Project to the University of Michigan. Microarray data have been deposited to the Gene Expression Omnibus (GSE64602).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Y.S. performed most experiments with help from R.C., A.L., and A.K.. R.C. and A.L. contributed equally. Y.S. and D.N. designed the experiments, analyzed the results, and wrote the manuscript.
The authors declare no competing financial interests.
REFERENCES
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR, Siri M, Huang RH, Noble S, Lei XH, Clemons PA, Wagner BK. Assay of the multiple energy-producing pathways of mammalian cells. PLoS ONE. 2011;6:e18147. doi: 10.1371/journal.pone.0018147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21:2775–2787. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science (New York, NY. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry NL, Mino-Kenudson M, Oliver TG, Yilmaz OH, Yilmaz VO, Moon JY, Jacks T, Sabatini DM, Kalaany NY. Pten-null tumors cohabiting the same lung display differential AKT activation and sensitivity to dietary restriction. Cancer discovery. 2013;3:908–921. doi: 10.1158/2159-8290.CD-12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Ayton PM, Carapeti M, Kutok JL, Snyder CS, Williams IR, Cross NC, Glass CK, Cleary ML, Gilliland DG. MOZ-TIF2-induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2-mediated recruitment of CBP. Cancer Cell. 2003;3:259–271. doi: 10.1016/s1535-6108(03)00051-5. [DOI] [PubMed] [Google Scholar]
- Ertl RP, Chen J, Astle CM, Duffy TM, Harrison DE. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111:1709–1716. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nature reviews Immunology. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS chemical biology. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Archives of pharmacal research. 2015;38:346–357. doi: 10.1007/s12272-015-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol. 2014;32:267–273. doi: 10.1038/nbt.2800. [DOI] [PubMed] [Google Scholar]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends in pharmacological sciences. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Sabatini DM, Yilmaz OH. Dietary and metabolic control of stem cell function in physiology and cancer. Cell Stem Cell. 2014;14:292–305. doi: 10.1016/j.stem.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering SJ, Bushnell T, Sozer S, Ashton J, Rossi RM, Wang PY, Bell DR, Heinrich D, Bottaro A, Jordan CT. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 2007;110:2578–2585. doi: 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakada D. The Role of the Lkb1/AMPK Pathway in Hematopoietic Stem Cells and Leukemia. Critical reviews in oncogenesis. 2014;19:383–397. doi: 10.1615/critrevoncog.2014011765. [DOI] [PubMed] [Google Scholar]
- Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014 doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sykes SM, Lane SW, Bullinger L, Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H, Brumme KM, et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell. 2011;146:697–708. doi: 10.1016/j.cell.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science (New York, NY. 2014a;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Israelsen WJ, Lee D, Yu VW, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014b;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science (New York, NY. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegue E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.