Abstract
Background
Influenza vaccines available for children in the United States include inactivated influenza vaccine (IIV) and live, attenuated influenza vaccine (LAIV). Objectives of this study were to quantify proportions of IIV and LAIV received by vaccinated children, and examine associations between vaccine type received and demographic characteristics.
Methods
National Immunization Survey-Flu (NIS-Flu) parental reported data for the 2011-12 through 2013-14 influenza seasons were used to estimate proportions of vaccinated children 2-17 years who received IIV and LAIV. Tests of association between vaccination type and demographic variables were conducted using Wald chi-square tests and pair-wise comparison t-tests. Multivariable logistic regression was used to determine variables independently associated with receipt of LAIV versus IIV.
Results
In the 2013-14 season, 33.3% of vaccinated children received LAIV, similar to the proportion in the 2011-12 (32.2%) and 2012-13 (32.1%) seasons. Across all seasons studied, the strongest observed association was between vaccination type and child's age, with children 2-8 years (Adjusted Prevalence Ratio (95% confidence interval) [APR(95% CI)] 1.41(1.27-1.56), 1.46(1.34-1.59), and 1.50(1.38-1.63) for 2011-12, 2012-13, and 2013-14) and 9-12 years (APR(95% CI) 1.37(1.23-1.54), 1.38(1.26-1.51), and 1.50(1.38-1.63) for 2011-12, 2012-13, and 2013-14) being more likely to have received LAIV than children 13-17 years. Among those vaccinated, whites were more likely to have received LAIV compared to blacks (APR(95% CI) 1.19(1.05-1.35), 1.24(1.10-1.39), and 1.22(1.11-1.34) for 2011-12, 2012-13, and 2013-14), and children living above poverty (annual income >$75,000) were more likely to have received LAIV than those living at or below poverty (APR(95% CI) 1.43(1.23-1.67), 1.13(1.02-1.26), and 1.16(1.06-1.28) for 2011-12, 2012-13, and 2013-14).
Conclusions
This study provides a baseline of the extent and patterns of LAIV uptake that can be used to measure the impact of relevant public health policy. Additional research is needed to investigate parental and provider preferences and barriers regarding LAIV.
Keywords: Influenza vaccines, Vaccination, Child, LAIV vaccine, Health surveys
Introduction
Influenza is a serious disease that can lead to hospitalization and death. Rates of influenza infection are highest among children, with children <5 years and especially those <2 years at high risk for complications, hospitalizations, and deaths [1-9]. Vaccination is the most effective strategy for preventing influenza infection and its potentially serious complications [10]. The Advisory Committee on Immunization Practices (ACIP) recommended influenza vaccination for children 6-23 months in 2004, expanded the age range to include children 6-59 months in 2006, and further expanded the recommendation to include children 6 months-18 years in 2008 [11-13].
Two types of influenza vaccine are available for children in the United States, inactivated influenza vaccine (IIV) and live, attenuated influenza vaccine (LAIV). IIV has been available for many years and is administered by intramuscular injection (i.e. a shot). A variety of IIV products are available from several different manufacturers. Age indications for IIV have no upper limit for children, but the lower limit varies by vaccine with some approved for children as young as 6 months [10;14]. LAIV first became available and recommended for use in healthy persons 5-49 years in 2003, and then expanded for use in healthy children 2-4 years in 2007 [15;16]. Only one LAIV product is available, and it is administered intranasally (i.e. the nasal spray) [10;14]. LAIV and IIV products were all trivalent, containing two influenza A and one influenza B viral antigens, until the 2013-14 influenza season when trivalent LAIV was replaced by a quadrivalent LAIV formulation, containing an additional influenza B viral antigen, and IIVs became available in both trivalent and quadrivalent formulations [10]. At the June 2014 meeting, the ACIP voted to include a preference for the use, when immediately available and there are no contraindications, of LAIV for healthy children 2-8 years in their recommendations for the 2014-15 influenza season based on studies that appeared to demonstrate superior efficacy of LAIV as compared with IIV among children, particularly younger children [17-20]. At the February 2015 meeting, the ACIP voted to remove this preferential recommendation when other study data showed that LAIV may not be superior to IIV [14;21].
The objectives of this study were to quantify the proportion of children vaccinated against influenza who received LAIV in recent seasons, and to examine associations between vaccine type received and demographic characteristics. The results of this study provide baseline data for vaccine policy considerations, and serve as essential input into vaccine impact, cost-effectiveness models, and vaccine safety analyses.
Methods
Data from the National Immunization Survey-Flu (NIS-Flu) from 2011-2014 were analyzed to assess type of influenza vaccination received by vaccinated children 2-17 years during the 2011-12, 2012-13, and 2013-14 influenza seasons [22]. The NIS-Flu is an ongoing, national list-assisted random-digit-dialed dual frame land line and cellular telephone survey of households with children. It includes three components: the NIS for children 19–35 months, the NIS-Teen for children 13–17 years, and the NIS child influenza module for children 6–18 months and 3–12 years identified during the screening of households for the NIS and NIS-Teen [22-27]. Data were collected by parental report, and interviews conducted September through June for the 2011-12 season and October through June for the 2012-13 and 2013-14 seasons from all 50 states and the District of Columbia were included in the analysis. The Council of American Survey and Research Organizations (CASRO) response rates ranged from 51.8%-63.4% for landline and 18.1%-33.5% for cellular telephones [28-31].
The NIS-Flu sample included 102,254, 107,550, and 130,409 children for the 2011-12, 2012-13, 2013-14 seasons, respectively. The study sample used to examine LAIV uptake was limited to a subset of data (n=34,025, n=42,331, and n=55,256 for the 2011-12, 2012-13, and 2013-14 seasons, respectively) that included children who were at least 2 years old (at October 1st of each season), had received at least one dose of influenza vaccine, and had information about influenza vaccination type available. Survey respondents were asked if their child had received an influenza vaccination and, if so, during which month and year; for vaccinated children with missing month and year of vaccination (2.3%, 6.3%, and 7.7% for the 2011-12, 2012-13, and 2013-14 seasons, respectively), this information was imputed from donor pools matched for week of interview, age group, state of residence, and race/ethnicity. For children who received an influenza vaccination, respondents were asked “Was this a shot or the spray in the nose?”; children missing this information were excluded from the study (4.3%, 4.3%, and 5.7% for the 2011-12, 2012-13, and 2013-14 seasons, respectively). Information on child, maternal, and household socio-demographic characteristics were also collected during the NIS-Flu interviews.
Children were considered vaccinated if they were reported to have received an influenza vaccination August through May for the 2011-12 season and July through May for the 2012–13 and 2013-14 seasons. State level and national influenza vaccination coverage estimates and methods were published previously for children 6 months and older, and were calculated for this study using the same methodology but for children who were at least 2 years old (at October 1st) [22;29-31]. Tests of association between vaccination type received and demographic variables were conducted using Wald chi-square tests followed by pair-wise comparison t-tests. Multivariable logistic regression was used to determine variables independently associated with receipt of LAIV versus IIV. The dependent variable in the multivariable model was receipt of LAIV, and independent variables included the following: child's age, sex, race-ethnicity, mother's education, poverty/annual household income, number of children in the household, urban-rural residence, region of residence, and vaccination facility type. Adjusted prevalence ratios (APR) based on predicted marginals from the logistic regression model are reported.
Although some children in the study had received two doses of influenza vaccine in an influenza season, this study focused on the first (or only) dose received. A sub-analysis was done among children who received two doses in a season to quantify the consistency in vaccination type received, excluding those with missing information on vaccination type for one or both of their vaccinations (2.3%, 5.3%, and 2.8% for the 2011-12, 2012-13, and 2013-14 seasons, respectively).
To assess accuracy of parental reported type of influenza vaccination, NIS and NIS-Teen parental and provider reported vaccinations during the study vaccination period for the 2012-13 influenza season, the most recent available season, were summarized, and status over one or more vaccinations was classified as delivered by IIV only, LAIV only, or both IIV and LAIV for children with available type information. The sample for this analysis included children 2 years and older (at October 1st) with both a parent and provider reported influenza vaccination (n=2,685 for NIS and n=2,918 for NIS-Teen). Children were excluded if type information was missing (2.8% for NIS and 4.2% for NIS-Teen) or if type was reported as both IIV and LAIV (1.0% for NIS and 0.4% for NIS-Teen) from either source. The difference between the percent of children who received the nasal spray according to parental versus provider report was calculated.
A two-sided significance level of 0.05 was adopted for all statistical tests. Reported percentages and corresponding 95% confidence intervals (95% CI) were weighted, while reported sample sizes were unweighted. All analyses were weighted to population totals and to adjust for households having multiple telephone lines, unit non-response, and non-coverage of non-telephone households. Analyses were conducted using SAS (version 9.3) and SUDAAN (version 11.0.0) statistical software to account for the complex design.
Results
The sample characteristics are presented in Table 1. The distribution across the various groups of children remained consistent for all three seasons studied.
Table 1. Demographic characteristics of the study population of children 2 - 17 years who received an influenza vaccination, United States, National Immunization Survey-Flu (NIS-Flu), 2011-12 through 2013-14 influenza seasons.
| 2011-2012 | 2012-2013 | 2013-2014 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | unweighted n | weighted % (± 95% CI*) | unweighted n | weighted % (± 95% CI) | unweighted n | weighted % (± 95% CI) |
| Overall | 34,025 | 42,331 | 55,256 | |||
| Child's age | ||||||
| 2 – 8 years | 16,693 | 51.4 (± 1.3) | 21,410 | 48.7 (± 1.0) | 27,800 | 47.6 (± 0.9) |
| 2 - 4 years | 7,547 | 22.0 (± 1.2) | 9,549 | 19.9 (± 0.8) | 11,965 | 19.5 (± 0.8) |
| 5 – 8 years | 9,146 | 29.4 (± 1.1) | 11,861 | 28.8 (± 0.9) | 15,835 | 28.1 (± 0.8) |
| 9 - 12 years | 9,079 | 25.9 (± 1.1) | 11,416 | 25.3 (± 0.9) | 16,261 | 25.7 (± 0.8) |
| 13 - 17 years | 8,253 | 22.7 (± 1.1) | 9,505 | 26.0 (± 0.9) | 11,195 | 26.7 (± 0.9) |
| Child's sex | ||||||
| Male | 17,551 | 51.5 (± 1.3) | 21,885 | 51.3 (± 1.0) | 28,234 | 51.0 (± 0.9) |
| Female | 16,474 | 48.5 (± 1.3) | 20,446 | 48.7 (± 1.0) | 27,022 | 49.0 (± 0.9) |
| Child's race/ethnicity | ||||||
| White, non-Hispanic | 20,638 | 54.9 (± 1.3) | 25,401 | 53.3 (± 1.1) | 32,869 | 52.3 (± 1.0) |
| Black, non-Hispanic | 3,724 | 15.4 (± 1.1) | 4,235 | 13.1 (± 0.8) | 5,482 | 13.1 (± 0.7) |
| Hispanic | 5,817 | 23.9 (± 1.3) | 7,603 | 24.1 (± 1.0) | 10,254 | 24.7 (± 1.0) |
| Other or multiple race, non-Hispanic | 3,846 | 5.7 (± 0.4) | 5,092 | 9.4 (± 0.5) | 6,651 | 9.9 (± 0.5) |
| Mother's education | ||||||
| < High school | 3,000 | 14.1 (± 1.2) | 3,810 | 12.6 (± 0.8) | 5,229 | 14.0 (± 0.8) |
| High school or equivalent | 6,119 | 19.7 (± 1.1) | 7,285 | 19.6 (± 0.9) | 8,998 | 18.0 (± 0.8) |
| Some college | 8,145 | 24.7 (± 1.3) | 9,889 | 24.3 (± 0.9) | 12,892 | 23.6 (± 0.9) |
| ≥ College degree | 15,379 | 41.5 (± 1.3) | 19,393 | 43.6 (± 1.0) | 25,866 | 44.4 (± 1.0) |
| Poverty/annual household income† | ||||||
| Above poverty (> $75,000) | 14,048 | 35.6 (± 1.2) | 17,280 | 35.8 (± 1.0) | 23,114 | 36.4 (± 0.9) |
| Above poverty (≤ $75,000) | 11,551 | 32.5 (± 1.2) | 13,883 | 31.2 (± 1.0) | 17,350 | 29.8 (± 0.9) |
| At or below poverty | 5,203 | 22.6 (± 1.3) | 6,643 | 22.4 (± 1.0) | 8,785 | 22.6 (± 1.0) |
| Unknown | 3,223 | 9.3 (± 0.7) | 4,525 | 10.6 (± 0.6) | 6,007 | 11.2 (± 0.5) |
| Number of children in household | ||||||
| 1 | 10,749 | 22.9 (± 1.0) | 13,921 | 24.0 (± 0.8) | 16,969 | 22.9 (± 0.7) |
| 2 - 3 | 20,402 | 65.1 (± 1.2) | 25,037 | 64.5 (± 1.0) | 33,663 | 64.8 (± 0.9) |
| ≥ 4 | 2,848 | 12.1 (± 0.9) | 3,333 | 11.5 (± 0.8) | 4,559 | 12.3 (± 0.8) |
| Urban-rural residence | ||||||
| Urban (MSA, principle city) | 11,412 | 33.5 (± 1.3) | 14,745 | 33.5 (± 1.0) | 15,175 | 26.9 (± 0.9) |
| Suburban (MSA, not principle city) | 15,759 | 52.2 (± 1.3) | 18,674 | 50.1 (± 1.0) | 29,943 | 59.5 (± 1.0) |
| Rural (non-MSA) | 6,854 | 14.2 (± 0.8) | 8,912 | 16.3 (± 0.7) | 10,138 | 13.6 (± 0.6) |
| Region of residence | ||||||
| Northeast | 7,211 | 18.6 (± 0.8) | 9,827 | 19.8 (± 0.7) | 12,813 | 18.5 (± 0.6) |
| Midwest | 6,723 | 20.6 (± 0.8) | 8,623 | 21.2 (± 0.7) | 11,215 | 20.9 (± 0.6) |
| South | 13,201 | 36.9 (± 1.2) | 14,883 | 35.8 (± 1.0) | 19,672 | 37.4 (± 0.9) |
| West | 6,890 | 23.8 (± 1.4) | 8,998 | 23.2 (± 1.1) | 11,556 | 23.1 (± 1.0) |
| Vaccination facility type | ||||||
| Doctor's office | 21,678 | 64.0 (± 1.4) | 26,983 | 64.9 (± 1.0) | 32,501 | 64.8 (± 1.0) |
| Hospital | 1,135 | 3.8 (± 0.5) | 1,592 | 3.9 (± 0.4) | 1,961 | 3.2 (± 0.3) |
| Clinic or health center/other medical | 5,322 | 18.2 (± 1.2) | 6,720 | 17.1 (± 0.9) | 7,934 | 17.9 (± 0.9) |
| Local health department | 1,096 | 3.9 (± 0.7) | 1,260 | 3.2 (± 0.4) | 1,432 | 2.8 (± 0.4) |
| Pharmacy or store | 1,202 | 3.4 (± 0.5) | 1,760 | 4.2 (± 0.4) | 1,800 | 4.6 (± 0.4) |
| School | 2,917 | 5.1 (± 0.4) | 3,283 | 5.4 (± 0.5) | 3,292 | 5.4 (± 0.4) |
| Other non-medical/work | 604 | 1.6 (± 0.3) | 620 | 1.3 (± 0.2) | 656 | 1.3 (± 0.2) |
CI = Confidence Interval half-width
Poverty level was defined based on the reported number of people living in the household and annual household income, and the U.S. Census poverty thresholds
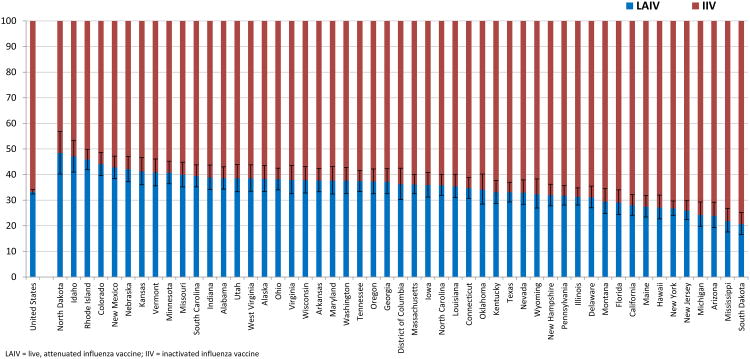
National and state level estimates for receipt of LAIV by children 2-17 years are shown in Table 2 and Figure 1. In the United States, 33.3% of vaccinated children received LAIV and the rest (66.7%) received IIV during the 2013-14 season. At the state level, the proportion of children vaccinated during the 2013-14 season that received LAIV ranged from 20.6% (South Dakota) to 48.4% (North Dakota).
Table 2. Weighted prevalence (%) of children 2 - 17 years vaccinated against influenza who received live, attenuated influenza vaccine (LAIV) and overall influenza vaccination coverage among children 2 - 17 years by state, United States, National Immunization Survey-Flu (NIS-Flu), 2011-12 through 2013-14 influenza seasons.
| 2011-2012 | 2012-2013 | 2013-2014 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| State of residence | n* | Received LAIV† % (± 95% CI‡) | Total Vaccinated§ % (± 95% CI) | n | Received LAIV % (± 95% CI) | Total Vaccinated % (± 95% CI) | n | Received LAIV % (± 95% CI) | Total Vaccinated % (± 95% CI) |
| United States | 34,025 | 32.2 (± 1.2) | 49.6 (± 1.0) | 42,331 | 32.1 (± 1.0) | 55.1 (± 0.9) | 55,256 | 33.3 (± 0.9) | 57.7 (± 0.8) |
| Alabama | 578 | 34.7 (± 6.5) | 46.5 (± 5.2) | 593 | 30.6 (± 6.5) | 50.3 (± 5.3) | 993 | 38.6 (± 4.4) | 59.8 (± 4.3) |
| Alaska | 415 | 30.7 (± 7.5) | 36.1 (± 4.9) | 632 | 37.2 (± 6.4) | 44.4 (± 4.4) | 813 | 38.3 (± 5.1) | 49.8 (± 4.4) |
| Arizona | 543 | 32.8 (± 9.5) | 45.5 (± 5.8) | 722 | 27.8 (± 5.2) | 47.7 (± 4.1) | 793 | 23.9 (± 5.0) | 46.2 (± 4.3) |
| Arkansas | 707 | 41.7 (± 7.8) | 63.3 (± 5.1) | 780 | 35.0 (± 5.6) | 61.1 (± 4.4) | 1,001 | 37.7 (± 4.6) | 68.8 (± 3.8) |
| California | 619 | 23.2 (± 5.0) | 51.9 (± 4.7) | 854 | 28.7 (± 4.6) | 55.0 (± 4.1) | 1,104 | 28.0 (± 4.1) | 62.3 (± 4.3) |
| Colorado | 618 | 45.4 (± 6.6) | 50.8 (± 5.5) | 851 | 41.0 (± 4.9) | 57.2 (± 3.9) | 1,186 | 44.1 (± 4.5) | 60.1 (± 3.4) |
| Connecticut | 722 | 28.6 (± 5.7) | 58.2 (± 4.7) | 1,011 | 29.1 (± 4.2) | 64.0 (± 3.9) | 1,174 | 34.7 (± 4.1) | 69.2 (± 3.9) |
| Delaware | 644 | 26.4 (± 6.3) | 54.6 (± 6.1) | 923 | 27.9 (± 5.2) | 65.0 (± 6.7) | 1,158 | 31.1 (± 4.3) | 65.5 (± 4.0) |
| District of Columbia | 616 | 31.5 (± 8.5) | 63.8 (± 6.7) | 742 | 37.3 (± 7.8) | 71.5 (± 6.6) | 948 | 36.2 (± 6.2) | 65.7 (± 4.5) |
| Florida | 427 | 35.1 (± 9.0) | 42.3 (± 6.8) | 507 | 28.7 (± 5.5) | 44.8 (± 5.1) | 722 | 29.0 (± 4.8) | 47.8 (± 3.9) |
| Georgia | 546 | 35.2 (± 7.2) | 42.7 (± 5.5) | 588 | 40.5 (± 6.0) | 51.9 (± 4.8) | 727 | 37.2 (± 4.9) | 50.5 (± 4.1) |
| Hawaii | 789 | 30.9 (± 7.6) | 66.1 (± 8.3) | 985 | 27.4 (± 7.1) | 68.5 (± 6.8) | 1,176 | 27.1 (± 4.7) | 69.7 (± 5.3) |
| Idaho | 403 | 42.4 (± 8.0) | 40.2 (± 4.9) | 487 | 42.5 (± 6.4) | 43.0 (± 4.6) | 646 | 47.1 (± 6.2) | 44.3 (± 4.5) |
| Illinois | 1,106 | 29.3 (± 5.1) | 43.1 (± 3.9) | 1,406 | 29.3 (± 4.2) | 50.5 (± 3.6) | 2,005 | 31.3 (± 3.4) | 52.8 (± 2.7) |
| Indiana | 558 | 38.9 (± 6.8) | 45.4 (± 4.6) | 618 | 29.3 (± 5.2) | 51.8 (± 4.1) | 995 | 38.8 (± 4.8) | 49.4 (± 4.0) |
| Iowa | 567 | 36.9 (± 5.5) | 47.5 (± 4.3) | 604 | 31.7 (± 5.0) | 51.1 (± 4.4) | 846 | 35.9 (± 4.8) | 52.3 (± 4.4) |
| Kansas | 553 | 40.1 (± 6.4) | 45.3 (± 4.7) | 569 | 32.5 (± 5.1) | 44.2 (± 3.9) | 697 | 41.2 (± 5.3) | 55.8 (± 4.1) |
| Kentucky | 582 | 38.3 (± 6.1) | 46.1 (± 4.7) | 658 | 33.7 (± 5.7) | 57.2 (± 5.6) | 749 | 33.1 (± 4.5) | 52.3 (± 4.3) |
| Louisiana | 730 | 30.3 (± 5.8) | 54.8 (± 5.0) | 809 | 36.8 (± 5.0) | 56.3 (± 4.6) | 961 | 35.4 (± 4.5) | 56.5 (± 3.8) |
| Maine | 608 | 24.4 (± 5.2) | 58.5 (± 4.8) | 719 | 26.8 (± 5.2) | 62.2 (± 4.5) | 1,028 | 27.4 (± 4.2) | 59.9 (± 3.7) |
| Maryland | 1,004 | 38.0 (± 5.5) | 62.0 (± 7.5) | 980 | 36.3 (± 6.5) | 66.2 (± 5.6) | 1,157 | 37.6 (± 5.4) | 64.8 (± 4.7) |
| Massachusetts | 638 | 28.7 (± 4.9) | 62.0 (± 4.7) | 980 | 27.7 (± 4.0) | 74.4 (± 3.5) | 1,246 | 36.1 (± 3.8) | 71.0 (± 3.5) |
| Michigan | 552 | 26.7 (± 5.1) | 43.9 (± 4.1) | 655 | 27.8 (± 5.3) | 49.0 (± 4.2) | 844 | 24.2 (± 4.7) | 52.6 (± 4.2) |
| Minnesota | 524 | 39.6 (± 6.8) | 50.6 (± 5.2) | 674 | 47.8 (± 5.2) | 59.0 (± 4.7) | 847 | 40.7 (± 4.4) | 61.7 (± 4.1) |
| Mississippi | 482 | 18.5 (± 6.0) | 41.4 (± 5.4) | 557 | 21.6 (± 5.5) | 44.8 (± 4.9) | 694 | 21.8 (± 4.6) | 42.9 (± 4.2) |
| Missouri | 424 | 35.4 (± 6.9) | 43.4 (± 5.2) | 633 | 37.2 (± 5.0) | 49.2 (± 4.3) | 712 | 39.9 (± 4.8) | 52.3 (± 3.8) |
| Montana | 508 | 36.1 (± 8.1) | 40.5 (± 4.7) | 636 | 30.5 (± 6.4) | 43.9 (± 4.4) | 658 | 29.4 (± 4.9) | 48.3 (± 4.4) |
| Nebraska | 443 | 43.8 (± 6.4) | 49.1 (± 5.3) | 635 | 35.6 (± 5.0) | 58.5 (± 4.8) | 795 | 42.1 (± 5.0) | 60.7 (± 4.2) |
| Nevada | 460 | 44.2 (±12.5)‖ | 44.1 (± 6.8) | 646 | 35.7 (± 6.0) | 49.4 (± 4.3) | 935 | 32.9 (± 4.7) | 48.3 (± 4.1) |
| New Hampshire | 650 | 28.1 (± 5.6) | 51.9 (± 5.0) | 854 | 24.8 (± 4.0) | 57.5 (± 4.7) | 1,119 | 31.9 (± 4.2) | 62.6 (± 3.9) |
| New Jersey | 665 | 18.2 (± 4.2) | 60.3 (± 4.6) | 946 | 24.0 (± 5.2) | 65.3 (± 4.1) | 1,169 | 25.9 (± 3.7) | 66.1 (± 3.5) |
| New Mexico | 763 | 53.5 (± 6.1) | 59.0 (± 4.8) | 801 | 49.6 (± 5.7) | 66.2 (± 4.7) | 1,265 | 42.8 (± 4.4) | 65.0 (± 4.0) |
| New York | 1,052 | 23.5 (± 3.8) | 52.3 (± 3.7) | 1,657 | 24.3 (± 2.8) | 59.6 (± 2.7) | 1,990 | 26.8 (± 2.8) | 63.0 (± 2.6) |
| North Carolina | 559 | 39.2 (±10.4)‖ | 55.0 (± 6.4) | 848 | 35.5 (± 4.5) | 56.7 (± 4.1) | 1,031 | 35.8 (± 4.1) | 60.2 (± 3.6) |
| North Dakota | 417 | 50.9 (± 8.9) | 51.9 (± 6.0) | 810 | 43.0 (± 5.8) | 60.3 (± 5.3) | 977 | 48.4 (± 8.4) | 61.8 (± 5.1) |
| Ohio | 534 | 44.2 (± 6.3) | 48.6 (± 5.4) | 766 | 39.2 (± 5.0) | 52.7 (± 4.5) | 934 | 38.2 (± 4.2) | 52.8 (± 3.6) |
| Oklahoma | 480 | 26.0 (± 7.0) | 52.6 (± 6.0) | 611 | 27.0 (± 6.2) | 48.4 (± 5.3) | 883 | 34.1 (± 5.9) | 54.0 (± 4.3) |
| Oregon | 512 | 35.6 (± 6.0) | 43.9 (± 5.0) | 759 | 31.6 (± 4.9) | 46.8 (± 4.0) | 884 | 37.3 (± 4.9) | 52.0 (± 4.1) |
| Pennsylvania | 1,436 | 32.4 (± 4.2) | 51.6 (± 3.8) | 1,828 | 32.4 (± 4.7) | 63.9 (± 5.1) | 2,649 | 31.8 (± 3.8) | 59.0 (± 3.7) |
| Rhode Island | 908 | 45.3 (± 6.1) | 72.9 (± 5.0) | 1,015 | 46.1 (± 4.6) | 80.6 (± 4.3) | 1,344 | 45.9 (± 4.0) | 73.5 (± 4.6) |
| South Carolina | 564 | 26.5 (± 6.4) | 48.6 (± 6.0) | 694 | 30.8 (± 5.4) | 51.8 (± 4.7) | 1,038 | 39.4 (± 4.3) | 56.3 (± 4.0) |
| South Dakota | 534 | 25.7 (± 6.0) | 57.8 (± 5.2) | 623 | 19.1 (± 4.6) | 71.6 (± 7.1) | 774 | 20.6 (± 4.3) | 67.6 (± 4.4) |
| Tennessee | 642 | 35.6 (± 6.0) | 48.4 (± 5.4) | 697 | 31.1 (± 5.7) | 54.2 (± 4.7) | 1,015 | 37.5 (± 4.1) | 59.5 (± 3.9) |
| Texas | 3,368 | 34.4 (± 3.4) | 50.5 (± 2.7) | 3,438 | 33.7 (± 3.6) | 54.6 (± 3.8) | 4,570 | 33.1 (± 3.8) | 61.2 (± 3.1) |
| Utah | 365 | 30.2 (± 8.6) | 46.3 (± 6.0) | 504 | 33.3 (± 5.7) | 47.3 (± 4.6) | 689 | 38.5 (± 5.3) | 47.5 (± 4.0) |
| Vermont | 532 | 26.5 (± 6.1) | 55.1 (± 5.7) | 817 | 34.7 (± 5.5) | 59.8 (± 5.1) | 1,094 | 40.8 (± 5.2) | 57.6 (± 4.2) |
| Virginia | 742 | 39.2 (± 6.4) | 48.4 (± 5.8) | 817 | 40.0 (± 6.7) | 59.4 (± 5.9) | 1,137 | 37.9 (± 5.5) | 61.5 (± 4.7) |
| Washington | 479 | 36.1 (± 6.6) | 43.0 (± 4.6) | 607 | 31.9 (± 5.4) | 55.3 (± 4.9) | 775 | 37.6 (± 5.1) | 55.9 (± 4.3) |
| West Virginia | 530 | 26.7 (± 7.3) | 49.0 (± 5.8) | 641 | 31.8 (± 5.5) | 53.2 (± 4.7) | 888 | 38.5 (± 5.2) | 52.6 (± 4.1) |
| Wisconsin | 511 | 39.7 (± 6.5) | 49.1 (± 4.6) | 630 | 34.3 (± 5.1) | 53.4 (± 4.3) | 789 | 37.8 (± 5.1) | 56.6 (± 4.1) |
| Wyoming | 416 | 20.4 (±11.3)‖ | 44.0 (± 7.0) | 514 | 28.3 (±12.2)‖ | 44.4 (± 6.0) | 632 | 32.3 (± 5.8) | 40.3 (± 4.8) |
n = unweighted sample size of vaccinated children 2-17 years.
The proportion of children 2 - 17 years vaccinated against influenza who received LAIV. The proportion of vaccinated children who received inactivated influenza vaccine (IIV) can be calculated by subtracting the proportion who received LAIV from 100.
CI = confidence interval half-width.
Influenza vaccination coverage estimates for children 6 months – 17 years and methods have been published previously on FluVaxView (http://www.cdc.gov/flu/fluvaxview/). The same methodology was used to calculate these estimates for children 2 – 17 years.
Estimates might not be reliable because the confidence interval half-width is >10.
Figure 1. Influenza vaccination type among vaccinated children by state, United States, National Immunization Survey-Flu (NIS-Flu), 2013-14 influenza season.
In bivariate analysis, child's age and race-ethnicity, mother's education, poverty status/annual household income, number of children in the household, region of residence, and place of vaccination were all found to be associated with influenza vaccination type received across all three seasons studied (Table 3). Vaccinated children 2-8 years were more likely than children in the other age groups, such as 13-17 years, to have received LAIV (34.6%, 35.4%, and 36.5% versus 24.7%, 24.2%, and 24.3% for the 2011-12, 2012-13, and 2013-14 seasons, respectively). A higher percentage of whites received LAIV (37.0%) than blacks (28.1%), Hispanics (30.1%), and children of other or multiple races (28.5%), who all had similar proportions, in the 2013-14 season; racial/ethnic differences in influenza vaccination type received remained constant across all seasons studied. Children of mothers who had at least a college degree were more likely to have received LAIV across all three seasons compared to children of mothers who were less educated. Children living in households above poverty with an annual income >$75,000 were consistently more likely to receive LAIV than children living in households above poverty with an annual income ≤$75,000, who were more likely to receive LAIV than children living at or below poverty. Children living in a household with only one child were less likely to receive LAIV than children living in households with multiple children. Across all seasons, fewer children living in the Northeast region received LAIV compared to the other regions. Finally, children who received their influenza vaccination at school were more likely to have received LAIV, especially compared to children who were vaccinated at a pharmacy. Influenza vaccination coverage estimates by selected characteristics related to the child and the child's household, are provided in Table 3.
Table 3. Weighted prevalence (%) of live, attenuated influenza vaccine (LAIV) received among children 2 - 17 years vaccinated against influenza and overall influenza vaccination coverage among children 2 - 17 years by selected demographic characteristics, United States, National Immunization Survey-Flu (NIS-Flu), 2011-12 through 2013-14 influenza seasons.
| 2011-2012 | 2012-2013 | 2013-2014 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | Received LAIV* % (± 95% CI†) | Total Vaccinated‡ % (± 95% CI) | Received LAIV % (± 95% CI) | Total Vaccinated % (± 95% CI) | Received LAIV % (± 95% CI) | Total Vaccinated % (± 95% CI) |
| Overall | 32.2 (± 1.2) | 49.6 (± 1.0) | 32.1 (± 1.0) | 55.1 (± 0.9) | 33.3 (± 0.9) | 57.7 (± 0.8) |
| Child's age | ||||||
| a. 2 – 8 years | 34.6 (± 1.7)§,c | 59.0 (± 1.5) | 35.4 (± 1.4)c | 63.3 (± 1.4) | 36.5 (± 1.3)c | 65.6 (± 1.3) |
| 2 - 4 years | 29.5 (± 2.6) | 63.0 (± 2.3) | 30.2 (± 2.1) | 65.7 (± 2.0) | 32.1 (± 2.1) | 68.1 (± 1.8) |
| 5 – 8 years | 38.5 (± 2.1) | 56.4 (± 1.9) | 38.9 (± 1.8) | 61.7 (± 1.8) | 39.5 (± 1.6) | 64.0 (± 1.7) |
| b. 9 - 12 years | 34.1 (± 2.4)c | 51.9 (± 2.1) | 33.9 (± 1.8)c | 55.4 (± 1.7) | 36.8 (± 1.6)c,‖ | 57.9 (± 1.4) |
| c. 13 - 17 years | 24.7 (± 2.2)a,b | 33.7 (± 1.6) | 24.2 (± 1.8)a,b | 42.5 (± 1.5) | 24.3 (± 1.6)a,b | 46.4 (± 1.5) |
| Child's sex | ||||||
| a. Male | 31.9 (± 1.6) | 49.4 (± 1.3) | 31.9 (± 1.3) | 54.5 (± 1.2) | 32.0 (± 1.2)b | 57.2 (± 1.1) |
| b. Female | 32.6 (± 1.8) | 48.7 (± 1.5) | 32.3 (± 1.3) | 54.6 (± 1.3) | 34.7 (± 1.3)a,‖ | 57.6 (± 1.2) |
| Child's race/ethnicity | ||||||
| a. White, non-Hispanic | 35.5 (± 1.5)b,c,d | 45.1 (± 1.1) | 35.2 (± 1.2)b,c,d | 51.9 (± 1.0) | 37.0 (± 1.1)b,c,d,‖ | 53.4 (± 0.9) |
| b. Black, non-Hispanic | 27.0 (± 3.0)a | 51.9 (± 3.1) | 26.5 (± 2.9)a | 54.7 (± 2.8) | 28.1 (± 2.4)a | 56.1 (± 2.3) |
| c. Hispanic | 28.8 (± 2.9)a | 56.8 (± 2.7) | 29.5 (± 2.2)a | 58.9 (± 2.3) | 30.1 (± 2.3)a | 64.8 (± 2.3) |
| d. Other or multiple race, non-Hispanic | 29.8 (± 3.0)a | 50.9 (± 2.9) | 28.6 (± 2.6)a | 58.7 (± 2.7) | 28.5 (± 2.5)a | 63.5 (± 2.2) |
| Mother's education | ||||||
| a. < High school | 27.7 (± 4.1)d | 58.2 (± 3.7) | 28.9 (± 2.9)d | 55.5 (± 3.1) | 28.9 (± 3.0)d | 63.6 (± 3.2) |
| b. High school or equivalent | 26.8 (± 2.3)c,d | 48.0 (± 2.3) | 29.3 (± 2.4)d | 54.1 (± 2.2) | 29.3 (± 2.2)d | 54.9 (± 1.9) |
| c. Some college | 32.5 (± 2.8)b,d | 44.9 (± 2.0) | 28.5 (± 1.9)d,¶ | 49.9 (± 1.7) | 30.3 (± 1.8)d | 51.3 (± 1.6) |
| d. ≥ College degree | 36.9 (± 1.6)a,b,c | 49.2 (± 1.4) | 36.8 (± 1.4)a,b,c | 58.0 (± 1.4) | 38.3 (± 1.2)a,b,c | 60.4 (± 1.1) |
| Poverty/annual household income** | ||||||
| a. Above poverty (> $75,000) | 37.3 (± 1.8)b,c,d | 48.9 (± 1.4) | 36.3 (± 1.5)b,c,d | 57.6 (± 1.4) | 38.2 (± 1.3)b,c,d | 59.1 (± 1.2) |
| b. Above poverty (≤ $75,000) | 32.9 (± 2.3)a,c | 44.8 (± 1.7) | 31.3 (± 1.7)a,c | 50.3 (± 1.5) | 31.8 (± 1.6)a,c | 52.5 (± 1.3) |
| c. At or below poverty | 24.1 (± 2.5)a,b,d | 55.6 (± 2.7) | 28.2 (± 2.3)a,b,¶ | 57.9 (± 2.2) | 28.5 (± 2.2)a,b,†† | 61.6 (± 2.3) |
| d. Unknown | 30.4 (± 3.4)a,c | 50.1 (± 3.0) | 28.5 (± 2.7)a | 52.3 (± 2.5) | 31.0 (± 2.4)a | 58.0 (± 2.5) |
| Number of children in household | ||||||
| a. 1 | 28.5 (± 2.2)b,c | 46.3 (± 1.7) | 26.0 (± 1.5)b,c | 53.0 (± 1.6) | 26.3 (± 1.4)b,c | 54.3 (± 1.4) |
| b. 2 - 3 | 32.9 (± 1.5)a | 50.6 (± 1.3) | 33.9 (± 1.2)a | 55.8 (± 1.2) | 35.5 (± 1.1)a,†† | 59.1 (± 1.0) |
| c. ≥ 4 | 35.4 (± 3.6)a | 46.5 (± 3.3) | 34.7 (± 3.5)a | 51.2 (± 2.9) | 35.1 (± 3.0)a | 55.0 (± 3.2) |
| Urban-rural residence | ||||||
| a. Urban (MSA, principle city) | 30.9 (± 2.0) | 52.7 (± 1.9) | 31.5 (± 1.7) | 57.6 (± 1.6) | 32.1 (± 1.8) | 61.0 (± 1.7) |
| b. Suburban (MSA, not principle city) | 33.4 (± 1.7) | 48.4 (± 1.4) | 32.7 (± 1.4) | 54.0 (± 1.3) | 34.3 (± 1.2)c | 57.4 (± 1.1) |
| c. Rural (non-MSA) | 31.0 (± 2.4) | 43.9 (± 1.8) | 31.4 (± 2.1) | 50.3 (± 1.8) | 31.6 (± 2.1)b | 51.0 (± 1.8) |
| Region of residence | ||||||
| a. Northeast | 26.1 (± 1.9)b,c,d | 54.5 (± 1.9) | 27.5 (± 1.8)b,c,d | 63.7 (± 1.8) | 30.2 (± 1.5)b,c,‖,†† | 63.6 (± 1.5) |
| b. Midwest | 36.2 (± 2.1)a,d | 45.7 (± 1.7) | 34.1 (± 1.7)a | 51.6 (± 1.5) | 35.3 (± 1.5)a,d | 53.7 (± 1.3) |
| c. South | 34.6 (± 2.0)a,d | 48.7 (± 1.6) | 33.8 (± 1.6)a | 53.2 (± 1.5) | 34.7 (± 1.5)a,d | 56.8 (± 1.3) |
| d. West | 29.9 (± 3.1)a,b,c | 48.8 (± 2.7) | 31.5 (± 2.6)a | 52.8 (± 2.3) | 31.8 (± 2.4)b,c | 57.5 (± 2.4) |
| Vaccination facility type | ||||||
| a. Doctor's office | 33.9 (± 1.4)b,c,e,f | 33.0 (± 1.2)b,c,e,f,g | 34.9 (± 1.1)b,c,e,f,‖ | |||
| b. Hospital | 24.9 (± 5.8)a,e,f | 26.3 (± 4.5)a,e,f | 24.9 (± 4.0)a,e,f | |||
| c. Clinic or health center/other medical | 24.8 (± 2.9)a,d,e,f | 29.8 (± 2.7)a,e,f,g,¶ | 28.1 (± 2.4)a,e,f | |||
| d. Local health department | 36.6 (± 10.8)c,e,f,‡‡ | 28.0 (± 4.9)e,f,g | 30.6 (± 5.4)e,f | |||
| e. Pharmacy or store | 10.9 (± 3.4)a,b,c,d,f,g | 9.2 (± 2.7)a,b,c,d,f,g | 15.3 (± 2.8)a,b,c,d,f,g,‖ | |||
| f. School | 55.5 (± 4.2)a,b,c,d,e,g | 56.2 (± 4.4)a,b,c,d,e,g | 54.2 (± 4.0)a,b,c,d,e,g | |||
| g. Other non-medical/work | 27.4 (± 9.0)e,f | 19.9 (± 5.9)a,c,d,e,f | 28.4 (± 8.3)e,f | |||
The proportion of children 2 - 17 years vaccinated against influenza who received LAIV. The proportion of vaccinated children who received inactivated influenza vaccine (IIV) can be calculated by subtracting the proportion who received the nasal spray from 100.
CI = confidence interval half-width.
Influenza vaccination coverage estimates for children 6 months – 17 years and methods have been published previously on FluVaxView (http://www.cdc.gov/flu/fluvaxview/). The same methodology was used to calculate these estimates for children 2 – 17 years, and included the following additional demographic characteristics: mother's education, poverty/annual household income, number of children in household, urban-rural residence, and region of residence.
The presence or absence of superscripted letters denotes whether that estimate was statistically significantly different at P < 0.05 from another row, and denotes which row it differed from (a, b, c, d, e, f, or g), based on pair-wise comparison t-test. For example, the percentage of males (a) who received LAIV (32.0%) was statistically significantly different from the percentage of females (b) who received LAIV (34.7%) in the 2013-14 season; this statistically significant difference is also true with regard to the percentage who received IIV.
There was a significant difference between the proportion of vaccinated children 2 - 17 years who received LAIV in the 2012-13 season as compared to the 2013-14 season.
There was a significant difference between the proportion of vaccinated children 2 - 17 years who received LAIV in the 2011-12 season as compared to the 2012-13 season.
Poverty level was defined based on the reported number of people living in the household and annual household income, and the U.S. Census poverty thresholds.
There was a significant difference between the proportion of vaccinated children 2 - 17 years who received LAIV in the 2011-12 season as compared to the 2013-14 season.
Estimates might not be reliable because the confidence interval half-width is >10.
The results of the multivariable analysis, presented in Table 4, were generally consistent with the bivariate analysis. The strongest association observed among vaccinated children across all three seasons was between vaccination type and child's age, with children 2-8 years (APR 1.41, 1.46, and 1.50 for the 2011-12, 2012-13, and 2013-14 seasons, respectively) and 9-12 years (APR 1.37, 1.38, and 1.50 for the 2011-12, 2012-13, and 2013-14 seasons, respectively) being more likely to have received LAIV than children 13-17 years.
Table 4. Association of receiving live, attenuated influenza vaccine (LAIV) (versus inactivated influenza vaccine (IIV)) with demographic characteristics among children 2 - 17 years vaccinated against influenza, United States, National Immunization Survey-Flu (NIS-Flu), 2011-12 through 2013-14 influenza seasons.
| Characteristics | 2011-2012 | 2012-2013 | 2013-2014 |
|---|---|---|---|
|
| |||
| APR* ± 95% CI† | APR ± 95% CI | APR ± 95% CI | |
| Child's age | |||
| 2 - 8 years | 1.41 (1.27-1.56) | 1.46 (1.34-1.59) | 1.50 (1.38-1.63) |
| 9 - 12 years | 1.37 (1.23-1.54) | 1.38 (1.26-1.51) | 1.50 (1.38-1.63) |
| 13 - 17 years | Referent | Referent | Referent |
| Child's sex | |||
| Male | Referent | Referent | Referent |
| Female | 1.02 (0.95-1.10) | 1.01 (0.96-1.07) | 1.08 (1.02-1.13) |
| Child's race/ethnicity | |||
| White, non-Hispanic | 1.19 (1.05-1.35) | 1.24 (1.10-1.39) | 1.22 (1.11-1.34) |
| Black, non-Hispanic | Referent | Referent | Referent |
| Hispanic | 1.11 (0.96-1.29) | 1.11 (0.96-1.28) | 1.10 (0.98-1.23) |
| Other, non-Hispanic | 1.06 (0.90-1.23) | 1.01 (0.87-1.17) | 0.96 (0.84-1.08) |
| Mother's education | |||
| < High school | 0.97 (0.82-1.15) | 1.07 (0.95-1.22) | 1.02 (0.91-1.15) |
| High school or equivalent | 0.88 (0.78-0.99) | 1.05 (0.95-1.17) | 1.01 (0.92-1.11) |
| Some college | Referent | Referent | Referent |
| ≥ College degree | 1.02 (0.93-1.12) | 1.22 (1.13-1.32) | 1.19 (1.11-1.27) |
| Poverty/annual household income‡ | |||
| Above poverty (> $75,000) | 1.43 (1.23-1.67) | 1.13 (1.02-1.26) | 1.16 (1.06-1.28) |
| Above poverty (≤ $75,000) | 1.33 (1.15-1.53) | 1.06 (0.96-1.18) | 1.07 (0.97-1.17) |
| At or below poverty | Referent | Referent | Referent |
| Unknown | 1.37 (1.15-1.63) | 1.03 (0.90-1.18) | 1.05 (0.93-1.18) |
| Number of children in household | |||
| 1 | Referent | Referent | Referent |
| 2 - 3 | 1.10 (1.01-1.20) | 1.24 (1.15-1.32) | 1.23 (1.16-1.31) |
| ≥ 4 | 1.29 (1.14-1.46) | 1.34 (1.19-1.51) | 1.31 (1.19-1.44) |
| Urban-rural residence | |||
| Urban (MSA, principle city) | 1.08 (0.97-1.20) | 1.00 (0.92-1.09) | 1.07 (0.98-1.17) |
| Suburban (MSA, not principle city) | 1.08 (0.98-1.20) | 1.00 (0.92-1.08) | 1.07 (0.99-1.16) |
| Rural (non-MSA) | Referent | Referent | Referent |
| Region of residence | |||
| Northeast | Referent | Referent | Referent |
| Midwest | 1.35 (1.23-1.48) | 1.20 (1.11-1.31) | 1.17 (1.10-1.26) |
| South | 1.36 (1.24-1.49) | 1.25 (1.16-1.36) | 1.20 (1.12-1.28) |
| West | 1.16 (1.03-1.32) | 1.15 (1.03-1.28) | 1.12 (1.03-1.22) |
| Vaccination facility type§ | |||
| Medical | Referent | Referent | Referent |
| Non-medical | 1.16 (1.04-1.29) | 1.03 (0.95-1.12) | 1.06 (0.99-1.14) |
APR = Adjusted Prevalence Ratio. Estimates in bold are statistically significantly different from the referent (P < 0.05). All variables listed in the table were included in the model.
CI = Confidence Interval.
Poverty level was defined based on the reported number of people living in the household and annual household income, and the U.S. Census poverty thresholds.
Place of vaccination was collapsed into two categories for the model: 1. Medical (doctor's office, hospital, clinic or health center/other medical), 2. Non-medical (local health department, pharmacy or store, school, or other non-medical/work).
Among children who received two doses of influenza vaccination, we found that vaccination type remained consistent (IIV for both doses or LAIV for both doses) in the majority of these children (85.3%, 85.2%, and 82.4% for the 2011-12, 2012-13, and 2013-14 seasons, respectively). The percentages of children who received IIV for the first dose and LAIV for the second dose were 6.7%, 8.2%, and 9.3%, while the percentages of children who received LAIV for the first dose and IIV for the second dose were 7.9%, 6.6%, and 8.3% for the three seasons, respectively.
In the comparison of provider versus parent reported type of influenza vaccination for the 2012-13 season, the percentage of vaccinated children 24-40 months (NIS) receiving LAIV was 17% by provider report and 22% by parent report. Based on NIS-Teen data, 28.4% and 29.0% of vaccinated adolescents 13-17 years were reported to have received LAIV by provider versus parent report, respectively.
Discussion
This is the first study, to our knowledge, to estimate the proportion of influenza vaccination type received (LAIV vs. IIV) by children using a national sample. We found that 33.3% of children 2-17 years vaccinated against influenza received LAIV during the 2013-14 influenza season, and that this proportion remained similar across all seasons studied. Although not representative of all children in the United States, a study of outpatient pediatric offices across the country, who provide influenza vaccination, also demonstrated that approximately 30% of vaccinated children received LAIV, during the 2008-2009 season [32]. In another study of immunization information systems (IIS) of 6 sentinel sites, which contain approximately 10% of the U.S. population of children 2-12 years, exclusive use of LAIV among vaccinated children was reported to be 38.0% for children 2-8 years during the 2013-14 influenza season, as compared to 36.5% of vaccinated children 2-8 years who were found to have received LAIV in this study [33].
As is typically seen with overall seasonal vaccination coverage estimates, we found that the proportion of vaccine type received varied considerably by state [29-31]. The estimated coverage rate for LAIV and IIV can be calculated for each state by multiplying the proportion of vaccinated children who received LAIV or IIV by the overall proportion of children who received an influenza vaccination. For example, the proportion of vaccinated children 2-17 years who received LAIV during the 2013-14 season in North Dakota was 48.4%, and the proportion of children who were vaccinated against influenza was 61.8%. Therefore, the estimated coverage rate for LAIV in North Dakota was 29.9%; conversely, the estimated coverage rate for IIV was 31.8%. These LAIV and IIV coverage estimates are very different from those for South Dakota, 13.9% and 53.7%, respectively.
The strongest association observed in the study was between vaccine type and child's age. Younger children, 2-8 years and 9-12 years, were more likely to have received LAIV than older children, 13-17 years. It is likely that children prefer the nasal spray over receiving a shot. One study that utilized an Internet panel survey of children 8-12 years found that the majority of children would choose the nasal spray over a shot due to reasons such as an expectation of limited discomfort from the nasal spray, not liking shots, and the perception that the nasal spray is easier than a shot [34]. It could be assumed that younger children would also prefer the nasal spray to a shot, possibly even more. As children get older, they likely become less fearful and more tolerant of receiving shots. It's possible that providers may be more likely to offer LAIV to younger children, as opposed to adolescents, because of this. In addition, as mentioned previously, some earlier studies found LAIV to be superior to IIV for younger children, which may have influenced provider recommendation [17-20].
Another finding from our study was that children living in households with higher incomes (>$75,000) were more likely to have received LAIV than those living at or below poverty. This could be due to the higher cost of LAIV [35]. However, LAIV is available through the Vaccines for Children Program (VFC), a federally funded program that provides vaccines to children who might otherwise not be vaccinated due to inability to pay [36]. In addition, the Affordable Care Act (ACA) helps make preventive services, including routinely recommended immunizations, affordable and accessible for all Americans by requiring private health plans to cover and eliminate cost sharing for these services when given by an in-network provider [37].
We also found that white children were more likely to have received LAIV than black children. Our study did not address the reasons why a particular type of vaccine was selected. Although conjecture, it is possible that providers who serve a higher proportion of black children or low income children may be less likely to recommend and offer LAIV to their patients, and this could be due to a variety of reasons such as the cost of LAIV, availability of LAIV, storage capacity, the overall proportion of their patient population who are eligible to receive LAIV, and provider beliefs and attitudes regarding LAIV. It is also possible that parents of black or low income children may be less likely to choose LAIV due to safety concerns and a lack of knowledge regarding efficacy. A separate study is needed to verify these speculations. It is important for all providers, when possible, to offer a variety of influenza vaccines to help increase vaccination coverage. Furthermore, it is important for providers to effectively communicate with their patients regarding the safety and efficacy of LAIV and other influenza vaccines. Future studies are needed to address racial and socioeconomic differences in LAIV vaccination coverage.
With regard to place of vaccination, we found that the majority of children received their influenza vaccination at their doctor's office, and that 34.9% of those children received LAIV, similar to the overall proportion of vaccinated children (33.3%). One strategy known to increase influenza vaccination coverage, in general, is to expand access through the use of non-traditional settings for vaccination, such as pharmacies and school venues, to reach individuals who may not visit a traditional physician's office during the influenza season [38]. We found the highest proportion of children to receive LAIV was among those who received their vaccine in a school setting (54.2%), but only 5.4% of children were vaccinated at a school. A low proportion of children were found to receive LAIV at a pharmacy (15.3%), and only 4.6% of vaccinated children received their vaccination at a pharmacy. It is important for places where children are commonly vaccinated, and for non-traditional settings such as pharmacies, to stock a variety of vaccine (LAIV and IIV) so that they can receive the most optimal or desirable vaccination.
This study is subject to the following limitations. First, type of influenza vaccination received was based on parental report, not validated with medical records, and, thus, is subject to recall bias. However, upon comparison, we found similar prevalence of LAIV based on provider and household reported data, with parental report possibly overestimating the share of vaccinations that were LAIV among the youngest children. Second, the NIS-Flu is a telephone survey and selection and non-response bias is possible and may remain even after weighting adjustments designed to reduce these types of bias. Third, we assessed influenza vaccination type for the first dose of vaccine administered, but some children received more than one dose. However, we found that the majority of children who received more than one dose of influenza vaccine received the same type of vaccination. Fourth, the NIS-Flu does not capture whether children have high risk conditions which would preclude them from receiving LAIV, and so we could not control for this in the model. This may partially explain the difference by age in the proportion of LAIV uptake, as older children are more likely to be diagnosed with chronic conditions, such as asthma [39].
The results of this study can be used to estimate LAIV use for children in the United States. The results of this study also provide baseline data that can be used to measure the impact of relevant public health policy. In addition, they can inform policy makers and other stakeholders of the socio-demographic characteristics associated with influenza vaccination type received by children, important in the development and tailoring of programs and messaging that will help to ensure that children receive the most optimal protection against influenza disease.
Abbreviations
- ACIP
Advisory Committee on Immunization Practices
- IIV
Inactivated Influenza Vaccine
- LAIV
Live, Attenuated Influenza Vaccine
- NIS-Flu
National Immunization Survey-Flu
- NIS
National Immunization Survey
- NIS-Teen
National Immunization Survey-Teen
- CASRO
Council of American Survey and Research Organizations
- APR
Adjusted Prevalence Ratio
- CI
Confidence Interval
- IIS
Immunization Information Systems
- VFC
Vaccines for Children
- ACA
Affordable Care Act
- MSA
Metropolitan Statistical Area
Footnotes
Author's contribution: KEK, TAS, and JAS conceived the study, KEK wrote the first draft of the manuscript and lead revisions of all subsequent versions. KEK had access to all data and takes responsibility for their integrity. KEK and YZ performed the statistical analyses. TAS and JAS participated in data interpretation and writing of the manuscript, and advised on the data analysis. All authors have reviewed and approved the submitted version of the manuscript.
Disclosure: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest statement: The authors have no financial relationships relevant to this article.
References
- 1.Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118(6):2409–17. doi: 10.1542/peds.2006-1475. [DOI] [PubMed] [Google Scholar]
- 2.Glezen PF, Couch RB. Interpandemic influenza in the Houston area, 1974--76. N Engl J Med. 1978;298:587–92. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 3.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6):1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 4.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–9. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS, Kioumehr F. The Tecumseh study of respiratory illness. IX. Occurence of influenza in the community, 1966--1971. Am J Epidemiol. 1975;102(6):553–63. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 6.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. Am J Public Health. 1982;72(9):1008–16. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 8.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 9.Thompson WW, Shay DK. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2013-2014. MMWR. 2013 Sep 20;62(RR07):1–43. [PubMed] [Google Scholar]
- 11.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2004 May 28;53(RR06):1–40. [PubMed] [Google Scholar]
- 12.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2006 Jul 28;55(RR-10):1–42. [PubMed] [Google Scholar]
- 13.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR. 2008 Aug 8;57(RR07):1–60. [PubMed] [Google Scholar]
- 14.CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014-15. MMWR. 2014 Aug 15;63(32):691–7. [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Using live, attenuated influenza vaccine for prevention and control of influenza: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2003 Sep 26;52(RR13):1–8. [PubMed] [Google Scholar]
- 16.CDC. Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2--4 years and other FluMist changes for the 2007--08 influenza season. MMWR. 2007 Nov 23;56(46):1217–9. [Google Scholar]
- 17.Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–9. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 18.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 19.Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860–9. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 20.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Trivalent live attenuated intranasal influenza vaccine administered during the 2003-2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics. 2007;120:e553–64. doi: 10.1542/peds.2006-2836. [DOI] [PubMed] [Google Scholar]
- 21.CDC. Advisory Committee on Immunization Practices (ACIP), ACIP meeting information. [accessed Oct 3, 2014]; Last updated September 18, 2014. Available at: http://www.cdc.gov/vaccines/acip/meetings/meetings-info.html.
- 22.CDC. Surveillance of influenza vaccination coverage - United States, 2007-08 through 2011-12 influenza seasons. MMWR. 2013 Oct 25;62(ss04):1–29. [PubMed] [Google Scholar]
- 23.CDC. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2013. MMWR. 2014 Jul 25;63(29):625–33. [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. National, state, and selected local area vaccination coverage among children aged 19-35 months - United States, 2013. MMWR. 2014 Aug 29;63(33):741–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009;124(5):642–51. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith PJ, Battaglia MP, Huggins VJ, et al. Overview of the sampling design and statistical methods used in the National Immunization Survey. Am J Prev Med. 2001;20(4S):17–24. doi: 10.1016/s0749-3797(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 27.Smith PJ, Hoaglin DC, Battaglia MP. Statistical Methodology of the National Immunization Survey, 2994-2002. National Center for Health Statistics; 2005. Report No.: 138. [PubMed] [Google Scholar]
- 28.Frankel LR. The report of the CASRO task force on response rates. In: Wiseman F, editor. Improving data quality in sample surveys. Cambridge, MA: Marketing Science Institute; 1983. [Google Scholar]
- 29.CDC. Flu Vaccination Coverage, United States, 2011-12 Influenza Season. [accessed Jun 17, 2014];2012 Available at: http://www.cdc.gov/flu/fluvaxview/coverage_1112estimates.htm.
- 30.CDC. Flu Vaccination Coverage, United States, 2012-13 Influenza Season. [accessed Jun 17, 2014];2013 Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm.
- 31.CDC. Flu Vaccination Coverage, United States, 2013-14 Influenza Season. [accessed Feb 27, 2015];2014 Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1314estimates.htm.
- 32.Bhatt P, Block SL, Toback SL, Ambrose CS. A prospective observational study of US in-office pediatric influenza vaccination during the 2007 and 2009 influenza seasons: use and factors associated with increased vaccination rates. Clin Pediatr. 2010 doi: 10.1177/0009922810370868. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers LA, Pabst LJ, Chaves SS. Increasing uptake of live attenuated influenza vaccine among children in the United States, 2008–2014. Vaccine. 2015;2015(33):22. doi: 10.1016/j.vaccine.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flood EM, Ryan KJ, Rousculp MD, et al. A survey of children's preferences for influenza vaccine attributes. Vaccine. 2011;29:4334–40. doi: 10.1016/j.vaccine.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Vaccines for children program (VFC), CDC vaccine price list, pediatric influenza vaccine price list. [accessed Apr 13, 2015];2014 Aug 1; Available at: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html#flu.
- 36.CDC. Vaccines for children program (VFC) [accessed Oct 2, 2014];2014 Apr 24; Available at: http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 37.HHS. The Affordable Care Act and Immunization Fact Sheet. [accessed Oct 3, 2014];2012 Jan 20; Available at: http://www.hhs.gov/healthcare/facts/factsheets/2010/09/The-Affordable-Care-Act-and-Immunization.html.
- 38.Murphy PA, Frazee SG, Cantlin JP, Cohen E, Rosan JR, Harshburger DE. Pharmacy provision of influenza vaccinations in medically underserved communities. J Am Pharm Assoc. 2012;52(1):67–70. doi: 10.1331/JAPhA.2012.10070. [DOI] [PubMed] [Google Scholar]
- 39.CDC. Summary Health Statistics for US Children: National Health Interview Survey, 2012. 2013 Report No.: 258. [PubMed] [Google Scholar]