Affecting more than 20 million Americans every year, major depressive disorder (MDD) is a major burden on society. A 2011 World Health Organization report predicted that depression will be the leading cause of disease burden worldwide by 2030. With multiple distinct combinations of diverse signs and symptoms that can lead to a diagnosis, MDD is extremely heterogeneous. This heterogeneity suggests that dysfunction occurs across several different brain regions. Consistent with this idea, human imaging studies implicate areas such as the prefrontal cortex and the hippocampus. More specifically, several magnetic resonance imaging studies and meta-analyses have consistently found volumetric reductions in prefrontal cortex and hippocampus (1). These volumetric effects can be partially reversed with antidepressant treatment. However, very few clinical studies have attempted to dissect these brain regions further and determine, for example, which subfields of the hippocampus are most sensitive to the course of MDD.
The subfields of the hippocampus are traditionally described as components of a trisynaptic circuit. The main input into the hippocampus is the perforant path, which is a bundle of axons emanating from layer II entorhinal cortex neurons. These axons synapse on the dendrites of granule cells in the dentate gyrus, which project axons (mossy fibers) to the proximal apical dendrites of pyramidal cells in CA3. CA3 pyramidal cells project to ipsilateral CA1 pyramidal cells through Schaffer collaterals. The circuitry is much more complex in reality. These distinct subfields play different roles in behavior and physiology. For example, pattern separation is believed to occur in the dentate gyrus, whereas CA3 may be more important for pattern completion (2). Accumulating evidence indicates that there are also functional differences along the dorsal/ventral axis of the rodent hippocampus. The dorsal (posterior in primates) hippocampus primarily performs cognitive functions such as learning and memory, whereas the ventral (anterior in primates) hippocampus is more related to stress and emotion (3, 4). Studies that attempt to dissect further the hippocampal subfields and anterior and posterior regions in patients would be informative because the development of successful new treatments will likely rely on precise manipulations of the appropriate subfields or regions that are dysfunctional in MDD. A few postmortem studies have found decreased cellular density in the hippocampus, including one study that showed patients with MDD have fewer anterior dentate gyrus granule cells than control subjects (5). However, functional imaging studies at this resolution in patients with MDD are lacking.
One potential contributing factor to MDD is stressful life events or chronic exposure to stress. However, MDD is characterized by recurrent episodes, and several lines of evidence from both prospective and epidemiologic studies indicate that as the number of depressive episodes increases, the role of stress in episode onset decreases (6). This phenomenon is described by the kindling or stress sensitization hypothesis. However, it is unclear how the relationship between stress and recurrence relates specifically to the volume of hippocampal subfields.
In contrast to clinical work, preclinical work has focused heavily on determining whether the hippocampus regulates mood and has provided abundant data on the effects of stress on hippocampal subfields. Initial preclinical studies in the 1980s and 1990s found that the hippocampus is extremely vulnerable to stressful experiences. Chronic stress leads to atrophy of apical dendrites in the CA1 and CA3 subfields. In addition, chronic stress suppresses adult neurogenesis in the dentate gyrus subfield of the hippocampus (4, 7). Adult hippocampal neurogenesis is required for the beneficial behavioral effects of antidepressant treatment (8). Also, the number of dentate gyrus granule cells is decreased after exposure to chronic stress. In addition to these effects, chronic stress results in anxiogenic and depressive-like behaviors (4). Long-term treatment with antidepressants can partially reverse all of these effects. Various chronic stress-related paradigms, including chronic mild stress, unpredictable chronic stress, social defeat, and chronic corticosterone treatment, are widely used to model MDD and treatment in rodents (4). Taken together, these results indicate that cytoarchitectural effects of stress are observed in all subfields of the hippocampus.
In their study in this issue of Biological Psychiatry, Treadway et al. (9) provide one of the first bridges between these preclinical results and clinical studies. The authors set out to evaluate brain morphology and stress levels in subjects with no history of MDD and in subjects with varied previous numbers of depressive episodes. The authors also used a recently developed method that permits definition of hippocampal subfields through coupling of 1.5-tesla magnetic resonance acquisition and detailed segmentation techniques. Similar to some previous findings, Treadway et al. found an inverse relationship between the number of depressive episodes and perceived stress. These data are consistent with the stress sensitization hypothesis. The authors next compared the number of prior depressive episodes with hippocampal subfield volumes. Although within-MDD-group comparisons found that all hippocampal subfields showed significantly decreased volume as the number of prior episodes increased, the strongest effects were found in the dentate gyrus. By contrast, when between-group comparisons considering all subjects were performed, only the dentate gyrus showed a significant volume reduction as the number of episodes increased (Figure 1). There was also a trend for an effect in CA2/3. The authors additionally found an association between cortical thinning in the left medial prefrontal cortex and the number of depressive episodes. By contrast, amygdala volume was not associated with the number of prior depressive episodes.
Figure 1.
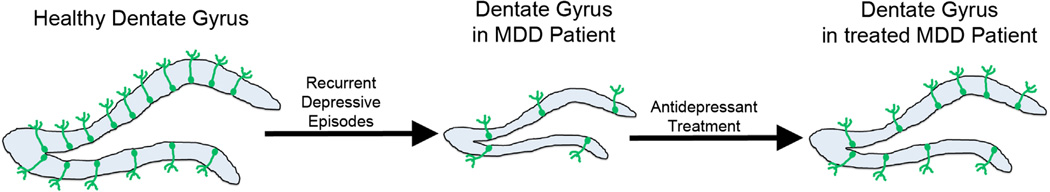
Recurrent depressive episodes lead to decreased dentate gyrus (blue) volume and possibly decreased adult hippocampal neurogenesis (green cells) levels. These changes are partially reversed by antidepressant treatment. MDD, major depressive disorder.
This novel study strongly correlates the dentate gyrus and possibly CA2/3 with the progression of recurrent depression. The dentate gyrus—and the hippocampus on the whole—exerts an inhibitory influence on the function of the hypothalamic-pituitary-adrenal axis. This negative feedback functions to terminate the stress response and is regulated by stress hormones produced in the adrenal gland that act on glucocorticoid and mineralocorticoid receptors, which are densely expressed in dentate gyrus granule cells. Stress hormones are also critical for dentate gyrus survival because adrenalectomy results in selective degeneration of granule cells (4, 7). Taken together, these data indicate that the dentate gyrus is one of the locations in the brain most sensitive to stress and that it also regulates the stress response. In addition, the subgranular zone of the dentate gyrus is one of only two locations in the brain where neurogenesis persists in adulthood.
Both stress and antidepressant treatment profoundly regulate the levels of adult hippocampal neurogenesis (4, 7). Adult hippocampal neurogenesis is necessary for some of the behavioral effects of antidepressants, and a recent study suggests that neurogenesis specifically in the ventral dentate gyrus is critical (4, 8, 10). Because preclinical work demonstrates that functional distinctions also occur along the dorsal/ ventral axis of the hippocampus (3) and patients with MDD have fewer granule cells than controls in the anterior dentate gyrus (5), it may be worthwhile for future imaging studies to assess segmentation along this axis. In addition, future studies should determine whether decreased dentate gyrus volume leads to long-term changes in the neural circuitry of the hippocampus and other limbic regions that receive dense inputs from the ventral (anterior) hippocampus, such as the prefrontal cortex, amygdala, and nucleus accumbens. Ultimately, it also will be critical to determine if long-term changes in the circuitry underlie recurrent depressive episodes independent of additional stressors.
Acknowledgments
This work was supported by the National Institute of Mental Health Grant Nos. K01 MH098188 (BAS), R01 MH091427 (EDL), and R37 MH068542 (RH); National Alliance for Research on Schizophrenia and Depression Young Investigator Award (BAS); and Hope for Depression Research Foundation Grant No. MPPN8883 (RH).
RH receives compensation as a consultant from Lundbeck and Roche.
Footnotes
Disclosures
BAS and EDL report no biomedical financial interests or potential conflicts of interest.
References
- 1.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- 5.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monroe SM, Harkness KL. Life stress, the "kindling" hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 7.Dranovsky A, Leonardo ED. Is there a role for young hippocampal neurons in adaptation to stress? Behav Brain Res. 2012;227:371–375. doi: 10.1016/j.bbr.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 9.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MV, Hen R. Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus. 2014;24:751–761. doi: 10.1002/hipo.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]


