Abstract
The current need for organ and tissue replacement, repair and regeneration for patients is continually growing such that supply is not meeting the high demand primarily due to a paucity of donors as well as biocompatibility issues that lead to immune rejection of the transplant. In an effort to overcome these drawbacks, scientists working in the field of tissue engineering and regenerative medicine have investigated the use of scaffolds as an alternative to transplantation. These scaffolds are designed to mimic the extracellular matrix (ECM) by providing structural support as well as promoting attachment, proliferation, and differentiation with the ultimate goal of yielding functional tissues or organs. Initial attempts at developing scaffolds were problematic and subsequently inspired a growing interest in 3D printing as a mode for generating scaffolds. Utilizing three-dimensional printing (3DP) technologies, ECM-like scaffolds can be produced with a high degree of complexity and precision, where fine details can be included at a micron level. In this review, we discuss the criteria for printing viable and functional scaffolds, scaffolding materials, and 3DP technologies used to print scaffolds for tissue engineering. A hybrid approach, employing both natural and synthetic materials, as well as multiple printing processes may be the key to yielding an ECM-like scaffold with high mechanical strength, porosity, interconnectivity, biocompatibility, biodegradability, and high processability. Creating such biofunctional scaffolds could potentially help to meet the demand by patients for tissues and organs without having to wait or rely on donors for transplantation.
1. Introduction
Each year, the number of people in the United States suffering from organ dysfunction or organ failure due to damaged or diseased tissue is increasing because of the aging population.[1] Illnesses or traumas, such as heart attacks[2], strokes[3], and joint degeneration[4] can drastically reduce the quality of life for the victims as well as causing levels of tissue damage that current medicine is incapable of adequately repairing. This lack of therapeutic efficacy is primarily due to the fact that current treatments are aimed at merely preventing or reducing further tissue damage rather than contributing to the repair or regeneration of the tissue. Medications such as anticoagulants (warfarin) and antiplatelet agents (aspirin) for heart attacks and strokes primarily function by preventing blood clots and do not contribute to any form of tissue regeneration[5]. Similarly, analgesics, such as acetaminophen (paracetamol)[6] and nonsteroidal anti-inflammatory drugs (e.g. aspirin and ibuprofen)[7], are given to patients suffering from osteoarthritis (degenerative joint disease) primarily for pain relief, however, they play a negligible role in tissue regeneration/repair. As a result, patients are obliged to live with chronically damaged tissues which leads to a lower quality of life and contributes to an increased healthcare cost[8]. The aim of regenerative medicine is to restore or replace damaged or diseased tissues with healthy, functioning tissue. Tissue engineering requires an understanding of the biological processes required for cellular proliferation and differentiation [9-12]. The process of tissue engineering often begins with a scaffold, which is a three-dimensional support medium essential for the appropriate proliferation and differentiation of cells embedded in, or infiltrating, the scaffold. Conventional techniques used for scaffold fabrication include solvent-casting particulate-leaching, gas foaming, fibre meshes/fibre bonding, phase separation, melt molding, emulsion freeze drying, solution casting, as well as freeze drying, and these are discussed further elsewhere[13, 14]. These conventional methods have many limitations since they are often inadequate at fabricating precise pore size, pore geometry, high levels of interconnectivity, and high mechanical strength [13, 14]. Other limitations of these conventional techniques also included suboptimal distribution of cells due to the inaccuracies inherent in the process of seeding cells manually. This becomes problematic since cells may need to be precisely arranged according to the need and function of the tissue such as endothelial cells aligning to form vessels, or osteoblasts forming mineralized clusters[14]. Three dimensional printing has been developed as an advanced technology to overcome the limitations of these conventional methods and may ultimately lead to the production of matrix scaffolds capable of more effectively promoting the regeneration of functional tissue. Three-dimensional printing technology has emerged as a promising tool to fabricate scaffolds with a high precision and accuracy, creating intricately detailed biomimetic 3D structures[15]. The techniques currently being used to achieve 3D printing of scaffolds, which involve a layer-by-layer process, include, but are not limited to, direct 3D printing, fused deposition modeling, stereolithography, and selective laser sintering. These techniques have been used to produce scaffolds ranging from millimeter to nanometer sized scaffolds. It is also important to note that solid freeform fabrication, additive manufacturing and 3D printing have become synonymous over the past decade and are now used interchangeably. Advantages of using 3D printing include the ability to fabricate versatile scaffolds with complex shapes capable of homogenous cell distribution, and the ability to imitate the extracellular matrix (ECM). However, the availability of biomaterials with the stability and desired properties for 3D printing of scaffolds is restricted depending on the printing technology used. Another disadvantage is the production time that it takes to fabricate scaffolds, which greatly increases as the scaffold design becomes more and more precise and intricate[16]. This is especially the case for conventional methods which involves a lot of manual labor compared to an automated process[17]. With increased research and understanding of 3D printing, the use of hybrid materials and multiple printing technologies may lead to the fabrication of ECM-like scaffolds capable of overcoming current disadvantages. Evolving from conventional techniques, 3D printing provides tissue engineers with a way to design scaffolds capable of mimicking the complex structures of the ECM and thereby providing a microenvironment for cell attachment, proliferation, distribution and differentiation with the potential to form functional tissue. In this review, we will assess the current 3D printing materials and technologies being implemented to design scaffolds.
2. Materials used for 3D printing of scaffolds
Important criteria to consider when fabricating suitable scaffolds are biocompatibility, biodegradability, pore interconnectivity, pore size, porosity, and mechanical properties. Biocompatibility and biodegradability are important properties for scaffold materials to possess, ensuring they are degraded into nontoxic products while leaving behind only the desirable living tissue. In addition, the material should have minimized inflammatory responses, thereby avoiding reducing the likelihood of rejection by the host's immune system. It would also be beneficial if scaffold materials could behave as substrates for cellular attachment, proliferation and differentiation. Furthermore, as cells proliferate and differentiate, the scaffold must be able to withstand the forces being applied by the cells otherwise its collapse would result in poor diffusion of oxygen, nutrients and waste, leading to inefficient tissue formation. Finally, the mechanical stability of the scaffold must be structurally sound so as to withstand daily activity and normal body movements[18]. Naturally derived materials such as alginate, chitosan, collagen, fibronectin, and hyaluronic acid have an advantage over synthetic materials as they provide more innately biological functions. Using naturally derived materials, that normally constitute or inhabit the ECM, results in a better mimicking of genuine ECM and this therefore enhances cell attachment and regulates cellular proliferation more efficiently than synthetic polymers[19]. Although natural materials are beneficial for cellular processes, the use of synthetic polymers such as poly(e-caprolactone) (PCL) and poly(D, L-lactic-co-glycolic acid) (PLGA) for scaffolding has yielded higher mechanical strengths, higher processability, and controllable degradation rates[19, 20]. However, these synthetic polymer scaffolds have relatively low biological activity, in terms of promoting tissue regeneration, compared to naturally derived- ECM polymers. In addition to being less biologically active, the intrinsic hydrophobicity of synthetic polymers, such as polyesters, generally results in poor cell adhesion[21], which results in suboptimal proliferation and differentiation, ultimately leading to substandard tissue formation[19].
For 3D printing systems utilizing powder beds, grain size and grain size distributions must be taken into account to produce porous scaffolds[22], as these factors have a direct influence on microporosity which has been seen to influence cell distribution, attachment, proliferation, and differentiation[23, 24]. To achieve biomimicry of the ECM, scaffolds need to be biologically active, have high mechanical strengths, be easy to process, and have controllable degradation rates. To create these complex scaffolds, hybrid systems comprising both synthetic and natural polymers have been used and are likely to be used in the future [25-27]. It is important to keep in mind that different powdered combinations, materials, and structure size have direct effects on the scaffold printability, as is the case for most materials in 3D printing. To be a viable option for tissue regeneration, it is important to keep in mind that the materials used for 3D printing of scaffolds for tissue engineering should be printable with a high degree of reproducibility. Such materials should also be cost-effective and malleable to form the desired morphology of the design scaffold.
2.1 Metals
Natural or synthetic biomaterials used for 3D printing of scaffolds can vary greatly depending on the type of printing technology used. These materials include metals, ceramics, polymers, and composites [28-30]. Metals with the potential for use in 3D printing of scaffolds include iron, cobalt, chromium, stainless steel and titanium alloy. Metals are attractive materials to use in the 3D printing of scaffolds because of their high mechanical strengths which have been shown to be similar to that of the bone, hence their common application to bone tissue regeneration[31, 32]. Aside from displaying high mechanical stabilities, metals are promising materials to explore since, to a limited degree, they are safe to use in vivo. To date, many of the aforementioned metals have been used to design 2D scaffolds, but only a few metals are actually used in 3D printing[31]. These metals include stainless steel, cobalt-chromium alloys, titanium and its alloys, nitinol, and others that are used for biomedical implants[33]. Further work would need to be performed to establish the feasibility of using certain metal materials as components of 3D printed scaffolds despite having been successfully used in 2D scaffolds. The current limitations include: 1) the 3D printing technology available, thus limiting the type of metals that can be used, and 2) the toxicity of metal ions caused by metal corrosion and degradation inside the body. This corrosion produces metal ions that may be toxic to the body at high concentrations and with the lack of a clearance pathway, a system designed to capture the metal ions or avoid systemic toxicity is necessary in addition to scaffold fabrication[34, 35]. Another parameter that hinders the use of metals is long degradation times, which results in functional tissue forming around the scaffold rather than ultimately replacing the scaffold. However, the use of minute or trace amounts of metals to increase the mechanical strength of current scaffolds has shown some promise[36-38]. The limitations of using metals is that they are not typically biodegradable, which provides a large disadvantage when trying to design an ECM-like scaffold for tissue regeneration. However, biodegradable metals have emerged as prominent candidates for 3D printing of scaffolds. Recently, the term“biodegradable metals” (BMs) has been used to describe degradable metallic biomaterials used for medical applications. Zheng et al. defines BMs as “metals expected to corrode gradually in vivo, with an appropriate host response elicited by released corrosion products, then dissolve completely upon fulfilling the mission to assist with tissue healing with no implant residues” [39]. Degradable metal materials need to fulfill two criteria in order to be legitimately classified as BMs. The first of these is the requirement for the metal material to possess suitable degradation rates in vivo, whilst the second criterion is that they must be comprised of an essential metallic element that can be readily metabolized by pathways in the human body. Examples of BMs include those that are magnesium-based, iron-based, zinc-based, or calcium-based and which consist of the pure metals themselves, alloys, or metal matrix composites. Specifically, Mg[40] and Fe[32, 41] based BMs have already started to be used for scaffold fabrication. A more expansive list of BMs and their feasibility for use in medical applications has been published elsewhere [39].
Fe-based BMs were amongst the first used in 3D printing of scaffolds for tissue engineering[32] [41]. Printed 3D scaffolds comprising BM have demonstrated promising in vitro results. In vitro studies have shown that an iron-manganese (Fe-Mn) alloy was suitable for bone scaffold fabrication[32]. Scaffolds were fabricated using an inkjet 3D printing technique, where Fe-Mn constituted the powder bed and an unspecified water-based organic solvent was used as the binder solution. The Fe-Mn scaffold showed strong tensile mechanical properties similar to bone, as well as biodegradability, which allowed the scaffold to adequately support cell proliferation and differentiation[32]. A similar method was implemented in a separate study using an inkjet 3D printer involving Mg3(PO4)2 powder with a binder solution containing 2 M K2HPO4, 0.5 M (NH4)2HPO4 or 20% H3PO4 to form a matrix of either struvite-(K) (MgKPO4.6H2O), struvite (MgNH4PO4.6H2O) or newberyite (MgHPO4.3H2O) by a hydraulic setting reaction[41]. It was demonstrated that hardening of the scaffold post-printing increased the compressive strength to 10 MPa for struvite and 35 MPa for newberyite scaffolds compared to the initial strengths of the printed samples (1.3 - 2.8 MPa)[41]. These compressive strengths are higher than the minimum strength required for scaffolds used for bone regeneration (2 MPa)[42]. However, the potential of these materials to be used for tissue engineering is currently uncertain due to a lack of data on the impact of these materials on cell viability as well as uncertainty as to their biocompatibility. Printing of 3D scaffolds using BMs for bone regeneration shows promise and should be further researched using a variety of metal materials in order to increase the availability of materials for 3D printing. Although these materials and scaffolds may be designed for bone replacement, they may have many other applications for other tissues since non-metallic scaffolds are less robust and can collapse under the contractile force applied by cells during cellular attachment and proliferation[43]. As the availability of BM steadily increases, the need for, and complications brought on by, permanent prosthetics and metal replacement parts can be reduced, thereby allowing patients to regenerate their own bone in the ultimate absence of non-physiological materials. The paucity of research involving metals as biomaterials for 3D printed scaffolds is primarily due to the traditional notion of non-biodegradability and limited processability (high temperatures are needed to form desired structures) attributed to metal materials [28, 44]. However, the implementation of BM aims to counter this notion and increase the practicality of using metals in 3D printing of more innovative and effective scaffolds. BMs are an untapped source for 3D printing of scaffolds for tissue engineering, as they could provide additional mechanical strength to current scaffolds to withstand most compressive and tensile forces.
2.2 Ceramics
Ceramics contain both metallic and nonmetallic elements and have been used as materials for 3D printed scaffolds due to their high mechanical strength and biocompatibility[45]. Ceramics are capable of scaffold fabrication for bone regeneration mainly due to their apatite-mineralization ability[46]. Hydroxyapatite (HA), itself a ceramic, is commonly found in human teeth and bones[47], thus making the use of HA, or similar ceramics, attractive materials for creating scaffolds with strong mechanical properties similar to that of natural bone. HA has garnered much attention in the field of regenerative medicine, and as such, is a commonly used material for 3D printed scaffolds. In one study, a rapid prototyping technique was used to create 3D printed scaffolds from HA with complex internal structures with slopes of 45° to allow for cell proliferation inside of the structure[48]. The interconnecting channels with pore sizes of 500μm in the designed scaffolds displayed the ability to facilitate mouse MC3T3-E1 cell proliferation, illustrating the potential of HA scaffolds to regenerate bone. Another study used computer-assisted 3D printing in a rapid prototyping technique to fabricate HA and tricalcium phosphate (TCP) scaffolds. The scaffolds were created using HA and tricalcium phosphate in a layer-by-layer process followed by sintering [49]. These scaffolds were seeded with human osteoblasts that were isolated from human iliac crest cancellous bone, and showed high biocompatibility and low cytotoxicity. The results provide further evidence that HA materials demonstrate biocompatibility and the ability to assist in cell growth and viability. More recently, the ceramic, calcium silicate (CaSiO3) was used to make scaffolds with higher bone healing capacity than that of tricalcium phosphate scaffolds[46]. The 3D printed scaffolds were printed through a printing device developed by the Fraunhofer Institute for Materials Research and Beam Technology. The device was able to print the CaSiO3 solution (in polyvinyl alcohol) in a controlled layer-by-layer plotting. Using this method it was demonstrated that pore morphology, pore size and porosity of the scaffold could be controlled[46]. It was also found that the CaSiO3 scaffolds could significantly improve bone regeneration compared to tricalcium phosphate scaffolds when implanted in vivo into femur defects in rats. Another ceramic material commonly used for 3D printed bone scaffolds is calcium phosphate [50-52], which, when combined with other ceramics, such as HA[50] and TCP[52], yields scaffolds with pore sizes of 300 μm that are large enough to allow nutrient transfer for cells. These scaffolds were also fabricated with >97.5% accuracy compared to the computer-aided design, allowing for future scaffolds to be intricately designed to mimic the ECM structure necessary for optimal cell attachment and proliferation[50]. This acquired pore size and the high resolution design makes calcium phosphate a suitable material for scaffold fabrication and cellular growth. Other studies have also shown the printability of calcium phosphate with other blended compounds, such as calcium sulfate, to create a powder composite with a water-based binder[51]. Using a combination of ceramic materials for 3D printing of scaffolds should be further investigated to create a material with high precision design, possessing adequate compressive strength, and the ability to promote cell proliferation and differentiation that can be applicable to both non-load bearing and load bearing orthopedic applications.
2.3 Polymers
Polymers represent a major category of materials with potential for use in the 3D printing of scaffolds for tissue engineering. Such polymers include synthetic poly(ethylene glycol) diacrylate (PEGDA) and natural gelatin methacrylate (GelMA)[53], which are both used in the formation of hydrogels[16]. Hydrogels are attractive biomaterials for tissue engineering since they possess adjustable mechanical properties, are biocompatible and have the ability to be hydrated while remaining insoluble and maintaining their 3D structure. In addition, the hydrating properties of hydrogels allow them to mimic those of biological tissue [54, 55]. Bothpoly(ε-caprolactone) (PCL) and poly(D, L-lactic-co-glycolic acid) (PLGA)scaffolds have been manufactured using rapid prototyping. The use of these scaffolds in the defects of rabbit tibias demonstrated their safety as well as their capacity to promote the generation of bone tissue[56]. An advantage of using synthetic polymer materials such as PLGA, and PCL is that both these synthetic polymers have been approved by the FDA for clinical use[57]. Another advantage of using PCL polymers and PLGA copolymers is the low toxicity of their degradation products, which feeds into metabolic pathways[13]. A disadvantage of using PLGA is that it can cause inflammatory responses when there is a build-up of acidic oligomers[58]. Inflammation plays a key role in tissue regeneration[59], but it is important to control or limit this response, as high levels of inflammation can lead to fibrosis resulting in poor tissue function, or even rejection of the implanted scaffold. Thus, it is crucial to understand the inflammatory effect of the particular biomaterial(s) used and the scaffold structure used to generate the desired tissue. The inflammatory response, generally mounted by the innate immune system, promotes the recruitment of cells (primarily neutrophils and monocytes) to the area of tissue damage in order to assist with tissue repair and regeneration[60]. In one study, 3D printed polylactic acid (PLA) and chitosan scaffolds were compared for their ability to induce inflammation and consequently impact on tissue regeneration[61]. Through the analysis of macrophage morphology and human monocyte cytokine profiles (tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, IL-12/23(p40) and transforming growth factor (TGF)-β1) it was concluded that the inflammatory properties of the scaffolds were determined by both scaffold geometry and composition. PLA-based scaffolds promoted a higher production of Il-6, IL-12/23, and IL-10 compared to chitosan scaffolds. Whilst chitosan scaffolds only promoted a higher secretion of TNF-α when compared to PLA-based scaffolds across the range of cytokines tested. It was also concluded that orthogonal scaffolds induced stronger inflammatory reactions compared to that of diagonal scaffolds due to an increased presence of multinucleated giant cells in the orthogonal scaffolds[61]. However, the ideal levels of inflammation to generate functional tissue were not tested and further studies involving other cells types and the formation of functional tissue are required.
Another interesting characteristic of these polymers is their rate of biodegradation, which is often too fast when PLGA is used and too slow when PCL is used. In a polymer degradation assay performed using scaffolds, it was shown that with comparable concentrations of PCL and PLGA, PLGA degraded by 18% at 14 days and 56% by 28 days compared to PCL whose degradation was 33% at 21 days, and 39% at 28 days[62]. Nevertheless both PLGA and PCL may still have beneficial regenerative traits depending on the type of injury[56, 63]. Long term healing may be necessary in open bone fractures[64, 65]. PCL is possibly a preferable choice for open fractures due to its slower degradation rate. A longer healing period is often required in open fractures because the bone has penetrated the skin which can, oftentimes, lead to infections that increase the time for healing. The slow degradation rate of PCL allows the scaffold to provide support for growing cells for a longer period of time enabling more dense tissue to form[56]. For cases such as closed fractures (broken bone that has not penetrated the skin), PLGA could be a potential candidate for bone regeneration.
PCL-based copolymers, such as PLGA-PCL-PLGA[66] and PCL-PEG-PCL[67], have been synthesized to control the degradation of PCL for controlled drug release applications. However, these copolymers have the potential to be used for tissue engineering applications as well. Other synthetic polymers used for scaffolding include polyglycolic acid (PGA), poly(propylene fumarate)(PPF) and poly(hydroxy butyrate)(PHB). Natural polymers, such as proteins and polysaccharides, have also been used for scaffold fabrication, and among these polymers, the most popular candidate for tissue engineering has been collagen type 1[13, 68]. Collagen scaffolds loaded with cationic PEI-pDNA complexes were recently used to create a patch capable of bone regeneration in calvarial defects in rats (Figure 1)[69].
Figure 1. In vivo bone formation in rat calvarial defects implanted with collagen scaffolds.
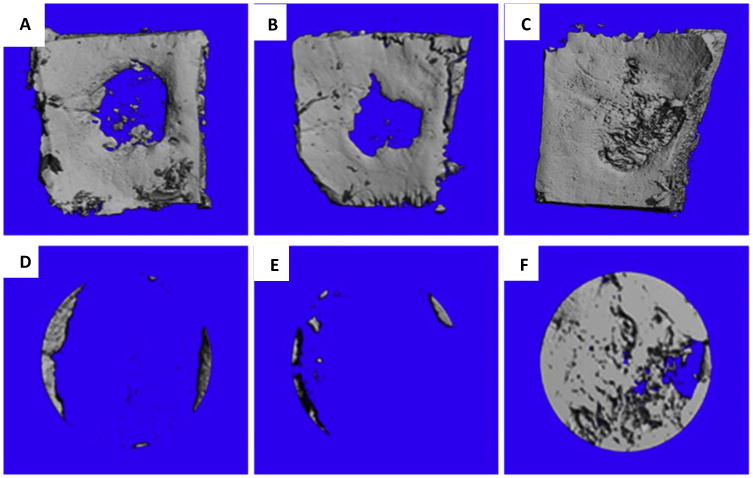
Representative microCT scans showing the level of regenerated bone tissue in calvarial defects 4 weeks after implantation with empty defects (no collagen scaffolds) (A, D), empty scaffolds (collagen scaffolds alone) (B, E,) and PEI-pPDGF-B complex-loaded scaffolds(collagen scaffold with complex) (C, F). Images D, E, F are close up images of the defects and shows the rate of bone regeneration[69]. Reprinted from Biomaterials, 35, Satheesh Elangovan, Sheetal R. D'Mello, Liu Hong, Ryan D. Ross, Chantal Allamargot, Deborah V. Dawson, Clark M. Stanford, Georgia K. Johnson, D. Rick Sumner, Aliasger K. Salem, The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor, 737-747, Copyright 2014, with permission from Elsevier.
This research illustrates the great potential for collagen to be used in 3D printing of implantable scaffolds. In another study, collagen was used with a phosphoric acid binder solution in a 3D ink-jet printing technique to yield scaffolds with improved mechanical strengths, cytocompatibility and bone regeneration capacities[68]. Other natural polymers that have been tested for their potential use in 3D printing are corn starch and dextran. These materials were used in a binder printing technique that utilized a water-based binder to form porous scaffolds. The scaffolds were not tested on cells for biocompatibility, however, with post-processing and additional fabrication methodologies, these materials could yield interconnected, high resolution scaffolds [70].
2.4 Composites
Composite materials, or composites, are important in the field of tissue engineering as they enable commonly used materials for 3D printing to have increased mechanical strength and more intricately designed scaffolds. One group made 3D printed scaffolds for bone regeneration comprising a composite of calcium phosphate and type I collagen using a ZPrinter® 450 printer to demonstrate the feasibility of the process and to enhance mechanical and cellular benefits in vitro[68]. In a separate study, a nozzle extrusion-based 3D printer was used to generate PLA-based composite scaffolds consisting of PLA and bioactive CaP glass. These scaffolds were made with two different layer designs: orthogonal layer configuration (ORTH) (Figure 2) and displaced double-layer design (DISPL) (Figure 3) to test the ability of nozzle-based rapid prototyping printing to print biodegradable scaffolds with different porosity and high mechanical properties[71]. The printed scaffolds were highly porous and possessed mechanical properties that were dependent on the layer design, where ORTH scaffolds yielded three times higher compression modulus strength at (90k+ MPa) compared to DISPL scaffolds (∼30k MPa). Composites also play a major role in increasing the mechanical properties of hydrogels. Composite hydrogels comprising ceramics, for example, can maintain their hydrophilic polymeric network to mimic that of innate tissue, whilst increasing their mechanical strength to withstand the compression forces caused by cell proliferation and differentiation[54]. Composites may be the key biomaterials for 3D printing of ECM-like scaffolds.
Figure 2. 3-D printed scaffolds with orthogonal layer configuration.
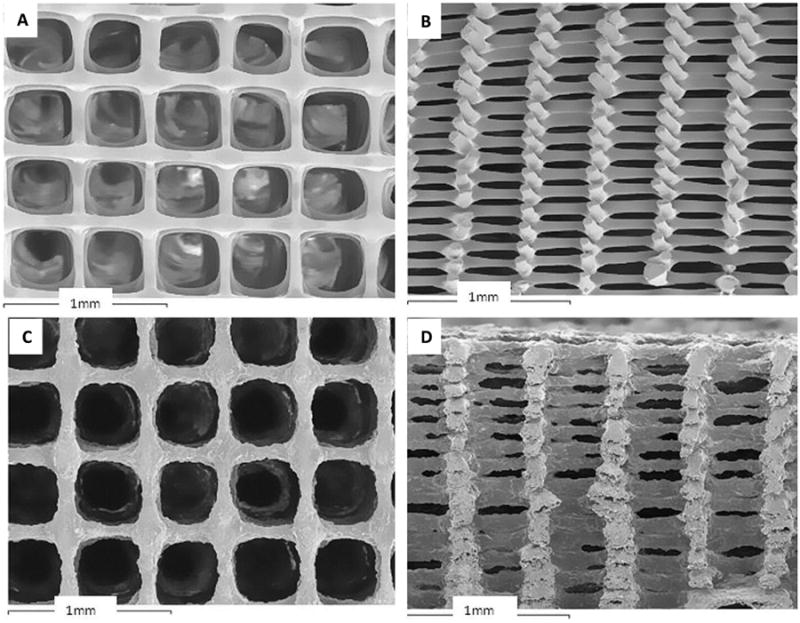
SEM images of scaffolds made from (A, B) PLA/PEG; (C, D) PLA/PEG/G5; (A, C) top view; (B, D) cross-section view[71]. Reprinted from Acta Biomaterialia, 9, T. Serra, J.A. Planell, M. Navarro, High-resolution PLA-based composite scaffolds via 3-D printing technology, 5521-5530, Copyright 2013, with permission from Elsevier
Figure 3. 3D printed scaffolds with displaced double-layer design.
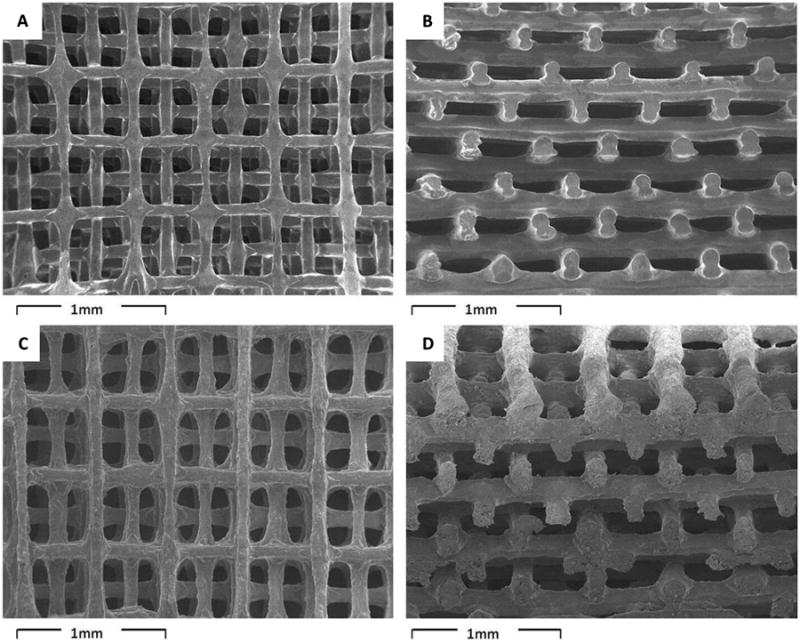
SEM images of scaffolds made from (A, B) PLA/PEG; (C, D) PLA/PEG/G5; (A, C) top view; (B, D) cross-section view[71]. Reprinted from Acta Biomaterialia, 9, T. Serra, J.A. Planell, M. Navarro, High-resolution PLA-based composite scaffolds via 3-D printing technology, 5521-5530, Copyright 2013, with permission from Elsevier
A more comprehensive review of 3D printed materials for bone tissue engineering has been published elsewhere [28]. The search for the optimal material or material blend for 3D printed scaffold fabrication is an ongoing challenge. This research is necessary, as different types of tissue replacements require different specifications such as specific pore sizes, scaffold morphologies, or mechanical strengths. Scientists are continuously searching for more effective scaffolds capable of mimicking the ECM for cellular attachment, proliferation, and differentiation resulting in the formation of functional tissue. To date, the majority of research on 3D printed scaffolds has been concerned with bone tissues, and therefore more research is necessary in the field of tissue engineering with respect to other tissues such as cardiac tissue. Newly designed composites and synthesized biomaterials may pave the way for 3D printed scaffolds with >99% precision, 100% interconnectivity, versatile pore size manipulation, and high mechanical strengths for a range of load-bearing and tissue formation applications.
3D Printing Techniques for Scaffold Fabrication
In the last decade, many different techniques have been used to form porous 3D biomimetic scaffolds, and have included phase-separation, self-assembly, electrospinning, freeze drying, solvent casting/particulate leaching, gas foaming, and melt molding. Using scaffolds, the architecture of native extracellular matrices can be mimicked at the nanoscale level and therefore provide the primary base for the regeneration of new tissue[72]. Originally a “top-down” approach was used as a tissue engineering method for scaffold fabrication. In this method, cells are seeded onto a biodegradable and biocompatible scaffold, and are predicted to migrate and fill the scaffold hence creating their own matrix. By using this technique, several avascular tissues such as bladder[73] and skin[74] have been engineered effectively. However, due to the limited diffusion properties of these scaffolds, this technique faces several challenges for fabrication of more complex tissues such as heart and liver.[75] Therefore, “bottom-up” methods have been developed to overcome this problem.[76] Bottom-up approaches include cell-encapsulation with microscale hydrogels, cell aggregation by self-assembly, generation of cell sheets, and direct printing of cells.[77] These tissue blocks can be assembled to form complex tissue constructs using various methods including microfluidics,[78] magnetic fields,[79] acoustic fields,[80] and surface tension.[81] These methods are relatively easy and have provided a solid foundation for the fabrication of scaffolds. However, as mentioned previously, these conventional methods suffer from several limitations including inadequate control over scaffold properties such as pore size, pore geometry, distribution of high levels of interconnectivity, and mechanical strength. As such, it is necessary to develop technologies with sufficient control so as to design more intricate tissue-specific scaffolds. In addition, scaffolds can be coated using surface modification techniques (such as introducing functional groups) to enhance cell migration, attachment and proliferation. Three-dimensional printing allowed scaffolds to become more precisely fabricated (similar to that of the computer-aided design (CAD)) with higher flexibility in the type of materials used to make such scaffolds. Three-dimensional printing uses an additive manufacturing process where a structure is fabricated using a layer-by-layer process. Materials deposited for the formation of the scaffold may be cross-linked or polymerized through heat, ultraviolet light, or binder solutions. Using this technology, 3D printed scaffolds can be prepared for optimized tissue engineering. For appropriate formation of tissue architecture, the seeding cells (often stem cells) require a 3D environment/matrix similar to that of the ECM. The ECM acts as a medium to provide proteins and proteoglycans among other nutrients for cellular growth. The ECM also provides structural support to allow for cellular functionality such as regulating cellular communication, growth, and assembly[82]. With this in mind, scientists and engineers originally attempted to replicate the ECM through conventional techniques, which consequently established a framework for using more advanced techniques, such as 3D printing, to yield higher quality scaffolds. The 3D printing technique can create defined scaffold structures with controlled pore size and interconnectivity and the ability to support cell growth and tissue formation [28, 83,84]. The current methods for 3D printing involve a CAD, which is then relayed to each 3D printing system to “print” the desired scaffold structure. Through various 3D printing technologies, discussed below, researchers are trying to fabricate biocompatible scaffolds that efficiently support tissue formation.
1.1. Computer Aided Design and Digital Imaging
The start of many 3D printing processes involves a CAD that must be drawn or taken from known organ structures. Generally 2D slices acquired from imaging instruments are compiled and stacked on top of one another to form a 3D structure[85, 86]. In tissue engineering, it is imperative to grow tissue similar to that of the native tissue and in order to accomplish this, imaging techniques can be used to produce scaffolds that closely mimic the structure of native tissues[87]. These images inform scaffold designs by providing morphology and size parameters to which scaffolds need to conform in order to fit into irregularly shaped defects/fractures where tissue formation is desired. The scaffold shape also helps to direct the growth of cells and provide shape for the final tissue[88]. It is also worth noting that scaffold shape can affect the type of tissue regenerated as can be seen in dentin tissue regeneration with differentially shaped scaffolds using dental pulp-derived cells[89]. The complexities in morphology and architecture of tissues can be delineated with imaging technologies such as magnetic resonance imaging (MRI) and computer tomography (CT). These imaging technologies help to take cross-sectional slices of organs and compile them into a 3D image, thus allowing the design of scaffolds to be a close representation of native organs[85].
MRI functions by using magnetic fields and pulsating radio waves to yield detailed pictures of organs and soft tissues. Using gradient coils to interpret energy signals produced by water molecules within the tissue, 2D images are generated[90]. These 2D images are then stacked to create a 3D image of the scanned area. Because MRI requires hydrogen molecules generally in water, they are best used for soft tissue imaging such as ligament and tendons and organs of the chest and abdomen (heart, spleen, pancreas, liver, kidneys). They are also used to image pelvic organs such as the bladder and reproductive organs.
CT, also known as computerized axial tomography, is a technology that uses X-rays to produce images from a scanned area. In a CT scanner, x-ray tubes are rotated around a patient's body producing signals that are taken up by digital x-ray detectors and sent to be processed by a computer to generate cross-sectional images of the body. These cross-sections are then stacked to create a 3D image of the scanned organ(s). CT scans are generally used for imaging bone due to its density while soft tissues can be problematic as they have varying abilities to inhibit x-ray penetration resulting in faint or undefined images. In order to image these soft tissues, contrasting agents such as iodine or barium-based compounds may be used to facilitate contrast and increase visibility[91].
MRI is preferable over CT when attempting to image soft tissue and other organs besides bones as the contrast of tightly placed organs can more readily seen when changes to radio waves and magnetic fields are applied. The radio waves and magnetic field enables the ability of the instrument to highlight the desired tissue in tightly knitted areas. However, CT scans create better quality images of bone structures than MRI due to the low concentration of water in bones resulting in less hydrogen atoms emitting energy to succinctly create a cross-sectional image. Creating a scaffold directly from the images is not always feasible due to the possibility of scanning diseased or damaged organs [85]. In this case, computer modeling may be necessary to recreate the missing parts of the organ or tissue. With MRI and CT imaging techniques, the reconstruction of both 2D and 3D images is a powerful tool to recreate the complexity of tissue structures. These tools allow researchers to be one step closer to fabricating a precise replica of the needed extracellular matrix to enhance functional tissue formation.
1.2. Direct 3D Printing
Three-D printing involves the fabrication of structures through successive layer deposition using a computerized process. The first “3D printer” was developed by investigators at the Massachusetts Institute of Technology (MIT) in the 1990s, and was based on the technology of an ordinary inkjet printer. This printing technique can sometimes be referred to as “binder jetting” or “drop-on-powder”[92]. In an ordinary 2D inkjet printer, the ink nozzle moves in a side to side motion incrementally along one plane such that the printed material has the 2 dimensions of length and width. A 3D printer uses the same technology, but as well as moving side to side along one plane, the printer has a platform capable of moving up and down (90 degrees to the side to side motion), hence adding the dimension of height and thereby printing in 3 dimensions. The 3D printer designed by MIT has similar characteristics to the 2D inkjet printer, however, instead of ink, the 3D printer uses a liquid binder solution that is selectively deposited on a powder bed instead of paper. The process begins with a powder bed, which could vary depending on materials used, that is spread onto the build platform and leveled using a roller system. The printer nozzle then dispenses binder solution in the designated powdered areas directed by the CAD. Once the binder solution and powder are combined, the excess powder is removed (blown off). The build platform is then lowered, and a new powder layer is deposited and leveled. This process is then repeated until the final structure is created (Figure 4). This technique also has the versatility to change the composition of binder and powder if it is deemed necessary where certain parts of the scaffold may require a material with higher mechanical strengths and/or smaller pore sizes. An example of this may be building a scaffold with larger pore sizes deep within the scaffold, while having smaller surface pores. With the increase in pore sizes, cells deep within the scaffold will be able to maintain their cellular processes as vital resources such as nutrients, oxygen, and waste are able to diffuse without difficulty compared to small pore sizes that may result in the nutrient deprivation of cells leading to cell death. Cell death on a large scale ultimately leads to the collapse of the scaffold and the inability to form functional tissue. The resulting desired effect of the fabricated scaffold will be to provide a medium that guarantees high proliferation and differentiation of cells to generate functional tissues. The advantages of this method are the expansive list of powder-binder solutions available to yield the desired scaffold. The use of binders, however, can lead to toxicity if they are not completely removed once the scaffold is ready to be implanted, as in the case of organic solvents that are used as binders for some powdered polymer materials. Another disadvantage for this printing technique is the post processing required, where heat treatment may be necessary to ensure durability[93].
Figure 4.
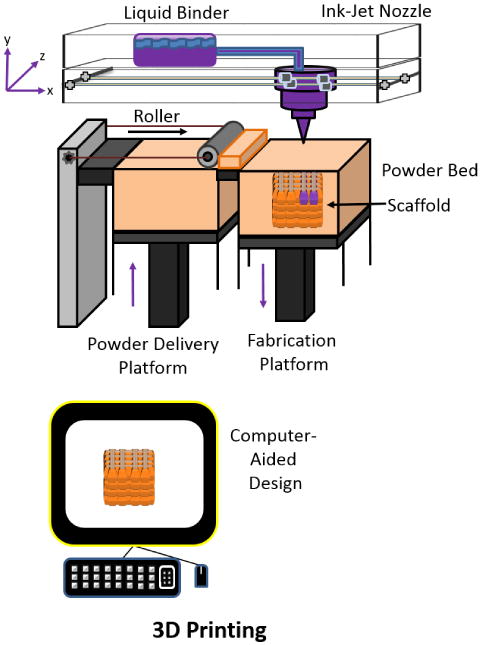
Schematic of direct 3D printing of a CAD scaffold. Adapted from “Porous scaffold design for tissue engineering” by Scott Hollister, Nature Materials 2006, 5, 590.[94]
Three-D printed scaffolds for tissue engineering are showing great promise as many new 3D printers become available through various companies. Using a Palmetto 3D printer, developed by investigators at the Medical University of South Carolina and Clemson University (South Carolina), the feasibility of fabricating alginate scaffolds using CaCl2 as the liquid binder was established[95] (Figure 5).
Figure 5. Fabrication of alginate scaffolds using 3D printing.
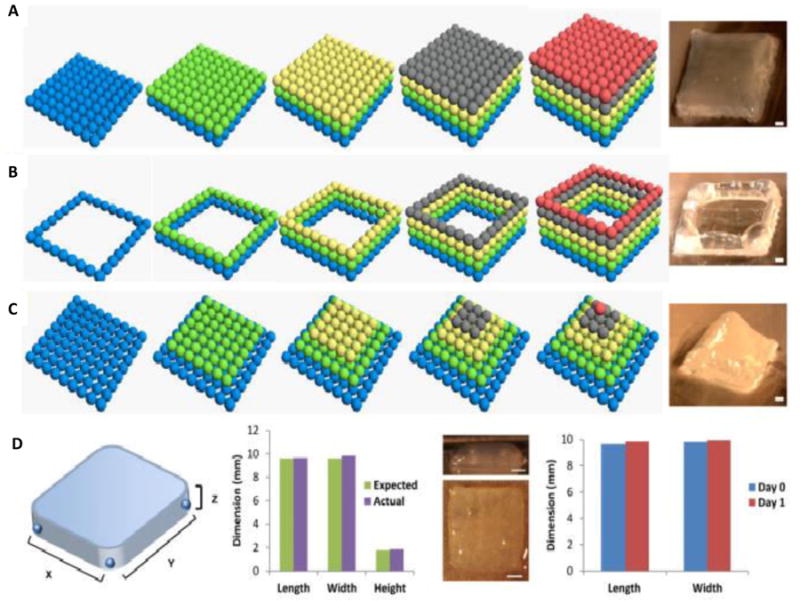
Formation of scaffold facilitated by 3D printed alginate hydrogels microdroplets with different geometries (cube (A), square frame (B) and pyramid (C)). The scale bar for (A)–(C) is 1 mm. The scale bar for (D) is 2 mm. The blue, green, yellow, gray and red represent the first, second, third, fourth and fifth layers of bioprinted alginate microdroplets, respectively[95]. Reprinted from Y. Tan, D. J. Richards, T. C. Trusk, R. P. Visconti, M. J. Yost, M. S. Kindy, C. J. Drake, W. S. Argraves, R. R. Markwald, Y. Mei, Biofabrication 2014, 6, 024111, Copyrighted 2014, with permission from IOP Publishing.
Biocomposite scaffolds comprising of chitosan and hydroxyapatite were 3D printed using a Spectrum 510 3D printer made by Z-Corp to yield dense (solid, nonporous) and porous cylindrical scaffolds[96]. These scaffolds were created by applying a binder solution consisting of 40 wt.% lactic acid to different chitosan/hydroxyapatite composites (20 wt.%, 25 wt.%, and 30 wt.% chitosan) followed by a post-hardening process. Optimal mechanical properties were observed with solid scaffolds printed using 25 wt.% chitosan as demonstrated by their high compression strength of 16.32 MPa and Young's modulus of 4.4 GPa[96]. However, the researchers were only capable of fabricating nonporous scaffolds with the desired high mechanical strength, which is problematic as porosity is an important property since it allows for diffusion of oxygen, nutrients, and cellular waste. The reason for the ineffectiveness of the porous scaffold is due to the post hardening process, which involves high concentrations of solvent immersion that ultimately led to the collapse of the porous scaffolds[96]. Therefore the fabrication of 3D printed chitosan/hydroxyapatite scaffolds for tissue engineering, whilst promising, still requires further optimization.
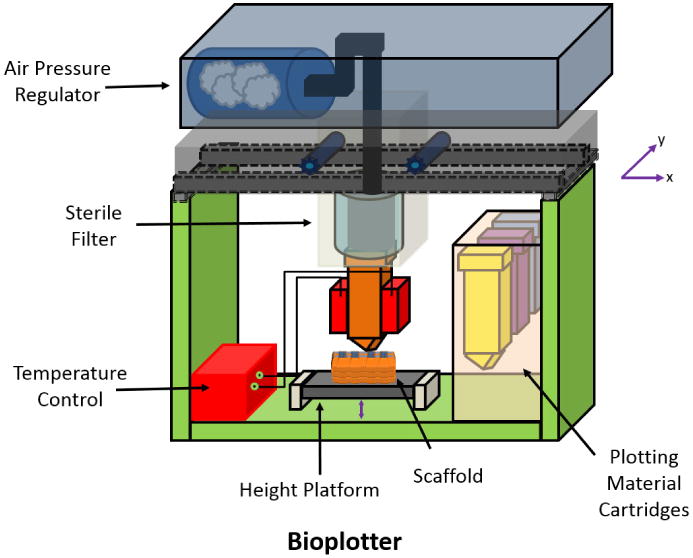
Bioplotter Printing
Bioplotter printing is a rapid manufacturing technique that uses a nozzle extrusion system of thermally or chemically treated materials (Figure 6). As with all 3D printing methods, a CAD is first created and then sent to the 3D printer. The materials are deposited in a layer-by-layer fashion, where each layer may contain a combination of different materials. Similar to that of ink cartridges in an inkjet printer, the Bioplotter printer is capable of using and changing “bioink” to develop the final scaffold structure. A key feature of Bioplotter systems is that they print cell-laden gels, often with other polymeric materials such as PCL, to yield viable and functional scaffolds[15, 97]. The printer utilizes a pneumatic pressurized system to extrude the material from the bioink cartridges. The disadvantage of using this system is the shear stress from the variously sized nozzles, which may impact negatively on cell viability during the printing process[98].
Figure 6. Schematic of Bioplotter Printer set-up.
Another valuable addition to 3D printing is the introduction of growth factors such as vascular endothelial growth factor (VEGF) or platelet-derived growth factor (PDGF) to enhance cell proliferation and differentiation and, in particular, to promote angiogenesis. The addition of these growth factors can increase the rate of tissue formation in scaffolds as well as generating a resilient tissue due to enhanced differentiation. In one study, in order to promote vascularization, a bioscaffolder pneumatic dispensing system was used to incorporate VEGF in a 3D printed matrigel-alginate scaffold [99]. The use of gelatin microparticles to control the release of VEGF in a sustained manner resulted in higher vascularization compared to scaffolds with no growth factors and scaffolds with fast release of VEGF when applied in murine models. The use of growth factors is still somewhat in its infancy in regards to 3D printing, as there have been fewer than 10 studies performed in last 5 years in regards to fabricating scaffolds with embedded growth factors, however, with their increased use, researchers will be able to create complex shaped scaffolds to more closely mimic the in situ ECM conditions required for optimal organogenesis.
In order to develop a more efficient all-in-one system, one group used a NovoGen MMX Bioprinter from Organovo which comprised two pumps and two nozzles[100]. This system was capable of dispensing gelatin methacrylate (GelMA) hydrogels, whilst simultaneously dispensing cells to seed the scaffold. This system enables the direct addition of cells into the scaffold rather than waiting to seed the cells after scaffold fabrication. This direct seeding has the advantage of homogenously distributing cells throughout the scaffold, as well as being less time consuming. A UV light guide was also added to the printer to allow for photopolymerization of the GelMA. This system generated HepG2 cell-laden scaffolds capable of retaining high cell viability for at least eight days in vitro. This study illustrates the viability of using a 3D printer to print scaffolds for complex tissue engineering processes. In a separate study, similar materials were used in a projection stereolithography system to 3D print GelMA scaffolds in a layer-by-layer process [101]. This technique allowed scaffolds to be fabricated down to microscale sizes while still being a viable option for supporting cell growth. The emergence of projection printing as a potential tool for 3D printing of scaffolds will allow researchers to design microsized scaffolds with high precision and be implanted directly into certain parts of the body without being overly intrusive. Researchers have seen success in the use of a 3D-Bioplotter® from Envision TEC to produce cell-laden 3D printed scaffolds. The 3D-Bioplotter® has been used for bone regeneration[102, 103] and soft tissue biofabrication[104].
Another Bioplotter printer is a nozzle-deposition tool used to fabricate 3D scaffolds called the Tissue Engineering 3Dn-300 that was designed by Sciperio/nScrypt Incorporated. This printer was used to yield PLA/PEG blends with 5, 10, and 20% (w/w) of PEG and PLA/PEG/bioactive calcium phosphate (CaP) glass composite scaffolds. The blend incorporated the use of PEG as a plasticizer to decrease the glass transition temperature of the blend and enable processing at low temperatures [105]. The addition of PEG improved scaffold processing, however, the ability of these scaffolds to support cell growth both in vitro and in vivo is yet to be explored. Using a modified Bioplotter printer, a multihead tissue/organ building system was used to print PCL and cell laden hydrogels, while using PEG as a sacrificial layer. This system utilized a hybrid system of inkjet printing (hydrogels) and fused deposition modeling (thermoplastics) to enhance the mechanical stability of the scaffold, as some hydrogels demonstrate poor mechanical properties[106]. Using this system, a complex-shaped scaffold for ear regeneration was fabricated[27]. The process involved the creation of a sacrificial layer as a base for the formulation of complex structures that was easily dissolved away. Similar to trying to build an inverse pyramid or bowl-shaped structures, there needs to be a support layer to allow the complex structure to take shape. Using these complex ear-shaped scaffolds (Figure 7), positive in vitro results of chondrogenesis and adipogenesis from the co-printed chondrocytes and adipocytes were obtained. The use of this hybrid system will allow direct 3D printing to increase its flexibility in designing scaffolds with even the most complex shapes.
Figure 7. Ear-shaped scaffolds.
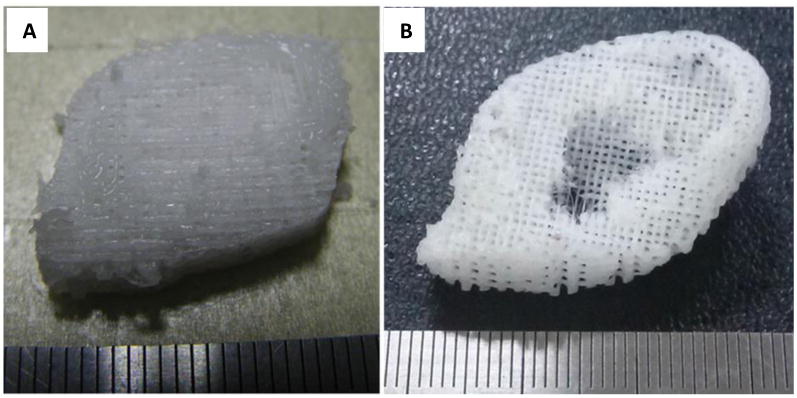
Photographs of ear shaped structures made from PCL using a PEG sacrificial layer (not shown) through a hybrid 3D printing system combining both inkjet printing and fused deposition modeling. (A) back of ear scaffold, (B) front of ear scaffold[27]. Reprinted from J. S. Lee, J. M. Hong, J. W. Jung, J. H. Shim, J. H. Oh, D. W. Cho, Biofabrication 2014, 6, 024103, Copyrighted 2014, with permission from IOP Publishing.
In a more recent study, a multihead tissue/organ building system was used to bioprint cell-laden scaffolds with decellularized extracellular matrix (dECM)[107]. The use of dECM is an attempt to closely mimic the complexities of the ECM by providing infiltrating or embedded cells with an environment similar to native tissue. Adipose ECM, cartilage ECM, and heart ECM were decellularized and used to create matrices capable of yielding high cell viability and functionality. The ability of scaffolds to yield tissue-specific gene expression in cell-laden gels and PCL frameworks was investigated. In this part of the study, human adipose-derived stem cells (hASCs) and human inferior turbinate-tissue derived mesenchymal stromal cells (hTMSCs) were used in scaffolds containing adipose ECM or cartilage ECM respectively to assess the ability of the scaffolds to provide an environment with the ability to effectively increase adipogenic or chondrogenic differentiation of adult stem cells[107]. Depending on the dECM used in the printing process, it can be specifically related to the increase in cellular differentiation of that specific type of tissue formation, as can be seen when the researchers cultured hASCs in decellularized adipose (adECM) or hTMSCS in decellularized cartilage (cdECM) to assess tissue-specific gene expression. The hASC culturing yielded increased expression of adipogenic markers over time and the adECM demonstrated an “adipoconductive” environment, while the hTMSC differentiation into a chondrogenic lineage was observed [107]. The researchers demonstrated for the first time, the ability of dECM bioink to be incorporated into 3D printed cell-laden scaffold constructs that were capable of harboring cells with high cell viability, cell differentiation, and generating functional tissue formation. The potential of using dECM increases the versatility of 3D scaffolds used for tissue engineering.
1.3. Fused deposition modeling
Fused deposition modeling (FDM) is a 3D printing technique that utilizes thermoresponsive polymers that have been heated beyond their glass transition temperatures and deposited onto a solid medium. This process works as a coiled thermoplastic polymer filament that is unwound and extruded through a heated nozzle onto a fabrication platform. Upon contact with the base (platform), the polymer hardens and sets. After a layer has been deposited, the process repeats itself in a layer-by-layer process until the CAD designed structure has been completed (Figure 8).
Figure 8.
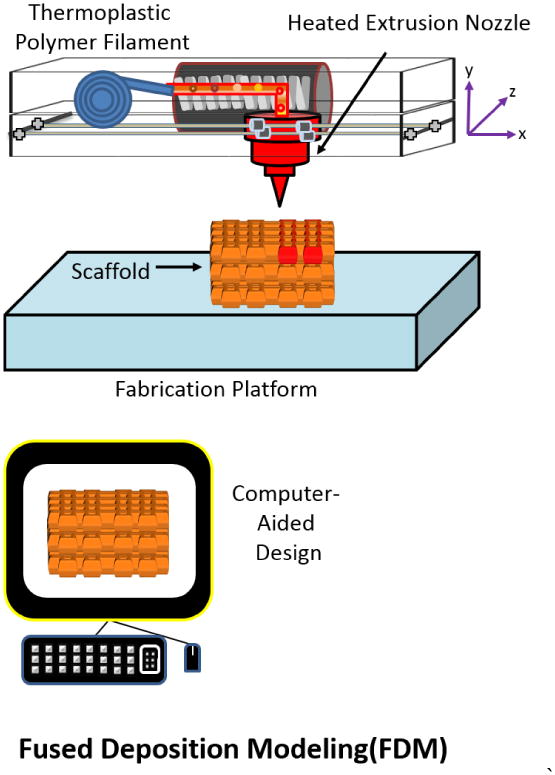
Schematic of Fused Deposition Modeling: a coil filament is printed through a heated extrusion nozzle and solidifies upon contact with the fabrication platform. Adapted from “Porous scaffold design for tissue engineering” by Scott Hollister, Nature Materials 2006, 5, 590.[94]
An advantage of using FDM is that it eliminates any potential toxicity caused by organic solvents that are often necessary to solubilize certain polymers, such as dichloromethane being used to solubilize PLGA[108]. The requirement of a thermoplastic material for this technology limits its application and versatility with respect to scaffold fabrication where the most commonly used material is acrylonitrile butadiene styrene (ABS). Other polymers, such as polycarbonate (PC), polyetherimide (PEI), and polyphenylsulfone (PPSF) have been used in FDM[109]. However, these materials are generally not used for tissue engineering purposes but rather for printing everyday objects such as most modern LEGOs. Further research is necessary to discover other thermoplastics, such as polyesters, that are viable options for scaffolding for tissue engineering. Even with this limitation, FDM has proven to be an important technique in fabricating scaffolds for tissue engineering. The primary option for FDM printed scaffold for tissue engineering is the polyester, PCL[110] as well as composites of PCL such as PCL-TCP[111, 112], and HA/PCL[113]. In a recent study, a multihead deposition system was used to mix PCL and PLGA to yield a blended scaffold with pore sizes of ∼300μm, and a high compressive strength of 3.2 MPa (Figure 9)[108]. The fabricated scaffolds, combined with mussel adhesive proteins as a functionalization material, yielded high cell attachment and proliferation of human adipose tissue-derived stem cells. This scaffold also yielded positive results in in vivo studies, where enhanced bone regeneration was seen in a calvarial defect rat model (Figure 10)[108].
Figure 9. SFF-based 3D PCL/PLGA scaffold.
SEM images of (A) whole structure of the SFF-based 3D PCL/PLGA scaffold. (B) Surface morphologies of (left) control, (middle) fp-151-, and (right) fp-151-RGD-coated scaffolds [108]. Reprinted from Acta Biomaterialia, 8, Jung Min Hong, Bum Jin Kim, Jin-Hyung Shim, Kyung Shin Kang, Ki-Joo Kim, Jong Won Rhie, Hyung Joon Cha, Dong-Woo Cho, Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins, 2578-2586, Copyright 2012, with permission from Elsevier
Figure 10. Effect of scaffolds on bone regeneration.
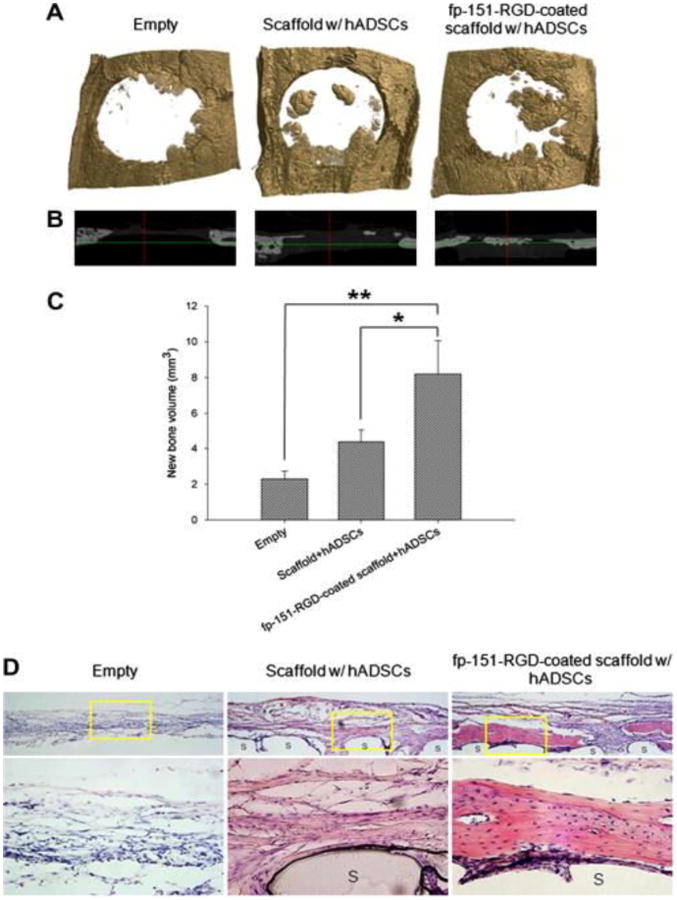
Calvarial defects in rats were implanted with various (indicated) PCL/PLGA blended scaffolds made using fused deposition modeling. (A) μCT scan 3-D and (B) X-ray 3-D axis images of rat calvaria and (C) quantified new bone volume after 8 weeks of implantation. (A) represent the calvarial defect margin. Values and error bars represent the means of quintuple samples and standard deviations with statistical significance (*p < 0.05 and **p < 0.01). (D) Histological analysis of in vivo bone regeneration 8 weeks after implantation. hADSC = human adipocyte-derived stem cells; fp-151-RGD = a genetically redesigned hybrid mussel adhesive proteins[108]. Reprinted from Acta Biomaterialia, 8, Jung Min Hong, Bum Jin Kim, Jin-Hyung Shim, Kyung Shin Kang, Ki-Joo Kim, Jong Won Rhie, Hyung Joon Cha, Dong-Woo Cho, Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins, 2578-2586, Copyright 2012, with permission from Elsevier
Another group further utilized FDM in a hybrid system to generate a scaffold with both natural and synthetic polymers in order to take advantage of the ability of the natural polymers to mimic the structure and biological function of the ECM[19]. In this study, PCL was processed by FDM as the base of the scaffold, which was then immersed into hyaluronic acid solution for 24 hours, followed by freeze drying. After the scaffold was dried, a coacervation reaction was completed by pipetting a methylated collagen solution into the dried scaffold. The newly coated PCL-HA-collagen scaffold was completed with a final coating of a 3% terpolymer solution consisting of hydroxylethyl methacrylate, methyl methacrylate, and methacrylic acid (HEMA–MMA–MAA). This hybrid scaffold, consisting of synthetic and natural polymers, allowed cells to penetrate through the entire structure. In vitro experiments were performed where cell suspensions were seeded into the scaffold during the addition of methylated collagen to the freeze-dried PCL-HA scaffolds. The researchers were able to support their hypothesis that self-assembled composite matrix and dynamic culture could enhance mesenchymal stem cell attachment, differentiation, and distribution compared to static cultures. In the dynamic cultures with the PCL composite scaffold, the scaffold promoted synergistic enhancement of osteogenesis in a telomerase reverse transcriptase gene-transduced cell population of human bone marrow-derived mesenchymal stem cells (hMSC-TERT) when compared to naked (non-coated PCL scaffolds) dynamic cultures and naked and embedded (coated) static cultures [19]. The enhancement of osteogenesis was define through calcium deposition levels and gene expression of osteogenic markers (alkaline phosphatase, osteocalcin, RunX2, Collagen, and BSP-1) which were noticeably higher in embedded dynamic cultures, indicating higher levels of mineralization[19].
A new approach that may warrant the increased use of FDM is the addition of electrospinning. One group was able to use an in-house melt electrospinning device to create a biphasic scaffold with a bone and periodontal compartment. Medical grade PCL-TCP membrane scaffolds, acting as the bone compartment, were fabricated using FDM and then coated with calcium phosphate, while the periodontal compartment was electrospun through a melt electrospinning device. A biphasic scaffold was then assembled by compressing a partially fused CaP-coated bone compartment (FDM scaffold) onto a periodontal compartment (melt electrospun mesh)[114]. The newly designed scaffold had pore sizes ranging from 100 – 400μm, which was large enough for cellular diffusion. In vivo testing of the scaffold confirmed tissue integration between both compartments, forming a tissue structurally resembling native periodontal tissues and establishing high levels of vascularization and tissue orientation in both bone and periodontal compartments[114]. The addition of multiple printing techniques and novel scaffold designs may give rise to advanced 3D printing technologies capable of fabricating higher quality scaffolds for tissue engineering.
1.4. Selective laser sintering (SLS)
In the fabrication of 3D scaffolds using the selective laser sintering (SLS) method, a layer of powder is smeared onto a surface, followed by the sintering of powdered particles together in a desired pattern using a laser beam (usually a CO2 laser). After deposition of the first layer, the process is repeated and another layer is applied to the pre-existing layer (Figure 11).
Figure 11.
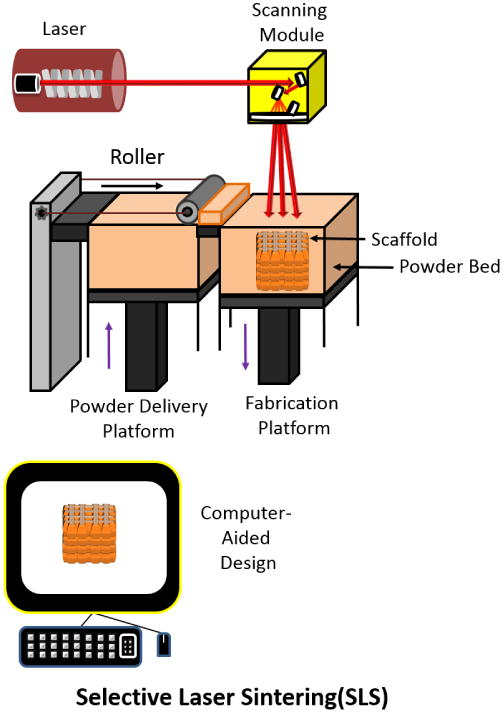
Schematic of Selective Laser Sintering technique. Adapted from “Porous scaffold design for tissue engineering” by Scott Hollister, Nature Materials 2006, 5, 590.[94]
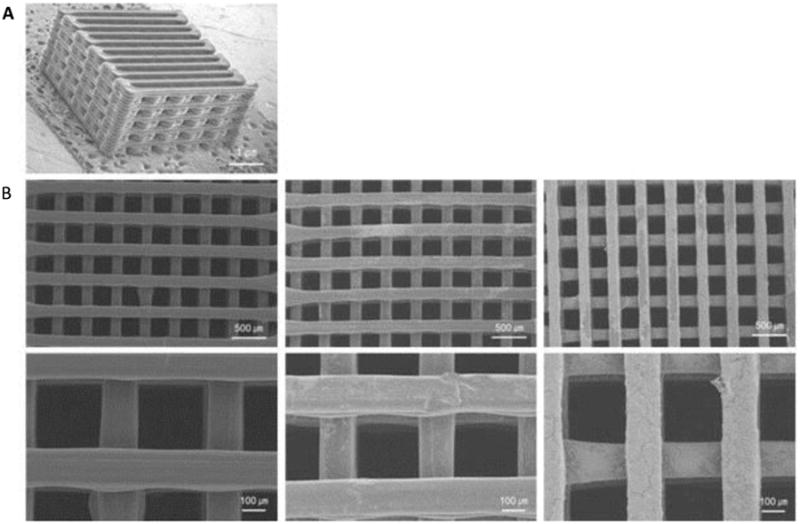
The SLS process has been used to prepare scaffolds from biocompatible and biodegradable polymers such as polyetheretherketone, polycaprolactone, polyvinyl alcohol, and poly(lactic acid). In one report, a biocomposite slurry consisting of hydroxyapatite (HA), silica sol, and sodium tripolyphosphate was used to generate scaffolds using SLS (Figure 12)[115]. These scaffolds demonstrated high mechanical strengths of up to 43.26 MPa but possessed low porosity. However, the in vitro studies indicated the feasibility of using these scaffolds for the growth of osteoblast-like cells[115]. Using SLS techniques can be beneficial when high mechanical strength and low porosity are required; however, a limitation of this technique is the requirement for powdered material to be capable of withstanding laser heat, as well as resisting shrinkage of the scaffold during the sintering process. Another potential disadvantage of using SLS is the pre- and post-heating treatments of the powdered material between the crystallization and glass transition or the melting temperatures to reduce the shrinkage caused by the laser to the scaffolds[116]. Another problem is that the material formulation of the scaffold must be able to withstand high temperatures reaching up to 1400°C[115] depending on the material being used. These criteria of high temperature treatments and maintenance are disadvantages due to time and cost.
Figure 12. Bone scaffolds generated by SLS.
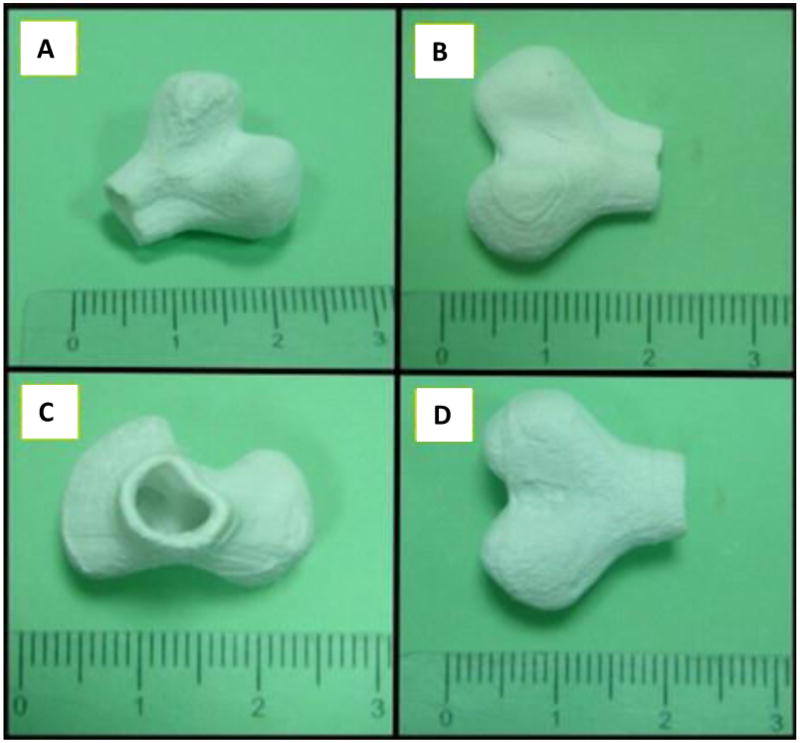
(A) Image of the scaffold, (B) front view, (C) top view, and (D) back view of bone scaffold parts[115]. Size is based on a centimeter ruler, with hatch marks indicating per millimeter. Reprinted from Applied Surface Science, 297, Fwu-Hsing Liu, Synthesis of biomedical composite scaffolds by laser sintering: Mechanical properties and in vitro bioactivity evaluation, 1-8, Copyright 2014, with permission from Elsevier
Selective laser sintering utilizes many parameters, such as powdered material, laser power, scan spacing, layer thickness, part bed temperature, scan speed, and roller speed, all of which can be optimized in order to fabricate complex and intricate scaffolds[117, 118]. Three of these parameters, laser power, scan spacing, and laser thickness have been optimized in the production of quality SLS scaffolds[118]. By optimizing these parameters the researchers were able to fabricate a nanocomposite scaffold made from poly(hydroxybutyrate-co-hydroxyvalerate)(PHBV) microparticles (oil-in-water method) and calcium phosphate/PHBV nanocomposite microspheres (solid-in-oil-inwater method). It was determined that the parameters investigated had a significant effect on mechanical properties (compressive properties), accuracy, and stability of the scaffolds[118]. Yielding higher quality scaffolds with predetermined properties can be achieved by optimizing the parameters of SLS, however, these scaffolds will need to be further investigated in vitro and in vivo to assess their functionality and efficacy.
Researchers have also shown the ability to use SLS printing to print scaffolds using PCL[119] or PCL/HA[120] powdered mixtures with precision(Figure 13). The scaffolds fabricated had high compression modulus ranging from 52-67 MPa and with the seeding of bone morphogenetic protein-7 transduced primary human gingival fibroblasts, illustrated generation of bone in vivo through histological staining and μCT data[119]. The studies show the potential use of SLS printing for bone tissue engineering.
Figure 13. SLS design file and actual fabricated scaffold.
(A) STL design file for the 1.75mm x=y=z porous scaffold. (B) 1.75mm x=y=z PCL scaffold fabricated by SLS[119]. Reprinted from Biomaterials, 26, Jessica M. Williams, Adebisi Adewunmi, Rachel M. Schek, Colleen L. Flanagan, Paul H. Krebsbach, Stephen E. Feinberg, Scott J. Hollister, Suman Das, Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering, 4817-4827, Copyright 2005, with permission from Elsevier
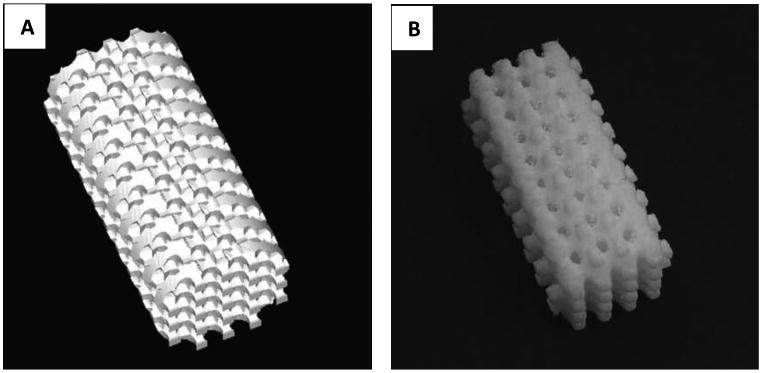
1.5. Stereolithography(SLA)
Stereolithography (SLA) is a process where 3D scaffolds are formed from a liquid polymer via a light-mediated chemical reaction. In this process, a photosensitive (photocurable) polymer is deposited onto a surface medium which is then exposed to light (UV range of 300 – 400nm)[121-124]. After the first layer is cured, this process is repeated, overlaying the previous layer, until the scaffold has been fully designed (Figure 14).
Figure 14.
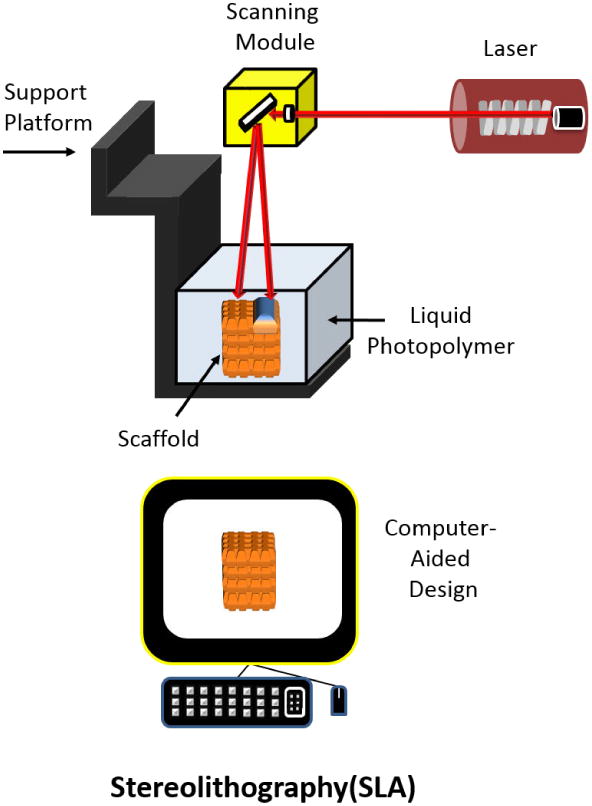
Schematic of Stereolithographical technique for manufacturing scaffolds. Adapted from “Porous scaffold design for tissue engineering” by Scott Hollister, Nature Materials 2006, 5, 590.[94]
Examples of biomaterials used for SLA are polypropylene fumarate (PPF) with photocrosslinkable bonds and polyethylene glycol acrylate. An advantage of using SLA is the ability of the user to control and create defined geometries of the scaffold with high resolution, which almost mimics that of the CAD design[125]. A disadvantage of using SLA is that the materials are required to be photopolymers and utilizes photoinitiators. The major categories of photoinitiators include radical photopolymerization through photocleavage, hydrogen abstraction, and cationic photopolymerization where cationic photoinitiator are not used for tissue engineering due to the generation of toxic byproducts[126, 127]. Researchers recently used a SLA 250/40 stereolithograph machine to print scaffolds made from PPF and using the photoinitiator, Irgacure 819124. The fabricated scaffolds had a pore size range of 150-800 μm and a porosity of 90%. Their study showed the feasibility of using PPF material with SLA for scaffolding purposes. Future in vitro and in vivo studies would need to be conducted to establish cytotoxicity and biocompatibility of the fabricated scaffolds.
Using an EnvisionTEC Per-factory Mini Multilens SLA machine, photocrosslinkable PCL macromers were printed into a 3D scaffold using Irgracure 369 as the photoinitiator. These scaffolds showed no material shrinkage during the fabrication process, as well as possessing porosity of ∼70.5%, with an average pore size of 465 μm. The method in which the scaffold was printed required additional solvents and showed the potential to be used for tissue engineering[128]. However, the researchers did not test the functionality and viability of the scaffolds. Although the ability to print scaffolds for tissue engineering using stereolithography has been demonstrated, future in vitro and in vivo experiments are needed to establish the potential of this method and to increase adoption of stereolithography for this purpose.
Using a continuous liquid interface production (CLIP) technique, Tumbleston et al. was able to utilize stereolithography in a manner that surpasses any 3D printing technique in terms production time for object fabrication[129]. Current layer-by-layer printing takes hours to construct, and even longer for finer resolution constructs. With a “dead zone” thickness of 20 μm, and optical absorption height of the resin of 100μm the speeds of fabrication was in excess of 300 mm/hour. When the optical absorption height was changed to 300μm, thereby sacrificing resolution, speeds of greater than 1000 mm/hour were achieved. This is compared to layer-by-layer processing that typically produces only a few millimeters per hour. The CLIP technique operates by projecting UV images through a UV transparent and oxygen-permeable window stationed underneath a liquid resin bath. A dead zone is created between the window and the continuously elevating curing part such that reactive liquid resin is always available for curing (Figure 15).[129] There is a tradeoff in this system where the increase in speed of production yields poorer resolution 3D objects (Figure 16). However, the novel implementation surpasses conventional stereolithography techniques in terms of production time and is only limited in terms of fabrication time based on resin cure rates and viscosity, rather than step by step layer formation seen in typical stereolithography[129].
Figure 15. CLIP schematic and printed objects.
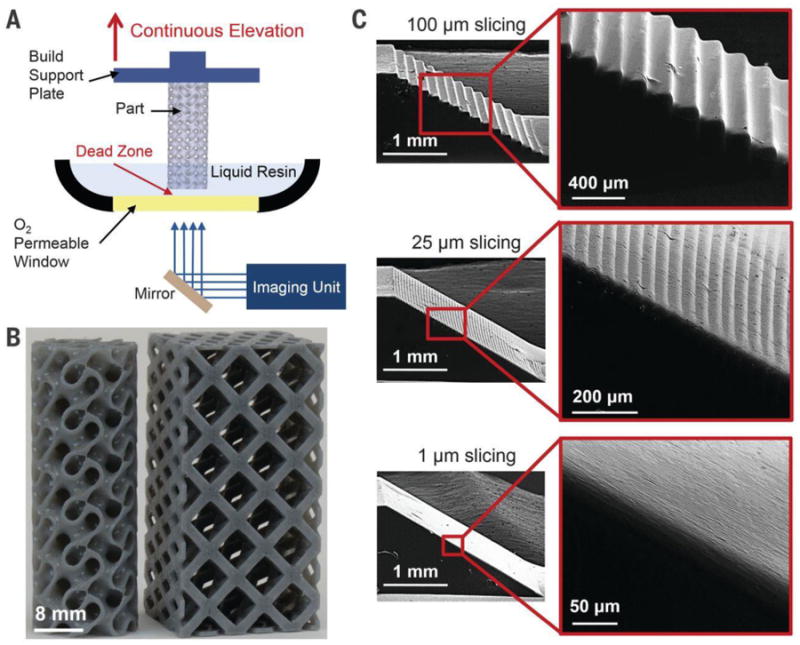
A) Schematic of CLIP printer displaying an oxygen permeable window and dead zone space (B) Scaffold design using CLIP with print speeds of 500 mm/hour (movies S1 and S2). (C) Ramp test analysis, illustrating that the same speed is achievable reagardlesss of 3D model slicing thickness (100 μm, 25 μm, and 1 μm). Reprinted with the permission from J. R. Tumbleston, D. Shirvanyants, N. Ermoshkin, R. Janusziewicz, A. R. Johnson, D. Kelly, K. Chen, R. Pinschmidt, J. P. Rolland, A. Ermoshkin, E. T. Samulski, J. M. DeSimone, Science 2015, 347, 1349. Reprinted with permission from AAAS
Figure 16. Resolution patterned test.
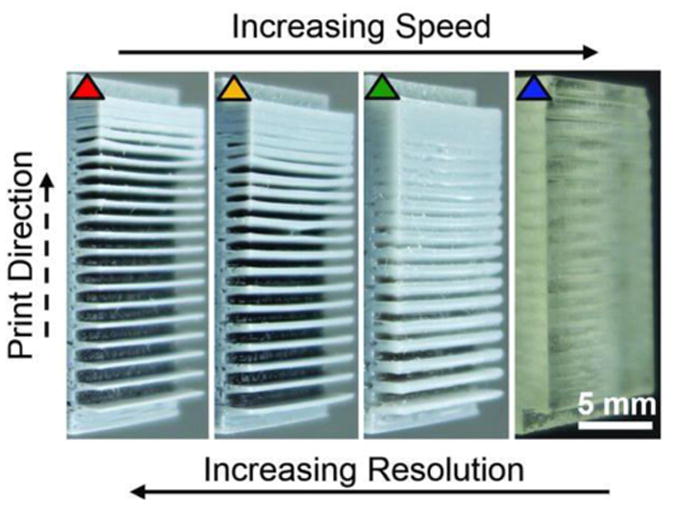
Reprinted with the permission from J. R. Tumbleston, D. Shirvanyants, N. Ermoshkin, R. Janusziewicz, A. R. Johnson, D. Kelly, K. Chen, R. Pinschmidt, J. P. Rolland, A. Ermoshkin, E. T. Samulski, J. M. DeSimone, Science 2015, 3. Reprinted with permission from AAAS
2. Nanofibrous scaffolds
2.1. Electrospinning
Electrospinning differs from SFF technologies by forming scaffolds through random orientations of nanofibers compared to a strategic layer-by-layer process. Electrospinning works by using electrostatic forces to produce fine fibers from polymer solutions or melts. The electrospinning process applies an electric field (high voltage) to a liquid polymer solution that is held by its surface tension to induce an electric charge on the liquid surface. Once this applied force reaches a critical point, the point where the repulsive electrical forces overcomes the surface tension, a jet of solution is ejected from the tip of the Taylor cone (point of eruption). The unstable and rapid whipping, caused by electrostatic repulsion, elongates the fibers which are then collected by a grounded collector. The jet of solution continually undergoes elongation and solvent evaporation, which eventually leads to the production of randomly oriented nanofiber constructs [130-132]. Electrospinning is capable producing interconnected nanofibers ranging from 5 nm to more than 1 μm in diameter. This method has been seen to form randomly oriented nanofibers possessing a high surface area with low density as well as high porosity with small pore size (∼100 μm)[130]. Recently, production of aligned nanofibers has been reported using synthetic and natural biomaterials that include collagen, chitosan, and gelatin. In a proof of concept experiment, microcontact printing was used in combination with biocompatible electrospun scaffolds[133]. Poly(ε-caprolactone) matrices were obtained by electrospinning, which was followed by the transfer of poly-L-lysine protein patterns from polydimethylsiloxane stamps onto the electrospun substrates. This scaffold was then tested using human bone-marrow-derived mesenchymal stromal cells and displayed the ability to be an effective substrate for cell adhesion. The use of electrospinning technologies to fabricate scaffolds for tissue engineering has attracted the interest of researchers due to the similarity in morphology to that of native ECM[133].
2.2 Bio-electrospraying and Cell Electrospinning
Another interesting approach to the development of a novel 3D printing method for the fabrication of scaffolds is through bio-electrospraying [134]. Bio-electrospraying works similarly to electrospinning, but small droplets are formed instead of fine fibers. Keat-Eng Ng et al. were able to use this technology to process living cells and overcome the disadvantages of ink-jet printing linked to the shear stress of the needle gauge affecting cell mortality. Using bio-electrospraying, primary cardiac cells could be printed to yield functional cardiac tissue, where myocytes were seen to beat spontaneously for a period of 5 weeks [135]. Further details on bio-electrospraying is available in a comprehensive review by Suwan Jayasinghe[136]. Cell electrospinning is another technique that uses electrospinning, but uses a coaxial system to generate cell laden scaffolds[137]. This technology has seen success in printing polydimethylsiloxane polymers with the immortalized human astrocytoma cell line, 1321N1. The cell laden fiber mesh/scaffold created using this method displayed no cellular damage during the nanofabrication process[138]. These results demonstrate the potential for this technique to provide functionalized scaffolds harboring living cells.
2.3 Self-assembling scaffolds
A novel approach to designing 3D printed scaffolds is through the use of self-assembled scaffolds. This method relies on non-covalent spontaneous interactions between hydrophilic and hydrophobic amino acid motifs to drive scaffold assembly[139]. Different environmental cues can trigger self-assembly of peptides or nucleic acids. One research team has developed three new threonine-based nanofibrous, self-assembling multidomain peptides (MDP) capable of forming porous hydrogels (Figure 17)[139]. The researchers tried to formulate peptides with the ability to self-assemble in a similar fashion to that of β-sheet nanofibers since β-sheet nanofibers are a good representation of biomimetic materials[139-141]. Stem cells from human exfoliated deciduous teeth were encapsulated in hydrogels formed by the MDPs and showed cytocompatibility. Compared to serine-based MDPs, the threonine-based MDPs were dependent on bioactive functionalization (RGDS sequence) for cell attachment to occur as the results suggest that the threonine-containing hydrogels are more selective. The use of a RGDS tetrapeptide vs. RGD is due RGDS appearing as the key cell-attachment component of fibronectin. The most popular polymers to have been used in self-assembled scaffolds include rationally designed nucleic acids and peptides[142]. The ability to create peptide-based assembled scaffolds that can mimic the structure and function of the ECM can be a huge advantage to allow cells to attach and proliferate at a much faster rate. This technology can improve current 3D printed scaffolds by increasing the recruitment of cells to the desired site.
Figure 17. Self-assembling scaffolds.
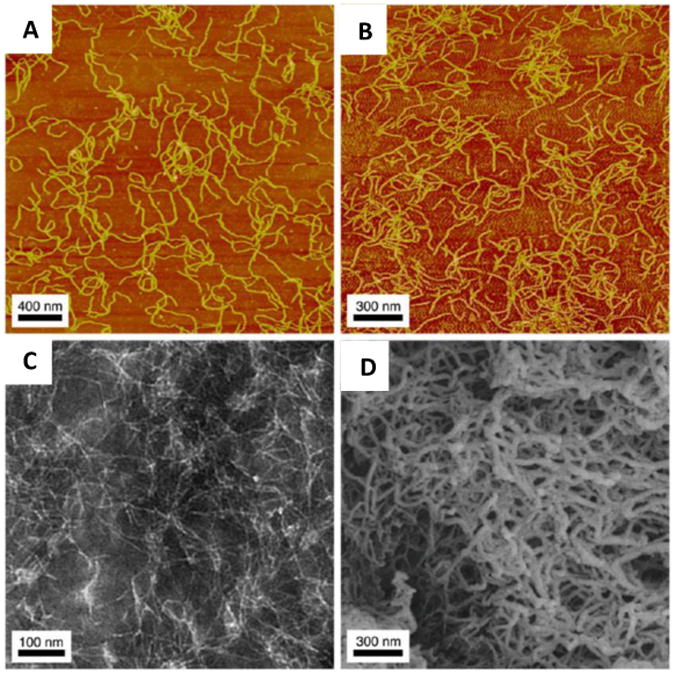
AFM of a 0.01% by weight peptide solution under nongelation conditions (A) and gelation conditions (B); TEM of a 0.01% by weight peptide solution under non-gelation conditions (C); and SEM of 1% by weight hydrogel (D) of K(TL)2SLRG(TL)3KGRGDS nanofibers illustrate the different morphology observed with the Threonine-Leucine (TL)-based nanofibers[139]. Reprinted with permission from M. K. Kang, J. S. Colombo, R. N. D'Souza, J. D. Hartgerink, Biomacromolecules 2014, 15, 2004.Copyright 2014 American Chemical Society.”
3. Indirect 3D Printing
With indirect 3D printing, a 3D printed scaffold is made by using a negative mold based on a scaffold design. Once the mold is made, the biomaterial is cast into the negative mold. After casting is complete, the negative mold is dissolved to obtain the desired scaffold. This technique is more conventional compared to the techniques discussed thus far for 3D printing of scaffolds and requires a solvent-based system to dissolve away support layers leading to longer scaffold fabrication times[143]. However, an advantage to this technique is that it is capable of creating customized scaffolds that precisely fit the patient's need, for example, researchers at the University of California were able to print a scaffold that mimic a human mandibular condyle[144]. In this study, the research group used indirect 3D printing to make biomimetic scaffolds composed of PCL and chitosan. The scaffold was prepared by adding PCL dissolved in chloroform to a gelatin mold made from 3D printing. Once the scaffold was dried, the gelatin mold was then removed by placing the PCL-gelatin scaffold in dH2O at 50°C for 6 h to obtain the PCL scaffold. After the PCL scaffold was acquired, an apatite coating was added to the scaffold and this addition was done to help support the cell growth of bone marrow stromal cells (BMSCs)[144]. The fabricated PCL apatite-coated scaffolds showed higher proliferation of BMSCs compared to non-coated PCL scaffolds. In another study involving indirect 3D printing, Park, Jung, and other colleagues created an advanced indirect 3D printing technique with projection-based micro-stereolithography and an injection molding system[145]. A sacrificial mold was made using this system and an alkali-soluble photopolymer. From this mold, PCL scaffolds were fabricated by both solvent-based methods and thermal molding using the injection molding system. This advanced indirect 3D printing method showed that the thermal molding process achieved a substantial reduction in scaffold fabrication time and higher mechanical properties compared to conventional indirect 3D printing. Thus, with combinatorial techniques, indirect 3D printing disadvantages can be negated. Indirect 3D printing has also been use to fabricate scaffolds with natural polymer such as collagen. These novel collagen scaffolds demonstrated the ability to support the growth of hMSCs for up to 4 weeks in vitro and have little to no cytotoxicity on the seeded cells[146]. Indirect 3D has the ability to print viable scaffolds and may be a useful and cheaper alternative to fabricate scaffolds at a laboratory scale when compared to purchasing a 3D printer.
Wax Printing
Wax printing is a form of indirect 3D printing, as the wax printer prints a negative mold from which the scaffold material solution is casted. The formation of a 3D printed scaffold from a wax printer starts with droplets of build wax and support wax being dispensed from a nozzle to build a layer on the surface. Upon completion of the printed layer, the printed surface is flattened and another layer is printed. Each layer is allowed a cool down period so that the wax will harden. After the structure is complete, it can be immersed in selective solvents to dissolve the support wax to form a porous structure which is the negative mold. The casting solution made from the scaffold material is then added to the negative mold and hardened. The negative mold is then either dissolved or melted to leave behind the scaffold only (Figure 18) [147].
Figure 18.
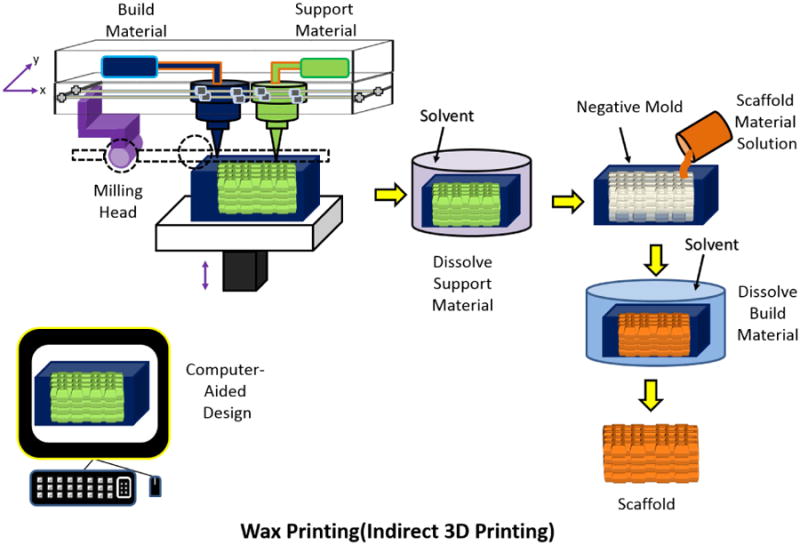
Schematic of Wax Printing process. Adapted from “Porous scaffold design for tissue engineering” by Scott Hollister, Nature Materials 2006, 5, 590.[94]
The disadvantage of using wax is that it was not originally designed to be biocompatible. This is problematic as the non-biodegradable wax residuals may contaminate the scaffold resulting in unsolicited effects[148]. However, the company TEOX Ltd. has developed new biocompatible waxes, BioBuild and BioSupport, to be used as mold materials for wax printing[149]. These proprietary waxes can be dissolved in either water heated to 35°C (BioSupport) or ethanol (BioBuild)[147, 149]. More recently, wax printing using paraffin spheres and wax was used to fabricate poly(L-lactide) (PLLA) nanofibrous scaffolds with controlled shape, pore size, and poor connectivity[150]. The wax support mold was printed with a Modelmaker II (Solidscape Inc.) in a layer-by-layer process and was then filled with paraffin spheres (build wax layer). The wax layer was then dissolved away with cyclohexane, and PLLA casting solution was cast in the mold filled with paraffin spheres. After the PLLA scaffold was formed, the paraffin spheres were also dissolved away with cyclohexane. In vitro studies for the fabricated nanofibrous scaffolds indicated osteoblast cell growth and differentiation, illustrating the potential for wax printing to be used to fabricate scaffolds with the capacity to regenerate bone tissues[150].
Although the advances in 3D printing have led to the fabrication of scaffolds with fine resolutions (down to 10 μm) suitable for a range of tissues, the translation of 3D printed scaffolds to the clinic has been slow. Current obstacles to using 3D printed scaffolds in clinical applications reside in issues of biologics, engineering, cost, and regulation/safety. For biologics, it is necessary to take into consideration the survival requirements of cells such as oxygen diffusion, cell migration and levels of vascularization which have often been suboptimal. In terms of engineering, the processability and reproducibility of the scaffolds is necessary to ensure consistency and homogenous application. As 3D printing is a novel approach to tissue engineering, the procurement of a 3D printer with the ability to print scaffolds with fine resolution can be an expensive investment (up to $750,000 for a single printer). In addition, the supply of approved and appropriate materials, cell culture facilities and, in many cases, recombinant factors will add to the financial burden. Finally, regulation and safety guidelines must be established and a standard must be set in order to ensure that scaffolds meet a certain criteria before being used[151]. Never-the-less, the abundance of preclinical in vivo studies using various 3D printing techniques demonstrates the feasibility of using scaffolds for tissue regeneration and is encouraging (Table 1). As scientists, engineers, pharmacists, dentists and physicians continue to collaboratively develop these 3D printed scaffolds for tissue regeneration, the likelihood of overcoming barriers to clinical applications is high.
Table 1. Comparison table and preclinical progress of various 3D printing techniques used to print scaffolds for tissue engineering.
| Printing Method | Advantages | Disadvantages | Preclinical progress |
|---|---|---|---|
| Direct 3D printing/Inkjet |
|
|
|
| w/electrospinning |
|
||
| Bioplotting |
|
||
| Fused Deposition Modeling | |||
| Selective Laser Sintering | |||
| Stereolithography | |||
| Electrospinning | |||
| Indirect 3D Printing |
|
Conclusion
Technologies for 3D printing of scaffolds for tissue engineering are an area of research undergoing rapid advances. A major aim in the development of 3D printed scaffolds is the creation of scaffolds that closely resemble the native microenvironmental properties at the site of implantation, such as ECM properties, load bearing mechanical properties, pore size arrangements to allow nutrient diffusion and cell migration, and the appropriate growth factor milieu for the promotion of angiogenesis and/or osteogenesis. As new materials and “bioinks” are synthesized and novel printing methods are discovered, the 3D printing of scaffolds to be used in tissue engineering continues to become more sophisticated and effective. The 3D printing techniques and materials discussed in this review are likely to contribute to improved approaches to generating functional tissue for replacement and repair. Composite materials and hybrid 3D printing approaches are likely to lead to the next generation of advanced 3D printed scaffolds for tissue engineering applications. These hybrid systems, as discussed, have the potential to mitigate the disadvantages of any one printing technique and even the limitations of the materials used. As current techniques are further fine-tuned and more bioink materials become available, the design of effective ECM-like scaffolds becomes increasingly possible. The focus of 3D printing techniques in medicine to date has generally been aimed at regenerating or replacing tissue in vivo, however, alternative approaches also being investigated include the printing of functional tissues in vitro. Such 3D printed tissues can be formed using a patient's own cells thereby potentially overcoming issues of rejection[191, 192]. Ultimately, 3D printing of scaffolds for tissue engineering may be the key to giving those suffering from organ failure and dysfunction caused by damaged or diseased tissue a chance at an improved quality of life.
Acknowledgments
We acknowledge financial support from a Deans Graduate Fellowship from the University of Iowa, the ITI Foundation for the Promotion of Implantology, Switzerland (ITI Research Grant No. 855 2012), the Osseointegration Foundation, the Osteology Foundation, the National Institutes of Health National Institute of Dental and Craniofacial Research (1R21DE02420601A1), the National Cancer Institute at the National Institutes of Health (5P30CA086862) and the Lyle and Sharon Bighley Professorship.
Footnotes
We have no conflicts of interest to declare.
References
- 1.Vol. 2014, U.S. Department of Health & Human Services, 2014.
- 2.Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G. Biomacromolecules. 2014;15:635. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Didenko VV, Ngo H, Minchew CL, Boudreaux DJ, Widmayer MA, Baskin DS. Molecular medicine (Cambridge, Mass) 2002;8:818. [PMC free article] [PubMed] [Google Scholar]
- 4.Laumonier T, Ménétrey J. The Knee Joint. Vol. 511 Springer; Paris: 2012. [Google Scholar]
- 5.A. H. Association, Vol. 2014, http://www.heart.org/HEARTORG/Conditions/HeartAttack/PreventionTreatmentofHeartAttack/Cardiac-Medications_UCM_303937_Article.jsp 2014.
- 6.Brandt KD, Mazzuca SA, Buckwalter KA. Rheumatology. 2006;45:1389. doi: 10.1093/rheumatology/kel100. [DOI] [PubMed] [Google Scholar]
- 7.Balmaceda CM. BMC Musculoskelet Disord. 2014;15:5. doi: 10.1186/1471-2474-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakesheff K. Future History Now Talks: Part I. Zurich, London: 2013. [Google Scholar]
- 9.Yang S, Leong KF, Du Z, Chua CK. Tissue engineering. 2001;7:679. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T, Ikeda K, Yamamoto K, Ishizaki H, Yoshizawa Y, Yanagiguchi K, Yamada S, Hayashi Y. BioMed research international. 2014;8 doi: 10.1155/2014/786892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 12.Hollister SJ. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 13.Sachlos E, Czernuszka JT. European cells & materials. 2003;5:29. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 14.Wust S, Muller R, Hofmann S. Journal of functional biomaterials. 2011;2:119. doi: 10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltola SM, Melchels FPW, Grijpma DW, Kellomäki M. Annals of Medicine. 2008;40:268. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 16.Hribar KC, Soman P, Warner J, Chung P, Chen S. Lab on a chip. 2014;14:268. doi: 10.1039/c3lc50634g. [DOI] [PubMed] [Google Scholar]
- 17.Leong KF, Cheah CM, Chua CK. Biomaterials. 2003;24:2363. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 18.International Journal of Polymer Science 2011, 2011.
- 19.Chen M, Le DQ, Baatrup A, Nygaard JV, Hein S, Bjerre L, Kassem M, Zou X, Bunger C. Acta Biomater. 2011;7:2244. doi: 10.1016/j.actbio.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Journal of biomedical materials research. 1998;43:422. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Gao C, Shen J. Biomaterials. 2002;23:4889. doi: 10.1016/s0142-9612(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 22.Spath S, Seitz H. Int J Adv Manuf Technol. 2014;70:135. [Google Scholar]
- 23.Cook JR, Crute BE, Patrone LM, Gabriels J, Lane ME, Van Buskirk RG. In vitro cellular & developmental biology: journal of the Tissue Culture Association. 1989;25:914. doi: 10.1007/BF02624004. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence BJ, Madihally SV. Cell adhesion & migration. 2008;2:9. doi: 10.4161/cam.2.1.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Ahn S, Bonassar LJ, Kim G. Macromolecular Rapid Communications. 2013;34:142. doi: 10.1002/marc.201200524. [DOI] [PubMed] [Google Scholar]
- 26.Shim JH, Kim JY, Park M, Park J, Cho DW. Biofabrication. 2011;3:034102. doi: 10.1088/1758-5082/3/3/034102. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Hong JM, Jung JW, Shim JH, Oh JH, Cho DW. Biofabrication. 2014;6:024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 28.Bose S, Vahabzadeh S, Bandyopadhyay A. Materials Today. 2013;16:496. [Google Scholar]
- 29.Liu WY, Li Y, Liu JY, Niu XF, Wang Y, Li DY. J Nanomater. 2013;7 [Google Scholar]
- 30.Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Biomaterials. 2003;24:181. doi: 10.1016/s0142-9612(02)00276-4. [DOI] [PubMed] [Google Scholar]
- 31.Vasconcellos LMRd, Oliveira MVd, Graça MLdA, Vasconcellos LGOd, Carvalho YR, Cairo CAA. Materials Research. 2008;11:275. [Google Scholar]
- 32.Chou DT, Wells D, Hong D, Lee B, Kuhn H, Kumta PN. Acta Biomaterialia. 2013;9:8593. doi: 10.1016/j.actbio.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Hansen DC. The Electrochemical Society Interface. 2008;17:31. [Google Scholar]
- 34.Wataha J, Hobbs D, Wong J, Dogan S, Zhang H, Chung KH, Elvington M. J Mater Sci: Mater Med. 2010;21:1289. doi: 10.1007/s10856-009-3941-8. [DOI] [PubMed] [Google Scholar]
- 35.Davis RR, Hobbs DT, Khashaba R, Sehkar P, Seta FN, Messer RL, Lewis JB, Wataha JC. Journal of biomedical materials research Part A. 2010;93:864. doi: 10.1002/jbm.a.32407. [DOI] [PubMed] [Google Scholar]
- 36.Jayakumar R, Ramachandran R, Divyarani VV, Chennazhi KP, Tamura H, Nair SV. International journal of biological macromolecules. 2011;48:336. doi: 10.1016/j.ijbiomac.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Balla VK, Bodhak S, Bose S, Bandyopadhyay A. Acta Biomater. 2010;6:3349. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dabrowski B, Swieszkowski W, Godlinski D, Kurzydlowski KJ. Journal of biomedical materials research Part B, Applied biomaterials. 2010;95:53. doi: 10.1002/jbm.b.31682. [DOI] [PubMed] [Google Scholar]
- 39.Zheng YF, Gu XN, Witte F. Materials Science and Engineering: R: Reports. 2014;77:1. [Google Scholar]
- 40.Zhuang H, Han Y, Feng A. Materials Science and Engineering: C. 2008;28:1462. [Google Scholar]
- 41.Vorndran E, Wunder K, Moseke C, Biermann I, Muller FA, Zorn K, Gbureck U. Adv Appl Ceram. 2011;110:476. [Google Scholar]
- 42.Mitsak AG, Kemppainen JM, Harris MT, Hollister SJ. Tissue engineering Part A. 2011;17:1831. doi: 10.1089/ten.tea.2010.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corin KA, Gibson LJ. Biomaterials. 2010;31:4835. doi: 10.1016/j.biomaterials.2010.01.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Ushida T, Tateishi T. Macromolecular Bioscience. 2002;2:67. [Google Scholar]
- 45.Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Journal of biomedical materials research Part B, Applied biomaterials. 2005;74:782. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Fan W, Zhou Y, Luo Y, Gelinsky M, Chang J, Xiao Y. Journal of Materials Chemistry. 2012;22:12288. [Google Scholar]
- 47.Della MROY, Sari Kurtossy L. Nature. 1974;247:220. [Google Scholar]
- 48.Leukers B, Gulkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H. Journal of materials science Materials in medicine. 2005;16:1121. doi: 10.1007/s10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- 49.Warnke PH, Seitz H, Warnke F, Becker ST, Sivananthan S, Sherry E, Liu Q, Wiltfang J, Douglas T. Journal of biomedical materials research Part B, Applied biomaterials. 2010;93:212. doi: 10.1002/jbm.b.31577. [DOI] [PubMed] [Google Scholar]
- 50.Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, Leukers B. Journal of biomaterials applications. 2011;26:359. doi: 10.1177/0885328210373285. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Z, Buchanan F, Mitchell C, Dunne N. Materials Science and Engineering: C. 2014;38:1. doi: 10.1016/j.msec.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Castilho M, Moseke C, Ewald A, Gbureck U, Groll J, Pires I, Teßmar J, Vorndran E. Biofabrication. 2014;6:015006. doi: 10.1088/1758-5082/6/1/015006. [DOI] [PubMed] [Google Scholar]
- 53.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. Biomaterials. 2014;35:49. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro J, Oyen M. JOM. 2013;65:505. [Google Scholar]
- 55.Zhu J, Marchant RE. Expert review of medical devices. 2011;8:607. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SH, Park DS, Shin JW, Kang YG, Kim HK, Yoon TR, Shin JW. Journal of materials science Materials in medicine. 2012;23:2671. doi: 10.1007/s10856-012-4738-8. [DOI] [PubMed] [Google Scholar]
- 57.Griffith LG. Acta Materialia. 2000;48:263. [Google Scholar]
- 58.Intra J, Glasgow JM, Mai HQ, Salem AK. Journal of Controlled Release. 2008;127:280. doi: 10.1016/j.jconrel.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Tissue engineering Part B, Reviews. 2011;17:393. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajan V, Murray R. Wound Practice and Research. 2008;16:122. [Google Scholar]
- 61.Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M. Acta Biomater. 2014;10:613. doi: 10.1016/j.actbio.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 62.Sung HJ, Meredith C, Johnson C, Galis ZS. Biomaterials. 2004;25:5735. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 63.Ma PX. Materials Today. 2004;7:30. [Google Scholar]
- 64.Oryan SAA, Moshiri A. Hard Tissue. 2013;2 [Google Scholar]
- 65.Aydin A, Memisoglu K, Cengiz A, Atmaca H, Muezzinoglu B, Muezzinoglu US. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2012;17:796. doi: 10.1007/s00776-012-0269-x. [DOI] [PubMed] [Google Scholar]
- 66.Choi SH, Park TG. Journal of biomaterials science Polymer edition. 2002;13:1163. doi: 10.1163/156856202320813864. [DOI] [PubMed] [Google Scholar]
- 67.Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Qian Z. International journal of pharmaceutics. 2009;365:89. doi: 10.1016/j.ijpharm.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 68.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. Biomaterials. 2014;35:4026. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elangovan S, D'Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR, Salem AK. Biomaterials. 2014;35:737. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam CXF, Mo XM, Teoh SH, Hutmacher DW. Materials Science and Engineering: C. 2002;20:49. [Google Scholar]
- 71.Serra T, Planell JA, Navarro M. Acta Biomaterialia. 2013;9:5521. doi: 10.1016/j.actbio.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 72.M P, Wei GB. Adv Funct Mater. 2008;18:3568. [Google Scholar]
- 73.B F, Korossis S, Southgate J, Ingham E, Fisher J. Biomaterials. 2009;30:266. doi: 10.1016/j.biomaterials.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 74.H M, Groeber F, Hampel M, Hinderer S, Schenke-Layland K. Clin Plast Surg. 2012;39:33. doi: 10.1016/j.cps.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Guoyou H, Lin W, Shu Qi W, Yulong H, Jinhui W, Qiancheng Z, Feng X, Tian Jian L. Biofabrication. 2012;4:042001. [Google Scholar]
- 76.Nichol JW, Khademhosseini A. Soft Matter. 2009;5:1312. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.C P, Napolitano AP, Dean DM, Morgan JR. Tissue Eng. 2007;13:2087. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 78.P W, Chung SE, Shin S, Lee SA, Kwon S. Nat Mater. 2008;7:581. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 79.W C, Xu F, Rengarajan V, Finley TD, Keles HO, Sung Y, Li B, Gurkan UA, Demirci U. Adv Mater. 2011;23:4254. doi: 10.1002/adma.201101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.F T, Xu F, Turkaydin M, Sung Y, Gurkan UA, Yavuz AS, Guldiken RO, Demirci U. Biomaterials. 2011;32:7847. doi: 10.1016/j.biomaterials.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu F, Finley TD, Turkaydin M, Sung Y, Gurkan UA, Yavuz AS, Guldiken RO, Demirci U. Biomaterials. 2011;32:7847. doi: 10.1016/j.biomaterials.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Nanomedicine (London, England) 2010;5:469. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee KW, Wang S, Lu L, Jabbari E, Currier BL, Yaszemski MJ. Tissue engineering. 2006;12:2801. doi: 10.1089/ten.2006.12.2801. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Cui R, Sun L, Aifantis KE, Fan Y, Feng Q, Cui F, Watari F. International Journal of Polymer Science. 2014;2014:13. [Google Scholar]
- 85.Murphy SV, Atala A. Nature biotechnology. 2014;32:773. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 86.Wu B, Klatzky RL, Stetten G. Journal of experimental psychology Applied. 2010;16:45. doi: 10.1037/a0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Nanomedicine (London, England) 2010;5:469. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.M. Talib, J. Covington, A. Dove, A. Bolarinwa, L. Grover, 2012.
- 89.Tonomura A, Mizuno D, Hisada A, Kuno N, Ando Y, Sumita Y, Honda MJ, Satomura K, Sakurai H, Ueda M, Kagami H. Annals of biomedical engineering. 2010;38:1664. doi: 10.1007/s10439-010-9910-z. [DOI] [PubMed] [Google Scholar]
- 90.Berger A. BMJ (Clinical research ed) 2002;324:35. doi: 10.1136/bmj.324.7328.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.N. I. o. B. I. a. Bioengineering, Vol. 2014, U.S. Departement of Health & Human Services, National Institute of Health, 2014.
- 92.Sachs EM, Haggerty JS, Cima MJ, Williams PA. Google Patents. 1993
- 93.Cima M, Sachs E, Fan T, Bredt JF, Michaels SP, Khanuja S, Lauder A, Lee SJJ, Brancazio D, Curodeau A. Google Patents. 1995
- 94.Hollister SJ. Nat Mater. 2006;5:590. [Google Scholar]
- 95.Tan Y, Richards DJ, Trusk TC, Visconti RP, Yost MJ, Kindy MS, Drake CJ, Argraves WS, Markwald RR, Mei Y. Biofabrication. 2014;6:024111. doi: 10.1088/1758-5082/6/2/024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chavanne P, Stevanovic S, Wuthrich A, Braissant O, Pieles U, Gruner P, Schumacher R. Biomedizinische Technik Biomedical engineering. 2013 doi: 10.1515/bmt-2013-4069. [DOI] [PubMed] [Google Scholar]
- 97.Yousefi AM, Gauvin C, Sun L, DiRaddo RW, Fernandes J. Polymer Engineering & Science. 2007;47:608. [Google Scholar]
- 98.Ozbolat IT, Chen H, Yu Y. Robot Comput -Integr Manuf. 2014;30:295. [Google Scholar]
- 99.Poldervaart MT, Gremmels H, van Deventer K, Fledderus JO, Oner FC, Verhaar MC, Dhert WJ, Alblas J. Journal of controlled release: official journal of the Controlled Release Society. 2014;184:58. doi: 10.1016/j.jconrel.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 100.Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A. Biofabrication. 2014;6:024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gauvin R, Chen YC, Lee JW, Soman P, Zorlutuna P, Nichol JW, Bae H, Chen S, Khademhosseini A. Biomaterials. 2012;33:3824. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao S, Zhu M, Zhang J, Zhang Y, Liu Z, Zhu Y, Zhang C. Journal of Materials Chemistry B. 2014;2:6106. doi: 10.1039/c4tb00838c. [DOI] [PubMed] [Google Scholar]
- 103.Neufurth M, Wang X, Schröder HC, Feng Q, Diehl-Seifert B, Ziebart T, Steffen R, Wang S, Müller WEG. Biomaterials. 2014;35:8810. doi: 10.1016/j.biomaterials.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Wang MD, Zhai P, Schreyer D, Zheng RS, Sun XD, Cui FZ, Chen XB. Front Mater Sci. 2013;7:269. [Google Scholar]
- 105.Serra T, Ortiz-Hernandez M, Engel E, Planell JA, Navarro M. Materials science & engineering C, Materials for biological applications. 2014;38:55. doi: 10.1016/j.msec.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert WJ. Tissue engineering Part A. 2008;14:127. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- 107.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Nat Commun. 2014;5 doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong JM, Kim BJ, Shim JH, Kang KS, Kim KJ, Rhie JW, Cha HJ, Cho DW. Acta Biomater. 2012;8:2578. doi: 10.1016/j.actbio.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 109.Wong KV, Hernandez A. ISRN Mechanical Engineering. 2012;2012:10. [Google Scholar]
- 110.Zein I, Hutmacher DW, Tan KC, Teoh SH. Biomaterials. 2002;23:1169. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 111.Rai B, Lin JL, Lim ZXH, Guldberg RE, Hutmacher DW, Cool SM. Biomaterials. 2010;31:7960. doi: 10.1016/j.biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Khojasteh A, Behnia H, Hosseini FS, Dehghan MM, Abbasnia P, Abbas FM. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013;101B:848. doi: 10.1002/jbm.b.32889. [DOI] [PubMed] [Google Scholar]
- 113.Xu N, Ye X, Wei D, Zhong J, Chen Y, Xu G, He D. ACS applied materials & interfaces. 2014;6:14952. doi: 10.1021/am502716t. [DOI] [PubMed] [Google Scholar]
- 114.Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW. Journal of clinical periodontology. 2014;41:283. doi: 10.1111/jcpe.12214. [DOI] [PubMed] [Google Scholar]
- 115.Liu FH. Applied Surface Science. 2014;297:1. [Google Scholar]
- 116.Kruth JP, Levy G, Klocke F, Childs THC. CIRP Annals - Manufacturing Technology. 2007;56:730. [Google Scholar]
- 117.Gibson I, Shi D. Rapid Prototyping Journal. 1997;3:129. [Google Scholar]
- 118.Duan B, Cheung WL, Wang M. Biofabrication. 2011;3:015001. doi: 10.1088/1758-5082/3/1/015001. [DOI] [PubMed] [Google Scholar]
- 119.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Biomaterials. 2005;26:4817. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 120.Eosoly S, Lohfeld S, Brabazon D. Key Engineering Materials. 2009;396-398:659. [Google Scholar]
- 121.Matsuda T, Mizutani M. Journal of biomedical materials research. 2002;62:395. doi: 10.1002/jbm.10295. [DOI] [PubMed] [Google Scholar]
- 122.Griffith ML, Halloran JW. Journal of the American Ceramic Society. 1996;79:2601. [Google Scholar]
- 123.Dhariwala B, Hunt E, Boland T. Tissue engineering. 2004;10:1316. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 124.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Journal of biomedical materials research Part B, Applied biomaterials. 2003;64:65. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 125.Kim K, Yeatts A, Dean D, Fisher JP. Tissue engineering Part B, Reviews. 2010;16:523. doi: 10.1089/ten.teb.2010.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dworak C. Biocompatible Phosphorus Containing Photopolymers. 2011 [Google Scholar]
- 127.Nguyen KT, West JL. Biomaterials. 2002;23:4307. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 128.Elomaa L, Teixeira S, Hakala R, Korhonen H, Grijpma DW, Seppälä JV. Acta Biomaterialia. 2011;7:3850. doi: 10.1016/j.actbio.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 129.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, Chen K, Pinschmidt R, Rolland JP, Ermoshkin A, Samulski ET, DeSimone JM. Science. 2015;347:1349. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 130.Vasita R, Katti DS. International journal of nanomedicine. 2006;1:15. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leach MK, Feng ZQ, Tuck SJ, Corey JM. Journal of Visualized Experiments: JoVE. 2011;2494 doi: 10.3791/2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhardwaj N, Kundu SC. Biotechnol Adv. 2010;28:325. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Giannitelli SM, Abbruzzese F, Mozetic P, De Ninno A, Businaro L, Gerardino A, Rainer A. Asia-Pacific Journal of Chemical Engineering. 2014;9:401. [Google Scholar]
- 134.Jayasinghe SN, Qureshi AN, Eagles PA. Small. 2006;2:216. doi: 10.1002/smll.200500291. [DOI] [PubMed] [Google Scholar]
- 135.Ng KE, Joly P, Jayasinghe SN, Vernay B, Knight R, Barry SP, McComick J, Latchman D, Stephanou A. Biotechnol J. 2011;6:86. doi: 10.1002/biot.201000125. [DOI] [PubMed] [Google Scholar]
- 136.Jayasinghe SN. Analyst. 2011;136:878. doi: 10.1039/c0an00830c. [DOI] [PubMed] [Google Scholar]
- 137.Jayasinghe SN. Analyst. 2013;138:2215. doi: 10.1039/c3an36599a. [DOI] [PubMed] [Google Scholar]
- 138.Townsend-Nicholson A, Jayasinghe SN. Biomacromolecules. 2006;7:3364. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 139.Kang MK, Colombo JS, D'Souza RN, Hartgerink JD. Biomacromolecules. 2014;15:2004. doi: 10.1021/bm500075r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matson JB, Stupp SI. Chemical communications (Cambridge, England) 2012;48:26. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowerman CJ, Nilsson BL. Biopolymers. 2012;98:169. doi: 10.1002/bip.22058. [DOI] [PubMed] [Google Scholar]
- 142.Pierschbacher MD, Ruoslahti E. Nature. 1984;309:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 143.Yeong WY, Chua CK, Leong KF, Chandrasekaran M, Lee MW. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2007;82B:260. doi: 10.1002/jbm.b.30729. [DOI] [PubMed] [Google Scholar]
- 144.Lee JY, Choi B, Wu B, Lee M. Biofabrication. 2013;5:045003. doi: 10.1088/1758-5082/5/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park JH, Jung JW, Kang HW, Cho DW. Biofabrication. 2014;6:025003. doi: 10.1088/1758-5082/6/2/025003. [DOI] [PubMed] [Google Scholar]
- 146.Liu CZ, Xia ZD, Han ZW, Hulley PA, Triffitt JT, Czernuszka JT. Journal of biomedical materials research Part B, Applied biomaterials. 2008;85:519. doi: 10.1002/jbm.b.30975. [DOI] [PubMed] [Google Scholar]
- 147.Robert L, Anita S, Michael JM, Fan Y, William LN, Jeffrey MK. Nanotechnology and Tissue Engineering. Vol. 3 CRC Press; 2008. [Google Scholar]
- 148.Sachlos E, Reis N, Ainsley C, Derby B, Czernuszka JT. Biomaterials. 2003;24:1487. doi: 10.1016/s0142-9612(02)00528-8. [DOI] [PubMed] [Google Scholar]
- 149.Sachlos E, Gotora D, Czernuszka JT. Tissue engineering. 2006;12:2479. doi: 10.1089/ten.2006.12.2479. [DOI] [PubMed] [Google Scholar]
- 150.Wang P, Hu J, Ma PX. Biomaterials. 2009;30:2735. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J, He L, Zhou C, Zhou Y, Bai Y, Lee FY, Mao JJ. MRS Bulletin. 2015;40:145. [Google Scholar]
- 152.Tamimi F, Torres J, Al-Abedalla K, Lopez-Cabarcos E, Alkhraisat MH, Bassett DC, Gbureck U, Barralet JE. Biomaterials. 2014;35:5436. doi: 10.1016/j.biomaterials.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 153.Tarafder S, Davies NM, Bandyopadhyay A, Bose S. Biomater Sci. 2013;1:1250. doi: 10.1039/C3BM60132C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fielding G, Bose S. Acta Biomater. 2013;9:9137. doi: 10.1016/j.actbio.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Strobel LA, Rath SN, Maier AK, Beier JP, Arkudas A, Greil P, Horch RE, Kneser U. J Tissue Eng Regen Med. 2014;8:176. doi: 10.1002/term.1511. [DOI] [PubMed] [Google Scholar]
- 156.Choi HJ, Kim JM, Kwon E, Che JH, Lee JI, Cho SR, Kang SK, Ra JC, Kang BC. J Korean Med Sci. 2011;26:482. doi: 10.3346/jkms.2011.26.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shim JH, Kim SE, Park JY, Kundu J, Kim SW, Kang SS, Cho DW. Tissue engineering Part A. 2014;20:1980. doi: 10.1089/ten.tea.2013.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poldervaart MT, Wang H, van der Stok J, Weinans H, Leeuwenburgh SC, Oner FC, Dhert WJ, Alblas J. PLoS One. 2013;8:e72610. doi: 10.1371/journal.pone.0072610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 160.Chang JW, Park SA, Park JK, Choi JW, Kim YS, Shin YS, Kim CH. Artif Organs. 2014;38:E95. doi: 10.1111/aor.12310. [DOI] [PubMed] [Google Scholar]
- 161.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Lancet. 2010;376:440. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee CH, Marion NW, Hollister S, Mao JJ. Tissue engineering Part A. 2009;15:3923. doi: 10.1089/ten.tea.2008.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ. Tissue Eng Part C Methods. 2012;18:33. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Tissue engineering Part A. 2014;20:1342. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. Stem Cells Transl Med. 2012;1:792. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Analytical Chemistry. 2014;86:3240. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 167.Jensen J, Rolfing JH, Le DQ, Kristiansen AA, Nygaard JV, Hokland LB, Bendtsen M, Kassem M, Lysdahl H, Bunger CE. Journal of biomedical materials research Part A. 2014;102:2993. doi: 10.1002/jbm.a.34970. [DOI] [PubMed] [Google Scholar]
- 168.Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amedee J, Fricain JC, Catros S. Biofabrication. 2010;2:014101. doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- 169.Zieber L, Or S, Ruvinov E, Cohen S. Biofabrication. 2014;6:024102. doi: 10.1088/1758-5082/6/2/024102. [DOI] [PubMed] [Google Scholar]
- 170.Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. J Tissue Eng Regen Med. 2013;7:631. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Killat J, Reimers K, Choi CY, Jahn S, Vogt PM, Radtke C. International journal of molecular sciences. 2013;14:14460. doi: 10.3390/ijms140714460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G. Biomaterials. 2011;32:9218. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 173.Stampfl J, Baudis S, Heller C, Liska R, Neumeister A, Kling R, Ostendorf A, Spitzbart M. Journal of Micromechanics and Microengineering. 2008;18:125014. [Google Scholar]
- 174.Lee JW, Kang KS, Lee SH, Kim JY, Lee BK, Cho DW. Biomaterials. 2011;32:744. doi: 10.1016/j.biomaterials.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 175.Park JH, Jung JW, Kang HW, Joo YH, Lee JS, Cho DW. Biofabrication. 2012;4:035004. doi: 10.1088/1758-5082/4/3/035004. [DOI] [PubMed] [Google Scholar]
- 176.Li X, He J, Bian W, Li Z, Li D, Snedeker JG. Biofabrication. 2014;6:015010. doi: 10.1088/1758-5082/6/1/015010. [DOI] [PubMed] [Google Scholar]
- 177.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Journal of biomedical materials research. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 178.Casper CL, Stephens JS, Tassi NG, Chase DB, Rabolt JF. Macromolecules. 2004;37:573. [Google Scholar]
- 179.J Nanomater. 2012;2012:13. [Google Scholar]
- 180.Li D, Xia Y. Advanced Materials. 2004;16:1151. [Google Scholar]
- 181.Jayasinghe SN, Warnes G, Scotton CJ. Macromol Biosci. 2011;11:1364. doi: 10.1002/mabi.201100131. [DOI] [PubMed] [Google Scholar]
- 182.Sampson SL, Saraiva L, Gustafsson K, Jayasinghe SN, Robertson BD. Small. 2014;10:78. doi: 10.1002/smll.201300804. [DOI] [PubMed] [Google Scholar]
- 183.Shin M, Yoshimoto H, Vacanti JP. Tissue engineering. 2004;10:33. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 184.Srouji S, Ben-David D, Lotan R, Livne E, Avrahami R, Zussman E. Tissue engineering Part A. 2011;17:269. doi: 10.1089/ten.TEA.2010.0250. [DOI] [PubMed] [Google Scholar]
- 185.Tillman BW, Yazdani SK, Lee SJ, Geary RL, Atala A, Yoo JJ. Biomaterials. 2009;30:583. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 186.Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Biomaterials. 2003;24:181. doi: 10.1016/s0142-9612(02)00276-4. [DOI] [PubMed] [Google Scholar]
- 187.H. Chia, 2014.
- 188.Lee M, Wu B. In: Computer-Aided Tissue Engineering. Liebschner MAK, editor. Vol. 868. Humana Press; 2012. p. 257. [Google Scholar]
- 189.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, Jia X, Grayson WL. Journal of biomedical materials research Part A. 2014;102:4317. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 190.Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV. Biomaterials. 2010;31:5945. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Lancet. 2011;377:1175. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, Lopez-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A. Lancet. 2014;384:329. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]


