Summary
This exploratory study assessed apoptosis in peripheral blood leucocytes (PBL) from β-thalassaemia patients receiving chronic transfusions and chelation therapy (deferasirox or deferoxamine) at baseline, 1, 6, and 12 months. At baseline, thalassaemic PBLs presented 50% greater levels of Bax (BAX), 75% higher caspase-3/7, 48% higher caspase-8 and 88% higher caspase-9 activities and 428% more nucleosomal DNA fragmentation than control subjects. Only neutrophils correlated significantly with apoptotic markers. Previously, we showed that over the treatment year, hepatic iron declined; we now show that the ratio of Bax/Bcl-2 (BCL2), (−27.3%/year), and caspase-9 activity (−13.3%/year) declined in both treatment groups, suggesting that chelation decreases body iron and indicators of PBL apoptosis.
Keywords: apoptosis, Bax, caspase, oxidant-stress, inflammation
Frequent transfusions in β-thalassaemia major patients cause cyclic changes in haemoglobin and increased non-transferrin-bound iron (NTBI). Despite improved chelation therapies there is risk for iron overload, organ failure, infection, oxidative stress and chronic inflammation, resulting in elevated levels of pro- and anti-inflammatory proteins such as tumour necrosis factor α, interleukin (IL)2, 10 and high sensitivity C-reactive protein (hsCRP) (Del Vecchio et al, 2002; Walter et al, 2008). These proteins participate in response and resolution to infection and tissue injury by balancing leukocyte proliferation and apoptotic cell death. Thalassaemia patients are chronically immuno-stimulated by transfusions, NTBI, chelation and organ injury and without resolution of inflammation, there is chronic inflammatory protein production. Some of these proteins are proliferative and some are resolving (apoptotic) in nature. Persistence or lack of immune cells at inflammatory sites or the development of chronic inflammation (Scheel-Toellner et al, 2004) observed in β-thalassaemia patients may result from dysregulation of the apoptotic cell death pathway. Furthermore, increased apoptosis reduces neutrophil function (Whyte et al, 1993) and could contribute to increased infection risk in thalassaemia patients.
Apoptosis initiated extrinsically by death receptor ligation eventually activates procaspase-8 (Scheel-Toellner et al, 2004), while increases in Bax (BAX) and/or decreases in Bcl-2 (BCL2) intrinsically activates the mitochondrial pathway (Brenner & Mak, 2009) with eventual activation of caspase-9. The balance between Bax and Bcl-2 forms an apoptotic rheostat that is regulated by oxidants (Korsmeyer et al, 1993). Executioner caspases 3 and 7 subsequently activate endonucleases cleaving DNA into nucleosomal fragments. Other pro-apoptotic stimuli include reactive oxygen species (ROS), DNA damage, and anti-inflammatory cytokines, such as IL10 (Turina et al, 2007).
Thalassaemia patients have increased markers for ROS (such as malondialdehyde, MDA), and inflammation (such as CRP and IL10) and these levels of MDA and CRP can be controlled by chelation (Walter et al, 2008). Iron overload increases leucocyte mitochondrial dysfunction (Walter et al, 2002) and may also be implicated in increased apoptotic mechanisms (Ichii et al, 2012). Young paediatric thalassaemia patients (<5 years, not chronically transfused), showed no increases in neutrophil apoptosis (Ören et al, 2003), however the impact of chronic iron burden and chelation on the expression of leucocyte apoptotic markers in older, chronically transfused thalassaemia patients has not been determined.
This study evaluated six markers of peripheral blood leucocyte (PBL) apoptosis in thalassaemia compared with control subjects, to assess whether improved control of iron overload with deferasirox or deferoxamine was associated with changes in the level of apoptotic markers.
Materials and methods
Patients and study design
Details of this ancillary study of the phase III deferasirox trial, (CICL670A0107) and trial subjects have been described (Walter et al, 2008). The study was approved by the institutional review board at each site and conducted under supervision of a Data and Safety Monitoring Board convened by the National Heart, Lung, and Blood Institute. Forty-nine β-thalassaemia subjects (from seven Thalassemia Clinical Research Network sites) (28 male, age = 22.4 ± 10.7, range 3–42 years), weighing ≥12 kg, with chronic iron overload [liver iron concentration (LIC) ≥2 mg Fe/g dw] from blood transfusions (≥8/year) were randomized to receive deferasirox (n = 24) or deferoxamine (n = 25). Thirty controls (15 male, 24.5 ± 9.0 years) frequency-matched for age, sex, race and vitamin supplement intake were enrolled for a single visit. All participants provided written informed consent.
Laboratory analyses
Fasting blood samples were collected from subjects that had abstained from medications, chelators and nutritional supplements for the previous 24 h, at 1, 6, and 12 months. Peripheral blood leucocytes were isolated from Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) gradients and the levels of Bax and Bcl-2 determined by enzyme immunometric assay (Assay Designs, Ann Arbor, MI, USA). Activity of caspase-3/7, -8 and -9, determined by luminescent enzyme activity assay (Caspase – Glo™ assay, Promega, Madison, WI, USA), is reported as average relative light units (RLU)/μg PBL protein, or RLU was converted to ng by comparison to a standard curve of recombinant caspase (BioVision, Mountain view, CA, USA). Nucleosomal DNA fragmentation was determined by enzyme-linked immunosorbent assay (Roche, Indianapolis, IN, USA). Vitamin E, iron measures, CRP, cytokines, LIC, NTBI and ferritin were measured as described (Walter et al, 2008).
Statistics
Analysis of covariance (ANCOVA) and repeated measures ANCOVA evaluated associations controlling for age, gender, race, treatment and dose, illness (previous 2 weeks), and baseline LIC. P < 0.05 was considered statistically significant. Outcomes were log-transformed in models, but back-transformed for figures. Outliers were removed (visual inspection) and associations were modelled by repeated measures regression with subject-specific intercepts and slopes. Fisher’s exact test and t-test compared demographics.
Results and discussion
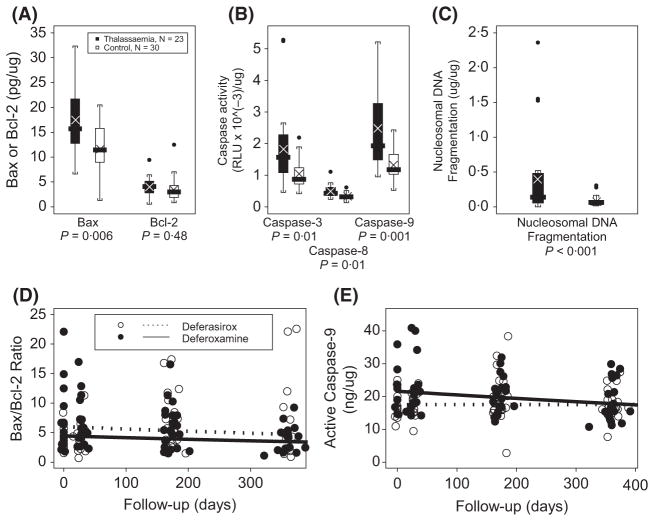
Thalassaemic PBLs had increased levels of Bax (P = 0.006), no change in Bcl-2 (P > 0.05), but greater caspase-3/7 (P = 0.01), -8 (P = 0.01), and -9 (P = 0.001) activities compared to controls (Fig 1A–C). Both neutrophils and lymphocytes are known to increase the translocation of pro-apoptotic Bax to mitochondria during intrinsic apoptosis (Scheel-Toellner et al, 2004; Brenner & Mak, 2009), but in our study only neutrophils correlated with Bax and other tested apoptotic markers (Table I). Neutrophils are sensitive to redox changes and upregulate Bax on exposure to increased oxidants (Geering & Simon, 2011). In parallel with our finding of increased Bax expression, others have reported elevated Bax and apoptosis in neutrophils during infection (Perskvist et al, 2002), which could reflect high levels of neutrophil turnover during inflammation or exposure to oxidants. Enhanced exposure of thalassaemic PBLs to NTBI could increase intracellular iron and cause nuclear oxidative DNA damage. This could upregulate p53 (TP53) leading to the observed increase in Bax expression. A high ratio of Bax/Bcl-2 indicates decreased stability of the mitochondrial outer membrane and increased potential for mitochondrial dysfunction and increased oxidant production. In the one year follow-up, the Bax/Bcl-2 ratio decreased 27.3%/year, (from 6.16 to 5.28, P = 0.033, Fig 1D) during treatment with either deferoxamine or deferasirox in parallel with decreased body iron burden. Given that we previously found enhanced markers for circulating levels of lipid peroxidation (MDA) (Walter et al, 2008) and now find enhanced expression of Bax and increased caspase-9 activity, our data also supports the work of others who showed that the Bax/Bcl2 ratio may constitute an ‘apoptotic rheostat’, which determines susceptibility to oxidant-induced cell death (Korsmeyer et al, 1993; Perskvist et al, 2002). Thus, the shift in balance of the Bax-Bcl-2 ratio may determine neutrophil fate, and although some doubt Bcl2 expression in neutrophils, recent reports have confirmed the expression of Bcl2 in neutrophils (Sarkar et al, 2012). What may be relevant to thalassaemic neutrophils is the increased expression of Bax, possibly because of their exposure to NTBI and oxidants. Also suggestive of the role of iron and oxidants in thalassaemic PBL apoptosis are the correlations of caspase-8 with transferrin saturation, γ-tocopherol with caspase-3/7 and α-tocopherol and hsCRP with nucleosomal DNA fragmentation (Table I).
Fig. 1.
A–C (top) Baseline peripheral blood leucocyte levels of Bax (A); caspase activities (B) and nucleosmal DNA fragmentation (C). The values for thalassaemia patients are means of both chelator groups with the P values indicated for each comparison. The boxplots show the median value with box around the interquartile range with whiskers for range and dotted outliers. The symbol ‘×’ marks the mean value. D,E (bottom) Distributions of the Bax/Bcl-2 ratio (D) and the amount of active Caspase 9 (E) versus time stratified by treatment group (deferasirox, open symbols; deferoxamine, filled symbols). Day zero (0) is the first day of treatment. Regression lines of the observed data are overlaid (deferasirox ● – ● – ●; deferoxamine —), with no significant interaction between time and chelator (P = 0.98 for Bax/Bcl-2; P = 0.14 for Caspase-9).
Table I.
Significant correlations between Biomarkers and: A – PBL Apoptotic biomarkers, B – Neutrophils, C – Inflammatory Indicator, D – Tocopherols and E – Transferrin saturation.
| Biomarker | Correlation | P-value | |
|---|---|---|---|
| A. PBL Apoptotic biomarkers | |||
| Caspase-9 | Bax | 0.45 | <0.001 |
| Caspase-8 | Bax | 0.47 | <0.001 |
| Nucleosomal DNA fragmentation | Bax | −0.37 | <0.001 |
| Caspase-3/7 | Bcl-2 | 0.075 | 0.010 |
| Caspase-9 | Bax/Bcl-2 ratio | 0.35 | 0.001 |
| Caspase-8 | Bax/Bcl-2 ratio | 0.38 | <0.001 |
| Nucleosomal DNA fragmentation | Bax/Bcl-2 ratio | −0.18 | 0.007 |
| B. Neutrophils | |||
| ANC | Bax/Bcl-2 ratio | 0.29 | 0.004 |
| ANC | Bcl-2 | −0.27 | 0.0035 |
| ANC | Bax | 0.21 | 0.045 |
| ANC | Nucleosomal DNA fragmentation | 0.19 | 0.04 |
| ANC | Caspase-9 | 0.16 | 0.048 |
| C. Inflammatory Indicator hsCRP | Nucleosomal DNA fragmentation | 0.25 | 0.01 |
| D. Tocopherols | |||
| α-tocopherol | Nucleosomal DNA fragmentation | 0.28 | 0.002 |
| γ-tocopherol | Caspase-3/7 | −0.23 | 0.02 |
| E. Transferrin saturation | Caspase-8 | −0.24 | 0.010 |
PBL, peripheral blood leucocyte; ANC, absolute neutrophil concentration; hsCRP, high sensitivity C-reactive protein; Note: alanine transaminase, aspartate transaminase, malondialdehyde, Vitamin C, non-transferrin bound iron, liver iron concentration, ferritin, γ-interferon, tumour necrosis factor-α, interleukin (IL)5 and IL8 did not correlate with PBL apoptotic markers.
Caspase-9 activity also declined during treatment (13.3%/year, P = 0.049, Fig 1E), from 19.2 to 17.9 ng/μg protein for both chelators (control levels = 14.9 ng/μg). Bax (and Bax/Bcl-2) and caspase-9 (and -8) were amongst our strongest correlations (Table I), which has also been found in other studies in both healthy neutrophils and lymphocytes during inflammation (Scheel-Toellner et al, 2004). Caspase-9 activity has also been found to be closely associated with changes in the expression of Bax (Scheel-Toellner et al, 2004). Another possible mechanism for enhanced apoptosis in thalassaemic PBLs concerns the high levels of IL10 that we have previously shown in the same study population (Walter et al, 2008). IL10 is an important regulatory anti-inflammatory cytokine involved in the resolution of acute inflammatory and oxidative stress responses (Haddad & Fahlman, 2002). It also promotes neutrophil apoptosis during resolution of inflammation perhaps by enhancing caspase-8 activity (Turina et al, 2007).
In support of this possibility, we found enhanced caspase-8 activity in thalassemic PBLs. Caspase-8 participates in extrinsic, receptor-mediated apoptosis. However, procaspase-8 can become activated not only by this extrinsically mediated pathway, but also by ROS (Scheel-Toellner et al, 2004) and/or possibly by IL10 (Turina et al, 2007). Thus, in thalassaemia patients, enhanced caspase-8 activity may be due to enhanced levels of ROS and/or anti-inflammatory cytokines. Thalassaemic PBLs also had increased caspase-3 activity relative to the controls, providing the final common pathway to both death receptor and mitochondria-dependent apoptotic mechanisms. Because we observed both the enhanced activity of caspase-9, and -8, our results suggest that both apoptotic pathways are stimulated during apoptosis of thalassaemic PBLs although we cannot rule out caspase-8 activated bid may be initiating the mitochondrial pathway. The increase in caspase-3 activity invokes the activity of downstream nucleases, as shown by the 5.3-fold elevation of nucleosomal fragmentation in patients compared to controls (P < 0.001, Fig 1C). Nucleosomal DNA fragmentation did not change during the one-year follow-up, however, Bax and the Bax/Bcl-2 ratio correlated with nucleosomal DNA fragmentation (Table I). This high level of nucleosomal DNA fragmentation confirms that circulating PBLs of thalassaemia patients had high levels of apoptotic proteins. This unusually high level of fragmentation is suggestive of reactive oxygen- or inflammatory-induced apoptosis occurring in thalassaemic PBLs.
We also explored the possible contribution of splenectomy and chelation to the enhanced levels of thalassaemic PBL apoptosis. 57% of the thalassaemia patients were splenectomized; however, analysis controlling for splenectomy showed no effect. Besides controlling iron load, it is also possible that the chelators themselves may affect PBL apoptosis; for example deferoxamine and deferiprone were found to reduce leucocyte cytokine production (Del Vecchio et al, 2002), although other iron chelators, such as lactoferrin, have recently been found to delay apoptosis (Francis et al, 2011). While we have seen an increase in apoptotic proteins in thalassemic PBLs, we have not seen a decrease in any one type of leukocyte that is similar to that seen with infection. This may seem paradoxical; however the increase in apoptotic proteins without a decrease in cell number could be due in part to the unresolved inflammation present in the disease and may become another marker of chronic inflammation in thalassemia.
In conclusion, this ancillary study found high levels of apoptotic markers in thalassaemic PBLs, including increased Bax expression, caspase activity and nucleosomal DNA fragmentation. Neutrophils were the only PBL type that consistently correlated with the measured apoptotic markers. In the one-year follow-up, iron chelation therapy with deferasirox or deferoxamine was equally effective in decreasing LIC (Walter et al, 2008), the ratio of Bax/Bcl-2 and caspase-9 activity. Thus, with effective chelation, it may be possible to decrease the PBL mitochondrial Bax/Bcl-2 ratio and the level of some pro-apoptotic proteins.
Acknowledgments
This work was supported by the following NIH-NHLBI cooperative agreements: U01-HL65232 to Children’s Hospital of Philadelphia, U01-HL65233 to University Health Network Toronto General Hospital, U01-HL65239 to Children’s Hospital & Research Center Oakland, U01-HL65244 to Weill Medical College of Cornell University, U01-HL65260 to Children’s Hospital Boston and U01-HL65238 to New England Research Institutes. The study was also supported, in part, at Children’s Hospital & Research Center Oakland by NIH-NIDDK grant R01-DK57778 and NIH-NCRR grant M01-RR01271, at Children’s Hospital of Philadelphia by NIH-NCRR grant UL1-RR024134 and at Children’s Hospital Boston by NIH-NCRR grant M01-RR02172.
Appendix I
The following institutions and researchers contributed to the Thalassemia Clinical Research Network Mitochondrial Ancillary Study reported in this paper (listed in alphabetical order). Children’s Hospital Boston: Ellis Neufeld, MD, PhD, Principal Investigator, Melody Cunningham, MD, Co-Principal Investigator, Jennifer Braunstein, RN, Study Coordinator, Joanna Hedstrom Study Coordinator. Children’s Hospital of Philadelphia: Alan R. Cohen, MD, Principal Investigator, Janet L. Kwiatkowski, MD, Co-Principal Investigator, Catherine S. Manno, MD, Coinvestigator, Debra Hillman, Study Coordinator, Marie Martin, RN, Nurse Coordinator. Children’s Hospital & Research Center Oakland: Elliott Vichinsky, MD, Principal Investigator, Sylvia Singer MD, Co-Principal Investigator, Paul Harmatz, MD, Co-Investigator, Patrick Walter, PhD, Co-Investigator, Nancy Sweeters RN PNP and Eun-Ha Pang, Study Coordinators, Dru Foote, RN PNP, Thalassemia Nurse, Annie Higa, Laboratory Technician; Satellite, Children’s Hospital of Los Angeles: Thomas Coates, MD, Principal Investigator, Robert Weihing, MD, Ph.D. Research Associate, Kerry Wymbs, Study Coordinator. Toronto General Hospital: Nancy Olivieri, MD, Principal Investigator, Laura Merson, Giulia Muraca, Clinical Research Managers, Melissa Stamplecoski, Study Coordinator. University College London: John Porter, MD, Principal Investigator, Michelle Cummins, Study Coordinator, Patricia Evans, Research Associate. Weill Medical College of Cornell: Patricia J. Giardina, MD, Principal Investigator, Robert W. Grady, PhD, Co-Investigator, Dorothy Kleinert, NP, MPH, MA, Thalassemia Nurse, Jeffrey E. Mait, Study Coordinator. Network Steering Committee Chair: David Nathan, MD. National Heart, Lung, and Blood Institute: Charles Peterson, MD, Project Officer. Data Coordinating Center: New England Research Institutes, Inc., Libby Wright, PhD, and Sonja McKinlay, PhD, Principal Investigators, Eric Macklin, PhD, Co-Principal Investigator, Ellen McCarthy, Project Director.
Footnotes
This is publication number eleven of the Thalassemia Clinical Research Network (TCRN).
A list of TCRN member institutions and staff appears in Appendix I.
Author contributions
PBW: designed and performed the research, analysed the data and wrote the paper; JP: designed the research, recruited and administered procedures to patients and wrote the paper; PE: designed and performed the research and wrote the paper; JLK: designed the research, recruited and administered procedures to patients and wrote the paper; EJN: designed the research, recruited and administered procedures to patients and wrote the paper; TC: designed the research, recruited and administered procedures to patients and wrote the paper; PJG: designed the research, recruited and administered procedures to patients and wrote the paper; RWG: designed and performed the research, analysed and the data; EV: designed the research, recruited and administered procedures to patients and wrote the paper; NO: designed the research, recruited and administered procedures to patients and wrote the paper; FT: designed the statistics, analysed the data and wrote the paper; DA: designed the research and wrote the paper; EF: analysed the data and wrote the paper; AH: performed the research and analysed the data; BA: helped with data acquisition, analysis and interpretation of data and wrote the paper; PH: designed the research, recruited and administered procedures to patients, analysed the data and wrote the paper.
Disclosures
PBW has received research support from Novartis Pharmaceuticals Corp. PH has received an educational grant and research support from Novartis Pharmaceuticals Corp; EV, JP and PJG received research funding from Novartis Pharmaceuticals Corp.; TC has received funding and honoraria from Novartis Pharmaceuticals Corp.; DA is an employee of Novartis Pharmaceuticals Corp. All authors gave final approval for the manuscript to be published.
References
- Brenner D, Mak TW. Mitochondrial cell death effectors. Current Opinion in Cell Biology. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Del Vecchio GC, Schettini F, Piacente L, De Santis A, Giordano P, De Mattia D. Effects of deferiprone on immune status and cytokine pattern in thalassaemia major. Acta Haematologica. 2002;108:144–149. doi: 10.1159/000064705. [DOI] [PubMed] [Google Scholar]
- Francis N, Wong SH, Hampson P, Wang K, Young SP, Deigner HP, Salmon M, Scheel-Toellner D, Lord JM. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochimica et Biophysica Acta. 2011;1813:1822–1826. doi: 10.1016/j.bbamcr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death and Differentiation. 2011;18:1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Fahlman CS. Redox- and oxidant-mediated regulation of interleukin-10: an anti-inflammatory, antioxidant cytokine? Biochemical and Biophysical Research Communications. 2002;297:163–176. doi: 10.1016/s0006-291x(02)02094-6. [DOI] [PubMed] [Google Scholar]
- Ichii H, Masuda Y, Hassanzadeh T, Saffarian M, Gollapudi S, Vaziri ND. Iron sucrose impairs phagocytic function and promotes apoptosis in polymorphonuclear leukocytes. American Journal of Nephrology. 2012;36:50–57. doi: 10.1159/000339285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Seminars in Cancer Biology. 1993;4:327–332. [PubMed] [Google Scholar]
- Ören H, şahin B, İrken G, Ateş H, Duman M, Yılmaz Ş, Türker M, Atabay B, Yaprak I. Neutrophil apoptosis in patients with β-thalassemia major. Pediatric Hematology-Oncology. 2003;20:237–243. doi: 10.1080/713842280. [DOI] [PubMed] [Google Scholar]
- Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. Journal of immunology (Baltimore, Md : 1950) 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Aga E, Bussmeyer U, Bhattacharyya A, Möller S, Hellberg L, Behnen M, Solbach W, Laskay T. Infection of neutrophil granulocytes with Leishmania major activates ERK 1/2 and modulates multiple apoptotic pathways to inhibit apoptosis. Medical Microbiology and Immunology. 2012 doi: 10.1007/s00430-012-0246-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Craddock R, Webb PR, McGettrick HM, Assi LK, Parkes N, Clough LE, Gulbins E, Salmon M, Lord JM. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–2564. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- Turina M, Hoth JJ, Turpen RM, Scott MJ, Cheadle WG. Alveolar interleukin-10 regulates neutrophil apoptosis in severely traumatized patients. The Journal of Trauma. 2007;63:733–739. doi: 10.1097/01.ta.0000240112.35246.ae. [DOI] [PubMed] [Google Scholar]
- Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2264–2269. doi: 10.1073/pnas.261708798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, Coates T, Giardina PJ, Vichinsky E, Olivieri N, Alberti D, Holland J, Harmatz P. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–825. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]
- Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. Journal of immunology (Baltimore, Md : 1950) 1993;150:5124–5134. [PubMed] [Google Scholar]