Abstract
Peritoneal dialysis (PD) is a modality for treatment of patients with end-stage renal disease (ESRD) that depends on the structural and functional integrity of the peritoneal membrane. However, long-term PD can lead to morphological and functional changes in the peritoneum; in particular, peritoneal fibrosis has become one of the most common complications that ultimately results in ultrafiltration failure (UFF) and discontinuation of PD. Several factors and mechanisms such as inflammation and overproduction of transforming growth factor-β1 have been implicated in the development of peritoneal fibrosis, but there is no effective therapy to prevent or delay this process. Recent studies have shown that activation of multiple receptor tyrosine kinases (RTKs) is associated with the development and progression of tissue fibrosis in various organs, and there are also reports indicating the involvement of some RTKs in peritoneal fibrosis. This review will describe the role and mechanisms of RTKs in peritoneal fibrosis and discuss the possibility of using them as therapeutic targets for prevention and treatment of this complication.
Keywords: Peritoneal dialysis, peritoneal membrane, peritoneal fibrosis, receptor tyrosine kinase
Peritoneal dialysis (PD) is an alternative therapy to hemodialysis for patients with end-stage renal disease (ESRD). This modality relies on the blood supply and semi-permeability of the peritoneal membrane to allow diffusion of solute down its concentration gradient from systemic circulation to dialysate dwelling in the peritoneal cavity as well as ultrafiltration of water down an osmotic gradient created by dextrose in the PD fluid. Thus, the success of PD depends on the structural and functional integrity of the peritoneal membrane (1,2).
The peritoneal membrane (PM) is a translucent structure composed of a mesothelial cell monolayer with some characteristics of epithelial cells, and a sub-mesothelial compact zone that includes extracellular matrix (ECM), a few fibroblasts, innate immune cells such as macrophages and mast cells, as well as peritoneal capillaries and lymphatic vessels (3,4). As the primary protection against chemical or biochemical insults, human peritoneal mesothelial cells (HPMCs) undergo a variety of injuries in long-term PD, mainly owing to the continuous exposure of the membrane to conventional hyper-osmolar bio-incompatible PD fluids (5) (high dextrose concentration, low pH, glucose degradation products (GDPs), advanced glycation end products (AGEs) and recurrent episodes of peritonitis (6–9). Thus, despite advantages such as the great improvement of life quality as well as convenience and economy, the duration of PD also leads to peritoneal membrane dysfunction, which is characterized by detachment of the mesothelial layer, increased sub-mesothelial ECM deposition, fibrosis, and angiogenesis (10–12). The peritoneal membrane may progressively experience fibro-proliferative changes, culminating in ultrafiltration failure (UFF) (13) and ultimately forcing the patient to dropout of PD therapy (9,12,14,15).
Development of peritoneal fibrosis is largely driven by fibroblasts, which are spindle-shaped, motile and contractile cells that respond to pro-fibrotic stimuli by synthesizing and organizing ECM (16–18). Besides the proliferation of local tissue fibroblasts, fibroblasts may also be derived from circulating bone marrow-derived precursors (fibrocytes) (19) and resident mesothelial cells by epithelial-to-mesenchymal transition (EMT) (20–23). Epithelial-to-mesenchymal transition has been generally recognized as an important mechanism for embryogenesis, wound healing, tumor invasiveness and metastasis (24). The process includes the disruption of intercellular junctions, loss of apical-basal polarity, and appearance of fibroblast-like phenotypes with migratory and invasive capacity (20,25). Likewise, EMT occurs in peritoneum impairment (26,27). It has been well documented that after subjection to PD, HPMCs progressively undergo a loss of epithelial morphology marked by a decrease in the expression of biomarkers such as cytokeratin and E-cadherin through induction of the regulatory protein snail (20); mesothelial cells acquire a migratory phenotype along with the up-regulation of integrin (20,28).
Accumulating evidence has shown that activation of receptor tyrosine kinases (RTKs) is associated with peritoneal fibrosis. Receptor tyrosine kinases are a large family of cell-surface receptors with 58 members in humans (29) that share a conserved architecture of an extracellular ligand-binding region, a single-pass transmembrane domain and an intra cellular tyrosine kinase domain (29–31). Growth factor binding to the ligand-binding domain initiates the intracellular signaling transduction that is involved in some vital cellular processes, such as cell proliferation, differentiation, migration and survival (30,31). The current review will mainly focus on the tyrosine growth factor receptors engaged in peritoneal fibrosis during PD, including epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), and hepatocyte growth factor receptor (c-Met). We will highlight the role and mechanisms of those RTKs in peritoneal fibrosis (Table 1) (Figure 1) and discuss their potential as therapeutic targets for prevention and treatment of this complication.
TABLE 1.
The Role and Mechanisms of RTKs in Peritoneal Fibrosis
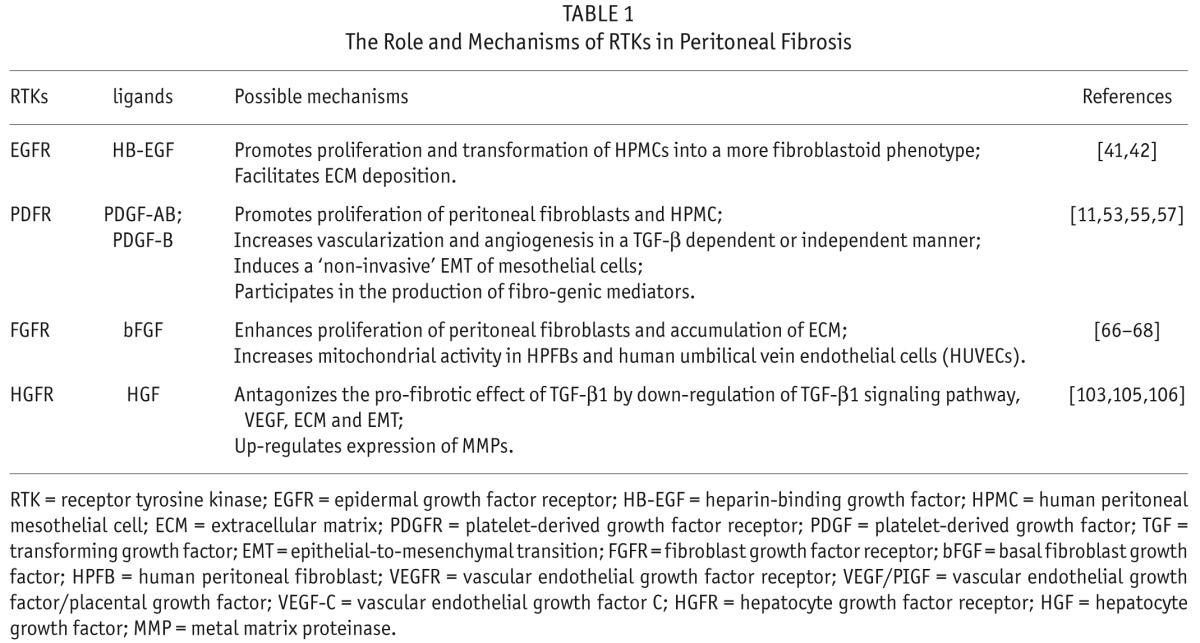
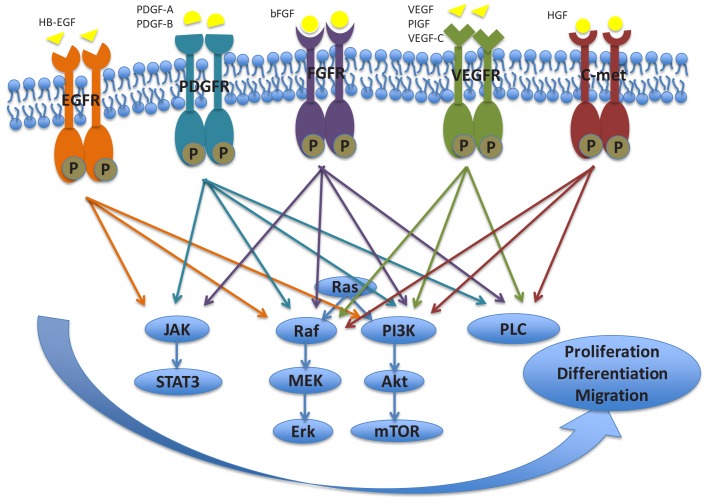
Figure 1 —
The major signaling pathways downstream of various growth factor receptors associated with development of peritoneal fibrosis. HB-EGF = heparin-binding growth factor; PDGF = platelet-derived growth factor; bFGF = basic fibroblast growth factor; VEGF = vascular endothelial growth factor; PIGF = Placental growth factor; HGF = hepatocyte growth factor; EGFR = epidermal growth factor receptor; PDGFR = platelet-derived growth factor receptor; FGFR = fibroblast growth factor receptor; VEGFR = vascular endothelial growth factor receptor; C-met = hepatocyte growth factor receptor; JAK = Janus kinase; STAT3 = signal transducer and activator of transcription 3; MEK = Mitogen-activated protein kinase kinase; Erk = extracellular signal-regulated kinase; PI3K = phosphatidylinositol 3-kinase; Akt = Protein kinase B; mTOR = mammalian target of rapamycin; PLC = phospholipase C.
EGFR
Epidermal growth factor receptor is a family of transmembrane proteins with intrinsic tyrosine kinase activity. The EGFR family has 4 distinct members: the EGFR (also known as ErbB-1/human epidermal growth factor receptor-1, HER1), ErbB-2 (neu/ER2), ErbB-3 (HER3) and ErbB-4 (HER4), which can be activated by growth factors of the epidermal growth factor (EGF) family (32). These EGF-related proteins are composed of EGF, transforming growth factor-α (TGF-α), amphiregulin, heparin-binding growth factor (HB-EGF) and epiregulin (33). Epidermal growth factor receptor activation can also be triggered indirectly by stimuli other than ligand-induced interaction with the EGFR ectodomain, such as by endothelin-1, angiotensin II (Ang II), and TGF-β1 (34). Such EGFR activation has been termed “transactivation,” in which the signal initiated by diverse stimuli can converge on EGFR, which in turn induces activation of downstream signaling pathways (35).
Epidermal growth factor receptor activation induces dimerization and phosphorylation of tyrosine residues in its cytosolic domains (36,37). Once these tyrosine residues are phosphorylated, they become the docking sites for intercellular kinases and then initiate activation of multiple intracellular signaling pathways, including the extracellular signal-regulated kinase (ERK) pathway, the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, and the phosphoinositide-3-kinase (PI3K)/Akt pathway (33). Activation of these pathways modulates a number of cellular responses, including cell proliferation and survival as well as protein expression (38,39).
Activation of the EGF/EGFR system is involved in peritoneal fibrosis. Heparin-binding growth factor, along with its cell receptors, is detectable in HPMCs and/or peritoneal macrophages, either in PD effluent or peritoneal membrane biopsies (40–42). Activation of EGFR by HB-EGF and the associated molecules on the cell surface (CD9, CD44 and integrin α3β1) also facilitates HPMC proliferation in vitro, with particularly high expression of HER1 and HER4 (42). In addition, EGF has been shown to be a potent mitogen and chemotactic factor for HPMCs (41). Upon stimulation with exogenous HB-EGF, HPMCs can be transformed into a more fibroblast-like phenotype, which produces more ECM proteins.
In contrast, Faull et al. argued that HB-EGF is normally present in the peritoneal cavity and is a helpful growth factor at this site, where it facilitates appropriate repair of the mesothelial cell layer and the peritoneal membrane (43). Since loss of mesothelial cells occurs during peritonitis or over time on PD, depletion of this source of HB-EGF may diminish its “beneficial” properties (43). Our recent studies have shown, however, that despite the fact that activation of EGFR is required for recovery of renal function and structure following acute kidney injury, its persistent activation contributes to development and progression of renal fibrosis in an animal model of ischemia/reperfusion-induced acute kidney injury (33). However, it remains unclear whether EGFR activation would be involved in the development of peritoneal fibrosis in response to diverse insults during PD.
PDGFR
The platelet-derived growth factors (PDGFs) family is made up of 5 different disulphide-linked homo- or hetero-dimeric cytokines from 4 different polypeptide chains, namely PDGF-AA, -AB, -BB, -CC, and –DD (44). These 5 isoforms bind to a dimer of tyrosine kinase receptors, which consists of 2 different receptor chains (PDGF-α and PDGF-β) with diverse binding specificities and affinities (45). Whereas PDGF-B binds to both receptor chains, PDGF-A and -C are specific ligands for the PDGFR-α chain. Platelet-derived growth factor-D binds mainly to the homo-dimeric PDGFR-β and, with lower affinity, to the hetero-dimeric PDGFR-αβ (46,47).
Interaction of PDGF with its receptor results in autophosphorylation of the cytoplasmic tyrosine kinase domain of the PDGFR and subsequently recruits adaptor proteins carrying Src homology 2 (SH2) and SH3 domains to this site. Platelet-derived growth factor receptors thereby engage several well-characterized signaling transduction pathways mainly via JAK/STAT, PI3K, phospholipase Cγ (PLC-γ), or mitogen-activated protein kinase (MAPK) pathways, promoting gene expression and mediating the biological functions of the PDGF isoforms, such as proliferation, migration, and survival (46,48).
Activation of PDGFRs has been implicated in a broad range of pathophysiologic events, ranging from cell proliferation and migration, to ECM accumulation and production of pro- and anti-inflammatory mediators, to tissue permeability and hemodynamics (49). As an important fibro-genic mediator, PDGF facilitates fibroblast chemotaxis, proliferation, and fibroblast-mediated tissue matrix contraction (50,51). In renal fibrosis, PDGFR-β and its ligands are mainly involved in glomerulosclerosis, while the PDGFR-α seems to dominantly drive interstitial fibrosis (49,52). Escalation of PDGF expression was observed in the effluent of PD patients with progressive fibrosis. Beavis et al. (53) observed a consistently strong proliferative effect of PDGF-AB on peritoneal fibroblasts that was partially inhibited with a PDGF blocking antibody. Platelet-derived growth factor-B over-expression in the rat peritoneum led to increased vascularization with increased solute transport, as in the peritoneum of patients on long-term PD (11), but significant fibrosis and EMT were absent. As such, investigators proposed that the phenomenon may result from a lack of TGF-β, and PDGF-B may play a role in response to peritoneal injury (54). On the other hand, it was observed that using an adenovirus expressing PDGF-B induced sustained angiogenesis in a TGF-β or Smad signaling dependent manner (55,56). In addition, PDGF-B also induces EMT in peritoneal mesothelial cells but not mobilization, a phenomenon described as a novel “non-invasive” EMT. Furthermore, it is evident that PDGF plays an important role in the production of a number of fibro-genic mediators, such as TGF-β1, interleukin-1β (IL-1β), tumor necrosis factor-α, bFGF and thrombin. Thus, it is possible that targeting PDGFR may have an anti-fibrotic effect in patients on PD.
The ERK cascade acts as a critical step in downstream signaling of PDGF stimulation. It was found that dipyridamole acting as an anti-proliferative and anti-fibrotic agent can inhibit PDGF-stimulated HPMC proliferation through attenuation of ERK activity and reduction of cell-cycle protein expression (57). This suggests that inhibition of ERK may also have therapeutic potential in attenuating peritoneal fibrosis.
FGFR
The FGFR family has 5 members: FGFR1, FGFR2, FGFR3, FGFR4, and FGFR5. They are highly conserved single-pass transmembrane tyrosine kinase receptors (58,59). While FGFR5 can bind FGFs with high affinity, it lacks an intracellular tyrosine kinase domain (58).
At least 22 members of human FGFs have been identified so far, 18 of which are secreted poly-peptidic growth factors that bind to receptors expressed at the cell surface of target cells; the other 4 FGFs (FGF11, FGF12, FGF13, and FGF14), more correctly referred to as FGF homologous factors, are not secreted and act intracellularly (59). Most FGF ligands function in a classic autocrine/paracrine style whereas some of them such as FGF19, FGF21, and FGF23 act as hormones with poor affinity to heparin sulphate proteoglycans (HSPGs) that diffuse from the source of production into the circulation (58). Fibroblast growth factor signaling through FGFRs exerts diverse physiological and pathological activation, depending on the stage of maturation (58). In embryonic development, FGFs are involved in proliferation, differentiation, migration, and survival, and in adults, they participate in angiogenesis, wound healing and carcinogenesis.
Interaction of FGFs with their receptors triggers the transphosphorylation of kinase domains, leading to the docking of adaptor proteins with SH2 domains such as FRS2 (fibroblast growth factor receptor substrate 2). Subsequently, 4 key downstream pathways are activated: RAS/RAF/MAPK, PI3K/AKT, STAT and PLCγ (58,60–62).
Basic fibroblast growth factor (bFGF) (namely FGF2) is known to participate in the development of many fibrotic and angiogenic diseases by encouraging the proliferation of various cultured cells including fibroblasts, endothelial cells, and vascular smooth muscle cells (63,64). Accordingly, bFGF was detectable in PD effluent (65), and increased expression of bFGF was also detected in cultivated HPMC with high-glucose medium in the early stage (66,67). Exogenous bFGF enhances the proliferation of cultured human peritoneal fibroblasts (HPFBs), which has a far stronger effect than TGF-β (68). Bicarbonate-containing dialysis fluids are superior to lactate-containing ones with regard to their influence on the production of vascular endothelial growth factor (VEGF) and bFGF, although lactate and bicarbonate are both toxic for HPMCs (69,70). An in vitro study demonstrated that bFGF from the conditioned medium of cultured HPMCs increased mitochondrial activity in HPFBs and human umbilical vein endothelial cells (HUVECs), and this activation was markedly suppressed by co-incubation with an anti-bFGF antibody (68). Basic FGF is mitogenic for HPFBs and increases fibronectin secretion by these cells when they are exposed to high concentrations of glucose. Thus, investigators speculated that bFGF released from damaged mesothelial cells may have an important role in promoting fibroblast proliferation and accumulation of ECM in peritoneal fibrosis (67). Additional studies are needed to examine the precise mechanism by which bFGF accelerates peritoneal fibrosis.
VEGFR
Vascular endothelial growth factor exerts its biological effect by binding to VEGFR, a family of transmembrane receptors that belongs to subclass V of the tyrosine kinase receptor superfamily and shares a similar molecular structure. The VEGFR family contains 3 main members: VEGFR-1/FLT1 (FMS-like-tyrosine kinase), VEGFR-2/KDR/FLK1 (fetal liver kinase 1), VEGFR-3/FLT3 (FMS-like-tyrosine kinase-3) (71,72). Vascular endothelial growth factor receptor-1 and VEGFR-2 are expressed in the cell surface of most blood endothelial cells (ECs) while VEGFR-3 is largely restricted to lymphatic EC (71). Each of those RTKs is composed of 3 identical domains: an extracellular part of seven Ig homology domains, a single transmembrane region and a tyrosine kinase sequence that is interrupted by a kinase-insert domain (73).
The VEGF family members are homo-dimeric glycoproteins with multiple isoforms such as VEGF121, VEGF165, VEGF189, and VEGF206 that are generated and bioavailable by alternative exon splicing (74). Vascular endothelial growth factor 121 is a non-heparin-binding acidic protein that is freely diffusible while VEGF165 has intermediate properties of a basic character and moderate affinity for heparin. Vascular endothelial growth factor 189 and VEGF206 are highly basic proteins that bind tightly to extracellular heparin-containing proteoglycans (71,74) and may become bioactive by cleavage after plasmin generation and ECM breakdown (75).
In response to a variety of stimuli, ligand binding to VEGFR-2 induces activation of several signaling pathways, including PI3K/Akt, RAF/MEK/Erk, and PLCγ and results in extensive EC mitogenesis and survival, as well as angiogenesis and microvascular permeability (76). Previous studies have shown that peritoneal mesothelial cells, macrophages or ECs have the capacity to produce VEGF in vitro and/or in vivo in response to a variety of stimuli such as GDPs, AGEs, and some other growth factors (77–79). Despite the denudation of these cells, mesothelial cells that have undergone EMT (80) are mainly responsible for the production of VEGF in PD patients and therefore may cause the elevated peritoneal transport rate (81); these cells also promote angiogenesis via VEGF and fibrosis via ECM formation (82). Evidence has shown that mesothelial cells (MCs) change the expression pattern of VEGFRs and co-receptors during mesothelial-to-mesenchymal transition (MMT), which determines a switch of the VEGF effect on MCs from a proliferative response to an invasive one (83).
Several lines of evidence indicate that peritoneal fibrosis is generated in animal models after injection with chlorhexidine gluconate (CG) and is characterized by thickening of the sub-mesothelial zone and increased number of vessels, myofibroblasts, and infiltrating macrophages (84–86). In addition, a large number of VEGF-, proliferating cell nuclear antigen-, and TGF-β-positive cells were observed in the thickening sub-mesothelial area (85,86). The induction of structural and functional microvascular alterations by hyperglycemia could be largely prevented by long-term treatment with a neutralizing anti-VEGF monoclonal antibody whereas treatment with an isotype-matched control antibody was invalid (77,87). Accordingly, these investigations offer compelling evidence to support the substantial connection between peritoneal fibrosis and angiogenesis.
Motomura and colleagues demonstrated that the gene transfer of soluble VEGF type I receptor (sFlt-1) attenuated peritoneal fibrosis formation and was accompanied by approximately 81% reduction of collagen deposition in mesenteric tissue. They suggested that this may be due to inhibition of the pro-inflammatory and angiogenic effect of VEGF/placental growth factor (88). Subsequently, treatment with TNP-470 and thalidomide, both known to prevent angiogenesis, can significantly ameliorate these fibrotic changes and decrease VEGF expression (84,85). All these studies suggest that the progression of peritoneal fibrosis can be attenuated by anti-angiogenesis with reduction of VEGF expression. Therefore, inhibition of VEGF expression may be an interesting therapeutic approach for prevention of peritoneal fibrosis.
Angiogenesis is also regulated by inhibitors including endostatin (89,90). Growth inhibition of angiogenesis by endostatin has also been identified in an experimental mouse model, where endostatin can suppress VEGF expression (91). In patients on long-term PD, angiogenesis and vasculopathy have been shown to closely relate to increased sub-mesothelial fibrosis (11,92). Angiogenesis increases exchange efficiency accompanied by vascular wall alterations such as increased permeability so that the expanded vascular network decreases the glucose-driven osmotic pressure gradient of the PD fluid by an increase in small solute transport, thus leading to UFF (12,93). Since angiogenesis is associated with peritoneal fibrosis (94) and VEGF is a key angiogenic mediator (71), expression of VEGF is indicative of peritoneum damage in PD patients.
Since lymphatic reabsorption may also contribute to UF failure, investigators studied the role of the lymph-angiogenesis mediator VEGF-C in human dialysis effluent, peritoneal tissues, and HPMCs, which indicated that lymph-angiogenesis is associated with fibrosis through the TGF-β/VEGF/C pathway (95).
Vascular endothelial growth factor can bind fibrinogen and fibrin specifically (96). Researchers have suggested that accumulating fibrin by increased VEGF mediates the development of encapsulating peritoneal sclerosis (EPS) from simple sclerosis (92). Consistent with previous studies, fibrin deposition plays an important role in tissue fibrosis by contributing to recruitment of inflammatory cells, macrophages, and fibroblasts, and leads to the synthesis of extracellular matrix (97).
The VEGF-VEGFR system is crucial for angiogenesis, and anti-VEGF-VEGFR molecules have considerable potential as shown in the clinical treatment of cancer patients (98). However, the molecular mechanisms of different VEGF/VEGFR isoforms in peritoneal fibrosis and angiogenesis require further investigation.
HGFR/C-MET
Hepatocyte growth factor (HGF), originally described as a potent mitogen for fully differentiated hepatocytes in the late 1980s, has been identified as a multifunctional polypeptide participating in a wide variety of cellular events such as mitogenesis, morphogenesis and angiogenesis (99,100). The biological effects of HGF are primarily mediated by the tyrosine kinase transmembrane receptor, hepatocyte growth factor receptor (HGFR), also known as cellular Met (c-Met), which is an oncogenic gene product belonging to the RTK superfamily. Cellular Met is expressed predominantly on the surface of endothelial and epithelial cells in many organs, including the liver, kidney, prostate, pancreas, muscle, and bone marrow (99). Upon bioactive HGF binding, c-Met undergoes receptor dimerization/multimerization and auto-phosphorylation of C-terminally clustered tyrosine residues of tyrosine kinase. This process can recruit intracellular adaptor proteins, including PI3K, growth-factor-receptor-bound protein 2 (Grb2), Grb2-associated binder 1 (Gab1), PLCγ, and SH2-domain-containing protein tyrosine phosphatase (Shp2) to initiate downstream signaling (101).
Studies have shown that HGF is an intrinsic anti-fibrotic factor that directly antagonizes the pro-fibrotic actions of TGF-β (102,103). As elucidated in renal fibrosis (102), HGF exerts its anti-fibrotic actions through suppression of myofibroblast activation and matrix overproduction, blockage of tubular EMT, modulation of cell proliferation and apoptosis to preserve normal kidney structure and function under pathological conditions and acceleration of matrix degradation. Since HPMCs play an important role in peritoneal function and are damaged by high glucose solution via the signal of TGF-β1 produced by HPMCs (104), the possible effect of HGF in rescuing HPMCs from peritoneal fibrosis induced by TGF-β1 also has been reported (105,106). Administration of recombinant HGF significantly inhibited the growth of HPMCs induced by high concentrations of D-glucose and/or TGF-β1. Moreover, the growth inhibition can be completely restored by transfecting HGF cDNA into HPMCs (105). It has also been shown that high glucose and TGF-β1 decreased HGF production from HPMCs, whereas addition of HGF restored HPMCs' viability when damaged by glucose. In addition, suppression of TGF-β1 production by HGF can induce up-regulation of metal matrix proteinase-2 (MMP-2) and decreased tissue inhibitor of metalloproteinase-2 (TIMP-2) production by HPMCs (105).
In addition, animal experiments indicated that HGF prevented the thickening of peritoneum induced by PD and down-regulated expression of TGF-β1, VEGF, and type I collagen (103). High concentrations of glucose induced EMT of HPMC associated with decreased production of HGF while exogenous treatment with HGF resulted in a dose-dependent prevention of high-glucose-induced EMT.
Several possible mechanisms may account for the anti-fibrotic effect of HGF. First, HGF counteracts the profibrotic action by down-regulating production of TGF-β1 (103,105). Second, HGF can intercept Smad signaling in various types of kidney cells. It has been reported that HGF signaling can block phosphorylated Smad-2/3 nuclear translocation in interstitial fibroblasts. Third, HGF affects cell-cell interaction and cell-extracellular matrix interaction and stimulates or activates proteolytic networks involved in the breakdown of ECM proteins by up-regulating expression of urokinase-type plasminogen activator and matrix metalloproteinase (101,106,107). Therefore, the reciprocal balance between HGF and TGF-β plays a decisive role in the pathogenesis of chronic fibrotic diseases, especially in peritoneal fibrosis.
Conclusion and Perspective
Fibrosis is a common and advanced pathological outcome in progressively injured organs, including lung, liver, kidney, and skin. In PD, chronic exposure to glucose (dextrose) in PD fluid can lead to peritoneal fibrosis and failure of the peritoneum as a dialysis membrane (108). Although exact mechanisms are not completely understood, knowledge of the molecular pathways leading to fibrosis is growing rapidly. Receptor tyrosine kinases have been recognized as important mediators in fibrogenic diseases (109). On this basis, some tyrosine kinase inhibitors (TKIs) have been tested for their anti-fibrotic efficacy in humans. For example, imatinib has been proven to be efficient in treating systemic sclerosis by blocking activation of the ATP-binding pocket of Abl on kinase via TGF-β signaling and PDGFR (110–112). Other studies have also demonstrated the anti-fibrotic effect of TKIs such as VEGFR and EGFR inhibitors in animals (110,112). Increasing evidence suggests that progressive peritoneal fibrosis is the predominant mechanism of UFF leading to discontinuation of PD; it is speculated that TKIs may also be promising drugs for prevention and treatment of peritoneal fibrosis. But it is still unclear how RTKs mediate peritoneal fibrosis and whether TKIs are effective in treating or preventing human peritoneal fibrosis. As more TKIs become commercially available, it will be interesting to further study the actions of TKIs in the animal model of peritoneal fibrosis and conduct clinical trials to investigate their efficacy in preventing and/or attenuating this complication in PD patients.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
This work was supported by grants from the National Institutes of Health (2RO1DK08506505A1 to SZ), the National Nature Science Foundation of China (81270778, 81470920 to SZ), and Pudong New District Foundation of China (PWZxk2014-06 to SZ). We thank Dr. George Bayliss for critically reading and revising this manuscript.
REFERENCES
- 1. Pletinck A, Vanholder R, Veys N, Van Biesen W. Protecting the peritoneal membrane: factors beyond peritoneal dialysis solutions. Nat Rev Nephrol 2012; 8:542–50. [DOI] [PubMed] [Google Scholar]
- 2. Devuyst O, Rippe B. Water transport across the peritoneal membrane. Kidney Int 2014; 85:750–8. [DOI] [PubMed] [Google Scholar]
- 3. Yung SS, Chan TM. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: the role of mesothelial cells. Mediators Inflamm 2012; 2012:484167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yung SS, Chan TM. Pathophysiology of the peritoneal membrane during peritoneal dialysis: the role of hyaluronan. J Biomed Biotechnol 2011; 2011:180594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Westrhenen R, Zweers MM, Kunne C, de Waart DR, van der Wal AC, Krediet RT. A pyruvate-buffered dialysis fluid induces less peritoneal angiogenesis and fibrosis than a conventional solution. Perit Dial Int 2008; 28:487–96. [PubMed] [Google Scholar]
- 6. Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int 2003; 23:530–41. [PubMed] [Google Scholar]
- 7. Tomino Y. Mechanisms and interventions in peritoneal fibrosis. Clin Exp Nephrol 2012; 16:109–14. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhary K, Khanna R. Biocompatible peritoneal dialysis solutions: do we have one? Clin J Am Soc Nephrol 2010; 5:723–32. [DOI] [PubMed] [Google Scholar]
- 9. Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 2010; 21:1077–85. [DOI] [PubMed] [Google Scholar]
- 10. Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int 2009; 29:605–17. [PubMed] [Google Scholar]
- 11. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9. [DOI] [PubMed] [Google Scholar]
- 12. Davies SJ, Mushahar L, Yu Z, Lambie M. Determinants of peritoneal membrane function over time. Semin Nephrol 2011; 31:172–82. [DOI] [PubMed] [Google Scholar]
- 13. Heimbürger O, Wang T, Lindholm B. Alterations in water and solute transport with time on peritoneal dialysis. Perit Dial Int 1999; 19(Suppl 2):S83–90. [PubMed] [Google Scholar]
- 14. Fusshoeller A. Histomorphological and functional changes of the peritoneal membrane during long-term peritoneal dialysis. Pediatr Nephrol 2008; 23:19–25. [DOI] [PubMed] [Google Scholar]
- 15. Saxena R. Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol 2008; 23:695–703. [DOI] [PubMed] [Google Scholar]
- 16. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007; 117:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18:1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kokubo S, Sakai N, Furuichi K, Toyama T, Kitajima S, Okumura T, et al. p38 MAPK activation promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int 2011; 31:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yanez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 2003; 348:403–13. [DOI] [PubMed] [Google Scholar]
- 21. Jimenez-Heffernan JA, Aguilera A, Aroeira LS, Lara-Pezzi E, Bajo MA, del Peso G, et al. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch 2004; 444:247–56. [DOI] [PubMed] [Google Scholar]
- 22. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007; 87:858–70. [DOI] [PubMed] [Google Scholar]
- 23. LeBleu VS, Kalluri R. Blockade of PDGF receptor signaling reduces myofibroblast number and attenuates renal fibrosis. Kidney Int 2011; 80:1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 2009; 119:1417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aroeira LS, Loureiro J, González-Mateo GT, Fernandez-Millara V, del Peso G, Sánchez-Tomero JA. Characterization of epithelial-to-mesenchymal transition of mesothelial cells in a mouse model of chronic peritoneal exposure to high glucose dialysate. Perit Dial Int 2008; 28(Suppl 5):S29–33. [PubMed] [Google Scholar]
- 27. Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, Jimenez-Heffernan JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18:2004–13. [DOI] [PubMed] [Google Scholar]
- 28. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol 2007; 19:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010; 141:1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103:211–25. [DOI] [PubMed] [Google Scholar]
- 32. Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006; 366:2–16. [DOI] [PubMed] [Google Scholar]
- 33. Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 2013; 83:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samarakoon R, Dobberfuhl AD, Cooley C, Overstreet JM, Patel S, Goldschmeding R. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal 2013; 25:2198–209. [DOI] [PubMed] [Google Scholar]
- 35. Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-beta1-stimulated smooth muscle cells is pp60(c-src)/MEK-dependent. J Cell Physiol 2005; 204:236–46. [DOI] [PubMed] [Google Scholar]
- 36. Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE, et al. Architecture and membrane interactions of the EGF receptor. Cell 2013; 152:557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanders JM, Wampole ME, Thakur ML, Wickstrom E. Molecular determinants of epidermal growth factor binding: a molecular dynamics study. PloS One 2013; 8:e54136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002; 110:669–72. [DOI] [PubMed] [Google Scholar]
- 39. Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 2012; 23:854–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iwamoto I, Imada A. Effects of growth factors on proliferation of cultured human peritoneal mesothelial cells. Nihon Jinzo Gakkai shi 1992; 34:1201–8. [PubMed] [Google Scholar]
- 41. Leavesley DI, Stanley JM, Faull RJ. Epidermal growth factor modifies the expression and function of extracellular matrix adhesion receptors expressed by peritoneal mesothelial cells from patients on CAPD. Nephrol Dial Transplant 1999; 14:1208–16. [DOI] [PubMed] [Google Scholar]
- 42. Faull RJ, Stanley JM, Fraser S, Power DA, Leavesley DI. HB-EGF is produced in the peritoneal cavity and enhances mesothelial cell adhesion and migration. Kidney Int 2001; 59:614–24. [DOI] [PubMed] [Google Scholar]
- 43. Faull RJ. Bad and good growth factors in the peritoneal cavity. Nephrology 2005; 10:234–9. [DOI] [PubMed] [Google Scholar]
- 44. Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 2004; 15:197–204. [DOI] [PubMed] [Google Scholar]
- 45. Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol 2008; 19:12–23. [DOI] [PubMed] [Google Scholar]
- 46. van Roeyen CR, Ostendorf T, Floege J. The platelet-derived growth factor system in renal disease: an emerging role of endogenous inhibitors. Eur J Cell Biol 2012; 91:542–51. [DOI] [PubMed] [Google Scholar]
- 47. Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J 2005; 272:5723–41. [DOI] [PubMed] [Google Scholar]
- 48. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22:1276–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol 2012; 27:1041–50. [DOI] [PubMed] [Google Scholar]
- 50. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004; 15:255–73. [DOI] [PubMed] [Google Scholar]
- 51. Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 2011; 80:1170–81. [DOI] [PubMed] [Google Scholar]
- 52. Eitner F, Bucher E, van Roeyen C, Kunter U, Rong S, Seikrit C, et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J Am Soc Nephrol 2008; 19:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beavis MJ, Williams JD, Hoppe J, Topley N. Human peritoneal fibroblast proliferation in 3-dimensional culture: modulation by cytokines, growth factors and peritoneal dialysis effluent. Kidney Int 1997; 51:205–15. [DOI] [PubMed] [Google Scholar]
- 54. Cina D, Patel P, Bethune JC, Thoma J, Rodriguez-Lecompte JC, Hoff CM, et al. Peritoneal morphological and functional changes associated with platelet-derived growth factor B. Nephrol Dial Transplant 2009; 24:448–57. [DOI] [PubMed] [Google Scholar]
- 55. Patel P, West-Mays J, Kolb M, Rodrigues JC, Hoff CM, Margetts PJ. Platelet-derived growth factor B and epithelial mesenchymal transition of peritoneal mesothelial cells. Matrix Biol 2010; 29:97–106. [DOI] [PubMed] [Google Scholar]
- 56. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005; 201:925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hung KY, Chen CT, Yen CJ, Lee PH, Tsai TJ, Hsieh BS. Dipyridamole inhibits PDGF-stimulated human peritoneal mesothelial cell proliferation. Kidney Int 2001; 60:872–81. [DOI] [PubMed] [Google Scholar]
- 58. Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res 2012; 18:1855–62. [DOI] [PubMed] [Google Scholar]
- 59. Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J 2011; 437:199–213. [DOI] [PubMed] [Google Scholar]
- 60. Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 2005; 16:107–37. [DOI] [PubMed] [Google Scholar]
- 61. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005; 16:139–49. [DOI] [PubMed] [Google Scholar]
- 62. Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005; 16:233–47. [DOI] [PubMed] [Google Scholar]
- 63. Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int 2000; 57:1521–38. [DOI] [PubMed] [Google Scholar]
- 64. Shi HX, Lin C, Lin BB, Wang ZG, Zhang HY, Wu FZ, et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PloS One 2013; 8:e59966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lai KN, Lai KB, Lam CW, Chan TM, Li FK, Leung JC. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 2000; 35:644–52. [DOI] [PubMed] [Google Scholar]
- 66. Cronauer MV, Stadlmann S, Klocker H, Abendstein B, Eder IE, Rogatsch H, et al. Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. Am J Pathol 1999; 155:1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ogata S, Yorioka N, Kohno N. Glucose and prednisolone alter basic fibroblast growth factor expression in peritoneal mesothelial cells and fibroblasts. J Am Soc Nephrol 2001; 12:2787–96. [DOI] [PubMed] [Google Scholar]
- 68. Ogata S, Naito T, Yorioka N, Kiribayashi K, Kuratsune M, Kohno N. Effect of lactate and bicarbonate on human peritoneal mesothelial cells, fibroblasts and vascular endothelial cells, and the role of basic fibroblast growth factor. Nephrol Dial Transplant 2004; 19:2831–7. [DOI] [PubMed] [Google Scholar]
- 69. Ogata S, Mori M, Tatsukawa Y, Kiribayashi K, Yorioka N. Expression of vascular endothelial growth factor, fibroblast growth factor, and lactate dehydrogenase by human peritoneal mesothelial cells in solutions with lactate or bicarbonate or both. Adv Perit Dial 2006; 22:37–40. [PubMed] [Google Scholar]
- 70. Ogata S, Yorioka N, Kiribayashi K, Naito T, Kuratsune M, Nishida Y. Viability of, and basic fibroblast growth factor secretion by, human peritoneal mesothelial cells cultured with various components of peritoneal dialysis fluid. Adv Perit Dial 2003; 19:2–5. [PubMed] [Google Scholar]
- 71. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669–76. [DOI] [PubMed] [Google Scholar]
- 72. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407:242–8. [DOI] [PubMed] [Google Scholar]
- 73. Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci 2003; 28:488–94. [DOI] [PubMed] [Google Scholar]
- 74. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 2001; 114:853–65. [DOI] [PubMed] [Google Scholar]
- 75. Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 1993; 4:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull 2011; 34:1785–8. [DOI] [PubMed] [Google Scholar]
- 77. De Vriese AS, Mortier S, Lameire NH. Glucotoxicity of the peritoneal membrane: the case for VEGF. Nephrol Dial Transplant 2001; 16:2299–302. [DOI] [PubMed] [Google Scholar]
- 78. Seo MJ, Oh SJ, Kim SI, Cho KW, Jo I, Schaub T, et al. High glucose dialysis solutions increase synthesis of vascular endothelial growth factors by peritoneal vascular endothelial cells. Perit Dial Int 2001; 21(Suppl 3):S35–40. [PubMed] [Google Scholar]
- 79. Szeto CC, Lai KB, Chow KM, Szeto CY, Wong TY, Li PK. Differential effects of transforming growth factor-beta on the synthesis of connective tissue growth factor and vascular endothelial growth factor by peritoneal mesothelial cell. Nephron Exp Nephrol 2005; 99:e95–104. [DOI] [PubMed] [Google Scholar]
- 80. Zhang J, Oh KH, Xu H, Margetts PJ. Vascular endothelial growth factor expression in peritoneal mesothelial cells undergoing transdifferentiation. Perit Dial Int 2008; 28:497–504. [PubMed] [Google Scholar]
- 81. Aroeira LS, Aguilera A, Selgas R, Ramirez-Huesca M, Perez-Lozano ML, Cirugeda A, et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am J Kidney Dis 2005; 46:938–48. [DOI] [PubMed] [Google Scholar]
- 82. Kim YL. Update on mechanisms of ultrafiltration failure. Perit Dial Int 2009; 29(Suppl 2):S123–7. [PubMed] [Google Scholar]
- 83. Perez-Lozano ML, Sandoval P, Rynne-Vidal A, Aguilera A, Jimenez-Heffernan JA, Albar-Vizcaino P, et al. Functional relevance of the switch of VEGF receptors/co-receptors during peritoneal dialysis-induced mesothelial to mesenchymal transition. PloS One 2013; 8:e60776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yoshio Y, Miyazaki M, Abe K, Nishino T, Furusu A, Mizuta Y, et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int 2004; 66:1677–85. [DOI] [PubMed] [Google Scholar]
- 85. Arai H, Furusu A, Nishino T, Obata Y, Nakazawa Y, Nakazawa M, et al. Thalidomide prevents the progression of peritoneal fibrosis in mice. Acta Histochem Cytochem 2011; 44:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fabbrini P, Schilte MN, Zareie M, ter Wee PM, Keuning ED, Beelen RH, et al. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol Dial Transplant 2009; 24:3669–76. [DOI] [PubMed] [Google Scholar]
- 87. De Vriese AS, Tilton RG, Stephan CC, Lameire NH. Vascular endothelial growth factor is essential for hyperglycemia-induced structural and functional alterations of the peritoneal membrane. J Am Soc Nephrol 2001; 12:1734–41. [DOI] [PubMed] [Google Scholar]
- 88. Motomura Y, Kanbayashi H, Khan WI, Deng Y, Blennerhassett PA, Margetts PJ, et al. The gene transfer of soluble VEGF type I receptor (Flt-1) attenuates peritoneal fibrosis formation in mice but not soluble TGF-beta type II receptor gene transfer. Am J Physiol Gastrointest Liver Physiol 2005; 288:G143–50. [DOI] [PubMed] [Google Scholar]
- 89. Kakuta T, Tanaka R, Satoh Y, Izuhara Y, Inagi R, Nangaku M, et al. Pyridoxamine improves functional, structural, and biochemical alterations of peritoneal membranes in uremic peritoneal dialysis rats. Kidney Int 2005; 68:1326–36. [DOI] [PubMed] [Google Scholar]
- 90. Gao D, Zhao ZZ, Liang XH, Li Y, Cao Y, Liu ZS. Effect of peritoneal dialysis on expression of vascular endothelial growth factor, basic fibroblast growth factor and endostatin of the peritoneum in peritoneal dialysis patients. Nephrology 2011; 16:736–42. [DOI] [PubMed] [Google Scholar]
- 91. Tanabe K, Maeshima Y, Ichinose K, Kitayama H, Takazawa Y, Hirokoshi K, et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int 2007; 71:227–38. [DOI] [PubMed] [Google Scholar]
- 92. Io H, Hamada C, Ro Y, Ito Y, Hirahara I, Tomino Y. Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int 2004; 65:1927–36. [DOI] [PubMed] [Google Scholar]
- 93. Stavenuiter AW, Schilte MN, Ter Wee PM, Beelen RH. Angiogenesis in peritoneal dialysis. Kidney Blood Press Res 2011; 34:245–52. [DOI] [PubMed] [Google Scholar]
- 94. Kaneko K, Hamada C, Tomino Y. Peritoneal fibrosis intervention. Perit Dial Int 2007; 27(Suppl 2):S82–6. [PubMed] [Google Scholar]
- 95. Kinashi H, Ito Y, Mizuno M, Suzuki Y, Terabayashi T, Nagura F, et al. TGF-beta1 promotes lymphangiogenesis during peritoneal fibrosis. J Am Soc Nephrol 2013; 24:1627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000; 96:3772–8. [PubMed] [Google Scholar]
- 97. Kishimoto C, Kitazawa M, Takada H. Interstitial fibrin-fibronectin deposition with T cell infiltrates precedes fibrosis in murine viral myocarditis. Int J Exp Pathol 1998; 79:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013; 153:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Venepalli NK, Goff L. Targeting the HGF-cMET axis in hepatocellular carcinoma. Int J Hepatol 2013; 2013:341636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol 2011; 26(Suppl 1):188–202. [DOI] [PubMed] [Google Scholar]
- 101. Blumenschein GR, Jr, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol 2012; 30:3287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 2004; 287:F7–16. [DOI] [PubMed] [Google Scholar]
- 103. Nakamura S, Niwa T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J Am Soc Nephrol 2005; 16:144–50. [DOI] [PubMed] [Google Scholar]
- 104. Loureiro J, Aguiler A, Selgas R, Sandoval P, Albar-Vizcaino P, Perez-Lozano ML, et al. Blocking TGF-beta1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 2011; 22:1682–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Matsuo K, Maeda Y, Naiki Y, Matsuoka T, Tamai Y, Yonekawa S, et al. Possible effects of hepatocyte growth factor for the prevention of peritoneal fibrosis. Nephron Exp Nephrol 2005; 99:e87–94. [DOI] [PubMed] [Google Scholar]
- 106. Naiki Y, Matsuo K, Matsuoka T, Maeda Y. Possible role of hepatocyte growth factor in regeneration of human peritoneal mesothelial cells. Int J Artif Organs 2005; 28:141–9. [DOI] [PubMed] [Google Scholar]
- 107. Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int 2001; 59:2023–38. [DOI] [PubMed] [Google Scholar]
- 108. González-Mateo GT, Loureiro J, Jiménez-Hefferman JA, Bajo MA, Selgas R, López-Cabrera M, et al. Chronic exposure of mouse peritoneum to peritoneal dialysis fluid: structural and functional alterations of the peritoneal membrane. Perit Dial Int 2009; 29:227–30. [PubMed] [Google Scholar]
- 109. Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 2014; 59:1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Iwamoto N, Distler JH, Distler O. Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials. Curr Rheumatol Rep 2011; 13:21–7. [DOI] [PubMed] [Google Scholar]
- 111. Distler JHW, Distler O. Intracellular tyrosine kinases as novel targets for anti-fibrotic therapy in systemic sclerosis. Rheumatology 2008; 47:v10–1. [DOI] [PubMed] [Google Scholar]
- 112. Beyer C, Distler JH, Distler O. Are tyrosine kinase inhibitors promising for the treatment of systemic sclerosis and other fibrotic diseases? Swiss Med Wkly 2010; 140:w13050. [DOI] [PubMed] [Google Scholar]