Abstract
Biodegradable polymer/hydroxyapatite (HA) composites are desired for skeletal tissue engineering. When engineered with thermal-responsive shape memory properties, they may be delivered in a minimally invasive temporary shape and subsequently triggered to conform to a tissue defect. Here we report the shape memory properties of thermoplastic amphiphilic poly(D,L-lactic acid-co-ethylene glycol-co-D,L-lactic acid) (PELA, 120 kDa) and HA-PELA composites. These materials can be cold-deformed and stably fixed into temporary shapes at room temperature and undergo rapid shape recovery (< 3 s) at 50 °C. Stable fixation (>99% fixing ratio) of large deformations is achieved at −20 °C. While the shape recovery from tensile deformations slows with higher HA contents, all composites (up to 20 wt% HA) achieve high shape recovery (>90%) upon 10-min equilibration at 50 °C. The permanent shapes of HA-PELA can be reprogramed at 50 °C, and macroporous shape memory scaffolds can be fabricated by rapid prototyping.
Keywords: biodegradable, hydroxyapatite, rapid prototyping, tissue engineering, shape memory polymer
1. Introduction
Shape memory polymers (SMPs) can be programed with a permanent shape, subsequently deformed into a stable temporary shape, and triggered to recover back to their permanent shape. In the case of thermal-responsive SMPs, the recovery trigger is a temperature above the transition temperature (Ttrans), either glass transition temperature (Tg) or melting temperature (Tm), of the polymer. Programming the permanent shape is accomplished by casting the polymer into a mold for thermoset SMPs, or by deforming the polymer at a temperature above the Tm for thermoplastic SMPs. For both thermoset and thermoplastic SMPs, deforming the polymer above the Tg may program the temporary shape. The netpoints (physical entanglement or chemical crosslinks) of a SMP network are responsible for setting the SMPs permanent shape while temperature sensitive switching domains fix the temporary shape.[1] Engineering synthetic biomaterials with shape memory properties has the potential to enable more effective in vivo delivery of “smart” implants or tissue engineering scaffolds. The SMP implant could be delivered in a minimally invasive temporary shape to a tissue defect and subsequently thermally triggered to recover to its pre-programmed permanent shape precisely fitting the defect.
In order for thermal responsive SMPs to be safely applied for biomedical applications, two basic requirements of the SMPs must be met: 1) biocompatibility, and 2) reasonably narrow Ttrans within a safe temperature range (<60°C).[2,3] In a bone environment, for example, exposure to temperatures above 45 °C for one minute or short exposure to temperatures above 70 °C induce necrosis.[3] Therefore, the Ttrans and rate of shape recovery are particularly important to reduce thermal damage to surrounding tissue. In addition, to facilitate clinical translation, versatile and scalable fabrication methods (e.g. a thermoplastic polymer would be more desired than thermoset in terms of the cost and ease of processing), bioactivity tailored for the specific application, physical properties enabling facile surgical handling (hydrophilicity, elasticity), and biodegradability are desired. Biodegradable SMPs have captivated the biomedical research community since they were exploited by Lendlein and Langer in 2001/2002 as resorbable self-tightening sutures.[4,5] A wide variety of SMPs have been since developed with varying mechanical properties, shape memory performance, and bioactivity.[1,6] We have previously shown that a degradable urethane-crosslinked SMP with GPa-glassy state storage modulus at body temperature can achieve stable temporary shape fixing at room or body temperature and full and rapid (<3 s) permanent shape recovery at ~50 °C.[7] This network was composed of polyhedral oligomeric silsesquioxane (POSS)-centered macromers grafted with 8 identical poly(D,L-lactic acid) (PLA) arms. However, while the POSS-PLA SMP is biocompatible, its degradation was shown to result in acute inflammation locally, which could be of a concern if it is used in large quantity in vivo.[8] This immune response is likely elicited by the acidic degradation byproducts of PLA.[9]
Calcium phosphates such as hydroxyapatite (HA), the main mineral component in bone, have been blended with biodegradable polyesters to improve their bioactivity and buffer acidic degradation byproducts.[10–13] This HA/polymer composite strategy can be applied to improve the biological performance of biodegradable SMPs. The shape memory performance of HA-PLA composites has been studied extensively.[14–16] While such composites have shape memory behavior, they tend to exhibit slow permanent shape recovery (e.g. 100 s) even at relatively high triggering temperatures (e.g. 70 °C). Overall, biodegradable polymer/HA composites exhibiting an optimal combination of shape memory properties and biological performance are lacking. In our prior work, we blended high molecular weight (>100,000 Da) poly(D,L-lactic acid-co-ethylene glycol-co-D,L-lactic acid) (PELA) amphiphilic triblock copolymer with HA and electrospun the stable suspension into nano/micro fibrous scaffolds.[17] The HA-PELA scaffolds exhibited significantly improved handling characteristics (elasticity, hydrophilicity) and bioactivity (e.g. ability to support potent osteogenic differentiation of stem cells) than electrospun HA-PLA counterparts. Both the amphiphilic PELA and the incorporation of HA were critical for achieving the ultimate performance of the electrospun scaffolds, with the hydrophilic PEG block of PELA responsible for binding and dispersing the HA while the HA enhancing the osteoconductivity and osteoinductivity of the composite. The shape memory performance of PELA and its HA composites, however, has not been evaluated.
The shape memory behavior of amphiphilic polymers is a result of the phase separation between the hydrophilic and hydrophobic blocks.[18] By design, the phase exhibiting a higher thermal transition may act as the physical crosslinks or hard segments, while the other phase exhibiting the lower thermal transition could act as the switching phase or soft segments.[19] Amphiphilic polymer networks are often crosslinked to achieve sufficient mechanical integrity for shape memory and stability in aqueous solutions. For example, thiol-ene cross-linked poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) foams have been shown to exhibit shape memory properties at biologically relevant temperatures (40–50 °C).[20] The shape memory performance of photo-crosslinked amphiphilic gels composed of PEG and PCL or PEG and poly(L-lactide-co-glycolide) have also been reported.[21,22] In general, photo-polymerized networks are not as amenable to polymer processing approaches such as extrusion or rapid prototyping as thermoplastics. When the amphiphilic polymers are of sufficiently high molecular weight to facilitate physical entanglement and achieve aqueous stability, tough and flexible thermoplastics can be fabricated.[23–27] Gu & Mather reported the shape memory behavior of high molecular weight (Mn>200,000 Da) PCL-PEG thermoplastic polyurethanes.[18] Such materials can be cold-deformed to high strains (e.g. ~1240% strain) by a combination of elastic and plastic deformations at room temperature, which is above the Tg but below the Tm of both PCL and PEG blocks. Upon the release of the external stress, the material immediately recovered its elastic strain (from ~1240% loaded to 800% strain unloaded) while the plastic deformations were fully recovered after 1 min at 70 °C, a temperature above the Tm for both blocks. The shape memory behavior of this system was a result of the physical crosslinks between the high molecular weight polymer chains (entanglements), acting as stable net points even above the Tm, and the recoverable stretching and recoiling of the PCL and PEG phases.
Here we hypothesized that a thermoplastic SMP can be generated with high molecular weight amphiphilic PELA and that HA can be incorporated with PELA while preserving shape memory properties. To test these hypotheses, we fabricated uniform composites of HA and PELA and examined their thermal mechanical properties and reprogrammable shape memory behavior around a physiologically relevant temperature, as a function of the HA content. To further assess their suitability for minimally invasive biomedical uses, the shape memory performance of rapid prototyped HA-PELA scaffolds was also examined.
2. Experimental Section
2.1. Materials
3,6-Dimethyl-1,4-dioxane-2,5-dione (D,L-lactide) was purchased from Sigma–Aldrich (St. Louis, MO), purified by recrystallization twice in anhydrous toluene, and dried under vacuum prior to use. PEG (BioUltra, 20,000 Dalton) was purchased from Fluka (Switzerland). Polycrystalline HA powder was purchased from Alfa Aesar (Ward Hill, MA). All other solvents and reagents were purchased from Sigma–Aldrich (St. Louis, MO) and used as received.
2.2. PELA synthesis and characterization
2.2.1. Synthesis
PELA tri-block copolymer was synthesized by melt ring-opening polymerization as previously described.[17] Briefly, PEG (20,000 Dalton, 4 g, 0.2 mmol) was heated to 100 °C in a Schlenk flask and stirred under vacuum for 1 h to remove residual water. The melt was cooled to room temperature before Sn(II) 2-ethylhexanoate (~95%, 24.18 mg, 0.06 mmol) in anhydrous toluene was introduced by syringe. The toluene was removed by heating the mixture under vacuum at 100 °C for 15 min. The mixture was cooled to room temperature before D,L-lactide (17.295 g, 0.12 mol) was added under argon purge. The melt polymerization proceeded at 130 °C for 5 h under argon. The crude PELA was dissolved in chloroform, purified by precipitation in methanol, and dried under vacuum.
2.2.2. Characterization
The molecular weight and polydispersity of PELA was determined by gel-permeation chromatography on a Varian Prostar HPLC system equipped with two 5 mm PLGel MiniMIX-D columns and a PL-ELS2100 evaporative light scattering detector (Polymer Laboratories). THF was used as an eluent at 0.3 ml/h at room temperature. Molecular weight and polydispersity calculations were calibrated with EasiVial polystyrene standards (Agilent, Santa Clara, CA). 1H NMR spectra were recorded on a Varian Mercury 400 MHz spectrometer at 298 K using CDCl3 containing tetramethylsilane as the solvent.
2.3. PELA and HA-PELA films
2.3.1. Fabrication
Dense PELA and HA-PELA films were prepared by solvent casting. HA (0 to 20% w/w relative to PELA) was bath-sonicated in 4 mL chloroform for 30 min. PELA (1.25 g) was added and the mixture was stirred overnight. The HA-PELA mixture was subsequently poured into Teflon molds. The chloroform was evaporated in a fume hood at room temperature overnight and subsequently in a vacuum oven at 60 °C for 24 h. PELA films were prepared by evaporating a chloroform solution of PELA without HA in the same mold followed by vacuum drying under identical conditions. Films were stored at −20 °C prior to use.
2.3.2. Scanning electron microscopy (SEM)
The bottom surface of the solvent-cast films and their cross-sections were sputter coated with Au (~4 nm thick) and imaged on a Quanta 200 FEG MKII scanning electron microscope (FEI Inc., Hillsboro, OR) under high vacuum at 10 kV.
2.4. Mechanical testing
2.4.1. Tensile modulus / strength
The tensile modulus of PELA and HA-PELA films at room temperature was determined on a MTS Bionix 370 mechanical testing system (MTS Systems Corporation, Minneapolis, MN). ASTM D882-97 guidelines were followed with the exception of the initial grip displacement due to sample size constraints.[28] Specimens (5.3 mm × 35 mm × ~0.2 mm, n=3) were loaded into the MTS machine with an initial grip separation of 10 mm and subjected to a grip separation of 100 mm/min. The resulting force was recorded with a 250 N load cell (Interface, Scottsdale, AZ). The elastic modulus was calculated from the initial linear region of the stress-strain curve (1–5% strain). The initial toe region, if present, was excluded from the modulus calculation.
The tensile modulus at 37 °C was measured with a Q800 Dynamic Mechanical Analyzer (DMA) equipped with a gas cooling accessory (TA Instruments, New Castle, DE). Specimens (5.3 mm × 35 mm × ~0.2 mm) were loaded into a film tension fixture with a grip separation of 10 mm (n=3), equilibrated at 37 °C, held isothermally 10 min, then subjected to a 1-mN pre-load force. Tensile modulus was measured by ramping the specimens at a strain rate of 1 N /min. The modulus was calculated from the initial linear region of the stress strain curve (1–5% strain). The initial toe region, if present, was excluded from the modulus calculation.
2.4.2. Temperature-dependent storage modulus
The tensile storage moduli of PELA and HA-PELA were measured on a Q800 DMA equipped with a gas cooling accessory. Specimens (5.3 mm × 35 mm × ~0.2 mm) were loaded into a film tension fixture and equilibrated at −40 °C for 10 minutes before the temperature was ramped from −40 °C to 70 °C at a heating rate of 2 °C/min. The storage modulus was recorded at a strain amplitude of 0.02% and a frequency of 1 Hz.
2.4.3. Strain-controlled cyclic thermal mechanical testing
Shape memory characterization was performed under tension on a DMA Q800 equipped with a gas cooling accessory. Specimens (5.3 mm × 35 mm × ~0.2 mm) were equilibrated at 50 °C for 5 min and cooled to 25 °C and equilibrated for another 5 min prior to testing. The specimens were subjected to a 50% strain, and cooled at 2 °C/min to −20 °C while the constant strain of 50% was maintained. This yielded the elongated temporary shape εl. After being held at −20 °C for 5 min, the force on the sample was released to 0.01N and the resulting strain was recorded as the unloaded temporary shape εu. Shape recovery was triggered by heating the specimens at a rate of 2 °C/min to 50 °C and holding at 50 °C for 10 min. The recovered sample strain was recorded as εp. Each specimen was subjected to 3 consecutive cycles of testing. The second cycle was used to calculate the strain fixing ratio (Rf) and the strain recovery ratio (Rr) reported in Table 1. The Rf and Rr in a given cycle N were determined by using the following formulas:
| (1) |
| (2) |
Table 1.
Shape memory properties of HA-PELA films with varying HA contents determined from strain-controlled cyclic thermal mechanical testing.
| HA content (wt%) | Shape fixing ratio (Rf) | Recovery ratio at 50 °C (Rr) (no hold) | Recovery ratio (Rr) after 10 min hold at 50 °C |
|---|---|---|---|
| 0 | 99.3% | 85.3% | 95.6% |
| 5 | 99.4% | 74.2% | 94.2% |
| 10 | 99.4% | 75.8% | 94.0% |
| 20 | 99.4% | 72.4% | 90.6% |
2.4.4. Stress-controlled cyclic thermal mechanical testing
Specimens (5.3 mm × 35 mm × ~0.2 mm) were equilibrated at 50 °C for 5 min and cooled to 25 °C prior to testing. After being equilibrated at 25 °C for 5 min, the specimens were subjected to a 0.8 MPa tensile stress, and cooled at 2 °C/min to −20 °C while the constant stress was maintained. This yielded the elongated temporary shape under stress εl. After being held at −20 °C for 5 min, the stress was released to a 1-mN pre-load force. The resulting strain was recorded as the unloaded temporary shape εu. Shape recovery was triggered by heating the specimens at a rate of 2 °C/min to 50 °C and holding at 50 °C for 5 min. The recovered sample strain was recorded as εp. Each specimen was subjected to 4 consecutive cycles of testing. The Rf and Rr in a given cycle N were determined using formulas (1) and (2).
2.5. Demonstration of reprogrammable shape memory
A flat bar (5.3 mm × 35 mm × 0.2 mm) of HA-PELA (20 wt% HA) was deformed into a temporary spiral shape at room temperature. Shape recovery (to permanent flat bar) was initiated by submerging the specimen in 50 °C deionized water (SI video 1). In order to reprogram the permanent shape into a spiral, the specimen was submerged in 50 °C deionized water and deformed into a spiral shape. The specimen was then allowed to cool to room temperature while the spiral shape was fixed. Subsequently, the specimen was deformed into a temporary flat bar shape at room temperature. Shape recovery (to reprogrammed permanent spiral) was initiated by submerging the specimen in 50 °C deionized water (SI video 2).
2.6. Demonstration of three-dimensional (3-D) rapid prototyped scaffold with shape memory behavior
2.6.1. Scaffold fabrication
A 3-D CAD model of a 16 mm × 16 mm × 2.4 mm square prism composed of a staggered arrangement of 0.4 mm lines was designed in 3-Matics (Materialise, Belgium, Fig. 6A) and converted into g-code instructions by MakerWare (MakerBot Industries, Brooklyn, New York). A MakerBot® Replicator™ 2X 3-D printer (MakerBot Industries, Brooklyn, New York) was used to fabricate the 3-D macroporous HA-PELA scaffolds (25 wt% HA) based on the CAD design, as we previously described.[29] A biopsy punch was used to core a 5-mm diameter specimen from the rapid prototyped prism.
Figure 6.

Shape memory properties of a rapid prototyped macroporous cylindrical HA-PELA (25 wt% HA) scaffold. (A) CAD image (left) and stereomicroscopy image (right) of the rapid prototyped HA-PELA scaffold. (B) Cold-pressing and fixation of the HA-PELA scaffold into a collapsed disc at room temperature. (C) Rapid shape recovery of the collapsed disc into the original cylindrical shape in a 50 °C water bath. Scale bars = 3 mm.
2.6.2. Shape memory demonstration
The rapid prototyped HA-PELA scaffold was deformed at room temperature by manual compression into a collapsed cylinder shape. Recovery of the permanent expanded shape was triggered by submerging the scaffold in 50 °C deionized water.
3. Results and Discussion
3.1. Characterization of PELA and HA-PELA composites
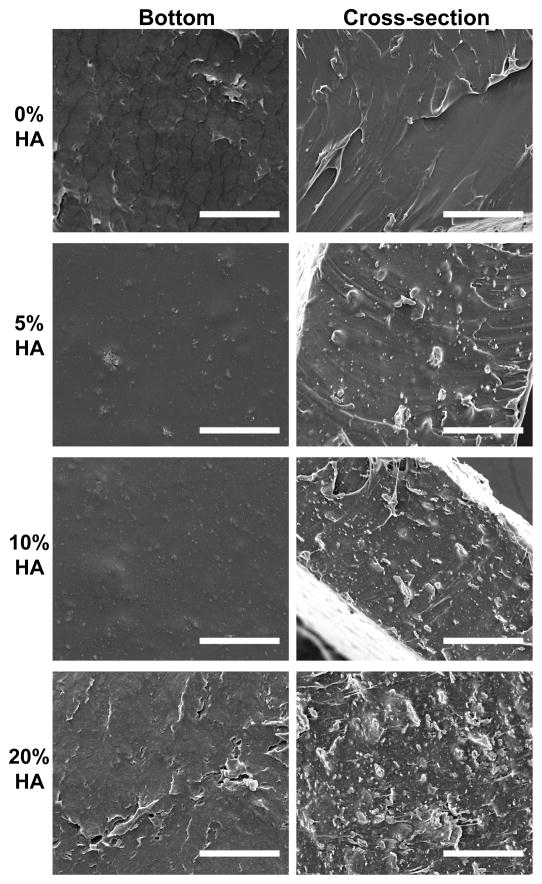
GPC and NMR characterizations confirmed the synthesis of high molecular weight PELA (Mn = 120,000 Da; Mw = 190,000 Da; Mw/Mn = 1.56; 1H NMR (400 MHz, CDCl3) δ 5.19 (m, 1126H), 3.65 (m, 1816H), 1.5 (m, 3921H) ppm.) We blended the PELA with various concentrations of HA (0 to 20 wt%), and solvent cast the composites into films. The favorable interaction between the hydrophilic PEG domain and hydrophilic HA mineral helped maintain the stable and uniform HA dispersion during the solvent casting procedure.[30] This was evidenced by the minimal settling of HA to the bottom face of the films (Figure 1A), and the uniformly distributed HA across the thickness of the films (Figure 1B) as revealed by SEM micrographs of the cross-sections.
Figure 1.
Scanning electron micrographs of the bottom surface (left) and cross-sections (right) of solvent-cast PELA films with 0–20 wt% of HA. Scale bars = 50 μm.
We examined the mechanical properties of the PELA and HA-PELA films by tensile testing. We aimed to test the films to failure under tension by following the ASTM D882-97 guidelines for thin plastic sheeting.[28] However, all films were highly elastic and either could not break within the limits of the MTS testing machine (~1000% strain) or broke at the grips. There was no significant difference in the elastic modulus of the films with lower HA contents (<10 wt% HA), however, the elastic modulus of the 20 wt% HA composite was significantly higher than all other groups (Figure 2A). We also examined the elastic modulus of the films at 37 °C to estimate their mechanical properties in vivo (Figure 2B). These films could not be strained to failure on the DMA (> 100% strain) at 37 °C, and their moduli were approximately an order of magnitude lower at 37 °C than those at 25 °C. The reinforcing effect of the structurally incorporated HA on the elastic modulus of the amphiphilic composites persisted at 37 °C, with the 20 wt% HA composite exhibiting significantly higher modulus.
Figure 2.
Elastic moduli (n=3) of PELA films with 0–20 wt% of HA at (A) 25 °C or (B) 37 °C. Specimens (5.3 mm × 35 mm × ~0.2 mm) were ramped at 100 mm/min (25 °C) on an MTS mechanical testing system or at 1 N/min (37 °C) on a Q800 dynamic mechanical analyzer. * p < 0.05 (One way ANOVA with Tukey post-hoc).
3.2. Thermal mechanical properties of PELA and HA-PELA composites
We examined the storage moduli of PELA and HA-PELA composites as a function of temperature to determine the suitable temperature range for programming shape memory. The storage modulus of PELA was expected to drop around its Tg (~ 19 °C, as we previously determined by differential scanning calorimetry).[17] In agreement with the tensile strength results, the initial glassy state GPa-storage moduli (−40 °C) of the HA-PELA composites increased with increasing HA content (Figure 3). The storage moduli dropped by an order of magnitude around 19 °C, making it possible to deform the composites into temporary shapes at room temperature. This feature could allow the surgeon to deform the PELA or HA-PELA implant into a temporary shape desired for minimally invasive implantation at the surgical table without heating. Above 40 °C, the storage moduli descended into an elastic state plateau, supporting the feasibility of triggering shape recovery at this temperature. The storage modulus at 50 °C increased with the addition of HA (10 and 20 wt%), indicating that the reinforcing effect of HA was maintained at elevated temperatures. This reinforcement is likely a result of the HA particles limiting polymer chain motion.[31,32]
Figure 3.
Temperature-dependent storage moduli of PELA films with 0–20 wt% of HA. Specimens (5.3 mm × 35 mm × ~0.2 mm) were subjected to 0.02% strain at a frequency of 1 Hz while temperature was ramped at 2 °C/min on a Q800 dynamic mechanical analyzer.
3.3. Shape memory performance of PELA and HA-PELA composites
Cyclic thermal mechanical testing was used to quantitatively assess the shape memory properties of PELA and HA-PELA composites. Prior to testing, we heated the films to 50 °C, and cooled them back to 25 °C to remove any potential thermal memory. Cyclic testing was performed under both strain-controlled (Figure 4A) and stress-controlled conditions (Figure 4B). The strain-controlled testing allows for a fair comparison of the shape memory behaviors among the samples with varying HA compositions by subjecting them to the same tensile strain. All calculations of shape fixation ratios and shape recovery ratios were based on the second cycle of strain-controlled testing (Table 1). The temporary shape of the films was programed by deforming the specimens at 25 °C followed by cooling to −20 °C to fix the shape. Under these programing conditions, all films exhibited a shape fixation ratio (Rf) of >99% regardless of the HA content (Table 1). Stable fixation of a substantially strained (50% stain) temporary shape could not be accomplished at room temperature, possibly due to the elastic recovery of the strained polymer chains above their Tg (~19 °C). Shape recovery was initiated by heating the films to 50 °C. While incorporation of 20% HA reduced the recovery ratio of PELA by ~5%, all films recovered to >90% of their initial strain within 10 min at 50 °C. The incorporation of 5% and 10% HA had a negligible effect on the recovery ratio (~1–2%). However, HA incorporation reduced the rate of shape recovery as evident by the lower recovery ratio prior to the 10-min hold at 50 °C. This is likely due to the HA particles slowing the movements of the polymer chains, as previously observed for HA/PLA composites.[14]
Figure 4.
Shape memory behavior determined by (A) strain-controlled and (B) stress-controlled cyclic thermal mechanical testing of PELA films with 0–20 wt% of HA. Three consecutive cycles for each specimen (5.3 mm × 35 mm × ~0.2 mm) are shown.
The cyclic testing under the stress-controlled mode takes into account the stiffening effect of the incorporated HA. The decreasing tensile strains achieved under the constant stress as a function of increasing HA content (Figure 4B) suggest that the polymer chain movement was restricted in the presence of HA. The shape fixation and recovery ratios of 20 wt% HA-PELA determined from strain-controlled (50% strain) and stress-controlled (~18% strain) testing were similar. The shape recovery ratio determined by the latter prior to and after a 5-min hold at 50 °C was 61.8% and 90.6%, respectively. This observation supports that good shape memory properties of PELA were largely retained despite the incorporation of HA content as high as 20 wt%. Taken together, the cyclic thermal mechanical testing shows that both PELA and HA-PELA films exhibited high shape fixation (>99%) and recovery ratios (>90%). For applications such as bone tissue engineering where the incorporation of osteoconductive HA is desired, up to 20 wt% HA can be blended with PELA while maintaining the shape memory behavior. However, to achieve optimal shape memory properties, the incorporation of < 10 wt% HA should be considered.
3.4. Demonstration of reprogrammable shape memory
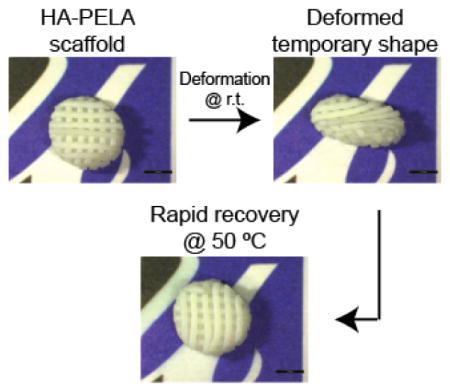
The high molecular weight of PELA enables the formation of physical netpoints within the polymer network, obviating the need for chemical crosslinking and permitting thermoplastic shape memory properties. The permanent shape of thermoplastic SMPs can be reprogrammed at elevated temperatures (>Tm).[5,33] This is advantageous because the permanent shape can be programed after polymer processing/formation rather than being limited to the shape of the mold, as the case for thermoset SMPs. This can enable reuse/recycling of the SMP and fine-tuning of the shapes based on end-users’ requirements. We demonstrated the ability to reprogram the permanent shape of HA-PELA (20 wt% HA) (Figure 5). The HA-PELA was first solvent cast into a flat bar shape, which defined its initial permanent shape. Cold-deforming the film into a spiral and fixing at room temperature programmed the temporary shape. Submerging the HA-PELA in 50 °C water triggered rapid recovery (< 3 s) back to the permanent flat bar shape (Figure 5A, SI Video 1). To reprogram the permanent shape, the flat bar of HA-PELA was submerged in 50 °C water and deformed into a spiral (Figure 5B, SI Video 2). The reprogrammed spiral shape was fixed upon cooling to room temperature. A stable temporary flat bar shape was programed by simply unrolling the spiral at room temperature. Submerging the flat bar in 50 °C water triggered rapid recovery (<3 s) to the reprogramed permanent spiral shape. Of note, while we needed to cool the samples in order to maintain the strained temporary shape during cyclic stress-controlled testing, the spiral or flat bar temporary shapes, without extensive tensile deformations, could be stably fixed at room temperature for over 24 h. This difference in shape fixation may be due to the varying amounts of plastic/elastic deformation between stretching the specimen under high strains vs. the spiral wrapping. When wrapping the PELA or HA-PELA specimen into a spiral or unwrapping a spiral into a flat bar, the polymer chains were only exposed to relatively low strains, thus allowing for the lower energy deformations be adequately fixed at room temperature. When straining the samples under tension, the relatively low level of PEG (~ 15 wt%) in PELA was insufficient to introduce enough crystallinity to prevent the release of stored energy within the polymer network and the spontaneous shape recovery at room temperature. In previously reported PCL-PEG composites, the semi-crystalline PCL combined with the higher content of crystalline PEG (>30 wt%) was sufficient to prevent complete elastic recovery at room temperature.[18]
Figure 5.

Reprogrammable shape memory of HA-PELA films (20 wt% HA). (A) Cold-deformation and fixation into a temporary spiral at room temperature (r.t.) and rapid shape recovery to permanent flat bar shape (as cast) at 50 °C. (B) Reprogramming the flat bar into a permanent spiral shape at 50 °C, cold-deformation and fixation into a temporary flat bar at r.t., and subsequent rapid recovery back to reprogrammed permanent spiral.
3.5. Shape memory rapid-prototyped macroporous HA-PELA scaffolds
Taking advantage of the thermoplastic nature of HA-PELA, we also prepared 3-D macroporous scaffolds by rapid prototyping. Tissue engineering scaffolds composed of a biodegradable SMP, delivered in a minimally invasive fashion to the defect site, could potentially minimize surgical morbidity. Here we show that the shape memory properties observed with the dense HA-PELA film are retained with the rapid prototyped 3-D scaffold (Figure 6). The macroporous HA-PELA cylindrical scaffolds were readily manually deformed into a compressed cylindrical shape at room temperature, and could rapidly (~ 3 s) recover to their original shape at 50 °C. Techniques such as solvent-casting/particulate leaching, [20,34] thermal induced phase separation, [35] and gas foaming[36] have been used to fabricate macroporous shape memory materials for tissue engineering applications. Comparing to these techniques, rapid prototyping offers more precise control over pore size, pore interconnectivity, and scaffold shape. To our knowledge, this is the first report of a rapid prototyped biodegradable polymer-mineral composite scaffold exhibiting thermal responsive shape memory behavior.
4. Conclusions
We demonstrated the shape memory behavior of uncrosslinked amphiphilic biodegradable thermoplastic polymer PELA and HA-PELA composites around physiologically relevant temperatures. Both PELA and HA-PELA composites were highly elastic and could be readily deformed at room temperature. Temporary shapes with small strain deformations could be stably fixed at room temperature while extensive tensile deformations required lower temperatures to fix. Both PELA and HA-PELA were able to rapidly recover their permanent shapes at a safe triggering temperature of 50 °C, around the Tm of the PEG component. Furthermore, the permanent shape of these thermoplastic materials could be readily reprogrammed at 50 °C, owing to the physical crosslinks within the high molecular weight PELA network. We demonstrated that the incorporation of HA (up to 10 wt% HA) had a minimal impact on the shape memory performance of PELA (e.g. no change in the nearly quantitative shape fixing ratio; a slight decrease in recovery ratio from 95.6% to 94.0–94.2%). Higher HA content (20 wt%) increased the tensile modulus of the composites while preserving good shape memory properties (99.4% shape fixation, 90.6% shape recovery). Finally, we showed that the attractive shape memory behavior of HA-PELA was retained with macroporous scaffolds fabricated by rapid prototyping. The shape memory properties demonstrated with the HA-PELA composites, combined with their previously established osteoconductivity and osteoinductivity, make them uniquely suited for guided skeletal tissue regeneration where the safe delivery and precise fitting of the scaffold within a complex defect is desired.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01GM088678 and a University of Massachusetts Commercial Ventures & Intellectual Property Technology Award. Core resources supported by the National Center for Research Resources Grant S10RR021043 were used.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.Behl M, Razzaq MY, Lendlein A. Adv Mater. 2010;22:3388. doi: 10.1002/adma.200904447. [DOI] [PubMed] [Google Scholar]
- 2.Rem AI, Oosterhuis JA, Journée-de Korver HG, van den Berg TJ, Keunen JE. Exp Eye Res. 2001;72:153. doi: 10.1006/exer.2000.0939. [DOI] [PubMed] [Google Scholar]
- 3.Berman AT, Reid JS, Yanicko DR, Sih GC, Zimmerman MR. Clin Orthop Relat Res. 1984;186:284. [PubMed] [Google Scholar]
- 4.Lendlein A, Schmidt AM, Langer R. Proc Natl Acad Sci U S A. 2001;98:842. doi: 10.1073/pnas.031571398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lendlein A, Langer R. Science. 2002;296:1673. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 6.Madbouly SA, Lendlein A. Adv Polym Sci. 2010;41 doi: 10.1007/12_2009_28. [DOI] [Google Scholar]
- 7.Xu J, Song J. Proc Natl Acad Sci U S A. 2010;107:7652. doi: 10.1073/pnas.0912481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filion TM, Xu J, Prasad ML, Song J. Biomaterials. 2011;32:985. doi: 10.1016/j.biomaterials.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suganuma J, Alexander H. J Appl Biomater. 1993;4:13. [Google Scholar]
- 10.Agrawal CM, Athanasiou KA. J Biomed Mater Res. 1997;38:105. doi: 10.1002/(sici)1097-4636(199722)38:2<105::aid-jbm4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Lin G, Cosimbescu L, Karin NJ, Tarasevich BJ. Biomed Mater. 2012;7:024107. doi: 10.1088/1748-6041/7/2/024107. [DOI] [PubMed] [Google Scholar]
- 12.Schiller C, Epple M. Biomaterials. 2003;24:2037. doi: 10.1016/s0142-9612(02)00634-8. [DOI] [PubMed] [Google Scholar]
- 13.Ji W, et al. Biomaterials. 2012;33:6604. doi: 10.1016/j.biomaterials.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, Zhou S, Li X, Weng J. Biomaterials. 2006;27:4288. doi: 10.1016/j.biomaterials.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, et al. J Biomed Mater Res B Appl Biomater. 2008;86:170. doi: 10.1002/jbm.b.31003. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, et al. Chem Mater. 2007;19:247. [Google Scholar]
- 17.Kutikov AB, Song J. Acta Biomater. 2013;9:8354. doi: 10.1016/j.actbio.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, Mather PT. Polymer. 2012;53:5924. [Google Scholar]
- 19.Lendlein A, Kelch S. Angew Chemie Int Ed. 2002;41:2034. [PubMed] [Google Scholar]
- 20.Baker RM, Henderson JH, Mather PT. J Mater Chem B. 2013;1:4916. doi: 10.1039/c3tb20810a. [DOI] [PubMed] [Google Scholar]
- 21.Nagata M, Kitazima I. Colloid Polym Sci. 2005;284:380. [Google Scholar]
- 22.Feng Y, Zhang S, Zhang L, Guo J, Xu Y. Polym Adv Technol. 2011;22:2430. [Google Scholar]
- 23.Bae YH, Huh KM, Kim Y, Park K. J Control Release. 2000;64:3. doi: 10.1016/s0168-3659(99)00126-1. [DOI] [PubMed] [Google Scholar]
- 24.Cohn D, Hotovely-Salomon A. Polymer. 2005;46:2068. [Google Scholar]
- 25.Cohn D, Younes H. J Biomed Mater Res. 1988;22:993. doi: 10.1002/jbm.820221104. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Rashkov I, Espartero J, Vert M, Manolova N. Macromolecules. 1996;29:50. [Google Scholar]
- 27.Saito N, Okada T, Toba S, Miyamoto S, Takaoka K. J Biomed Mater Res. 1999;47:104. doi: 10.1002/(sici)1097-4636(199910)47:1<104::aid-jbm15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 28.ASTM Standard D882-97. ASTM International; 1998. [Google Scholar]
- 29.Kutikov A, Gurijala A, Song J. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.TEC.2014.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Supová M. J Mater Sci Mater Med. 2009;20:1201. doi: 10.1007/s10856-009-3696-2. [DOI] [PubMed] [Google Scholar]
- 31.Nazhat SN, et al. J Mater Sci Mater Med. 2000;11:621. doi: 10.1023/a:1008957729512. [DOI] [PubMed] [Google Scholar]
- 32.Kalfus J, Jancar J. Compos Sci Technol. 2008;68:3444. [Google Scholar]
- 33.Wischke C, Schossig M, Lendlein A. Small. 2014;10:83. doi: 10.1002/smll.201202213. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Burkes WL, Schoener Ca, Grunlan Ma. Polymer. 2012;53:2935. doi: 10.1016/j.polymer.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauter T, Kratz K, Lendlein A. Macromol Chem Phys. 2013;214:1184. [Google Scholar]
- 36.Singhal P, et al. J Polym Sci B Polym Phys. 2012;50:724. doi: 10.1002/polb.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.