Abstract
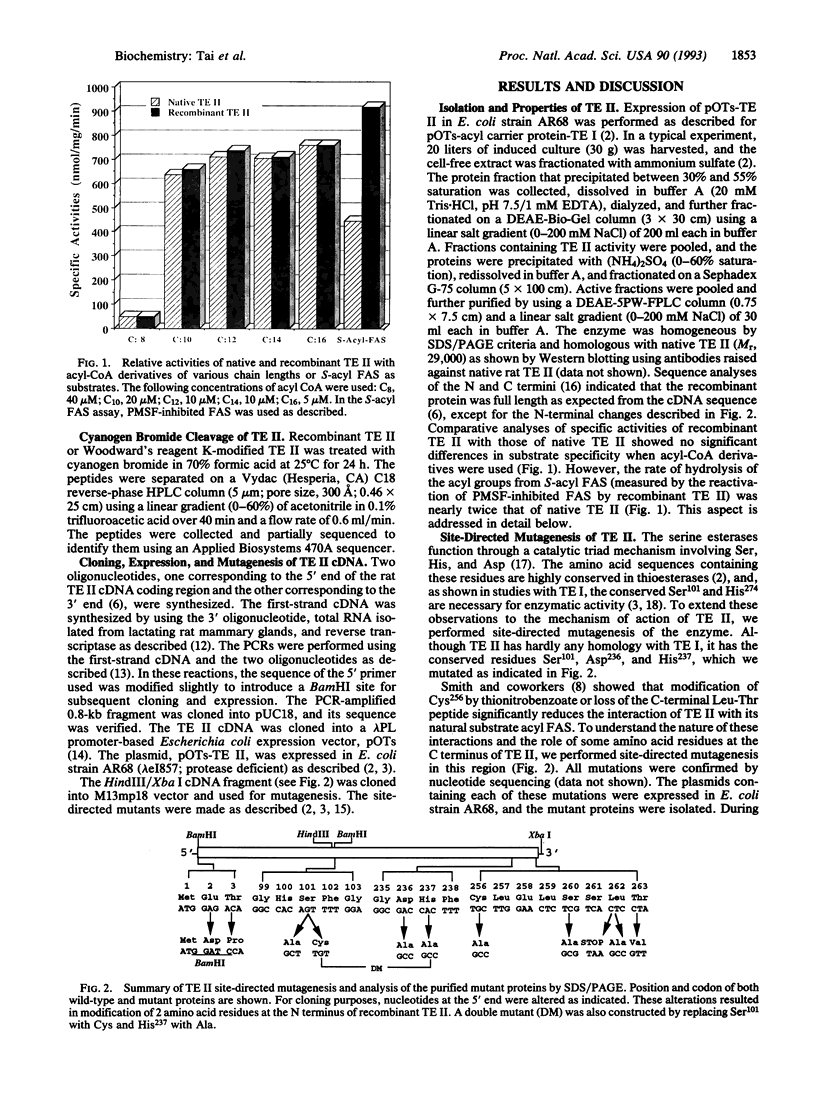
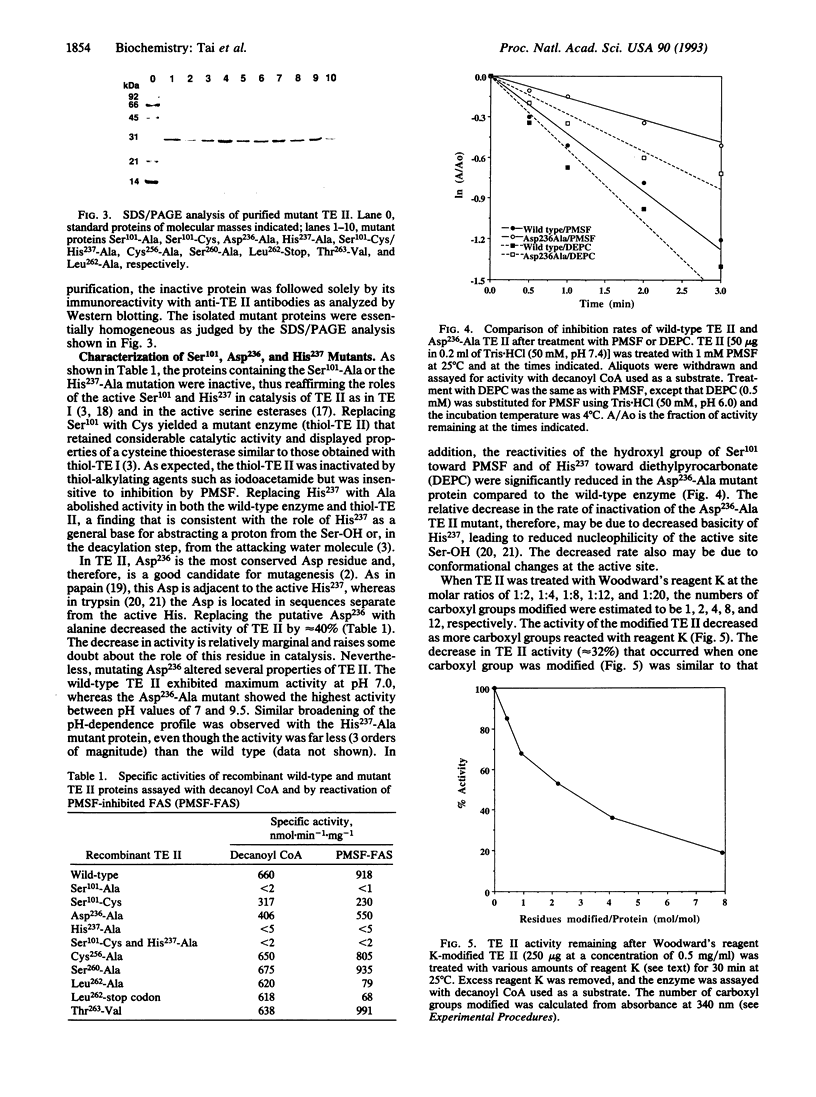
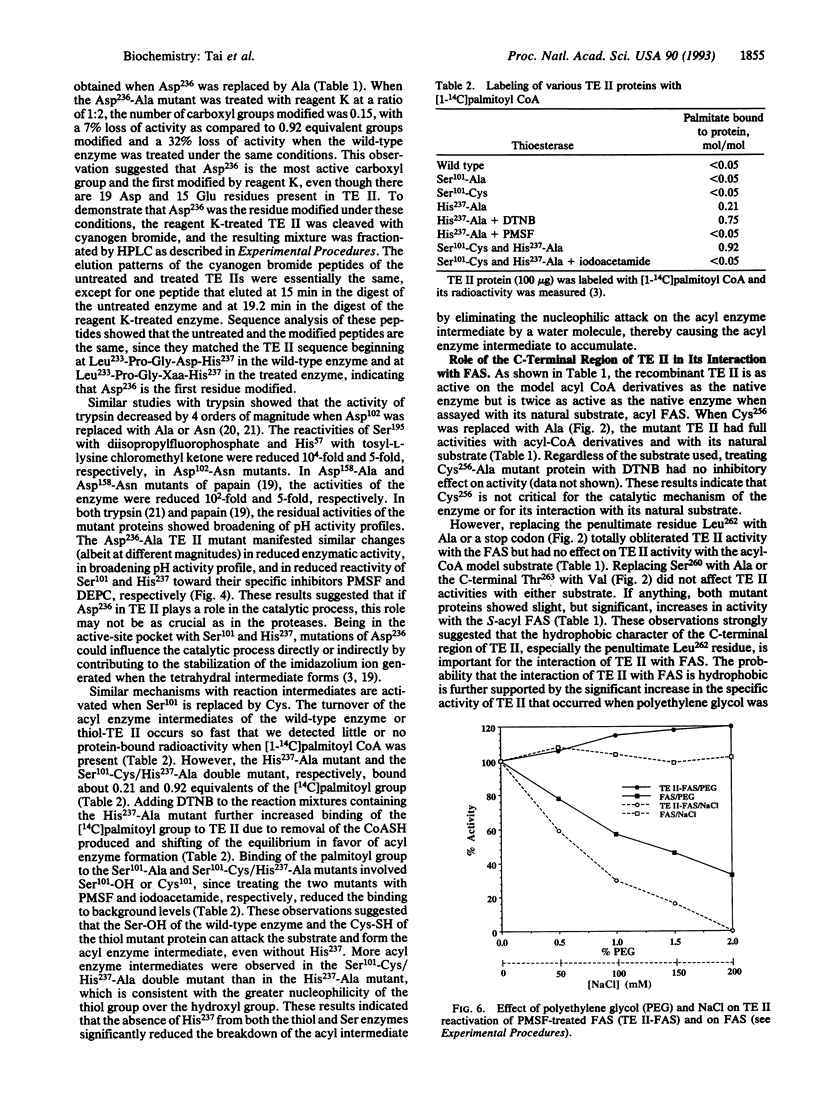
Thioesterase II (TE II), present in specialized tissues, catalyzes the chain termination and release of medium-chain fatty acids from fatty acid synthase [FAS; acyl-CoA:malonyl-CoA C-acyltransferase (decarboxylating, oxoacyl- and enoyl-reducing and thioester-hydrolyzing), EC 2.3.1.85]. We have expressed rat mammary gland TE II in Escherichia coli and created several site-directed mutants. Replacing both Ser101 and His237 with Ala yielded inactive proteins, suggesting that these residues are part of the catalytic triad as in FAS thioesterase (TE I). Mutating the conserved Asp236 or modifying it with Woodward's reagent K caused partial loss (40%) of TE II activity and reduced reactivity of Ser101 and His237 toward their specific inhibitors, phenylmethylsulfonyl fluoride and diethylpyrocarbonate, respectively. These results suggested that Asp236 enhances, but is not essential for, the reactivity of Ser101 and His237. Mutation analyses revealed that, at the C terminus, Leu262 is critical for TE II to interact with FAS. Hydrophobic interactions seem to play a role, since the interaction of TE II with FAS is enhanced by polyethylene glycol but reduced by salt. The Ser101 and His237 mutants and a synthetic C-terminal decapeptide did not compete in the interaction. These results suggest that a TE II-acyl FAS complex forms first, which then is stabilized by the interaction of the hydrophobic C terminus of TE II with FAS, leading ultimately to hydrolysis and release of fatty acid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirala S. S., Kasturi R., Pazirandeh M., Stolow D. T., Huang W. Y., Wakil S. J. A novel cDNA extension procedure. Isolation of chicken fatty acid synthase cDNA clones. J Biol Chem. 1989 Mar 5;264(7):3750–3757. [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- De Renobales M., Rogers L., Kolattukudy P. E. Involvement of a thioesterase in the production of short-chain fatty acids in the uropygial glands of mallard ducks (Anas platyrhynchos). Arch Biochem Biophys. 1980 Dec;205(2):464–477. doi: 10.1016/0003-9861(80)90129-0. [DOI] [PubMed] [Google Scholar]
- Foster R. J., Bonsall R. F., Poulose A. J., Kolattukudy P. E. Interaction of S-acyl fatty acid synthase thioester hydrolase with fatty acid synthase. Direct measurement of binding by fluorescence anisotropy. J Biol Chem. 1985 Feb 10;260(3):1386–1389. [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y in sequence determination of peptides. Methods Enzymol. 1977;47:84–93. doi: 10.1016/0076-6879(77)47010-1. [DOI] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem. 1978 Mar 10;253(5):1393–1401. [PubMed] [Google Scholar]
- Mikkelsen J., Witkowski A., Smith S. Interaction of rat mammary gland thioesterase II with fatty acid synthetase is dependent on the presence of acyl chains on the synthetase. J Biol Chem. 1987 Feb 5;262(4):1570–1574. [PubMed] [Google Scholar]
- Ménard R., Khouri H. E., Plouffe C., Laflamme P., Dupras R., Vernet T., Tessier D. C., Thomas D. Y., Storer A. C. Importance of hydrogen-bonding interactions involving the side chain of Asp158 in the catalytic mechanism of papain. Biochemistry. 1991 Jun 4;30(22):5531–5538. doi: 10.1021/bi00236a028. [DOI] [PubMed] [Google Scholar]
- Pazirandeh M., Chirala S. S., Huang W. Y., Wakil S. J. Characterization of recombinant thioesterase and acyl carrier protein domains of chicken fatty acid synthase expressed in Escherichia coli. J Biol Chem. 1989 Oct 25;264(30):18195–18201. [PubMed] [Google Scholar]
- Pazirandeh M., Chirala S. S., Wakil S. J. Site-directed mutagenesis studies on the recombinant thioesterase domain of chicken fatty acid synthase expressed in Escherichia coli. J Biol Chem. 1991 Nov 5;266(31):20946–20952. [PubMed] [Google Scholar]
- Safford R., de Silva J., Lucas C., Windust J. H., Shedden J., James C. M., Sidebottom C. M., Slabas A. R., Tombs M. P., Hughes S. G. Molecular cloning and sequence analysis of complementary DNA encoding rat mammary gland medium-chain S-acyl fatty acid synthetase thio ester hydrolase. Biochemistry. 1987 Mar 10;26(5):1358–1364. doi: 10.1021/bi00379a023. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sasaki G. C., Cheesbrough V., Kolattukudy P. E. Nucleotide sequence of the S-acyl fatty acid synthase thioesterase gene and its tissue-specific expression. DNA. 1988 Sep;7(7):449–457. doi: 10.1089/dna.1.1988.7.449. [DOI] [PubMed] [Google Scholar]
- Sinha U., Brewer J. M. A spectrophotometric method for quantitation of carboxyl group modification of proteins using Woodward's Reagent K. Anal Biochem. 1985 Dec;151(2):327–333. doi: 10.1016/0003-2697(85)90183-6. [DOI] [PubMed] [Google Scholar]
- Smith S. Medium-chain fatty acyl-s-4'-phosphopantetheine-fatty acid synthase thioester hydrolase from lactating mammary gland of rat. Methods Enzymol. 1981;71(Pt 100):188–200. doi: 10.1016/0076-6879(81)71027-9. [DOI] [PubMed] [Google Scholar]
- Sprang S., Standing T., Fletterick R. J., Stroud R. M., Finer-Moore J., Xuong N. H., Hamlin R., Rutter W. J., Craik C. S. The three-dimensional structure of Asn102 mutant of trypsin: role of Asp102 in serine protease catalysis. Science. 1987 Aug 21;237(4817):905–909. doi: 10.1126/science.3112942. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Ross P., Arslanian M. J., Aune K. C., Wakil S. J., Oliver R. M. Physicochemical studies of the rat liver and adipose fatty acid synthetases. J Biol Chem. 1979 Aug 10;254(15):7418–7426. [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Watt R. A., Shatzman A. R., Rosenberg M. Expression and characterization of the human c-myc DNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):448–456. doi: 10.1128/mcb.5.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowska H. E., Green B. N., Smith S. The carboxyl-terminal region of thioesterase II participates in the interaction with fatty acid synthase. Use of electrospray ionization mass spectrometry to identify a carboxyl-terminally truncated form of the enzyme. J Biol Chem. 1990 Apr 5;265(10):5662–5665. [PubMed] [Google Scholar]
- Witkowski A., Naggert J., Witkowska H. E., Randhawa Z. I., Smith S. Utilization of an active serine 101----cysteine mutant to demonstrate the proximity of the catalytic serine 101 and histidine 237 residues in thioesterase II. J Biol Chem. 1992 Sep 15;267(26):18488–18492. [PubMed] [Google Scholar]