Abstract
Organisms must be able to rapidly alter gene expression in response to changes in their nutrient environment. This review summarizes evidence that epigenetic modifications of chromatin depend on particular metabolites of intermediary metabolism, enabling the facile regulation of gene expression in tune with metabolic state. Nutritional or dietary control of chromatin is an often-overlooked, yet fundamental regulatory mechanism directly linked to human physiology. Nutrient-sensitive epigenetic marks are dynamic, suggesting rapid turnover, and may have functions beyond the regulation of gene transcription, including pH regulation and as carbon sources in cancer cells.
Rapid changes in gene expression in response to nutrient shifts
Nutritional influences on chromatin and gene regulation are readily observed in single-celled eukaryotes such as the budding yeast, which frequently encounter a wide variety of growth environments. Changes in nutrient availability promptly impact the expression of genes that regulate cell growth or survival. For example, glucose repletion to a starved yeast culture was found to rapidly induce massive changes in gene expression on a global scale [1,2]. Studies of yeast chemostat cultures growing under various nutrient limitations also revealed rapid changes in gene transcript levels, with certain groups of genes correlating either positively or negatively with growth rate [3–6]. These adjustments in mRNA abundance occurred very quickly in response to changes in growth rate. Moreover, continuous yeast cultures can also exhibit robust oscillations in oxygen consumption that are accompanied by periodic changes in transcript levels of the majority of genes. These oscillations had periods as short as 40 min [7], or on the time scale of hours [8]. In each scenario, the dynamic regulation of gene expression involves metabolic and nutritional influences on chromatin and chromatin-associated processes [9,10].
How can changes in the nutrient environment influence gene transcription so rapidly? Here we discuss emerging evidence that particular histone modifications depend on metabolites, either as cofactors or substrates, providing mechanisms by which fluctuating levels of specific metabolites directly and rapidly influence gene activity. As such, these metabolites may be viewed as ‘gatekeepers of chromatin’, enabling modulation of the chromatin landscape in response to key nutritional cues [11].
Dynamic histone acetylation and deacetylation
Histones are acetylated by a group of enzymes called histone acetyltransferases (HATs), which use acetyl-CoA as the acetyl donor. These enzymes are also known as lysine acetyltransferases (KATs) since they can also modify other (non-histone) proteins. Acetylation of the ε-amino group of histone tail lysyl residues neutralizes their positive charge and promotes a more ‘relaxed’ chromatin structure, in which DNA is more accessible for binding of various factors. The general view is that histone acetylation is controlled by transcription factor-mediated recruitment of HATs to gene promoters and regulatory regions [12,13]. However, numerous studies provide compelling evidence that histone acetylation is also regulated by fluctuations in the concentration of acetyl-CoA [9,14–16]. For example, various acetyltransferase enzymes have Km values in the low μM range [17,18], within the range of estimated intracellular concentrations of acetyl-CoA [15]. Studies of the yeast metabolic cycle (YMC) revealed changes in histone acetylation predominantly at genes involved in cell growth, precisely in phase with increased cellular levels of acetyl-CoA [15]. These observations provide the logic for models in which the regulation of cell growth genes is coupled to the level of acetyl-CoA, a key indicator of metabolic state (see Figure 1).
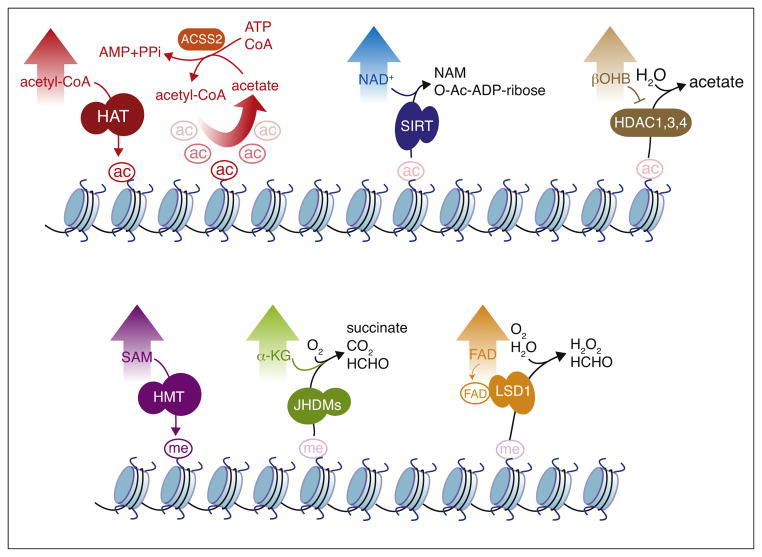
Figure 1.
Dynamic regulation of histone acetylation and methylation. Top: dynamic regulation of histone acetylation and deacetylation. Histone acetylation can be stimulated by intracellular levels of acetyl-CoA through HATs. Acetate can be converted into acetyl-CoA by the acetyl-CoA synthetase enzyme ACSS2. Acetylated histones can be deacetylated by sirtuins that use NAD+ as cofactor, or by other HDACs (1,3,4) that use activated water as nucleophile. The activity of HDACs (1,3,4) is inhibited by the ketone body βOHB. Bottom: dynamic regulation of histone methylation and demethylation. Histone methylation can be stimulated by intracellular levels of SAM through HMTs. Methylated histones can be demethylated by JHDMs dependent on α-KG, or by LSD1/amine oxidases dependent on FAD. Abbreviations: HAT, histone acetyltransferase; ACSS2, acyl-CoA synthetase short-chain family member 2; SIRT, Sirtuin; NAD+, nicotinamide adenine dinucleotide; NAM, nicotinamide; HDAC, histone deacetylase; βOHB, β-hydroxybutyrate; ac, acetylation; SAM, S-adenosylmethionine; αKG, α-ketoglutarate; JHDM, Jumonji C-terminal-domain-containing histone demethylase; LSD1, lysine (K)-specific demethylase 1; FAD, flavin adenine dinucleotide.
Histones and other proteins are deacetylated by enzymes known as histone deacetylases (HDACs) or lysine deacetylases (KDACs). Histone deacetylation typically results in a more condensed chromatin structure that correlates with repressed transcription. HDACs fall into two general groups, based on their catalytic mechanism [19]. Most HDACs (classes I, II, and IV) use activated water as the nucleophile, whereas class III HDACs (also known as ‘sirtuins’) use a cofactor, nicotinamide adenine dinucleotide (NAD+), to catalyze deacetylation [20]. NAD+ is a key electron carrier in the oxidation of hydrocarbon fuels. The discovery of sirtuins as NAD+-dependent deacylases suggested a link between cellular levels of NAD+ and the regulation of chromatin and gene expression [11]. Indeed, dietary restriction is proposed to promote health and longevity in model organisms by activating nuclear sirtuins [21,22]. Interestingly the other group of HDACs (water-as-nucleophile) may also be coupled to metabolism, since the ketone body β-hydroxybutryate (βOHB) is reported to function as an endogenous inhibitor of these enzymes [23••]. The concentration of βOHB in blood can reach low millimolar concentrations during fasting [24,25]. Furthermore, βOHB inhibits HDAC1 and HDAC3 (both class 1) and HDAC4 (class II) in vitro with an IC50 of 2–5 mM, suggesting HDAC activity may be physiologically inhibited by βOHB during fasting conditions. This mechanism may extend to our gut microbiome since butyrate, a product of bacterial fermentation, is proposed to inhibit HDAC activity in colonocytes [26].
Histones are so abundant that their acetylation and deacetylation may impact beyond chromatin. Each histone octamer, occupying ~146 bp of DNA, represents nearly 20 acetylatable lysines. Moreover, these acetyl moieties have very short half-lives, on the order of ~2–3 min [27,28]. These considerations led to the realization that substantial amounts of acetate might be ‘stored’ on histones and liberated by deacetylation [29,30••]. Released acetate could be re-captured by acetyl-CoA synthetase enzymes, which convert acetate to acetyl-CoA in an ATP-dependent reaction. Supporting this idea, there is strong evidence that acetate functions as an important carbon source for cancer cells, and is used to synthesize acetyl-CoA in challenging growth environments [30••,31]. In two models of hepatocellular carcinoma, adult mice that lacked the nucleocytosolic isoform of acetyl-CoA synthetase (ACSS2) had significantly reduced tumor burdens [30••]. There is also a strong correlation between ACSS2 expression and the uptake of [11C]acetate into liver tumors in mice [30••]. These observations suggest certain tumors can avidly capture acetate, including that released from histones, as fuel for growth and survival in vivo.
Interestingly, acetate present on histones is also proposed to regulate cellular pH [32••]. As intracellular pH decreases, histones are globally deacetylated and the released acetate anions are co-exported with protons out of the cell by monocarboxylate transporters (MCTs), providing a mechanism to stabilize intracellular pH [32••]. HDAC and MCT inhibitors reduced acetate export, and lowered intracellular pH, thereby interfering with pH maintenance in acidic environments [32••]. This unexpected cellular role for ‘global’ histone acetylation has important implications for the mechanism of action of HDAC inhibitors, which are used therapeutically to treat cancer. Moreover, by inhibiting histone deacetylation, HDAC inhibitors may also decrease the free acetate pool available as a fuel source for cancer cells.
Histones are also modified by malonylation, succinylation and crotonylation [33–35]. These modifications, also probably derived from corresponding acyl-CoA metabolites, suggest further intriguing links between chromatin regulation and metabolic state.
Dynamic histone methylation and demethylation
Histone methylation is another widespread modification that may be linked to nutrition. All four core histones can be methylated, and in some cases individual residues can be either mono-methylated, di-methylated, or tri-methylated [36–40]. Specific methylation marks can correlate with either active or repressed chromatin. Methylation is catalyzed by histone methyltransferase enzymes (HMTs), which use S-adenosylmethionine (SAM) as the methyl donor. HMTs are also known as lysine methyltransferases (KMTs) since they also target proteins other than histones. SAM is generated from methionine by methionine adenosyl transferase (MAT), also known as SAM synthetase. During methylation, SAM donates its methyl group and is converted to S-adenosylhomocysteine (SAH). SAH is then hydrolyzed to homocysteine, which is converted back to methionine in a reaction that requires folate and cobalamin (i.e., vitamin B12) [41]. Consequently, histone methylation status might be influenced by fluctuations in SAM or regulators of SAM synthesis. Moreover, arginine residues and DNA itself are also methylated, and may be similarly influenced by SAM levels. Examples include histone H2A methylation by protein arginine methyltransferase 5 (PRMT5) in Xenopus [42], and the PRMT-3 enzyme in Caenorhabditis elegans [43]. DNA methylation at cytosines, typically in the context of CpG dinucleotides, correlates with gene silencing [44], and appears to be regulated by SAM levels during osteoclast differentiation [45].
The idea that folate is used to regenerate methionine (hence, SAM), and sustain methylation, was recently tested in yeast [41]. Controls showed that H3K4 methylation in folate-synthesis-deficient yeast cells was rescued by exogenous folate; similarly, exogenous methionine rescued H3K4 methylation in methionine-synthesis-deficient yeast. High concentrations of folate also increased SAM levels in folate-synthesis-deficient cells, and folate or methionine limitation affected methylation at one site (H3K4) more severely than another (H3K79) [41], suggesting a potential hierarchy of histone methylation responses to nutritional limitation.
Immunofluorescence and cell fractionation assays suggest that the mammalian metabolic enzyme MATIIα, which uses methionine and ATP to generate SAM, actually localizes to chromatin and may directly supply substrates or cofactors to histone-modifying enzymes [46••]. SAM levels are also influenced by Tdh (threonine dehydrogenase), which oxidizes threonine, producing glycine and acetyl-CoA, which can contribute to SAM synthesis. Tdh influences the pluripotency of mouse embryonic stem cells [47,48]. The Tdh-dependent pool of SAM appears to be particularly important for H3K4 trimethylation (‘H3K4me3’) [48], which is critical for self-renewal of pluripotent stem cells [49]. These findings indicate that the threonine dehydrogenase-dependent pool of SAM is important for stem cell pluripotency.
Histone methylation was long thought to be irreversible because this modification, and histones themselves, had apparently similar half-lives [50]. This notion changed in 2004, when the first of several histone demethylases (HDMs) was identified [51–53]. These enzymes, also known as lysine demethylases (KDMs) because they also modify non-histone lysines, fall into two main classes: the JmjC (Jumonji C domain)-containing HDMs, which depend on Fe(II) and α-ketoglutarate, and the lysine-specific amine oxidases, which are depend on flavin adenine dinucleotide (FAD). The existence of HDMs implied that methylation was also dynamic. Estimates of the half-lives of methyl modifications on histones range from 0.3 to 4 days [54,55], significantly longer than acetylation modifications, which are on the order of minutes [27,28]. Consistent with this idea, histone methylation marks in yeast tend to be longer-lived than acetylation marks [56].
The JmjC HDMs require both α-ketoglutarate (a TCA cycle intermediate) and iron, Fe(II), to remove methyl groups from histones, yielding succinate, formaldehyde, and carbon dioxide [53]. Thus, fluctuations in α-ketoglutarate might influence histone methylation status. Indeed, mouse embryonic stem cells use both glucose and glutamine to maintain high levels of intracellular α-ketoglutarate [57••], as a proposed mechanism to maintain pluripotency by promoting both histone demethylation and DNA demethylation. This model is based on the experimental manipulation of intracellular α-ketoglutarate levels, which affected multiple chromatin modifications, including H3K27me3 and ten–eleven translocation (Tet)-dependent DNA demethylation, important for pluripotency-associated genes [57••]. On the other hand, fluctuations in the level of SAM appear to influence pluripotency by affecting H3K4 trimethylation [48]. The interplay between metabolites such as SAM and α-ketoglutarate, which appear to regulate opposing reactions that influence methylation status and stem cell pluripotency, is an important area for future investigation.
The lysine-specific amine oxidase class of histone demethylases (KDMs) require FAD as a cofactor to catalyze demethylation [51]. The founding member, LSD1/KDM1, regulates cellular energy balance by coupling FAD biosynthesis to gene regulation [58]. In adipocytes, LSD1 inhibits genes involved in energy expenditure and mitochondrial metabolism by demethylating H3K4 at these loci [58]. Interestingly, this regulation depends on FAD availability. In adipose tissue from mice on a high-fat diet (high FAD levels), LSD1-targeted genes were repressed. When FAD synthesis was inhibited, LSD1-targeted gene expression increased [58]. This evidence identifies FAD as another metabolite that influences histone methylation status and gene expression.
Histone demethylation notably generates formaldehyde, as well as hydrogen peroxide in the case of the FAD-dependent KDMs. Both byproducts are reactive and potentially damaging to DNA [59,60]. As such, there may be mechanisms by which these products are locally detoxified within the nucleus (e.g., by hypothetical nuclear aldehyde dehydrogenase or catalase-related enzymes). A more speculative possibility is that these toxic metabolites also influence chromatin-based signaling (e.g., through redox-based or covalent modification).
Summary and perspective
There is growing evidence that the epigenetic regulation of chromatin is coupled to the levels of metabolites that change in response to nutrient status. These metabolites may also influence the recruitment of transcriptional regulatory complexes to DNA. For example, acetyl-CoA promotes recruitment of the Gcn5p-containing Spt-Ada-Gcn5-acetyltransferase (SAGA) transcriptional coactivator complex to ribosomal genes and CLN3 (G1 cyclin) by inducing autoacetylation of SAGA subunits such as SPT7, ADA3, and SGF73 [15,61]. Histone modifiers are also themselves subject to metabolite-sensitive modifications [15,62]. A key outstanding question is whether particular classes of genes are specifically responsive to regulation by metabolites, or whether metabolites control chromatin purely at the bulk level. Nonetheless the dynamic chromatin landscape is clearly influenced by metabolic cues and more examples of the nutritional regulation of chromatin undoubtedly remain to be discovered.
Acknowledgments
We apologize to authors whose work could not be discussed due to space restrictions. BPT acknowledges funding support from R01GM094314 and R01CA185169.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Zaman S, Lippman SI, Zhao X, Broach JR. How Saccharomyces responds to nutrients. Annu Rev Genet. 2008;42:27–81. doi: 10.1146/annurev.genet.41.110306.130206. [DOI] [PubMed] [Google Scholar]
- 2.Zaman S, Lippman SI, Schneper L, Slonim N, Broach JR. Glucose regulates transcription in yeast through a network of signaling pathways. Mol Syst Biol. 2009;5:245. doi: 10.1038/msb.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavov N, Botstein D. Decoupling nutrient signaling from growth rate causes aerobic glycolysis and deregulation of cell size and gene expression. Mol Biol Cell. 2013;24:157–168. doi: 10.1091/mbc.E12-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boer VM, Amini S, Botstein D. Influence of genotype and nutrition on survival and metabolism of starving yeast. Proc Natl Acad Sci U S A. 2008;105:6930–6935. doi: 10.1073/pnas.0802601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauer MJ, Huttenhower C, Airoldi EM, Rosenstein R, Matese JC, Gresham D, Boer VM, Troyanskaya OG, Botstein D. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol Biol Cell. 2008;19:352–367. doi: 10.1091/mbc.E07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regenberg B, Grotkjaer T, Winther O, Fausboll A, Akesson M, Bro C, Hansen LK, Brunak S, Nielsen J. Growth-rate regulated genes have profound impact on interpretation of transcriptome profiling in Saccharomyces cerevisiae. Genome Biol. 2006;7:R107. doi: 10.1186/gb-2006-7-11-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klevecz RR, Bolen J, Forrest G, Murray DB. A genomewide oscillation in transcription gates DNA replication and cell cycle. Proc Natl Acad Sci U S A. 2004;101:1200–1205. doi: 10.1073/pnas.0306490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu BP, Kudlicki A, Rowicka M, McKnight SL. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme A synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Friis RM, Wu BP, Reinke SN, Hockman DJ, Sykes BD, Schultz MC. A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res. 2009;37:3969–3980. doi: 10.1093/nar/gkp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaochar S, Tu BP. Gatekeepers of chromatin: small metabolites elicit big changes in gene expression. Trends Biochem Sci. 2012;37:477–483. doi: 10.1016/j.tibs.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 13.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 14.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry RA, Kuo YM, Bhattacharjee V, Yen TJ, Andrews AJ. Changing the selectivity of p300 by acetyl-CoA modulation of histone acetylation. ACS Chem Biol. 2015;10:146–156. doi: 10.1021/cb500726b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr Opin Struct Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer MR, Fry CJ, Peterson CL, Denu JM. Modulating acetyl-CoA binding in the GCN5 family of histone acetyltransferases. J Biol Chem. 2002;277:27337–27344. doi: 10.1074/jbc.M203251200. [DOI] [PubMed] [Google Scholar]
- 19.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 20.Guedes-Dias P, Oliveira JM. Lysine deacetylases and mitochondrial dynamics in neurodegeneration. Biochim Biophys Acta. 2013;1832:1345–1359. doi: 10.1016/j.bbadis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27:2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. This study reported that the ketone body β-hydroxybutyrate (βOHB) produced by fasting or calorie restriction in mice can specifically inhibit class I histone deacetylases (HDACs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cahill GF, Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 26.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson V, Shires A, Chalkley R, Granner DK. Studies on highly metabolically active acetylation and phosphorylation of histones. J Biol Chem. 1975;250:4856–4863. [PubMed] [Google Scholar]
- 28.Waterborg JH. Dynamics of histone acetylation in vivo. A function for acetylation turnover? Biochem Cell Biol. 2002;80:363–378. doi: 10.1139/o02-080. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Pastor B, Cosentino C, Mostoslavsky R. A tale of metabolites: the cross-talk between chromatin and energy metabolism. Cancer Discov. 2013;3:497–501. doi: 10.1158/2159-8290.CD-13-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. This study showed that acetate can be captured as a source of acetyl-CoA which may promote the growth and survival of particular tumors. A substantial amount of the acetate pool may derive from histone deacetylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.McBrian MA, Behbahan IS, Ferrari R, Su T, Huang TW, Li K, Hong CS, Christofk HR, Vogelauer M, Seligson DB, et al. Histone acetylation regulates intracellular pH. Mol Cell. 2013;49:310–321. doi: 10.1016/j.molcel.2012.10.025. This paper reported that intracellular pH regulates histone acetylation. As pH decreased, histones were globally deacetylated, acetate anions were coexported with protons out of the cell by monocarboxylate transporters (MCTs) to prevent further reductions in intracellular pH. As intracellular pH increased, global histone acetylation increased. This regulation of chromatin acetylation by intracellular pH has important implications for the mechanism of action and therapeutic use of HDAC inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, Boeke JD, Zhao Y. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H, Sabari BR, Garcia BA, Allis CD, Zhao Y. SnapShot: histone modifications. Cell. 2014;159:458–458.e1. doi: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogure M, Takawa M, Saloura V, Sone K, Piao L, Ueda K, Ibrahim R, Tsunoda T, Sugiyama M, Atomi Y, et al. The oncogenic polycomb histone methyltransferase EZH2 methylates lysine 120 on histone H2B and competes ubiquitination. Neoplasia. 2013;15:1251–1261. doi: 10.1593/neo.131436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strahl BD, Grant PA, Briggs SD, Sun ZW, Bone JR, Caldwell JA, Mollah S, Cook RG, Shabanowitz J, Hunt DF, et al. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol Cell Biol. 2002;22:1298–1306. doi: 10.1128/mcb.22.5.1298-1306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109:745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 39.Edwards CR, Dang W, Berger SL. Histone H4 lysine 20 of Saccharomyces cerevisiae is monomethylated and functions in subtelomeric silencing. Biochemistry. 2011;50:10473–10483. doi: 10.1021/bi201120q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green EM, Mas G, Young NL, Garcia BA, Gozani O. Methylation of H4 lysines 5, 8 and 12 by yeast Set5 calibrates chromatin stress responses. Nat Struct Mol Biol. 2012;19:361–363. doi: 10.1038/nsmb.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadhu MJ, Guan Q, Li F, Sales-Lee J, Iavarone AT, Hammond MC, Cande WZ, Rine J. Nutritional control of epigenetic processes in yeast and human cells. Genetics. 2013;195:831–844. doi: 10.1534/genetics.113.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilczek C, Chitta R, Woo E, Shabanowitz J, Chait BT, Hunt DF, Shechter D. Protein arginine methyltransferase Prmt5-Mep50 methylates histones H2A and H4 and the histone chaperone nucleoplasmin in Xenopus laevis eggs. J Biol Chem. 2011;286:42221–42231. doi: 10.1074/jbc.M111.303677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi Y, Daitoku H, Yokoyama A, Nakayama K, Kim JD, Fukamizu A. The C elegans PRMT-3 possesses a type III protein arginine methyltransferase activity. J Recept Signal Transduct Res. 2011;31:168–172. doi: 10.3109/10799893.2011.555768. [DOI] [PubMed] [Google Scholar]
- 44.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med. 2015;21:281–287. doi: 10.1038/nm.3774. [DOI] [PubMed] [Google Scholar]
- 46••.Katoh Y, Ikura T, Hoshikawa Y, Tashiro S, Ito T, Ohta M, Kera Y, Noda T, Igarashi K. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol Cell. 2011;41:554–566. doi: 10.1016/j.molcel.2011.02.018. This study showed that a metabolic enzyme localizes on chromatin and can directly supply chromatin-modifying enzymes with substrates such as SAM. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Alexander P, Wu L, Hammer R, Cleaver O, McKnight SL. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109:801–806. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 53.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 54.Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12:917–930. doi: 10.1038/nrd4154. [DOI] [PubMed] [Google Scholar]
- 55.Zee BM, Levin RS, Xu B, LeRoy G, Wingreen NS, Garcia BA. In vivo residue-specific histone methylation dynamics. J Biol Chem. 2010;285:3341–3350. doi: 10.1074/jbc.M109.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuang Z, Cai L, Zhang X, Ji H, Tu BP, Boeke JD. High-temporal-resolution view of transcription and chromatin states across distinct metabolic states in budding yeast. Nat Struct Mol Biol. 2014;21:854–863. doi: 10.1038/nsmb.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2014;518:413–416. doi: 10.1038/nature13981. This paper showed that elevating the α-ketoglutarate (αKG) to succinate ratio in naive mouse embryonic stem cells, achieved by glucose and glutamine catabolism, promoted histone/DNA demethylation and maintains pluripotency. The intracellular αKG/succinate ratio regulated multiple chromatin modifications including H3K27me3 and ten–eleven translocation (Tet)-dependent DNA demethylation, and contributes to the regulation of pluripotency-associated gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hino S, Sakamoto A, Nagaoka K, Anan K, Wang Y, Mimasu S, Umehara T, Yokoyama S, Kosai K, Nakao M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat Commun. 2012;3:758. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stingele J, Schwarz MS, Bloemeke N, Wolf PG, Jentsch S. A DNA-dependent protease involved in DNA–protein crosslink repair. Cell. 2014;158:327–338. doi: 10.1016/j.cell.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 60.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi L, Tu BP. Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2013;110:7318–7323. doi: 10.1073/pnas.1302490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, et al. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308–315. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]