Abstract
Background
Even subtle impairments on cognitive test scores can be associated with future cognitive decline and dementia. We assayed the relationships between test score impairment and adverse outcomes.
Methods
Secondary analyses were performed on data from non-institutionalized participants, 50+ years of age (N = 30,038), from 12 countries taking part in the Survey of Health, Ageing and Retirement in Europe (SHARE) longitudinal study on aging. At baseline, participants’ cognition was tested using verbal fluency, immediate recall, and delayed recall tasks.
Results
Greater levels of cognitive impairment at baseline were strongly associated with future poor health outcomes and functional impairment. Controlling for age, sex and education, those with 1 (OR = 1.58, 95% CI = 1.34–1.87) or ≥ 2 (OR = 2.59, 95% CI = 2.17–3.09) impaired tests at baseline were more likely to die after an average of 40 months compared to individuals with no impairments. After selecting for participants who reported the absence of dementia initially, those with ≥ 2 cognitive impairments at baseline (OR = 3.34, 95% CI = 2.27–4.92) were more likely to report dementia at follow-up compared to those with no impairment.
Conclusions
People with impaired cognitive test scores at baseline are at greater risk to die or develop dementia within four years than their less impaired or unimpaired counterparts.
Keywords: Alzheimer’s disease, dementia, cognitive impairment, risk factors, longitudinal studies
INTRODUCTION
Cognitive decline and neurodegeneration (including Alzheimer’s disease (AD)) are both increasingly common features of aging. Baseline cognition is strongly associated with changes in cognition and functional impairment.(1) Similarly, the risk of institutionalization increases substantially with increasing cognitive and functional impairment. Functional impairment, or physical disability as measured by Instrumental and basic Activities of Daily Living (IADL; ADL), often proceeds in a hierarchical fashion(2) and indeed staging systems based entirely on function correlate well with more general staging schemes.(3,4) For example, severe dementia, which often manifests as difficulty with three or more ADLs (e.g., bathing, dressing, toileting), is a strong predictor of nursing home admission.(5) Impairments in IADLs (e.g., telephone use, financial management, housekeeping), which facilitate daily independent living, are often telltale signs of future cognitive decline(6) and risk of mild cognitive impairment (MCI) or conversion to dementia.(7) However, the level of cognitive impairment that implies increased risk of further mental or physical decline is not well defined, as it may vary greatly among individuals and populations.
The Survey of Health, Ageing and Retirement in Europe (SHARE) is the first longitudinal study to examine the various health, economic, and social factors that are associated with aging. It currently consists of more than 60,000 people from among the non-institutionalized population aged 50 and older, and their spouses/partners (independent of age), in 20 participating European countries. The cognition-related items included in the SHARE database have been used in some studies,(8,9,10) but there has not been an analysis of how cognitive status at baseline relates to subsequent adverse health outcomes associated with cognitive decline.
The first objective of the present analyses was to examine the relationships between cognition and health-related functional capacities (i.e., physical disability and difficulties with IADLs and ADLs) in the SHARE dataset. Secondly, this study examined how performance at baseline on three cognitive tasks relates to three adverse health outcomes at follow-up: dementia, institutionalization, and mortality.
METHODS
Study Sample
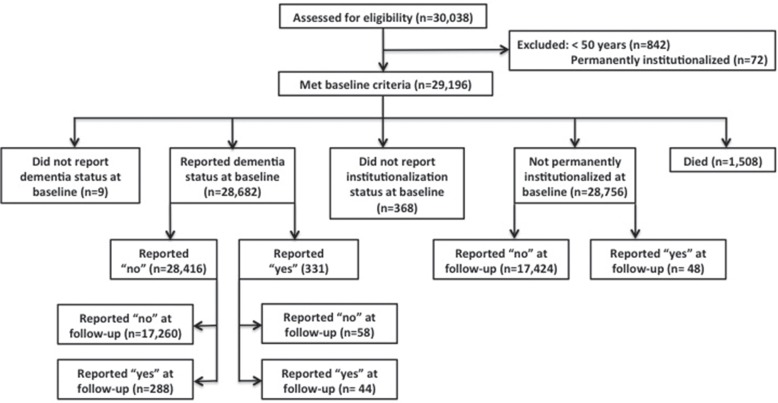
Secondary analyses were conducted on data from Waves 2 (baseline; 2006–2007) and 4 (2010–2011) from the SHARE database (releases 2.5.0 as of May 24th, 2011, and 1.1.1 as of November 30th, 2012, respectively; N = 30,038; Figure 1). Respondents from 12 countries (Austria, Germany, Sweden, Netherlands, Spain, Italy, France, Denmark, Switzerland, Belgium, Czech Republic, and Poland) who took part in the second and fourth waves of SHARE were chosen for our analyses. Data collection consisted of face-to-face interviews and mail-out surveys. At baseline, we excluded spouses/partners below the age of 50 (n = 842) and those individuals who were recorded as being permanently admitted to a nursing home in the last 12 months (n = 72). For the analyses examining reports of dementia, we made the same exclusion as above, but also excluded those who reported the presence of AD or dementia at baseline (n = 331). Dementia status was not recorded in Wave 1 of SHARE, hence our use of Wave 2 as baseline for these purposes. Health outcomes examined at Wave 4 included reports of AD or dementia, institutionalization, and mortality, after an average of 4 years and 3.6 months. Education level was standardized across participants in SHARE according to the ISCED-1997 code.(11) Approval for secondary analyses came from the Research Ethics Board of the Capital District Health Authority at Halifax, Nova Scotia, Canada.
FIGURE 1.
Participant flow diagram for baseline and follow-up outcomes: dementia status, institutionalization, and mortality.
Cognitive Tasks
The SHARE protocol included performance-based cognitive tests,(12) which were used to generate a cognition score for each participant during each wave, as previously described.(10) Only those cognitive tasks that were conducted in both Waves 2 and 4 were included in the score, namely, performance on verbal fluency, immediate recall, and delayed recall tasks. These tests are sensitive measures for discriminating between cognitively healthy individuals and those with MCI or dementia (i.e., AD).(2,13,14) Threshold performance scores for being coded as “1” (indicating impairment) were set in relation to scores previously shown to be indicative of MCI or AD, as follows: verbal fluency scores < 15; immediate recall scores < 5; and delayed recall scores < 4 (see Balthazar et al.,(13) Takayama,(14) and Xie et al.(15) for test details). A single code for recall ability was created by averaging the codes for immediate and delayed recall scores, such that “0” was considered unimpaired and “0.5” and “1” were considered impaired. Those reporting “don’t know” for any of these measures were coded as “1,” or impaired. Only those participants who completed all three cognitive tests in Wave 2 were used for the analyses (2.3% excluded). The cognition scores for each Wave were calculated by adding the total number of impairments (0–2) and are presented in relation to the number of tests demonstrating impairment, such that each subtest is weighted equally. Cognitive performance was classified as zero (n = 11,771), 1 (n = 10,423), or 2 impairments (n = 6,082).
Functional Capacity
Cognitive status in relation to various measures of functional capacity at baseline was examined, including ADLs, IADLs, mobility, and physical activity. Similar to a previous report,(2) cognitive performance was also examined in those with mild, moderate, and severe degrees of ADL and IADL functional impairment (Table 1; scored out of 13; not including the variables measuring mobility and physical activity independent from ADL and IADL questions). Individuals categorized as “mild” exhibited mild IADL impairment without ADL impairment and were grouped based on difficulty with any of the following: managing money, making telephone calls, taking medications, or using a map to navigate in a strange place. Individuals categorized as “moderate” exhibited difficulty with all of the IADLs in the “mild” group, plus ADLs that required prompts (i.e., impairment with bathing or showering); they may also exhibit difficulty with the preparation of a hot meal, shopping for groceries, or doing work around the house or garden. Individuals categorized as “severe” exhibited severe ADL and IADL impairment which included the same impairment as the “moderate” group, plus they must exhibit difficulty with the preparation of a hot meal, shopping for groceries, and doing work around the house or garden, as well as at least one other ADL impairment (i.e., dressing, walking across a room, eating, getting in or out of bed, or using the toilet).
TABLE 1.
Categories of functional impairment and corresponding criteria
| Category | Criteria | Total Number of Impairments |
|---|---|---|
| Mild |
|
1–4 |
| Moderate |
|
5–8 |
| Severe |
|
9–13 |
Dementia, Institutionalization, and Mortality
Diagnosis of AD or dementia (i.e., 0 = absent, 1 = present), was determined by responses to the following question in SHARE’s Wave 2 and 4 questionnaires: “Has a doctor every told you that you had/Do you currently have any of the conditions on this card? With this we mean that a doctor has told you that you have this condition, and that you are either currently being treated for or bothered by this condition?” The primary option of interest was “Alzheimer’s disease, dementia, organic brain syndrome, senility or any other serious memory impairment”. This health measure was reported by the participants themselves or a proxy respondent (e.g., spouse/partner).
Recent institutionalization status (i.e., 0 = not institutionalized, 1 = permanently institutionalized) was assessed using responses to the following question in Waves 2 and 4: “During the last twelve months, have you been in a nursing home overnight?” Clarifying information for the interviewer when coding responses included: “A nursing home provides all of the following services for its residents: dispensing of medication, available 24-hour personal assistance and supervision (not necessarily a nurse), and room and meals. Permanently means nonstop during the past 12 months”. Response options included: “no”, “yes, temporarily”, and “yes, permanently”. Those who reported being temporarily institutionalized at some point during the past 12 months were coded as not being currently institutionalized. Finally, survival was reported by relatives, friends, or neighbours (i.e., died; 0 = no, 1 = yes).
Statistical Analyses
The first objective was explored using one-way, between-subjects analyses of variance to examine whether at baseline various aspects of functional capacity differed between levels of cognitive impairment; independent samples t-tests were used to evaluate such differences for those who did or did not report AD or dementia at baseline. Post-hoc comparisons were conducted using Games-Howell. Due to potential overestimation of between-groups effects because of the large sample size, eta squared was calculated for the main effects models, and Cohen’s d was calculated for the differences in cognition among the mild, moderate, and severe functional performance groups, when possible. For the second objective, binary logistic regression was used to estimate the likelihood and 95% confidence intervals (CI) that 0, 1 or 2–3 cognitive impairments at baseline (Wave 2) predicted future reports of AD or dementia, permanent institutionalization, and mortality at Wave 4. Age, sex, and education were used as covariates to adjust the risk models. Means are reported as ± the standard deviation. SPSS (18.0.0, SPSS Inc.) was used to analyze the data, with statistical significance set at p = .05.
RESULTS
Baseline Demographics
At baseline (Wave 2), our sample had a mean age of 64.7 years (range 50–104), and 54.6% were women. Some 331 reported the presence of AD or dementia, and 16, 46, and 180 of these participants exhibited 0, 1, and ≥ 2 cognitive impairments at baseline. Some 1,508 were recorded as having died at follow-up (Figure 1).
Objective 1: Functional Capacity in Relation to Baseline Cognition and Dementia Status
Higher numbers of cognitive impairments were associated with higher average age, more difficulties with ADLs and IADLs, and more mobility impairments at baseline. Higher levels of cognitive impairment were also associated with lower levels of education and poorer self-perceived health (Table 2). Although these associations were significantly different at each level of cognitive impairment, larger (albeit moderate) effect sizes were associated primarily with differences between those with 1 and ≥ 2 impairments, while there were only small differences between those with 0 and 1 impairment (not reported here).
TABLE 2.
Functional capacity in relation to cognitive performance and dementia status at baseline (Wave 2)
| Health Factors | All (N=30,038) | Number of Cognitive Impairments in Those Non-Institutionalized at Baseline | Reported Dementia Status at Baseline | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 0 (n=11,771) | 1 (n=10,423) | ≥2 (n=6,082) | Without AD or Dementia (n=28,416) | With AD or Dementia (n=331) | ||
| Age (Mean ± SD) | 64.3 (10.4) | 61.2 (8) | 65.1 (9.6) | 70.3 (10.5) | 64.8 (10.3) | 77.6 (9.5) |
| Education (Mean ± SD; ISCED) | 2.6 (1.5) | 3.2 (1.4) | 2.5(1.4) | 1.7 (1.2) | 2.5 (1.5) | 1.8 (1.4) |
| Impairments in ADLs (Mean ± SD) | 0.2 (0.8) | 0.1 (0.4) | 0.2 (0.7) | 0.5 (1.2) | 0.2 (0.7) | 2.0 (2.3) |
| % More than 1 ADL | 10.7 | 4.9 | 9.1 | 21.7 | 9.9 | 58.3 |
| Impairments in IADLs (Mean ± SD) | 0.4 (1.1) | 0.1 (0.4) | 0.2 (0.7) | 0.9 (1.7) | 0.3 (0.9) | 3.8 (2.7) |
| % More than 1 IADL | 16.4 | 7.5 | 13.9 | 33.9 | 15.6 | 81.9 |
| % Self-rated health (very good or excellent health) | 26.5 | 30.6 | 23.5 | 8.1 | 17.4 | 2.7 |
| % Impaired activities | 44.7 | 36.5 | 44.3 | 61.4 | 44.6 | 89.7 |
| Impairments in mobility (Mean ± SD) | 1.5 (2.3) | 0.9 (1.6) | 1.4 (2.0) | 2.8 (2.9) | 1.5 (2.2) | 5.0 (3.3) |
| % Impaired on 1 or more aspects of mobility | 46.9 | 37.3 | 47.4 | 65.9 | 47.0 | 86.7 |
| % Impaired on 3 or more aspects of mobility | 23.3 | 13.4 | 21.8 | 44 | 23.0 | 72.5 |
| % Physically inactive | 11.6 | 4.9 | 9.3 | 27.1 | 11.3 | 58.9 |
| % Problem getting around with a map | 7.4 | 2.4 | 5.0 | 18.5 | 6.6 | 65.9 |
Functional Impairment Severity for All Participants at Baseline
Non-institutionalized individuals at baseline demonstrated a significant main effect of cognitive performance on functional capacity for those with a mild level of impairment (F [2, 2108] = 88.28, p < .001; η2 = .08; Table 3). Post hoc comparisons indicated that in those with mildly impaired function, the number of functional impairments was greater in the participants with ≥ 2 cognitive impairments at baseline, compared to those with 0 or 1 impairment. The effect sizes for comparisons of 1 vs. ≥ 2 (d = −.42) and 0 vs. ≥ 2 (d = −.54) were much larger than for the comparisons of 0 vs. 1 impairment (d = −.19). There was no influence of cognitive performance capacity on functional impairment among those with moderate (F [2, 22] = 2.25, p = .15) or severe levels of impairment (F [2,118] = 1.02, p = .36).
TABLE 3.
Functional status as measured by severity of functional impairment at baseline, in relation to cognitive and dementia status at baseline
| Number of Cognitive Impairments at Baseline (Wave 2) | Severity of Functional Impairment at Baseline | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mild | Moderate | Severe | ||||
| All participants (N=29,196) | N (%) | (M ± SD) | N (%) | M ± SD | N (%) | M ± SD |
| 0 | 342 (1) | 1.1±0.4 | 0 | --- | 1 | 13 |
| 1 | 656 (2) | 1.2±0.5 | 1 | 8 | 7 (0.02) | 11.3±1.5 |
| ≥2 | 1,213 (4) | 1.6±0.8 | 24 (0) | 7.1±0.6 | 117 (0.4) | 11.9±1.3 |
|
| ||||||
| Without AD or dementia (N=28,416) | ||||||
| 0 | 336 (1) | 1.1±0.4 | 0 | --- | 1 | 13 |
| 1 | 638 (2) | 1.2±0.5 | 1 | 8 | 7 (0.02) | 11.3±1.5 |
| ≥2 | 1,105 (4) | 1.5±0.7 | 17 (0.05) | 7±0.5 | 76 (0.3) | 11.9±1.3 |
|
| ||||||
| With AD or dementia (N=331) | ||||||
| 0 | 5 (2) | 1.4±0.9 | 0 | --- | 0 | --- |
| 1 | 18 (5) | 1.4±0.7 | 0 | --- | 0 | --- |
| ≥2 | 108 (33) | 2.2±1.0 | 7 (2) | 7.3±0.8 | 41 (12) | 11.9±1.4 |
The number of impairments in basic and instrumental activities of daily living (ADLs and IADLs) are shown as M ± SD.
Participants Chosen by Dementia Status
Participants who reported the presence of AD or dementia exhibited greater functional impairment than those who did not report AD or dementia (Table 2). Those who reported the absence of AD or dementia at baseline also exhibited a significant main effect of cognitive performance on functional capacity in those with mild level of functional impairment (F [2, 2076] = 68.57, p< .001; η2 = .06; Table 3). In those with mild functional capacity, the number of functional impairments was greater in the participants with ≥ 2 cognitive impairments at baseline, compared to those with 0 or 1 impairment. There was no effect of cognitive performance on those with moderate (F [2, 16] = 3.78, p = .07) or severe functional impairment (F [2, 78] = 0.98, p = .38). Similarly, among those with AD or dementia at baseline, poorer cognitive performance was related to worse functional impairment for those with mild functional impairment (F [2, 128] = 6.46, p < .01; η2 = .09; Table 3). The effect sizes for comparisons of 1 vs. ≥ 2 (d = −.66) and 0 vs. ≥ 2 (d = −.75) were much larger than for the comparisons of 0 vs. 1 (d = −.12) impairment.
Objective 2: Baseline Cognition in Relation to Health Outcomes
Risk of AD or Dementia
After selecting for individuals who did not report AD or dementia at baseline and taking into account age, sex, and education in our risk model, those who had ≥ 2 cognitive impairments at baseline (OR = 3.34, 95% CI = 2.27–4.92) were significantly more likely to report AD or dementia after ∼ 4 years, compared to those who did not exhibit any cognitive impairments. A trend towards greater dementia risk was found in those who exhibited 1 cognitive impairment, compared to those with no impairments (OR = 1.45, 95% CI = 0.99–2.14; p = .06; Tables 4 and 5).
TABLE 4.
Distribution of participants experiencing each outcome, based on cognitive performance at baseline in non-institutionalized individuals, and in those who reported the absence of AD or dementia at baseline.
| Outcomes | Number of Cognitive Impairments at Baseline | ||
|---|---|---|---|
|
| |||
| 0 N (%) | 1 N (%) | ≥2 N (%) | |
| Non-institutionalized at baseline | |||
| Institutionalization (n = 48) | 15 (31) | 14 (29) | 18 (37) |
| Mortality (n = 1,508) | 237 (16) | 521 (35) | 654 (43) |
| Non-institutionalized and without dementia at baseline | |||
| Self-reported dementia diagnosis (n = 288) | 42 (15) | 83 (29) | 153 (53) |
TABLE 5.
Logistic regression modeling of baseline cognition (i.e., number of cognitive impairments) in relation to health outcomes at follow-up.
| Cognitive Impairments at Baseline | Risk of AD or Dementia | Risk of Institutionalization | Risk of Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Model 1 | Reference category = 0 cognitive impairments | ||||||||
| 1 | 1.45 | 0.99–2.14 | .06 | 0.76 | 0.35–1.61 | 0.46 | 1.58 | 1.34–1.87 | < 0.001 |
| ≥2 | 3.34 | 2.27–4.92 | < 0.001 | 1.16 | 0.53–2.56 | 0.24 | 2.59 | 2.17–3.09 | < 0.001 |
Models adjusted for age, sex, and education.
Risk of Institutionalization
Baseline cognition was not associated with an increased risk of institutionalization in those participants who were recorded as not being permanently institutionalized at baseline (Table 4).
Risk of Mortality
Of the non-institutionalized participants, those who exhibited 1 (OR = 1.58, 95% CI = 1.34–1.87) or ≥ 2 cognitive impairments at baseline (OR = 2.59, 95% CI = 2.17–3.09), were at a greater risk of mortality after ∼ 4 years, than those who did not have impaired test performance (Tables 4 and 5).
DISCUSSION
These analyses are the first to characterize the relationship between cognitive performance, health status, and functional capacity, in the SHARE database. Specific attention was given to Waves 2 and 4 due to the lack of information regarding dementia status in Wave 1. The analyses demonstrate that cognitive performance impairments were strongly related to poorer health status and impaired functional capacity. Those with more than one cognitive impairment were at highest risk, but even one cognitive impairment at baseline predicted increased risk of adverse health outcomes and impaired functional capacity compared to those without cognitive impairments, and both effects were more pronounced in the group with even mild impairments in IADLs at baseline. The larger effect sizes for those with ≥ 2 cognitive impairments suggest a clinically important difference in impact on health outcomes between this group and those with one or no cognitive impairments.(16) These findings suggest that in otherwise healthy, non-institutionalized individuals, impairments in ≥ 2 cognitive domains may be strongly associated with future cognitive decline (e.g., MCI, AD), poorer health status, and greater functional impairment.
Although SHARE included cognitive tasks that are typically part of standard dementia screening tools (e.g., orientation to time, mathematical ability, verbal fluency, and immediate and delayed recall), not all tasks were measured within each Wave, and those that were did not constitute a comprehensive assessment. However, tasks measuring specific cognitive domains have been shown to stand alone in differentiating healthy individuals from those with MCI or AD.(13,14) Unfortunately, clinical diagnostic evidence for dementia (e.g., neuropsychological assessment, neuroimaging, biomarkers) was not obtained in SHARE, which constitutes a significant weakness for our analyses. That said, the cognitive domains that were included did provide some insight into the cognitive status of the SHARE sample, and they were shown here to be highly associated with subsequent dementia reports. In consequence, it seems reasonable to use the measures here to evaluate how dementia might arise over the course of the SHARE study, even if the measures do not allow for more than a broad definition of dementia (cognitive impairment in more than one domain that is sufficiently severe to interfere with social or occupational functioning) to be assayed.
In addition, SHARE data were collected directly from participants and their proxies using the same line of questioning. This increases the consistency among individual respondents, and subsequently the internal validity of the dementia measure. Clinical diagnosis of dementia is a time-consuming process and, even after extensive testing, inconsistent and inaccurate diagnoses based on clinical features remain common.(17,18) Diagnosis depends both on the diagnostic measures used and especially on the knowledge and experience of the clinician. Consistent self-reporting in surveys such as SHARE avoids this issue, although its weakness is a lack of extensive evaluation.
Various performance-based cognitive tests have been assessed for their utility in identifying those who are at high risk for cognitive decline or dementia and subsequent institutionalization.(19,20) Of these, the Mini-Mental State Exam (MMSE)(21) is used most frequently. Impairments on orientation to time, delayed recall (i.e., three-word recall task), and attention in the MMSE are the strongest predictors of conversion to dementia,(14) especially in combination with self- and informant ratings. Cognitive tasks assessing aspects of recall (i.e., word-list recall) and verbal fluency (i.e., animal naming) have been shown to be sensitive measures for discriminating between cognitively healthy individuals and those with MCI or AD.(13,14) We sampled both participant and proxy reports, as well as those cognitive measures known to be sensitive to identifying MCI or early AD. In addition, although there are other markers of cognitive status within Wave 2 of the survey (i.e., orientation to time, numeracy), only those tested here were administered during both Waves. Even so, it would seem unnecessary to include additional measures, since those who exhibited more than two impairments were found to be at a very high risk of adverse health outcomes.
Cognition and mental capacity play an important role in determining risk of future adverse health outcomes. Based on our previous findings,(2) three clusters of functional impairment (i.e., difficulty with ADLs and/or IADLs) were created, of which, the degree of cognitive impairment was associated with negative health outcomes in the “mild” functionally impaired group. Those exhibiting “moderate” and “severe” functional impairment, however, did not demonstrate a similar relationship between cognitive impairments and health outcomes, suggesting that cognitive performance is not useful in describing outcomes among individuals at later stages of functional decline. Not only is the establishment of clear guidelines for evaluating cognitive capability a valuable tool for aiding in the prediction of negative outcomes, such as dementia and mortality, but it appears also to be an important component in distinguishing healthy individuals from those with declining health status at early stages of functional decline.
SHARE provides an impressively large multinational characterization of health and aging in those over the age of 50. Not only does this database permit the longitudinal examination of the aging process and disease etiology, but it also provides insights into the quality of life and health of those in different nations. Because data collection is ongoing, characterization of cognitive impairments in relation to overall health on measures that are available within the most recent waves of SHARE will serve as a guide for future analyses that incorporate cognition into their models. Future analyses examining cognition in this cohort should take into consideration the clear delineation shown here between cognitive performance associated with future good health versus poor health and cognitive decline (e.g., a risk of MCI or dementia) so as not to exclude from subsequent analyses those who are not at a significant risk of declining health status (i.e., only choosing those with no cognitive impairment at baseline). As additional SHARE data become available, consistencies between measures and performance over an extended period of time may be examined.
Acknowledgments
Roxanne Sterniczuk is supported by the Canadian Institutes of Health Research (CIHR) and Sir Izaak Walton Killam (Dalhousie University) Postdoctoral Fellowships. Olga Theou is supported by CIHR Banting Postdoctoral Fellowship and the University Internal Medicine Research Foundation of Dalhousie University. Benjamin Rusak is supported by CIHR, Natural Sciences and Engineering Research Council of Canada, Capital Health Research Fund, Dalhousie Psychiatry Research Fund, and the Dalhousie Medical Research Foundation. Kenneth Rockwood is supported by operating grants from the Canadian Institutes of Health Research (MOP-209888) and the Nova Scotia Health Research Foundation (MED2006-2086), and by a fellowship from the Alzheimer Society of Canada. Kenneth Rockwood receives funding from the Dalhousie Medical Research Foundation as Kathryn Allen Weldon Professor of Alzheimer Research. This paper uses data from SHARELIFE release 1, as of November 24th, 2010, or SHARE release 2.5.0, as of May 24th, 2011. The SHARE data collection has been primarily funded by the European Commission through the 5th Framework Programme (project QLK6-CT-2001- 00360 in the thematic programme Quality of Life), through the 6th Framework Programme (projects SHARE-I3, RII-CT- 2006-062193, COMPARE, CIT5-CT-2005-028857, and SHARELIFE, CIT4-CT-2006-028812) and through the 7th Framework Programme (SHARE-PREP, 211909 and SHARE-LEAP, 227822). Additional funding from the U.S. National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, Y1-AG-4553-01 and OGHA 04-064, IAG BSR06-11, R21 AG025169) as well as from various national sources is gratefully acknowledged (see www.share-project.org for a full list of funding institutions).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors report no conflicts of interest.
REFERENCES
- 1.Mitnitski A, Fallah N, Rockwood NR, et al. Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J Nutrition Health Aging. 2011;15(10):863–67. doi: 10.1007/s12603-011-0066-9. [DOI] [PubMed] [Google Scholar]
- 2.Thomas SV, Rockwood K, McDowell I. Multidimensionality in instrumental and basic activities of daily living. J Clin Epidemiol. 1998;51(4):315–21. doi: 10.1016/S0895-4356(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 3.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–39. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 4.Reisberg B. Functional assessment staging (FAST) Psychopharmacol Bull. 1988;24(4):653–59. [PubMed] [Google Scholar]
- 5.Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the U.S.: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luck T, Riedel-Heller SG, Luppa M, et al. A hierarchy of predictors for dementia-free survival in old-age: results of the AgeCoDe study. Acta Psychiat Scand. 2014;129(1):63–72. doi: 10.1111/acps.12129. [DOI] [PubMed] [Google Scholar]
- 7.Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Experim Neuropsychol. 2012;34(1):11–34. doi: 10.1080/13803395.2011.614598. [DOI] [PubMed] [Google Scholar]
- 8.d’Uva TR, O’Donnell O, van Doorslaer E. Differential health reporting by education level and its impact on the measurement of health inequalities among older Europeans. Int J Epidemiol. 2008;37(6):1375–83. doi: 10.1093/ije/dyn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leist AK, Glymour MM, Mackenback JP, et al. Time away from work predicts later cognitive function: differences by activity during leave. Ann Epidemiol. 2013;23(8):455–62. doi: 10.1016/j.annepidem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterniczuk R, Theou O, Rusak B, et al. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10(7):767–75. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Educational, Scientific and Cultural Organization . International Standard Classification of Education, 1997. Montreal: UNESCO Institute for Statistics; 1997. [Google Scholar]
- 12.Ofstedal MB, Fisher GG, Herzog RA. Documentation of cognitive functioning measures in the health and retirement study HRS Documentation Report DR-006 [online] Ann Arbor, MI: University of Michigan, HRS Health Working Group; 2005. Available from: http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf. Accessed January 31st, 2015. [DOI] [Google Scholar]
- 13.Balthazar ML, Cendes F, Damasceno BP. Category verbal fluency performance may be impaired in amnestic mild cognitive impairment. Dement Neuropsychol. 2007;2:161–65. doi: 10.1590/s1980-57642008dn10200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayama Y. A delayed recall battery as a sensitive screening for mild cognitive impairment: Follow-up study of memory clinic patients after 10 years. J Med Dental Sci. 2010;57:177–84. [PubMed] [Google Scholar]
- 15.Xie H, Mayo N, Koski L. Predictors of future cognitive decline in persons with mild cognitive impairment. Dement Geriatr Cogn Disorders. 2011;32(5):308–17. doi: 10.1159/000334996. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, MacKnight C. Assessing the clinical importance of statistically significant improvement in anti-dementia drug trials. Neuroepidemiol. 2001;20(2):51–56. doi: 10.1159/000054761. [DOI] [PubMed] [Google Scholar]
- 17.MacKnight C, Graham J, Rockwood K. Factors associated with inconsistent diagnosis of dementia between physicians and neuropsychologists. J Am Geriatr Soc. 1999;47(11):1294–99. doi: 10.1111/j.1532-5415.1999.tb07428.x. [DOI] [PubMed] [Google Scholar]
- 18.Van den Dungen P, van Marwijk HW, van der Horst HE, et al. The accuracy of family physician’s dementia diagnoses at different stages of dementia: a systematic review. Int J Geriatr Psych. 2012;27(4):342–54. doi: 10.1002/gps.2726. [DOI] [PubMed] [Google Scholar]
- 19.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol Med Sci. 1992;47(4):M106–M110. doi: 10.1093/geronj/47.4.M106. [DOI] [PubMed] [Google Scholar]
- 20.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 21.Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psych. 2010;25(2):111–20. doi: 10.1002/gps.2306. [DOI] [PubMed] [Google Scholar]