Abstract
Infection with the helminth Schistosoma mansoni results in hepato-intestinal granulomatous inflammation mediated by CD4 T cells directed against parasite eggs. The severity of disease varies greatly in humans and mice; however, the genetic basis of such a heterogenous immune response remains poorly understood. Here we show that, despite their close genetic relationship, C57BL/10SnJ (B10) mice developed significantly more pronounced immunopathology and higher Th17-cell responses than C57BL/6J (B6) mice. Similarly, live egg-stimulated B10-derived dendritic cells (DCs) produced significantly more IL-1β and IL-23, resulting in higher IL-17 production by CD4 T cells. Gene expression analysis disclosed a heightened proinflammatory cytokine profile together with a strikingly low expression of Ym1 in B10 vs. B6 mice, consistent with failure of B10 DCs to attain alternative activation. To genetically dissect the differential response, we developed and analyzed congenic mouse strains that capture major regions of allelic variation, and found that the level of inflammation was controlled by a relatively small number of genes in a locus mapping to chromosome 4 117-143 MB. Our study has thus identified novel genomic regions that regulate the severity of the schistosome infection by way of controlling the mode of DC activation and consequent CD4 T-cell subset development.
Keywords: Dendritic cell activation, Host/pathogen interactions, B6/B10 mice, Genetics, Th17, Schistosoma mansoni
Introduction
Schistosomiasis is a complex parasitic disease that results from infection with trematode helminths. The World Health Organization estimates that there are over 200 million people with schistosomiasis, with 600 million at risk and an estimated 200,000 deaths per year (1). Despite effective treatment, schistosomiasis remains a significant public health risk and has a major socioeconomic impact throughout parts of Africa, Asia and South America. Following infection with the species Schistosoma mansoni, parasite eggs become trapped in the liver and intestine where they precipitate a CD4 T-cell mediated granulomatous inflammatory and fibrosing reaction directed against schistosome antigens (2,3). One of the most intriguing observations of S. mansoni infection involves the dramatically disparate disease pathologies that occur. Most humans develop the mild “intestinal” form of the disease, whereas 5–10% develop the severe hepatosplenic form, which can be life threatening (4). This marked heterogeneity in disease severity also exists in a murine model of schistosomiasis where C57BL/6J (B6) mice develop milder lesions in comparison with the pronounced hepatic granulomatous inflammation seen in CBA/J and SJL/J (SJL) mice (5, 6, 7). In low pathology strains, an initial proinflammatory response is promptly replaced by a dominant Th2 type environment and corresponding increase in the cytokines IL-4, IL-5, IL-10 and IL-13, whereas in high pathology strains a proinflammatory Th1 and Th17 response persists alongside the Th2 response (8). The immunopathology in schistosomiasis is the result of a CD4 T-cell hypersensitivity reaction and as such shares many mechanistic features with T-cell-mediated autoimmune diseases such as experimental autoimmune encephalomyelitis. For these reasons, a greater understanding of its mechanisms of pathogenesis is of vital interest.
Identification of quantitative trait loci (QTL), which harbor much of the genetic variation that leads to disease susceptibility, has led to the discovery of a number of molecular pathways that underlie disease processes (9). The marked phenotypic heterogeneity that develops following infection with S. mansoni, despite similar environments in humans or identical parasitic loads in experimental murine infection, indicates a profound genetic contribution to disease progression and thus makes schistosomiasis an excellent model with which to study the genetic basis of immune-mediated pathology. Previous studies in human schistosomiasis have reported an association between disease severity and HLA MHC haplotypes (10, 11, 12), additionally, two non-MHC loci, SM1 and SM2, have been linked with disease severity and intensity of infection, respectively (13, 14). By QTL analysis comparing the response of (B6 x SJL) F2 mice, we previously identified major loci mapping to chromosome 4 and 17 in mice that control granulomatous inflammation and IL-17 production (7). However, the complexities of QTL analysis comparing genetically disparate strains make it challenging to genetically dissect phenotypically complex traits.
Most inbred strains differ allelically at a large number of loci spread fairly uniformly throughout the genome (15). By contrast, strains such as C57BL/6J (B6) and C57BL/10SnJ (B10) share heritage to the degree they are essentially complex congenics of each other. In such cases, the vast majority of polymorphisms map to intervals that can be relatively easily captured in congenic strains. In this paper, we use this strategy to genetically and phenotypically dissect the host response to schistosome infection. We show that B10 mice develop severe immunopathology and produce high levels of Th1/Th17 associated cytokines compared with B6 mice, and with the aid of congenic mouse strains, we identified a locus on chromosome 4 that regulates complex interactions between the innate and adaptive immune system, which determine the level of pathology in the schistosome infection.
Results
B10 mice exhibit more severe hepatic granulomatous inflammation than B6 mice
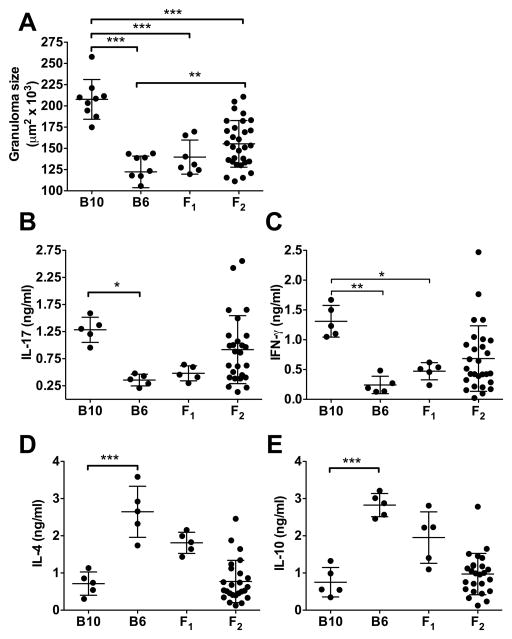
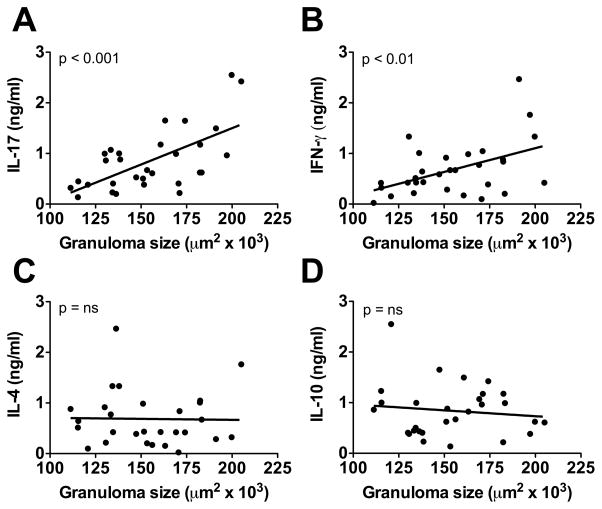
Seven weeks after infection with S. mansoni, B10 mice developed significantly larger liver granulomas than B6 mice. F1 mice developed small granulomas, similar to B6, indicating that low pathology was dominant. F2 mice displayed a wide range in granuloma size with some reaching those attained by either the B10 or B6 parental strains (Fig. 1A). Because proinflammatory cytokines, particularly IL-17, are associated with severe disease (8, 16), we analyzed cytokine production and found that B10 mice produced significantly higher levels of IL-17 and IFN-γ than B6 mice, while IL-17 and IFN-γ levels in F1 mice were closer to those of B6 mice (Fig. 1B, 1C), which, in turn, produced higher levels of IL-4 and IL-10 (Fig. 1D, 1E). F2 mice displayed wide variation in IL-17 and IFN-γ production, similar to granuloma formation, and linear regression analysis confirmed that these cytokines significantly correlated with granuloma size (Fig. 2A, 2B). In contrast, there was no statistically significant correlation between granuloma size and the Th2 cytokine IL-4 or the anti-inflammatory cytokine IL-10 (Fig. 2C, 2D). Taken together these results identify the B10 mouse as a model of high-pathology schistosomiasis in which granuloma size correlates with increased proinflammatory cytokine production.
Figure 1. Granuloma size and IL-17 and IFN-γ production by SEA-stimulated MLNCs from B10, B6, F1 and F2 mice.
(A) Granulomas were measured in liver sections obtained from 7-week-infected B10, B6, F1 and F2 mice, as described in Materials and Methods. A minimum of 20 granulomas were measured and averaged per mouse. Each dot represents an individual mouse (B10, n=9, B6, n=8, F1, n=7, F2, n=30). (B) IL-17, (C) IFN-γ, (D) IL-4 and (E) IL-10 production by SEA-stimulated MLNCs from these mice was measured in 48 h culture supernatants by ELISA. Each dot represents the mean cytokine level of triplicate determinations per mouse. (A–E) Black horizontal lines represent the mean of the group +/− SD between individual mice. Statistical analysis was determined by one-way ANOVA with Bonferroni’s post test using Prism software. Data shown for B10, B6 and F1 mice are representative of 1 out of 3 similar independent experiments. All data points shown for F2 mice are from 3 independent experiments. *** = p < 0.001, ** = p < 0.01 or * = p < 0.05, otherwise not significant.
Figure 2. Linear regression analysis of mean granuloma size vs cytokine production for individual F2 mice.
Linear regression analysis of (A) IL-17, (B) IFN-γ, (C) IL-4 and (D) IL-10 production from MLNCs with granuloma size in F2 mice as measured in Fig. 1. Each dot represents an individual F2 mouse (n=29 for each group). Statistical analysis was performed by linear regression using Prism software. ns = not significant.
Genetic variation of B6 and B10 mice is consistent with ancestral haplotype blocks
Given the substantial phenotypic differences in response to schistosome infection in B6 and B10 mice, we analyzed their genotypes. To establish chromosomal patterns of allelic variation, Mouse Phenome Database (MPD) SNP data sets were analyzed for B6/B10 polymorphism patterns to determine genetic regions of variation. Consistent with their common heritage, only 1.4% of CGD-MDA1 SNPs, as accessed via MPD, are polymorphic between B6 and B10. This limited variation differed substantially from comparisons of other common mouse strains with B6 (e.g., B6 vs. SJL were variant at 19.5% of CGD-MDA1 SNPs), and rather than being randomly distributed across the chromosomes, as would be expected if they arose by mutations after strain separation, ~97% of B6/B10 polymorphic SNPs clustered in 21 differential haplotype blocks on 11 chromosomes, representing ~5% of the total genome (Supporting Information Table 1, Supporting Information Figure 1). SSLP markers were designed to map to these haplotype blocks and 42 of 98 tested were polymorphic between B6 and B10. By contrast, 0 of 187 SSLP markers which mapped outside of these haplotype blocks were B6/B10 polymorphic, serving as further evidence that the haplotype blocks are regions of disparate strain heritage, inherently acting as ‘congenic’ segments between the two strains. We then used the informative SSLP markers to direct the construction of 15 B6.B10 congenic stocks that isolate different ‘congenic’ intervals for experimental testing (Supporting Information Table 1, Supporting Information Figure 1).
Distinct genetic segments control immunopathology and cytokine production after S. mansoni infection
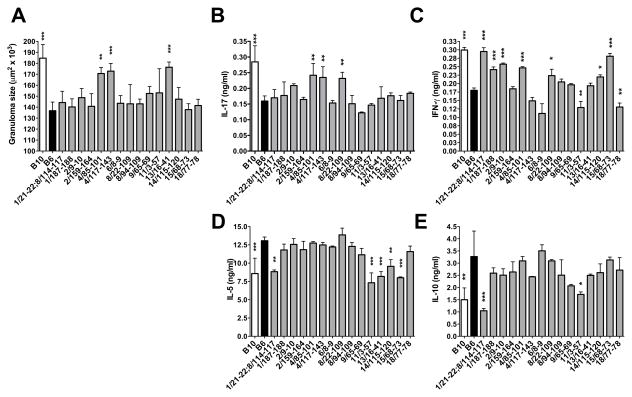
To genetically dissect the host response after S. mansoni infection, we infected cohorts of mice from 15 congenic lines (Table S1). Following infection, three lines containing congenic segments at chr 4 85-101 Mb (B6.B10-4/85-101), chr 4 117-143 Mb (B6.B10-4/117-143) and chr 13 16-41 Mb (B6.B10-13/16-41) exhibited significant increase in granuloma size when compared with B6 mice (Fig. 3A). Among these congenics, B6.B10-4/85-101 and B6.B10-4/117-143 also displayed significantly increased production of IL-17 by SEA-stimulated MLNCs (Fig. 3B); B6.B10-4/85-101 included an increase in IFN-γ (Fig. 3C) and B6.B10-13/16-41 a reduction in IL-5 (Fig. 3D); IL-10 levels were not affected by any of these congenic intervals (Fig. 3E). Interestingly, B6.B10-8/22-109 had significantly increased IL-17 production, which was uncoupled with increased granuloma size. Independent from effects on granuloma size and severe disease, several other congenic lines also appeared to differentially regulate IFN-γ, IL-5 and IL-10 cytokine production between B6 and B10 mice (Fig. 3). Taken together these results identify two major congenic intervals that control severe immunopathology and IL-17 production in murine schistosomiasis and also reveal multiple other intervals involved in cytokine regulation.
Figure 3. Granuloma size and cytokine production from B10, B6 and B6.B10 congenic mice.
Congenic mice contain segments of the B10 genome on an otherwise B6 background. Designations consist of the chromosome followed by the position of the congenic limits in Mb. (A) Granuloma size was measured by morphometric analysis as described in Materials and Methods. At least 15 granulomas were measured per mouse with at least 5 mice per group. Error bars represent SD between individual mice within an experiment. For cytokine analysis, SEA-specific IL-17 (B), IFN-γ (C) IL-5 (D) and IL-10 (E) production by MLNCs from 7wk infected mice was measured by ELISA. Cytokine levels are expressed as means +/− SD between individual mice within an experiment. Results are representative of 3 (granuloma size, IL-17 and IFN-γ) or 2 (IL-5 and IL-10) independent experiments. Statistically significant differences with respect to B6 were determined by one-way ANOVA with Bonferroni’s post test using Prism software. *** = p < 0.001, ** = p < 0.01 or * = p < 0.05, otherwise not significant.
Markers of alternative activation differ significantly between infected B6 and B10 mice
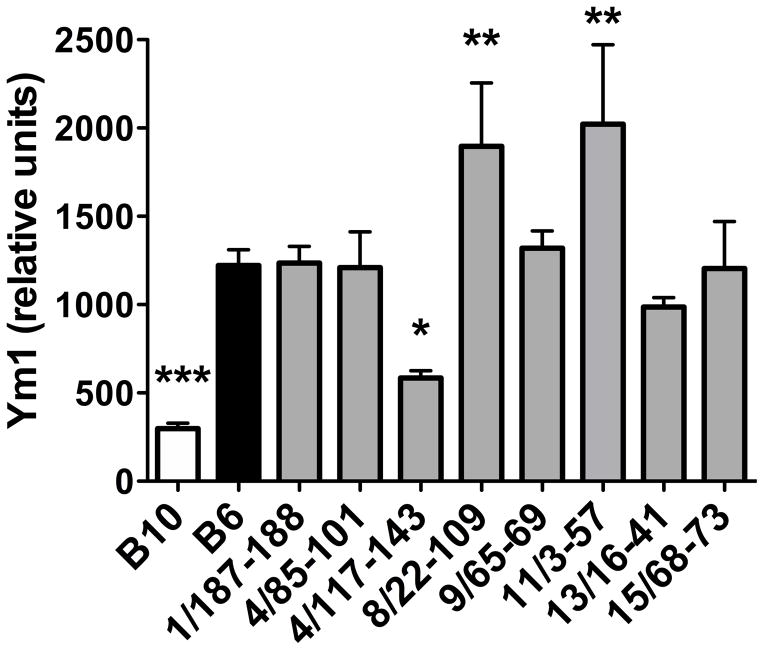
To further define the molecular mechanisms that underlie these genetic differences in phenotype between B6 and B10 mice, and thus provide insight for the identification of potential candidate genes, we designed a custom RT2 Profiler PCR Array (Qiagen) targeting genes associated with immunopathology in schistosomiasis. Analysis of expression profiles comparing MLNs from 7-wk infected B6 and B10 mice confirmed an increase in the Th17 cell-associated cytokines IL-17 and IL-23p19 in B10 MLNs, as well as in IFN-α and IFN-β, which are associated with increased susceptibility to Systemic Lupus Erythematosus in B10 mice (Fig 4) (17). Interestingly, Ym1, which is a marker of anti-inflammatory, alternatively activated macrophages (AAMs) (18) and critical to averting severe pathology and death in murine schistosomiasis (19), was greatly increased in B6 MLNs, compared with B10. Ym1 was also the most differentially regulated gene in our assay (Fig. 4). In addition, several other markers associated with the alternative activation pathway were upregulated in B6 MLNs, including IL-4, IL-13, IL-17e and CCL2 (Fig. 4) (20). We next examined Ym1 expression in MLNs from 7-wk infected B6.B10 congenic mice and found that, of the congenics tested, only B6.B10-4/117-143 had significantly reduced Ym1 expression compared with B6, and similar to B10 controls (Fig. 5). These data suggest that a locus on chromosome 4, 4/117-143, regulates the alternative activation pathway in response to schistosome infection leading to differential disease outcome between B6 and B10 mice.
Figure 4. Analysis of gene expression in B10 and B6 mice.
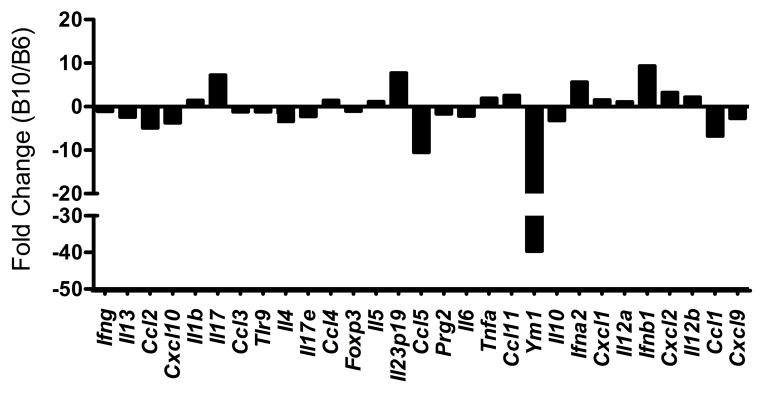
MLNs were isolated from infected B10 and B6 mice and total RNA was isolated and processed for cDNA. To determine gene expression differences, PCR Array analysis using SYBR green was performed as described in materials and methods. The y-axis represents the fold change in gene expression between B10/B6 mice, with individual genes displayed along the x-axis. Fold change was determined using the ΔΔCT method. Data were normalized to two housekeeping genes, gapdh and actb. Black bars represent the average of three biological replicates from one experiment, which is representative of three independent experiments.
Figure 5. Expression of Ym1 in MLNs from infected B10, B6 and B6.B10 congenic mice.
MLNs were isolated from infected B10, B6 and congenic mice and total RNA was isolated and processed for cDNA. TaqMan RT-PCR was performed using primers and probes for Ym1 from Applied Biosystems. All data were normalized to Gapdh and are presented as relative units as described in Materials and Methods. Each bar represents the mean mRNA level +/− SD from two individual mice. Data are representative of two independent experiments. Statistically significant differences with respect to B6 were determined by one-way ANOVA with Bonferroni’s post test analysis performed by Prism software. *** = p < 0.001, ** = p < 0.01, * = p < 0.05, otherwise not significant.
A locus in chromosome 4 biases B10-, but not B6-, derived DCs towards a proinflammatory phenotype
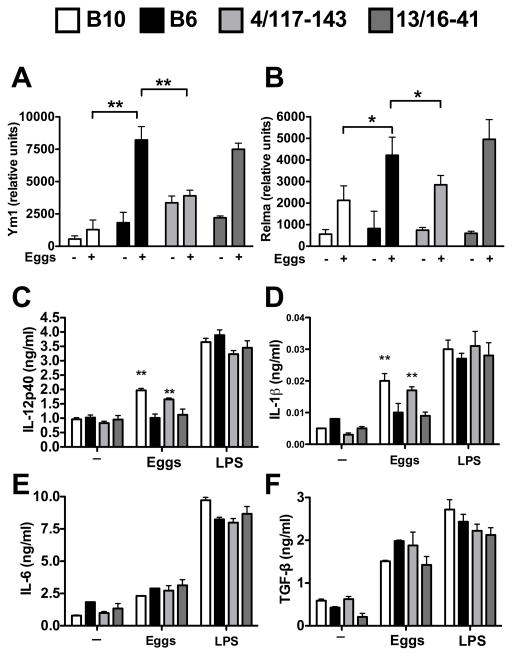
Along with macrophages, DCs are also capable of expressing markers of alternative activation resulting in a regulatory phenotype (21, 22) and previous work from our lab has shown that proinflammatory cytokine production by DCs in response to schistosome eggs is critical for Th17 cell differentiation and the development of severe disease (8, 23). To examine if DCs from B6 mice, but not B10, develop a regulatory phenotype in response to schistosome eggs, we used a previously established model in which BMDCs are exposed to live schistosome eggs, and cytokine levels in culture supernatants are measured after 24 hours (16). Egg-stimulated DCs from B6 and control B6.B10-13/16-41 mice indeed expressed high levels of Ym1 and RELMα, another marker of alternative activation (18), while DCs from both B10 and B6.B10-4/117-143 congenic mice expressed significantly less Ym1 and RELMα (Fig. 6A, B). Differential expression of Ym1 and RELMα was associated with DC proinflammatory cytokine production as egg-stimulated B10 and B6.B10-4/117-143-derived DCs produced significantly more IL-12p40 and IL-1β than B6 and control B6.B10-13/16-41 DCs (Fig. 6C, D). There were no significant differences in IL-6 or TGF-β production by egg-stimulated DCs (Fig. 6E, F) and LPS stimulation elicited strong cytokine responses throughout with no significant differences among the strains. These findings indicate that the B6.B10-4/117-143 locus, which regulates severe immunopathology, also controls DC expression of alternative activation markers and proinflammatory cytokine production.
Figure 6. Expression of Ym1 and RELMα and cytokine production by B10, B6 and congenic DCs stimulated with live eggs.
BMDCs from B10, B6, B6.B10-4/117-143 and B6.B10-13/16-41 mice were cocultured with or without 300 live schistosome eggs as described in materials and methods, and (A) Ym1 and (B) RELMα mRNA expression was measured by quantitative RT-PCR. (C–F) BMDCs from B10, B6, B6.B10-4/117-143 and B6.B10-13/16-41 mice were cocultured with or without 300 live schistosome eggs, or with LPS (10 ng/ml), as described in Materials and Methods. (C) IL-12p40, (D) IL-1β, (E) IL-6 and (F) TGF-β levels in 24 h supernatants were measured by ELISA. For A–B, all data were normalized to Gapdh and are presented as relative units as described in Materials and Methods. Each bar represents the mean mRNA level +/− SD between two independent experiments. For C–F, cytokine levels are expressed as means +/− SD of 3 individual samples within an experiment. Results shown are from one of three experiments with similar results. Statistically significant differences were determined by student’s t test analysis performed by Prism software. ** = p < 0.01, * = p < 0.05, otherwise not significant.
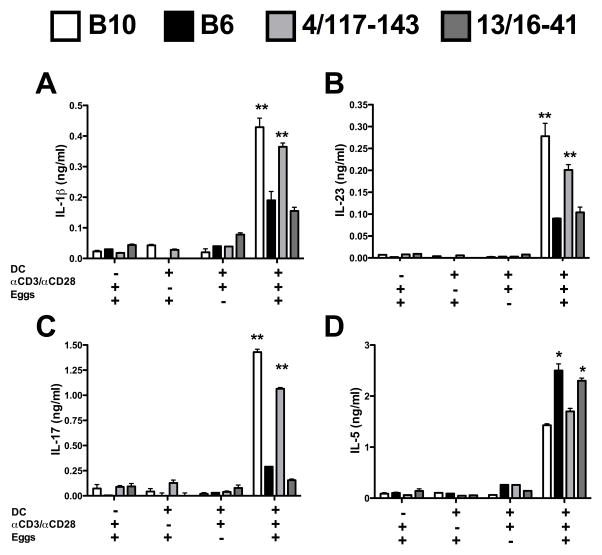
B10 and B6.B10-4/117-143 egg-stimulated DC-T cell cocultures produce more proinflammatory cytokines than B6
We next determined cytokine production by DC in coculture with syngeneic CD4 T cells stimulated with anti-CD3/CD28 coated beads and live schistosome eggs. B10 DC-CD4 T cell cocultures produced significantly more IL-1β and IL-23 than the corresponding B6 cocultures (Fig 7A, B). Consistent with the important role of these cytokines in Th17 cell differentiation (16, 23, 24, 25), B10 DC-CD4 T cell cocultures produced significantly more IL-17 than their B6 counterparts (Fig. 7C); in turn, B6 DC-CD4 T cell cocultures produced more IL-5 (Fig. 7D). Importantly, cytokine production by B6.B10-4/117-143 DC-CD4 T cell cocultures was similar to B10 cocultures in all cases, indicating that the presence of this locus sufficed to induce Th17 cell responses in this system; significantly less IL-17 was produced by the B6.B10-13/16-41 controls. All cocultures produced equivalent amounts of IL-17 when stimulated with an optimized Th17 cell-inducing cytokine mixture composed of IL-6, TGF-β and IL-23 (data not shown). Taken together, these findings further support the notion that the B6.B10-4/117-143 interval on chromosome 4 regulates the DC and CD4 T cell cytokines that determine the severity of disease.
Figure 7. Cytokine production by B10, B6 and congenic DC-CD4 T cell cocultures stimulated with live eggs.
(A–D) Naïve CD4 T cells from B10, B6, B6.B10-4/117-143 and B6.B10-13/16-41 mice were cocultured with syngeneic BMDCs in the presence or absence of αCD3/CD28 coated beads and 200 eggs as described in Materials and Methods. (A) IL-1β, (B) IL-23, (C) IL-17 and (D) IL-5 levels in 4 day culture supernatants were measured by ELISA. Cytokine levels are expressed as means +/− SD of 3 individual samples within an experiment. Results shown are from one experiment representative of 2 (IL-1, IL-23, IL-5) or 3 (IL-17) with similar results. Statistically significant differences with respect to B6 were determined by the Student’s t test performed by Prism software. ** = p < 0.01, * = p < 0.05, otherwise not significant.
Discussion
Despite ample evidence that the course and magnitude of disease in schistosomiasis are profoundly affected by the host genome, genetic factors that control this process have been difficult to identify. We hypothesized that this may be due in part to the complex interaction of a large number of immune response-related elements, which are subject to allelically driven phenotypic variability. Here we show that in a mouse model of schistosomiasis, B6 and B10 mice develop significantly different immunopathology despite their close genetic relationship. B10 mice exhibited pronounced hepatic egg-induced granulomatous inflammation and produced substantially higher levels of IL-17 and IFN-γ when compared with B6 mice. MPD SNP database surveys indicate that B6 and B10 share strain heritage throughout ~95% of their genomes and that the vast majority of their allelic differences can be accounted for by ancestry prior to the separation of the substrains. We therefore considered it most likely that the QTLs mapped to the ~5% of the genome which they inherited from different ancestral sources, rather than to the rare mutations which occurred since strain separation. This combination of clear phenotypic together with limited genetic differences afforded us a rare opportunity to interrogate the genetic basis of the schistosome egg-induced immunopathology in a focused manner. As a first pass, (B6 x B10) F1 and F2 mice were analyzed. F1 mice developed mild pathology, indicating that the B6 background is dominant. F2 progeny demonstrated a significant correlation between granuloma size and SEA-induced IL-17 and IFN-γ production, but not IL-4 and IL-10. These observations confirm that severe immunopathology occurs in a proinflammatory cytokine environment dominated by Th17 and Th1 cell subsets and, importantly, identify B10 mice as an effective new model to study high pathology. QTL analysis of a limited cohort of F2 mice did not detect any significant peaks associated with granuloma size or cytokine production (data not shown), but the spread in values of F2 granuloma size, as well as in IL-17 and IFN-γ levels, served as supporting evidence for complex genetic interaction in the B6/B10 infection response model.
To further dissect the genetic contribution to the disparate pathologies in B6 and B10 mice, we produced 15 different B6.B10 congenic mouse lines, each containing all or part of the 20 trackable congenic intervals defined above. This allowed us to determine the contributions of subsets of congenic intervals. The use of congenic mice has the advantage of potentially identifying regions of interest where F2 mapping fails due to genetically complex phenotypes, as well as confirming the contribution of a single locus to the outcome of a particular phenotype. Here we identified three B6.B10 congenic strains with exacerbated schistosome egg-induced immunopathology. The first, B6.B10-4/85-101, encompassing punctate polymorphic intervals extending from 85.4 to 101.2 Mb on chromosome 4, exhibited significantly increased granuloma size together with enhanced production of proinflammatory cytokines IL-17 and IFN-γ. The second congenic strain, B6.B10-4/117-143, developed significantly larger liver granulomas and increased IL-17 production but not elevated IFN-γ levels compared with B6 mice, supporting a more critical role of IL-17 in driving the hepatic immunopathology (26, 27). This locus contains overlapping QTL with several autoimmune diseases, including experimental autoimmune encephalomyelitis and insulin dependent diabetes (28, 29). The third congenic, B6.B10-13/16-41, also exhibited significantly larger granulomas than B6 mice but no increase in IL-17 or IFN-γ production, suggesting that other factors may also play a role in the development of severe liver pathology. This locus contains several genes of the Serpin family of protease inhibitors (e.g., Serpinb1c, Serpinb6b, Serpinb9), which serve to control deleterious proteolytic cascades in numerous physiologically important systems (30). Additionally, this region has been implicated in neutrophil infiltration, as well as in wound healing (31, 32). Interestingly, congenic strain B6.B10-8/22-109 upregulates IL-17 production following infection but does not develop severe hepatic lesions. These data combined with the development of severe disease in the absence of IL-17 in B6.B10-13/16-41 congenic mice suggests uncoupling of the cytokine profile with immunopathology possibly due to dissimilar leukocyte recruitment.
Given that expression differences in genes that lead to functional regulatory variation are important for a variety of complex traits, including schistosomiasis (9, 33), we designed a custom PCR array to determine expression differences among a number of genes known to contribute to the schistosome infection. Using this array, we identified Ym1 as the most overexpressed gene in MLNs from B6 compared with B10 mice. Other cytokines important for the development of AAMs were similarly upregulated in B6 mice (Fig. 4), further demonstrating that this pathway may play a major role in the differential outcomes of the schistosome infection in these strains.
AAMs are induced by IL-4 and IL-13, leading to an increased expression of several proteins, including Ym1, which serves as an identifying marker (34). Due to their association with Th2 responses AAMs are critical for protection against helminths, and are specifically required for host survival during schistosome infection (19, 34). DCs have also been shown to develop a regulatory phenotype, characterized by expression of Ym1 and induce the production of Th2 cytokines when cultured with CD4 T cells. (21, 35). More recently, IL-4 and RELMα were shown to drive alternative activation of DCs in the context of helminth infection (22). Our lab has shown that Th2 cell induction by egg-stimulated DCs is an attribute of low-pathology B6 mice; in contrast, DCs from high-pathology mice typically induce Th17 and Th1 responses. DCs thus play a decisive role in dictating CD4 T cell phenotype and outcome of disease severity in schistosomiasis (16).
Further investigation of alternative activation in DCs using an in vitro DC-CD4 T cell coculture system revealed that DCs derived from B10 mice fail to upregulate Ym1 and RELMα upon egg stimulation, which resulted in increased production of IL-12p40, IL-1, IL-23 and IL-17, but not of IL-6 and TGF-β. These findings are in agreement with previous results showing that IL-23 and IL-1 are critically involved in promoting pathogenic Th17 cells, including in schistosomiasis (23, 36, 37, 38, 39). On the other hand, Ym1-expressing DCs in B6 DC-CD4 T cell cocultures responded to egg stimulation with an increase in IL-5 secretion, denoting a dominant Th2 response typical of the B6 mouse. Importantly, and unlike B6 mice, in egg-stimulated B6.B10-4/117-143 cocultures Ym1 and RELMα expression was very low and resulted in increased amounts of IL-1β, IL-23 and IL-17. The contribution of other cell types to cytokine production cannot be completely excluded as the derivation of DCs from bone marrow cells does not result in a completely homogenous culture. These findings suggest that, despite the fact that the structural genes for Ym1 and RELMα are not contained within the 4/117-143 interval, this genetic interval nevertheless plays an important role in prompting the failure of alternative activation that in B10 mice results in elevated Th17 responses and the development of severe disease.
Large scale sequence-based analyses have revealed that among inbred mouse strains, ~97% of the genetic variation is ancestral and it is unlikely that these shared regions will contain causal polymorphisms that underlie identified QTL (40, 41). We have previously identified a locus on chromosome 4 from ~ 120–143 Mb as a critical regulator of pathology in a genetic cross between B6 and SJL mice (7). Therefore we posited that a causal gene(s) in this locus, which is/are responsible for the control of severe immunopathology during schistosome infection, may be contained in a shared haplotype block between B10 and SJL strains that differs from B6. We searched the CGD-MDA1 (42) dataset, available from the mouse phenome database at Jackson labs, for genetic regions containing at least 40 SNPs in which both B10 and SJL mice showed variation from B6 mice, and identified 12 of such haplotype blocks (Table I). After filtering out predicted and pseudogenes, there remained 38 potential candidate genes (Table I), among which ppt1 is expressed in CD8+ DCs and Themis2 is expressed in CD8− DCs. (BioGPS). Interestingly, upon activation, the CD8+ DCs produce large amounts of IL-12 and direct the development of Th1 (43) and possibly Th17 CD4 T cells. Another candidate gene is Stg3gal3, which is a member of the ST3GAL family catalyzing the transfer of sialic acid to O-linked glycans in an alpha2,3 linkage. Sialyltransferases undergo significant variation in gene expression during the differentiation and maturation of DCs and this significantly impacts DC function, including their ability to prime T cell responses (44). It is possible that the identified B6.B10-4/117-143 locus regulates sialylation patterns that distinctively affect B10 vs. B6 DC activation and consequent T cell subset selection and disease outcome.
Table I.
B6.B10-4/117-143 Haplotype blocks and associated candidate genes controlling DC alternative activation and severe disease
| Haplotype Block | Start Position (Mb) | Stop Position (Mb) | Total Genes In Interval | Candidate Genesa |
|---|---|---|---|---|
| 1 | 116.73 | 116.76 | 2 | Tesk2, Ccdc153 |
| 2 | 117.87 | 118.01 | 8 | Artn, Atp6v0b, Dph2, Ccdc24, Ipo13, Slc6a9, St3gal3, B4galt2 |
| 3 | 118.37 | 118.48 | 6 | Med8, Tie1, Mpl, Cdc20, Elovl1, Szt2 |
| 4 | 118.63 | 118.71 | 1 | Ebna1bp2 |
| 5 | 121.02 | 122.84 | 5 | Tmco2, Col9a2, Ppt1, Zmpste24, Rlf |
| 6 | 122.85 | 122.98 | 2 | Cap1, Mfsd2a |
| 7 | 131.11 | 131.25 | 0 | |
| 8 | 131.37 | 131.56 | 0 | |
| 9 | 132.23 | 132.31 | 4 | Taf12, Rab42, Trnau1ap, Gmeb1 |
| 10 | 132.37 | 132.50 | 3 | Phactr4, Med18, Sesn2 |
| 11 | 132.78 | 132.94 | 4 | Stx12, Ppp1r8, Fam76a, Themis2 |
| 12 | 133.62 | 133.82 | 3 | Pigv, Arid1a, Zdhhc18 |
Does not include predicted or pseudogenes
In summary, our study mapped genetic intervals that significantly contribute to severe hepatic egg-induced immunopathology in B10 mice, with the underlying mechanism pointing to a defect in the development of the alternative activation pathway controlled by a locus on chromosome 4. Although we have cited potentially relevant genes within this locus, additional ones are likely and cannot be excluded. Our observations provide new insight into the genetic basis of alternative activation of DCs, and pave the way to further identifying the causal genes exerting control over the pathology in schistosomiasis.
Materials and Methods
Mice and infection
Female C57BL/6J (B6) and C57BL/10SnJ (B10) mice, aged 5–6 weeks old, were purchased from The Jackson Laboratory (TJL, Bar Harbor, ME). (B6 x B10) F1 and F2 mice were bred at the Animal Facility at Tufts University School of Medicine. B6.B10 congenic lines were developed in the authors’ colonies at TJL as described below. Progeny from specific lines (Supporting Information Table 1) were shipped to Tufts for inclusion in experiments. All mice were maintained in accordance with the American Association for the Assessment and Accreditation of Laboratory Animal Care Guidelines. All mice were age-matched and infected between 6–11 weeks of age by intraperitoneal injection with 85 cercariae of S. mansoni (Puerto Rico strain). Cercariae were obtained from infected Biomphalaria glabrata snails, provided to us by the Biomedical Research Institute, through National Institutes of Health/National Institute of Allergy and Infectious Diseases Contract NO1-AI-55270. All mice were studied after seven weeks of infection.
SNP analysis identifies chromosomal regions by which B6 and B10 differ
SNP data sets were queried for B6 and B10 alleles across the genome using the Mouse Phenome Database (MGD; phenome.jax.org) portal. These included, in order of availability and increasing SNP density, JAXSNP1 (45), Chicago1 (46) and CGD-MDA1 (cgd.jax.org) (47). Mainly, the CGD-MDA1 data set was queried by chromosome for all SNPs which include both C57BL/6J and C57BL/10J alleles and percent polymorphism for sequential groups of 20 consecutive SNPs (hereafter called 20SNPs) was determined. 20SNPs with >5% polymorphism clearly clustered together and were interpreted as regions of differing strain heritage. To determine if clustering of polymorphic SNPs was statistically significant and to identify the significant regions, the following strategy was used: The full set of CGD-MDA1 SNPs across the autosomes and X chromosome with calls for both B6 and B10 were divided into groups of 20SNPs along each chromosome and percent polymorphism was determined for each group. Sum of the percentages was divided by block count to determine average polymorphism across the genome as a baseline. Next, for each group, we performed a chi-squared test comparing observed versus baseline percent polymorphism. To correct for multiple testing, a Bonferroni corrected p-value was calculated as alpha/total number of blocks; alpha = 0.05 and the total number of groups is 26890. This results in a Bonferroni p-value significance threshold of 1.86E-06.
Development of congenic strains
In regions identified as B6/B10 congenic (Supporting Information Table 1), intervals of dinucleotide repeats were identified either by search of Ensembl C57BL/6J reference sequence or occasionally by previously existing MIT markers already identified as B6/B10 polymorphic. In all cases, primers were designed to bracket the dinucleotide repeats, have a 58–60°C annealing temperature and have ~100 bp products using Primer Express software (Applied Biosystems Inc.). PCR products were visualized on 0.7% agarose 1.5% Synergel (BioExpress) gels with Ethidium Bromide. Using this method we were able to generate SSLP markers polymorphic between B6 and B10 for all the putative congenic intervals (Supporting Information Tables 1 and 2).
B6.B10 congenics were produced by an initial intercross of B6 and B10, followed by repeated backcross to B6. A ‘speed congenic’ approach was used which included typing for markers to be excluded as well as those to be retained (Supporting Information Table 1), such that after back cross 2–7 all congenic lines were known to carry B10 alleles at the indicated markers and intervals and B6 allele at all others. Lines were bred to homozygosity for the indicated intervals and maintained at TJL, while progeny were sent to Tufts for experimentation. Congenic lines which showed particularly interesting results were further backcrossed to N11 to ensure no genetic contamination was present. Congenics used in these studies are listed in Supporting Information Table 1.
Cell preparations, cultures and cytokine determinations
Livers and mesenteric lymph nodes (MLNs) were removed aseptically from 7-week infected mice. Single cell suspensions from MLNs were prepared as previously described (48). For purification of CD4 T cells, MLN cells (MLNCs) were negatively selected using a CD4+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA) following the manufacturer’s instructions. The resulting cell preparations were > 97% CD4+ cells as determined by flow cytometry. Granuloma cells (GC) were obtained by homogenization of the livers in a Waring blender, isolation of granulomas by 1 g sedimentation, extensive washing and enzymatic digestion with 1 mg/ml of collagenase type H, from Clostridium histolyticum (Sigma Chemicals) as previously described (6).
Bulk MLNC and GC suspensions (5 × 106 cells/ml) or purified CD4 T cells from MLNs (1 × 106 cells/ml) plus normal irradiated syngeneic splenocytes serving as antigen presenting cells (4 × 106 cells/ml), were incubated in the presence or absence of 15 μg/ml of soluble schistosome egg antigens (SEA) prepared as previously described (49). After 48 hours, the culture supernatants were removed, filtered and stored at −36°C until analysis by ELISA. For IL-4, IL-5 and IL-10 antibodies, standard cytokines and protocols were obtained from BD-Pharmingen (San Diego, CA) and for IFN-γ, IL-17, IL-6 and TNF-α from R&D Systems, Inc. (Minneapolis, MN).
Liver histopathology assessment
Liver samples from all mice were fixed in 10% buffered formalin and processed for routine histopathological analysis; 5-μm sections were stained with hematoxylin and eosin. The extent of hepatic granulomatous inflammation around schistosome eggs was measured by computer-assisted morphometric analysis using Image-Pro Plus software (Media Cybernetics) as described previously (6). Lesions were assessed blindly. To accurately reflect the magnitude of the granulomatous inflammation only those granulomas with a single visible central egg were counted. A minimum of 15 granulomas were measured per section. Two sections were appraised per liver. Mean granuloma size was measured in square micrometers ± SEM.
Bone marrow-derived DCs (BMDCs)
Femurs and tibias were removed from uninfected mice and bone marrow cells were flushed into 10cm petri dishes with cold PBS. Isolated bone marrow cells were cultured at a concentration of 5 × 105 cells/ml in 10 ml of complete RPMI medium containing 10% fetal calf serum (Aleken Biologicals, Nash, TX) and granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF-containing supernatant isolated from the B cell hybridoma J558L (provided by Dr. Nir Hacohen, Broad Institute, Cambridge, MA) was added to bone marrow cells at an optimized concentration of 1:10. On days 4 and 7 an additional 10ml of GM-CSF-containing medium was added to the cultures. Non-adherent cells, which were > 75% CD11c+ as determined by FACS analysis, were harvested on day 10 and used for experiments.
Schistosome egg isolation
Schistosome eggs were isolated under sterile conditions from 7 to 8 week-infected female Swiss Webster mice obtained from Charles River Laboratories, Kingston NY. To isolate eggs, livers were blended and eggs were separated from the tissue using a series of sieves and washes with saline solution as previously described (16).
BMDC-egg cocultures
BMDCs (1 × 106 cells/ml) were cultured in the presence of the indicated number of live eggs or LPS (10ng/ml Sigma-Aldrich). After 24 h, culture supernatants were collected, sterile filtered and assayed by ELISA for IL-1β, IL-12p40, IL-6 and TGF-β (Reagents and protocols from BD-Pharmingen or R&D systems). Potential contamination of egg-containing cultures with LPS was ruled out as previously described (16).
BMDC-CD4 T cell-egg cocultures
Purified CD4 T cells (1 × 106) were isolated from uninfected spleens, cultured with syngeneic BMDCs (2.5 × 105) and stimulated with the indicated number of live eggs plus anti-CD3/CD28 coated beads (3 × 105, Dynal/Invitrogen, Carlsbad, CA). After 4 days, culture supernatants were removed, sterile filtered and assayed by ELISA for IL-17, IL-6, IL-23 and IFN-γ using mAB, standards and protocols from R&D systems. For IL-5 ELISA, mAB, standards and protocols were obtained from eBioscience (San Diego, CA).
Quantitative Real-time RT-PCR
Total RNA was isolated from individual samples using TRIzol reagent (Invitrogen) as per manufacturer’s instructions. RNA (1–5μg) was subjected to DNASE I treatment (Roche) and reverse-transcribed using the high capacity cDNA reverse synthesis kit (Applied Biosystems). Real-time quantitative RT-PCR was performed on 10ng of cDNA/sample by TaqMan analysis using an ABI 7300 instrument per manufacturer’s instructions. GAPDH levels were used to normalize the data. TaqMan real-time probes for Chil3 (Ym1) and Retnla (RELMα) were obtained from Applied Biosystems. Using the average mean cycle threshold (Ct) value for GAPDH and the gene of interest for each sample, the equation 1.8 e (Ct (GAPDH) − Ct (GOI)) × 104 was used to obtain normalized values (50). For the RT2 Profiler PCR Array (SuperArray Bioscience, a Qiagen company), 20ng of cDNA was added to each well of the PCR array along with RT2 SYBR green/Rox PCR master mix (PA-012-24 SuperArray Bioscience, a Qiagen company). PCR cycling and data analysis were performed according to manufacturer’s instructions. For each independent experiment, biological triplicates were averaged to provide a mean Ct value. Data were normalized using the housekeeping genes gapdh and actb and analyzed by comparing 2^-ΔCt of the normalized samples.
Statistical analyses
One-way ANOVA, student’s t test and linear regression analysis were used to determine the statistical significance of differences between samples and were calculated with Prism Software (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service Grant R01 18919 (to M.J.S.) and R01 DK56597 and R01 AI28802 (to D.C.R).
Abbreviations used in this paper
- B10
C57BL/10SnJ
- B6
C57BL/6
- DC
dendritic cell
- QTL
quantitative trait loci
- Th17
T helper 17
- MLNC
mesenteric lymph node cell
- GC
granuloma cell
- AAM
alternatively activated macrophage
Footnotes
Conflict of Interest Disclosure
The authors declare no conflict of interests.
References
- 1.Schistosomiasis Fact Sheet. World Health Organization; Feb, 2014. Retrieved 15 March 2014. [Google Scholar]
- 2.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 3.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, Hamer DH, Stadecker MJ. Hepatic schistosomiasis. Infect Dis Clin North Am. 2000;14:583–604. viii. doi: 10.1016/s0891-5520(05)70122-7. [DOI] [PubMed] [Google Scholar]
- 5.Cheever AW, Duval RH, Hallack TA, Jr, Minker RG, Malley JD, Malley KG. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- 6.Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98:13243–8. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PM, Shainheit MG, Bazzone LE, Rutitzky LI, Poltorak A, Stadecker MJ. Genetic control of severe egg-induced immunopathology and IL-17 production in murine schistosomiasis. J Immunol. 2009;183:3317–23. doi: 10.4049/jimmunol.0901504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol. 2012;34:873–88. doi: 10.1007/s00281-012-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, et al. The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet. 2003;4:911–6. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May J, Kremsner PG, Milovanovic D, Schnittger L, Löliger CC, Bienzle U, Meyer CG. HLA-DP control of human Schistosoma haematobium infection. Am J Trop Med Hyg. 1998;59:302–6. doi: 10.4269/ajtmh.1998.59.302. [DOI] [PubMed] [Google Scholar]
- 11.McManus DP, Ross AG, Williams GM, Sleigh AC, Wiest P, Erlich H, Trachtenberg E, et al. HLA class II antigens positively and negatively associated with hepatosplenic schistosomiasis in a Chinese population. Int J Parasitol. 2001;31:674–80. doi: 10.1016/s0020-7519(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 12.Secor WE, del Corral H, dos Reis MG, Ramos EA, Zimon AE, Matos EP, Reis EA, et al. Association of hepatosplenic schistosomiasis with HLA-DQB1*0201. J Infect Dis. 1996;174:1131–5. doi: 10.1093/infdis/174.5.1131. [DOI] [PubMed] [Google Scholar]
- 13.Abel L, Marquet S, Chevillard C, elWali NE, Hillaire D, Dessein A. Genetic predisposition to bilharziasis in humans: research methods and application to the study of Schistosoma mansoni infection. J Soc Biol. 2000;194:15–8. [PubMed] [Google Scholar]
- 14.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, et al. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31–q33. Nat Genet. 2004;14:181–4. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 15.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–11. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–67. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozzo SJ, Vyse TJ, Menze K, Izui S, Kotzin BL. Enhanced susceptibility to lupus contributed from the nonautoimmune C57BL/10, but not C57BL/6 genome. J Immunol. 2000;164:5515–21. doi: 10.4049/jimmunol.164.10.5515. [DOI] [PubMed] [Google Scholar]
- 18.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh GhG. Differential expression of RELMα and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 19.Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–35. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S, Martinez FO. Alernative Activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–82. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci USA. 2012;109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shainheit MG, Lasocki KW, Finger E, Larkin BM, Smith PM, Sharpe AH, Dinarello CA, et al. The pathogenic Th17 cell response to major schistosome egg antigen is sequentially dependent on IL-23 and IL-1beta. J Immunol. 2011;187:5328–35. doi: 10.4049/jimmunol.1101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HLL, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of Il-17. J Immunol. 2008;180:2486–95. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 26.Rutitzy LI, Smith PM, Stadecker MJ. T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur J Immunol. 2009;39:2470–81. doi: 10.1002/eji.200939325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutitzky LI, Stadecker MJ. Exacerbated egg-induced immunopathology in murine Schistosoma mansoni infection is primarily mediated by IL-17 and restrained by IFN-γ. Eur J Immunol. 2011;41:2677–87. doi: 10.1002/eji.201041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reifsnyder PC, Li R, Silveira PA, Churchill G, Serreze DV, Leiter EH. Conditioning the genome identifies additional diabetes resistance loci in Type I diabetes resistant NOR/Lt mice. Genes Immun. 2005;6:528–38. doi: 10.1038/sj.gene.6364241. [DOI] [PubMed] [Google Scholar]
- 29.Teuscher C, Doerge RW, Fillmore PD, Blankenhorn EP. eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in the winter. Genetics. 2006;172:1147–53. doi: 10.1534/genetics.105.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pike RN, Bottomley SP, Irving JA, Bird PI, Whisstock JC. Serpins: finely balanced conformational traps. IUBMB Life. 2002;54:1–7. doi: 10.1080/15216540213825. [DOI] [PubMed] [Google Scholar]
- 31.Matesic LE, Niemitz EL, De Maio A, Reeves RH. Quantitative trait loci modulate neutrophil infiltration in the liver during LPS-induced inflammation. FASEB J. 2000;14:2247–54. doi: 10.1096/fj.99-1051com. [DOI] [PubMed] [Google Scholar]
- 32.McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci USA. 1998;95:11792–7. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay TF. The genetic architecture of quantitative traits. Annu Rev Genet. 2001;35:303–39. doi: 10.1146/annurev.genet.35.102401.090633. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J Immunol. 2009;182:5393–9. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- 36.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 38.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 39.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan TRA, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 41.Wiltshire T, Pletcher MT, Batalov S, Barnes SW, Tarantino LM, Cooke MP, Wu H, et al. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proc Natl Acad Sci USA. 2003;100(6):3380–3385. doi: 10.1073/pnas.0130101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Bell TA, Churchill GA, Pardo-Manuel de Villena F. On the subspecific origin of the laboratory mouse. Nat Genet. 2007;39(9):1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- 43.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234(1):18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 44.Crespo HJ, Lau JTY, Videira PA. Dendritic cells: a spot on sialic acid. Front Immunol. 2013 doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petkov PM, Wiles MV. MPD:JAXSNP1. Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, Maine USA: Mar, 2014. SNP data, 2000+ locations for 107 inbred strains of mice. http://phenome.jax.org. [Google Scholar]
- 46.Palmer A. MPD:Chicago1. Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, Maine USA: Mar, 2014. SNP data, 8200+ locations for 58 inbred strains of mice. http://phenome.jax.org. [Google Scholar]
- 47.Center for Genome Dynamics (CGD) MPD:CGD-MDA1. Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, Maine USA: Mar, 2014. SNP data from Mouse Diversity Genotyping Array, 545,000+ locations for 123 strains of mice. http://phenome.jax.org. [Google Scholar]
- 48.Rutitzky LI, Hernandez HJ, Yim YS, Ricklan DE, Finger E, Mohan C, Peter I, et al. Enhanced egg-induced immunopathology correlates with high IFN-gamma in murine schistosomiasis: identification of two epistatic genetic intervals. J Immunol. 2005;174:435–40. doi: 10.4049/jimmunol.174.1.435. [DOI] [PubMed] [Google Scholar]
- 49.Boros DL, Warren KS. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:448–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein WT, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–26. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.