Abstract
♦ Background:
Outcomes for peritoneal dialysis (PD) patients are affected by the characteristics of the peritoneal membrane, which may be determined by genetic variants. We carried out a systematic review of the literature to identify studies which assessed the association between genetic polymorphisms, peritoneal membrane solute transport, and clinical outcomes for PD patients.
♦ Methods:
The National Library of Medicine was searched using a variety of strategies. Studies which met our inclusion criteria were reviewed and data abstracted. Our outcomes of interest included: high transport status peritoneal membrane, risk for peritonitis, encapsulating peritoneal sclerosis (EPS), patient and technique survival. We combined data from studies which evaluated the same genetic polymorphism and the same outcome.
♦ Results:
We evaluated 18 relevant studies. All studies used a candidate gene approach. Gene polymorphisms in the interleukin (IL)-6 gene were associated with peritoneal membrane solute transport in several studies in different ethnic populations. Associations with solute transport and polymorphisms in endothelial nitric oxide synthase and receptor for advanced glycation end product genes were also identified. There was evidence of a genetic predisposition for peritonitis found in 2 studies, and for EPS in 1 study. Survival was found to be associated with a polymorphism in vascular endothelial growth factor and technique failure was associated with a polymorphism in the IL-1 receptor antagonist.
♦ Conclusions:
There is evidence that characteristics of the peritoneal membrane and clinical outcomes for PD patients have genetic determinants. The most consistent association was between IL-6 gene polymorphisms and peritoneal membrane solute transport.
Keywords: Gene polymorphism, peritoneal membrane, solute transport, encapsulating peritoneal sclerosis, peritonitis, interleukin-6, endothelial nitric oxide synthase, receptor for advanced glycation end products
Characteristics of the peritoneal membrane have an impact on patient outcomes and these characteristics are partially determined by genetics. For example, increased peritoneal membrane solute transport has been shown to be a risk factor for mortality (1). The largest study to date to investigate this association reported on over 3,000 incident peritoneal dialysis (PD) patients in Australia and New Zealand (2). In this study, Rumpsfeld et al. found that increased age and racial origin were associated with increased solute transport. These authors concluded that there is likely a genetic component to intrinsic solute transport based on the association with ethnicity. Further, in a recent review by Davies et al., the effect of known clinical variables on intrinsic peritoneal membrane function was felt to be fairly modest, suggesting that genetics may play a prominent role in variability in peritoneal membrane solute transport (3).
Properties of the peritoneal membrane can be categorized as intrinsic or acquired. Both are likely driven by genetic and non-genetic factors. The non-genetic factors of intrinsic peritoneal membrane function include uremia and inflammation (4), while acquired peritoneal membrane changes are related to exposure to glucose (5), peritonitis (6), and loss of residual renal function (7).
Aside from solute transport, other peritoneal membrane-related factors such as encapsulating peritoneal sclerosis (EPS) and risk for peritonitis may have a genetic component. Encapsulating peritoneal sclerosis is a complication of PD therapy that manifests as progressive, massive fibrosis and cocooning of the intra-abdominal organs (8). Although there is controversy surrounding the etiology of EPS, a genetic predisposition has been hypothesized (9). Peritonitis remains an important cause of PD technique failure (10,11). An extensive review of peritonitis risk factors has recently been published by Kerschbaum et al. (12). These authors identified ‘ethnicity’ as a non-modifiable risk factor for PD peritonitis, but it was not clear if this represents a genetic etiology, or a socioeconomic effect. It is clear that there is genetic susceptibility to many human infectious diseases (13), so it follows that there may be a genetic susceptibility to PD-related peritonitis.
Genetic variants are alterations in an individual's genetic code and range from single nucleotide polymorphisms (SNP), to small-scale structural changes (insertions or deletions), to large-scale chromosomal abnormalities including copy number variations or variable number of terminal repeats (VNTR) (14). In order to investigate whether there is an association between a trait and a genetic polymorphism, several approaches can be used. The simplest method is the ‘candidate gene approach’ where a particular known genetic polymorphism is identified in cases and controls. More complex evaluations require genome-wide approaches including genome-wide association scans (GWAS), and full genome, or exome, sequencing.
The aim of this study was to review and synthesize all published information concerning genetic variants, properties of the peritoneal membrane, and outcomes for PD patients.
Methods
We carried out a systematic review of studies that evaluated gene polymorphisms and association with PD-specific traits including solute transport, peritonitis, EPS, patient and technique survival.
Study Selection
The National Library of Medicine was searched. We used the following search terms: (‘gene polymorphism’ or ‘genetic polymorphism’ or ‘variant’ or ‘genotype’) and (‘peritoneal dialysis’). The database was searched from January 1976 to January 2013. We reviewed the reference list of all identified relevant papers. We carried out the search using OVID and Embase search engines. Bibliographies of included papers and review papers were scanned to identify any further studies.
Abstracts of all identified papers were screened in duplicate. Our pre-specified criteria for study inclusion were: (1) the report described patients who were on PD, and (2) an association between a gene polymorphism and a specified trait (solute transport, peritonitis, EPS, and mortality) was evaluated. Studies were excluded if original research was not described.
Data Abstraction
Two investigators independently reviewed each full length article that met the predetermined criteria. Data extracted included the gene of interest, specific polymorphism, study location, sample size, timing of peritoneal equilibrium test (PET), genotype frequency, main results, and statistical methods. Studies that evaluated cross-sectional comparisons between solute transport and genotype or longitudinal changes in peritoneal membrane function were identified. Ancillary data on any phenotypic changes (changes in protein concentration associated with gene polymorphisms) were also recorded.
Statistical Evaluation
We used the data presented in each paper to confirm the reported findings where possible. Genotypes can be grouped as dominant or recessive dichotomous variables (i.e. AA and Aa vs aa) and in this situation the most common comparison used for genotype association studies is the Pearson χ2 statistic (15). With the allelic, or additive approach, the 3 genotypes are considered separately as a ranked variable; for this approach, the Cochran-Armitage modification of the χ2 test is more appropriate (16). For data sets where the number of samples in each category is low, the Fisher's exact test is indicated (17), and the statistical analysis was repeated if necessary. In the case where 2 or more studies reported on the same outcome and the same genetic polymorphism, these data were combined and we applied the appropriate statistical test to evaluate for a significant association. We used R software for statistical analysis (18). P < 0.05 was considered significant throughout.
Results
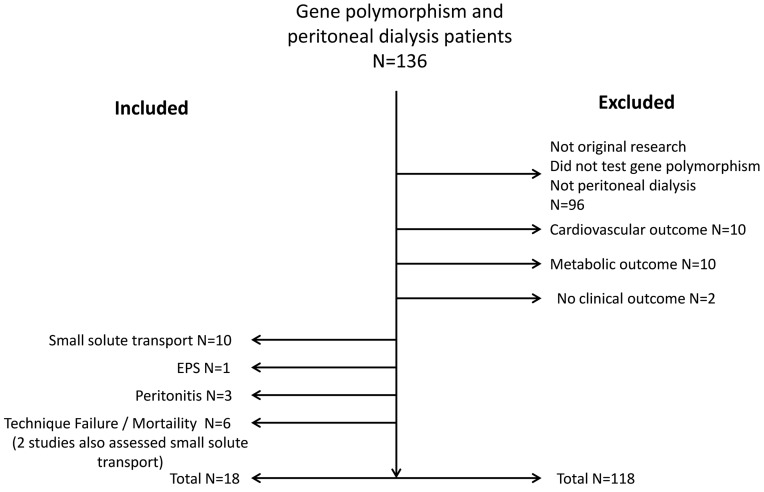
We recovered 136 studies from our initial search strategy. No further studies were identified through our review of the bibliographies of included papers. Of the 136 papers identified for abstract review, 18 papers were included in the final analysis (Figure 1). Of the included studies, all used a candidate gene polymorphism approach to assess for genotype/phenotype association. Ten studies evaluated small solute transport (all used D/P creatinine as the phenotype). Four of these studies included both a cross-sectional analysis and a longitudinal analysis for changes in peritoneal membrane function over time. Six studies evaluated mortality or technique failure (including 2 which also evaluated solute transport). Three studies evaluated the risk for peritonitis by genotype, and 1 study evaluated the risk for EPS by genotype.
Figure 1 —
Consort diagram outlining study inclusion and exclusion.
Solute Transport
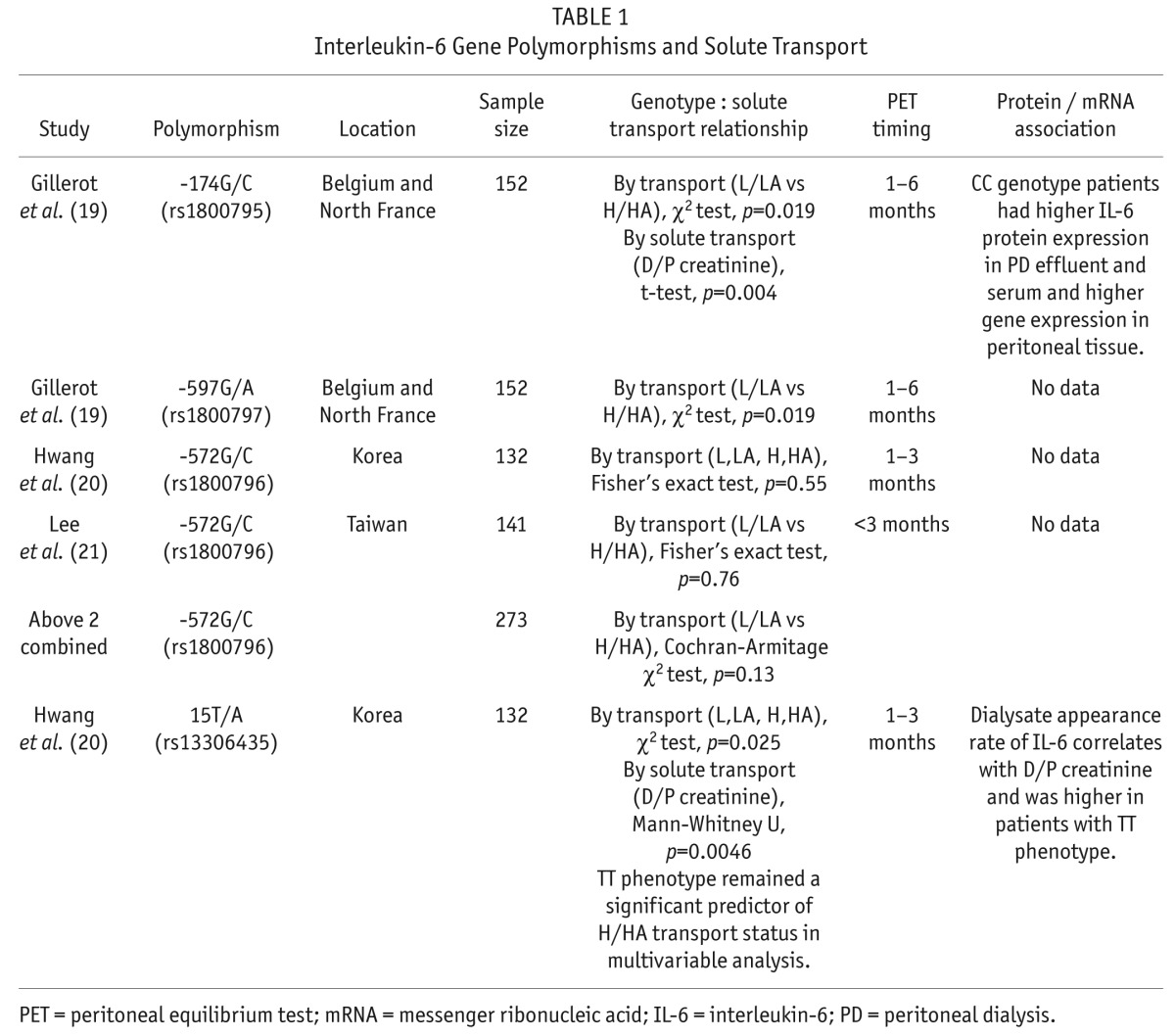
We found 3 separate reports that compared interleukin (IL)-6 gene polymorphisms and peritoneal membrane solute transport (Table 1) (19–21). In a study of 152 northern European PD patients, the IL-6 promoter polymorphisms 174G/C and -572G/C both correlated with increased solute transport (19). This -572G/C polymorphism was reported in 2 other PD populations: Korea (20) and Taiwan (21). No association was found between this polymorphism and solute transport in either of these studies. When we combined results from all 3 studies, there was still no significant association (Table 1). The IL-6 15T/A polymorphism was found to be positively associated with solute transport in the study by Hwang et al. in a population of Korean PD patients (20). The observed association between the IL-6 gene polymorphism and peritoneal membrane solute transport was supported by significant differences in IL-6 protein concentration for both the -174G/C genotype (19) and the 15T/A genotype (20).
TABLE 1.
Interleukin-6 Gene Polymorphisms and Solute Transport
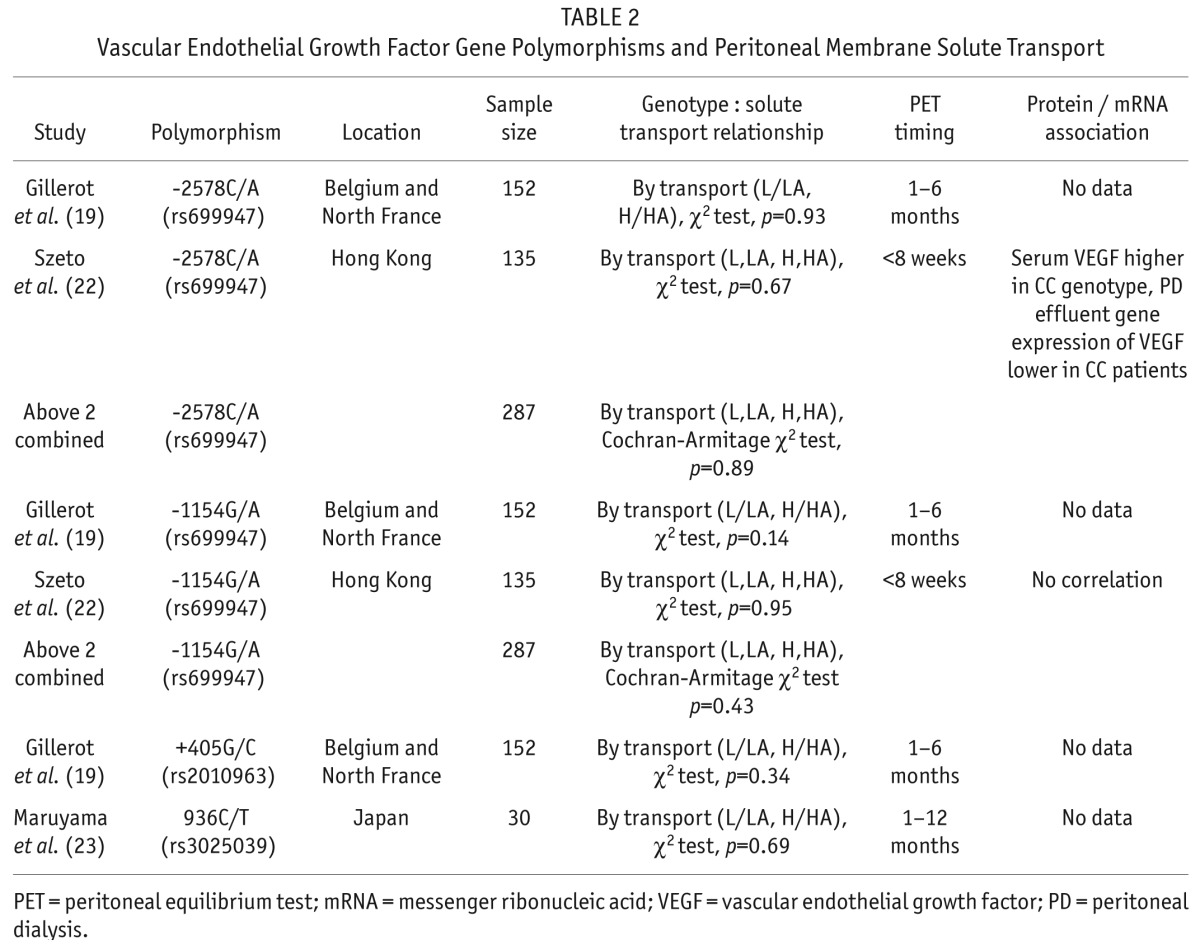
Associations between vascular endothelial growth factor (VEGF) gene polymorphisms and peritoneal membrane solute transport have been reported in 3 papers (19,22,23). Four different polymorphisms were studied; none were associated with solute transport (Table 2). Szeto et al. demonstrated that serum VEGF was higher in patients with the CC genotype associated with the -2578C/A polymorphism, whereas peritoneal effluent VEGF concentration was not associated with genotype (22).
TABLE 2.
Vascular Endothelial Growth Factor Gene Polymorphisms and Peritoneal Membrane Solute Transport
Endothelial nitric oxide synthase (eNOS) gene polymorphisms were studied in 4 reports (Table 3). A VNTR in intron-4 of the eNOS gene involves either 4 or 5 repeats of a 27-base pair sequence. This polymorphism was studied in 3 papers. This intron-4 VNTR polymorphism was associated with solute transport in 2 of these reports, in a population from Turkey (24) and Hong Kong (25). No association was found in a third report from northern Europe (19). Combining these 3 heterogenous results yielded a non-statistical association (p = 0.071). Wong et al. did not find a correlation between the intron-4 VNTR polymorphism and serum nitrate levels (25). Two other polymorphisms were also reported with no significant association with solute transport (Table 3).
TABLE 3.
Endothelial Nitric Oxide Synthase and Interleukin-10 Gene Polymorphisms and Peritoneal Membrane Solute Transport
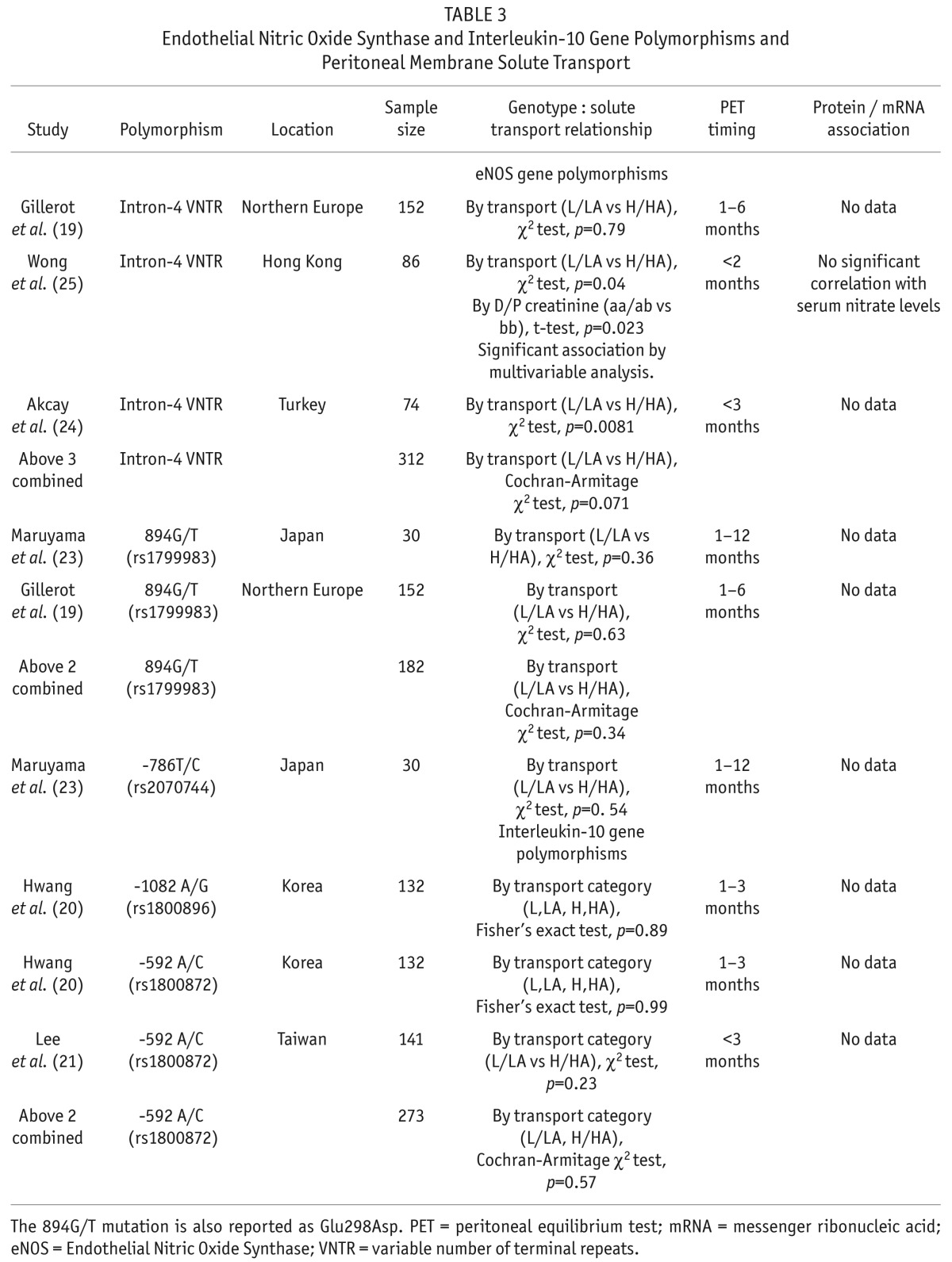
Two different IL-10 gene polymorphisms were not associated with solute transport (20,21) (Table 3).
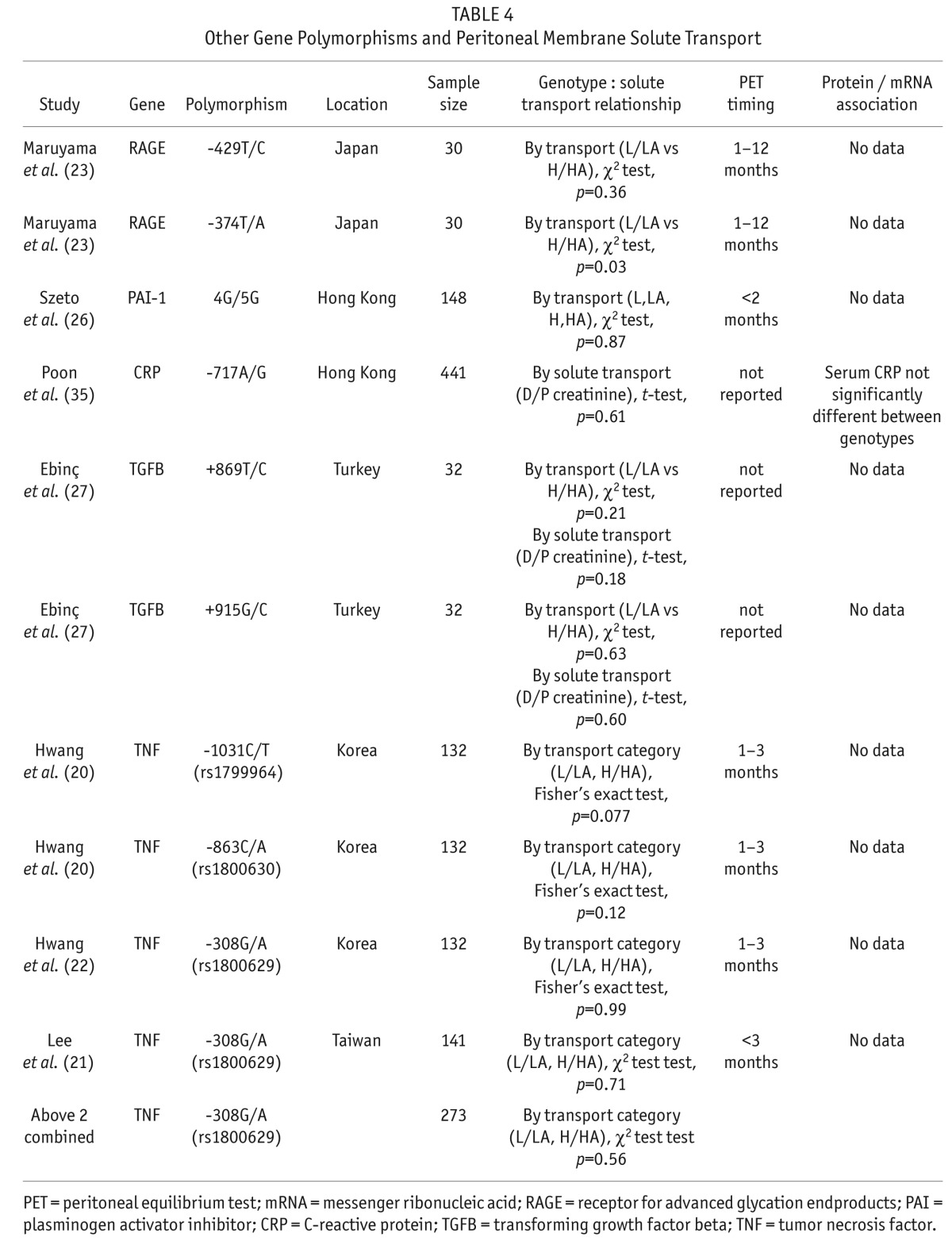
Studies evaluating other gene polymorphisms and association with solute transport are outlined in Table 4. From these studies, an association between receptor for advanced glycation end products (RAGE) gene polymorphism -374T/A and solute transport was identified (23). Polymorphisms associated with plasminogen activator inhibitor (PAI)-1 (26), transforming growth factor beta (TGFB) (27), and tumor necrosis factor (TNF) (20,21) did not predict solute transport.
TABLE 4.
Other Gene Polymorphisms and Peritoneal Membrane Solute Transport
Longitudinal Changes in Solute Transport
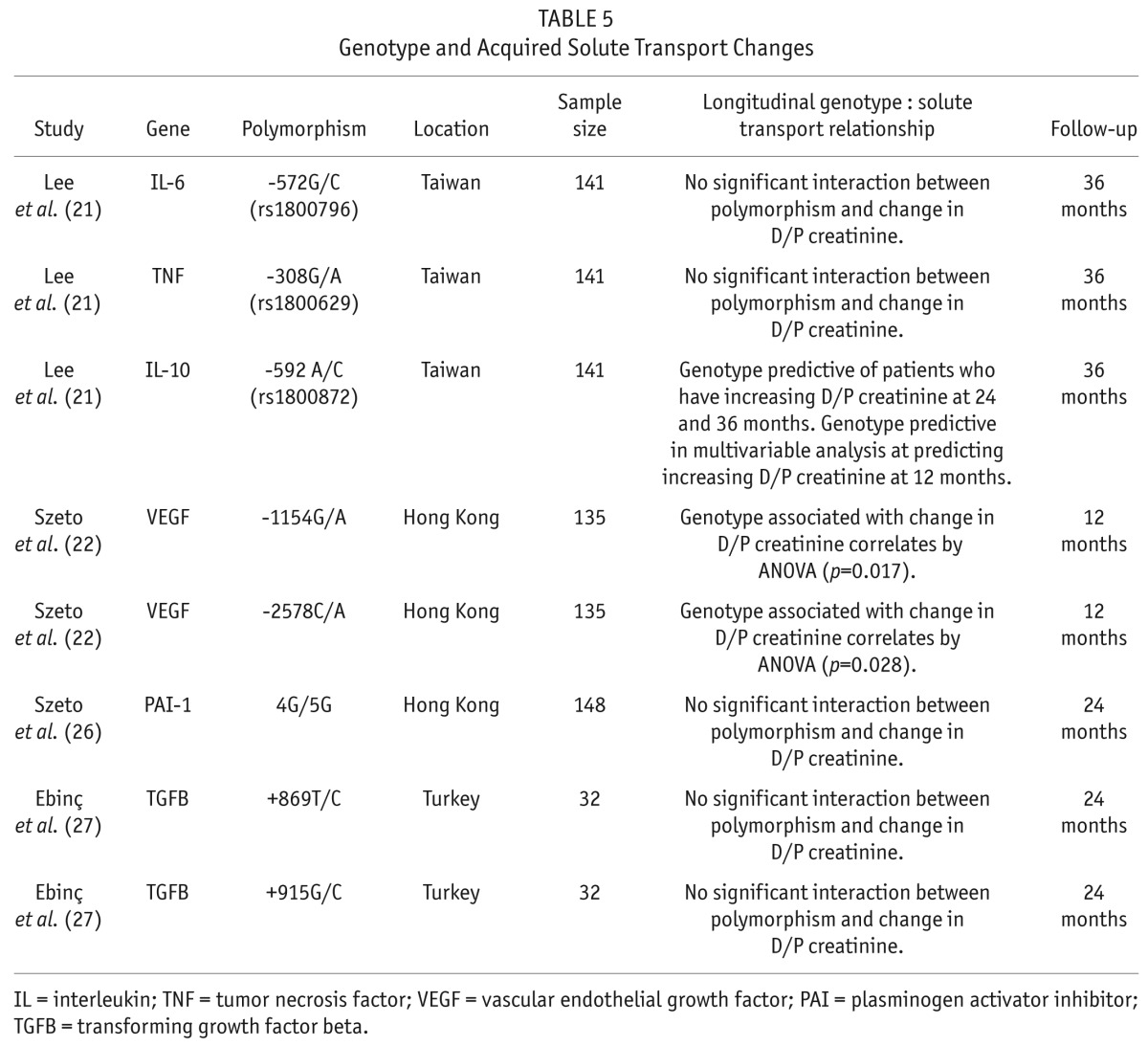
Three studies evaluated the association of gene polymorphism and longitudinal changes in solute transport (Table 5). In the paper by Lee et al., patients with the ‘A’ allele of the -592A/C IL-10 promoter polymorphism were at higher risk of increasing solute transport. This was significant in a univariate analysis at 2 and 3 years and in a multivariable analysis at 12 months (21). Szeto et al. demonstrated that 2 different VEGF promoter polymorphisms predicted for increased solute transport over the 12 months of follow-up (22). The IL-6 (21), TNF (21), IL-10 (21), VEGF (22), PAI-1 (26), and TGFB (27) -associated polymorphisms were not associated with longitudinal changes in solute transport (Table 5).
TABLE 5.
Genotype and Acquired Solute Transport Changes
Encapsulating Peritoneal Sclerosis
Only 1 study to date assessed the role of gene polymorphisms in EPS. Numata et al. found that a polymorphism in the promoter of the RAGE gene was significantly more common in 20 patients with EPS compared to 20 control PD patients (28). Other polymorphisms associated with RAGE, eNOS, and VEGF were not associated with EPS.
Peritonitis
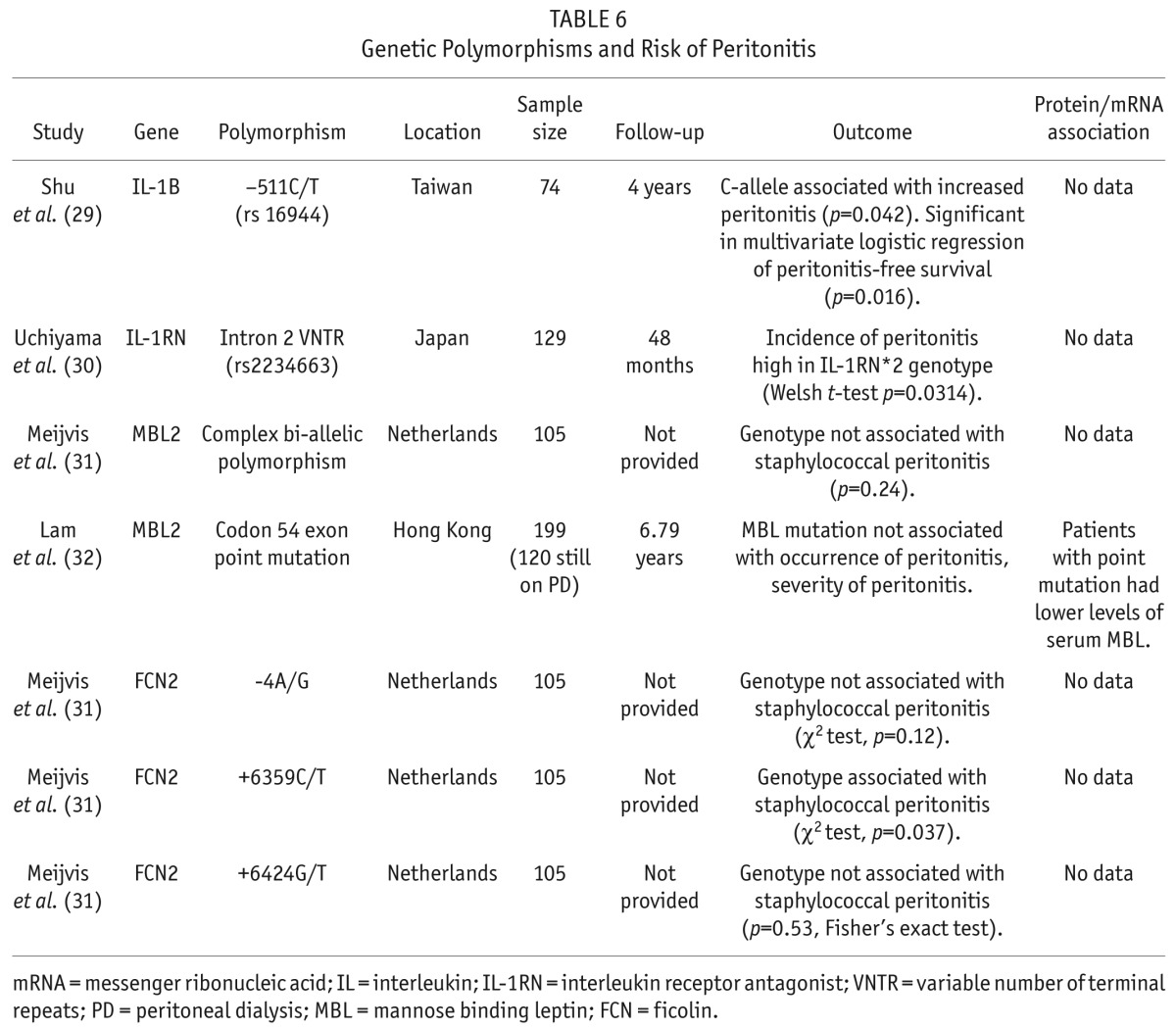
Four studies have evaluated the role of gene polymorphism and the risk for peritonitis (Table 6). Polymorphisms in the IL-1B (29) and IL-1 receptor antagonist (IL-1RN) (30) genes were both associated with an increased risk for peritonitis. Polymorphisms in the genes for 2 pattern recognition receptors which are components of the innate immune response have been assessed. Polymorphisms in mannose binding lectin were evaluated in 2 papers (31,32) and were found not to be associated with peritonitis. One of 3 polymorphisms associated with ficolin was found to associate with the incidence of staphylococcal peritonitis (31).
TABLE 6.
Genetic Polymorphisms and Risk of Peritonitis
Survival and Technique Failure
Seven papers evaluated the effect of gene polymorphisms on overall survival and PD technique failure (Table 7). A significant association was found between overall survival and polymorphisms of the promoter region of the VEGF gene (22). The CA or AA genotype was associated with a hazard ratio of 3.04 for mortality. Vascular endothelial growth factor concentration in the serum was significantly lower in patients with CA or AA genotypes. The angiotensin converting enzyme insertion/deletion polymorphism was studied in 453 patients starting dialysis and recruited to the Netherlands Cooperative Study on the Adequacy of Dialysis (Necosad) (33). In the entire cohort that included hemodialysis and PD patients, the DD genotype was associated with increased mortality. Peritoneal dialysis patients comprised 143 of the 453 patients enrolled, and in this subgroup, the effect of the DD genotype on mortality was no longer significant. The effect of the IL-6 -174G/C polymorphism on mortality in the Necosad PD cohort was also not significant (34). No effect on mortality was seen in several other studies of IL-1RN (30), glutathione S-transferase (GST) (35), carnosinase (36), and apolipoprotein (APO) -a (37). In the study by Poon et al. (35), an association between survival and GST M1 gene polymorphism was seen in a subgroup of patients over the age of 70 years. Also, Iliescu et al. found that lipoprotein a-levels were predictive of mortality, and the APO-a gene polymorphism correlated with lipoprotein a-levels, but APO-a gene polymorphism alone was not associated with mortality (37).
TABLE 7.
Genetic Polymorphisms and Risk of Mortality and Technique Failure
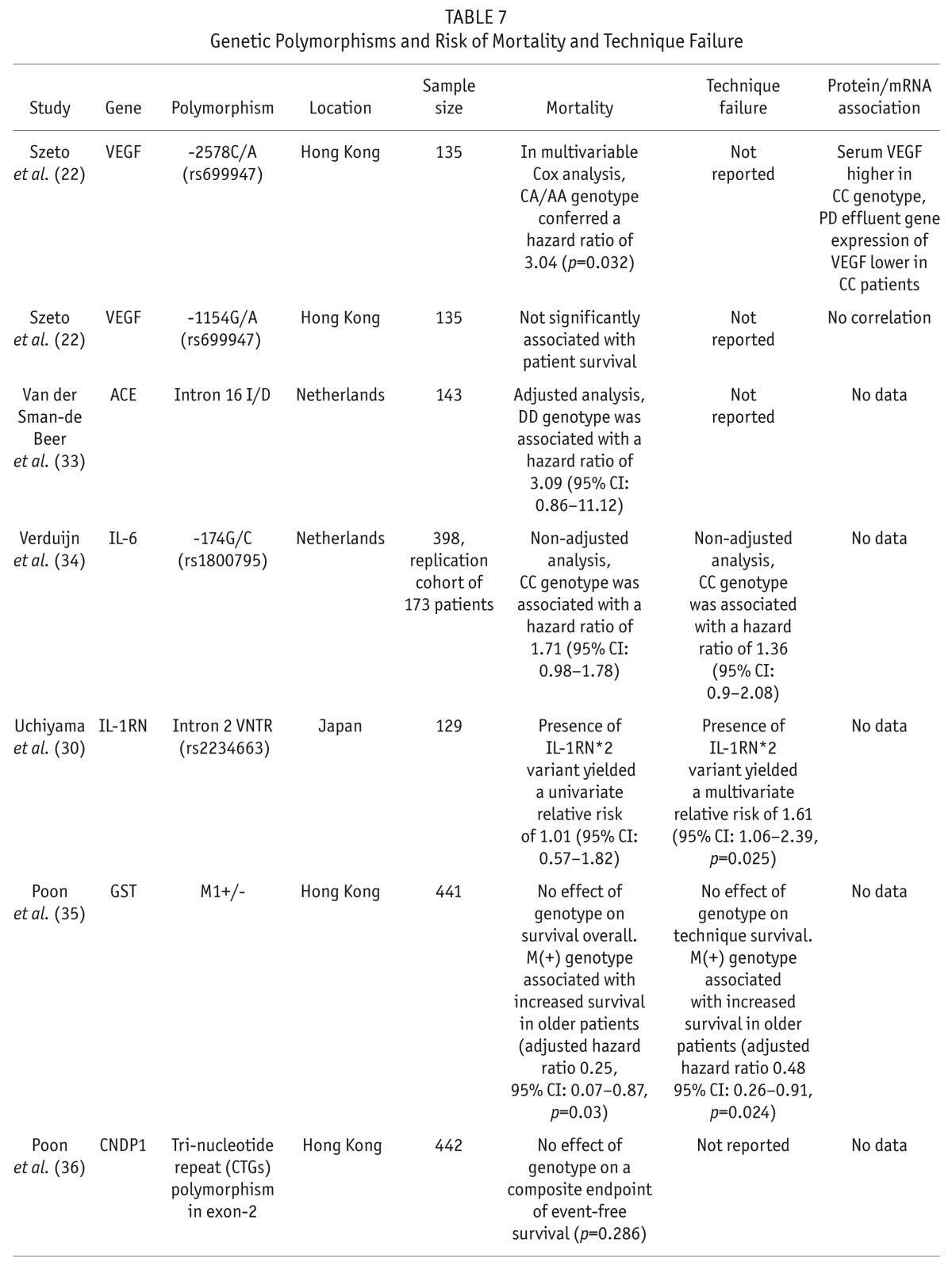
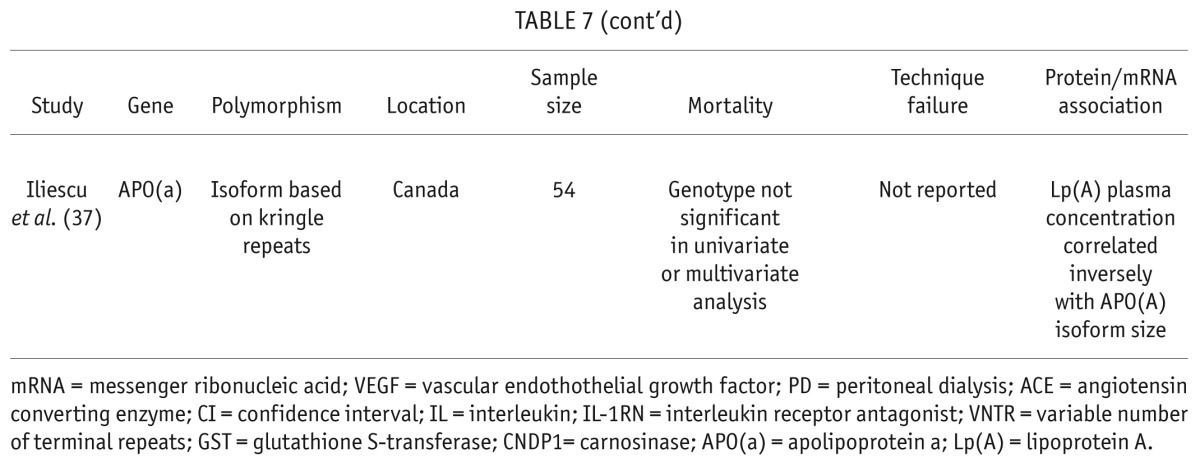
Technique survival was reported in 3 papers and was found to be significantly associated with polymorphisms in IL-1RN in a multivariable analysis (30). Similar to overall survival, Poon et al. found that the GST M1 polymorphism predicted technique survival, but only in a subgroup of patients over the age of 70 (35).
Discussion
Important clinical features and outcomes for PD patients, including peritoneal membrane solute transport, peritonitis, EPS, patient and technique survival, are influenced by genetic polymorphisms. Studies to date have used a candidate gene approach. Most of the polymorphisms studied were promoter sequence SNPs, but also included were insertion/deletion polymorphisms (33), repeated sequences (26), and other larger structural alterations (31). To date, no genome-wide screening study has been carried out specifically targeting PD patients. These screening studies, such as genome-wide association studies, allow for the assessment of SNP polymorphisms across the entire genome, but require a much larger cohort of patients to guard against false positive results from multiple testing. The candidate gene approach supports our understanding of the mechanism of peritoneal membrane injury and lends evidence to the idea that there is a genetic basis for PD patient outcomes. Genome-wide association scans would allow for the identification of potentially novel pathways and mechanisms.
Single nucleotide polymorphisms are genetic markers. Depending on the position, an SNP may or may not affect the protein structure or may influence the expression level of the protein (14). Although associated with a particular gene, these SNPs may in fact be a marker that is linked with another structural genetic variant in a completely different gene. A good example of this was the genetic variant found in black populations that increased the risk for focal segmental glomerulosclerosis. The original SNP was associated with the MYH9 gene, but was in fact linked to the actual polymorphism in the APOL1 gene (38).
Data demonstrating that protein structure or concentration of the target gene is altered in patients with a particular polymorphism are strongly supportive that the polymorphism is playing a causative role in the clinical outcome. The majority of studies we reviewed did not include these data. Some showed a positive correlation between gene polymorphism and protein level (19,20,22,32,37), whereas others looked and did not find a correlation (22,25,39).
Ethnicity clearly plays a role in genetic predisposition to disease. In the papers we reviewed, the intron-4 VNTR polymorphism in eNOS was found more commonly in patients with high transport peritoneal membranes in Turkish and Hong Kong populations, but not in a sample from northern France and Belgium (Table 3). Furthermore, some polymorphisms are simply not identified in certain ethnic populations. For instance, the IL-6 -174 C/G polymorphism is rare in Asian populations (20).
There is strong and consistent evidence that IL-6 gene polymorphisms have an effect on peritoneal membrane solute transport. This was seen in multiple studies in different ethnic populations and is supported by changes in protein concentration in 2 studies (19,20). This corresponds with increasing clinical evidence that IL-6 has a significant impact on peritoneal membrane solute transport and clinical outcomes. In the Global Fluid Study, PD effluent and plasma from 959 PD patients were analyzed. In a multivariable analysis, Lambie et al. found that dialysate, but not plasma, concentration of IL-6 strongly correlated with peritoneal solute transport (40). Furthermore, plasma, but not dialysate, concentration of IL-6 significantly predicted survival in this cohort. Therefore, local peritoneal inflammation, but not systemic, is an important determinant of peritoneal membrane function.
Endothelial nitric oxide synthase was strongly associated with solute transport in 2 independent studies (24,25) but not a third (19), and not supported by differences in protein concentration (25). One smaller study from Japan suggested an association between solute transport and the RAGE gene polymorphism (23). A separate polymorphism in the RAGE gene promoter was more commonly found in a small group of patients who developed EPS (28).
These studies were cross-sectional in nature, and it is perhaps more informative to understand the effect of gene polymorphisms on longitudinal changes in peritoneal membrane solute transport (Table 5). Interestingly, an IL-6 gene polymorphism was not associated with longitudinal changes in solute transport (21), but this same polymorphism was not associated with solute transport in 2 cross-sectional studies (20,21). It would be interesting to see if other IL-6 polymorphisms, such as -174G/C or 15T/A, associate with longitudinal changes in peritoneal membrane function. It is important to note that longitudinal changes in peritoneal membrane function represent classic gene-environment interactions. Genes associated with longitudinal changes may be related to cellular mechanisms of response to environmental injury such as glucose exposure and peritonitis.
Risk for infections have a strong genetic basis (13). It is not surprising that elements of the innate immune response such as IL-1B (29) and its receptor antagonist (30) are associated with increased incidence of peritonitis (Table 6). IL-1 receptor antagonist is particularly interesting as Uchiyama et al. found a polymorphism in IL-1RN to be associated with increased risk of peritonitis and with technique survival (30). There are many factors that influence patient and technique survival—comorbidity, social factors, etc.—so it is likely that a single genetic polymorphism will have minimal effects on this heterogenous outcome, and observed associations may be spurious.
This systematic review highlights the impact of gene polymorphisms on features of the peritoneal membrane and outcomes for PD patients. These studies provide supporting evidence of the role of genetics, and of the defined pathways, in the mechanism of peritoneal membrane injury. Future studies using genome-wide techniques will allow for the elucidation of novel pathways and mechanisms to increase our understanding of the peritoneal membrane.
REFERENCES
- 1. Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 2006; 17:2591–8. [DOI] [PubMed] [Google Scholar]
- 2. Rumpsfeld M, McDonald SP, Purdie DM, Collins J, Johnson DW. Predictors of baseline peritoneal transport status in Australian and New Zealand peritoneal dialysis patients. Am J Kidney Dis 2004; 43:492–501. [DOI] [PubMed] [Google Scholar]
- 3. Davies SJ, Mushahar L, Yu Z, Lambie M. Determinants of peritoneal membrane function over time. Semin Nephrol 2011; 31:172–82. [DOI] [PubMed] [Google Scholar]
- 4. Margetts PJ, McMullin JP, Rabbat CG, Churchill DN. Peritoneal membrane transport and hypoalbuminemia: cause or effect? Perit Dial Int 2000; 20:14–8. [PubMed] [Google Scholar]
- 5. Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 2001; 12:1046–51. [DOI] [PubMed] [Google Scholar]
- 6. del Peso G, Fernández-Reyes MJ, Hevia C, Bajo MA, Castro MJ, Cirugeda A, et al. Factors influencing peritoneal transport parameters during the first year on peritoneal dialysis: peritonitis is the main factor. Nephrol Dial Transplant 2005; 20:1201–6. [DOI] [PubMed] [Google Scholar]
- 7. Matsuda A, Matsumura O, Ogawa T, Tayama Y, Motojima M, Maeda T, et al. Contribution of residual renal function on peritoneal solute transport in dialysis patients. Am J Nephrol 2010; 32:187–93. [DOI] [PubMed] [Google Scholar]
- 8. Brown MC, Simpson K, Kerssens JJ, Mactier RA. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korte MR, Sampimon DE, Betjes MG, Krediet RT. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol 2011; 7:528–38. [DOI] [PubMed] [Google Scholar]
- 10. Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol 2003;14:2948–57. [DOI] [PubMed] [Google Scholar]
- 11. Kolesnyk I, Dekker FW, Boeschoten EW, Krediet RT. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int 2010; 30:170–7. [DOI] [PubMed] [Google Scholar]
- 12. Kerschbaum J, Konig P, Rudnicki M. Risk factors associated with peritoneal-dialysis-related peritonitis. Int J Nephrol 2012:483250 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorensen TI, Nielsen GG, Andersen PK, Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med 1988; 318:72–32. [DOI] [PubMed] [Google Scholar]
- 14. Pollex RL, Hegele RA. Copy number variation in the human genome and its implications for cardiovascular disease. Circulation 2007; 115:3130–8. [DOI] [PubMed] [Google Scholar]
- 15. Lunetta KL. Genetic association studies. Circulation 2008; 118:96–101. [DOI] [PubMed] [Google Scholar]
- 16. Hothorn LA, Hothorn T. Order-restricted scores test for the evaluation of population-based case-control studies when the genetic model is unknown. Biom J 2009; 51:659–69. [DOI] [PubMed] [Google Scholar]
- 17. Agresti A. A survey of exact inference for contingency tables. Statistical Science 1992; 7(1):131–53. [Google Scholar]
- 18. R Core Team R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 19. Gillerot G, Goffin E, Michel C, Evenepoel P, Biesen WV, Tintillier M, et al. Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int 2005; 67:2477–87. [DOI] [PubMed] [Google Scholar]
- 20. Hwang YH, Son MJ, Yang J, Kim K, Chung W, Joo KW, et al. Effects of interleukin-6 T15A single nucleotide polymorphism on baseline peritoneal solute transport rate in incident peritoneal dialysis patients. Perit Dial Int 2009; 29:81–8. [PubMed] [Google Scholar]
- 21. Lee YT, Tsai YC, Yang YK, Hsu KT, Liao SN, Wu CH, et al. Association between interleukin-10 gene polymorphism -592 (A/C) and peritoneal transport in patients undergoing peritoneal dialysis. Nephrology (Carlton) 2011; 16:663–71. [DOI] [PubMed] [Google Scholar]
- 22. Szeto CC, Chow KM, Poon P, Szeto CY, Wong TY, Li PK. Genetic polymorphism of VEGF: impact on longitudinal change of peritoneal transport and survival of peritoneal dialysis patients. Kidney Int 2004; 65:1947–55. [DOI] [PubMed] [Google Scholar]
- 23. Maruyama Y, Numata M, Nakayama M, Matsuo N, Nordfors L, Hosoya T, et al. Relationship between the -374T/A receptor of advanced glycation end products gene polymorphism and peritoneal solute transport status at the initiation of peritoneal dialysis. Ther Apher Dial 2007; 11:301–5. [DOI] [PubMed] [Google Scholar]
- 24. Akcay A, Micozkadioglu H, Atac FB, Agca E, Ozdemir FN. Relationship of ENOS and RAS gene polymorphisms to initial peritoneal transport status in peritoneal dialysis patients. Nephron Clin Pract 2006; 104:c41–6. [DOI] [PubMed] [Google Scholar]
- 25. Wong TY, Szeto CC, Szeto CY, Lai KB, Chow KM, Li PK. Association of ENOS polymorphism with basal peritoneal membrane function in uremic patients. Am J Kidney Dis 2003; 42:781–6. [DOI] [PubMed] [Google Scholar]
- 26. Szeto CC, Poon P, Szeto CY, Wong TY, Lai KB, Li PK. Plasminogen activator inhibitor-1 4G/5G genetic polymorphism does not affect peritoneal transport characteristic. Am J Kidney Dis 2002; 39:1061–7. [DOI] [PubMed] [Google Scholar]
- 27. Ebinç FA, Derici U, Gönen S, Reis KA, Erten Y, Bali M, et al. TGF-beta1 gene polymorphisms and peritoneal equilibration test results in CAPD patients. Ren Fail 2008; 30:15–9. [DOI] [PubMed] [Google Scholar]
- 28. Numata M, Nakayama M, Hosoya T, Hoff CM, Holmes CJ, Schalling M, et al. Possible pathologic involvement of receptor for advanced glycation end products (RAGE) for development of encapsulating peritoneal sclerosis in Japanese CAPD patients. Clin Nephrol 2004; 62:455–60. [DOI] [PubMed] [Google Scholar]
- 29. Shu KH, Chuang YW, Huang ST, Cheng CH, Wu MJ, Chen CH, et al. Association of interleukin-1beta gene polymorphism and peritonitis in uremic patients undergoing peritoneal dialysis. Blood Purif 2011; 32:156–60. [DOI] [PubMed] [Google Scholar]
- 30. Uchiyama K, Naito K, Tsuchida M, Takai K, Okayama N, Fujimura K, et al. Impact of a genetic polymorphism of the interleukin-1 receptor antagonist on technique survival in peritoneal dialysis patients. Blood Purif 2005; 23:450–8. [DOI] [PubMed] [Google Scholar]
- 31. Meijvis SC, Herpers BL, Endeman H, de Jong B, van Hannen E, van Velzen-Blad H, et al. Mannose-binding lectin (MBL2) and ficolin-2 (FCN2) polymorphisms in patients on peritoneal dialysis with staphylococcal peritonitis. Nephrol Dial Transplant 2011; 26:1042–5. [DOI] [PubMed] [Google Scholar]
- 32. Lam MF, Leung JC, Tang CC, Lo WK, Tse KC, Yip TP, et al. Mannose binding lectin level and polymorphism in patients on long-term peritoneal dialysis. Nephrol Dial Transplant 2005; 20:2489–96. [DOI] [PubMed] [Google Scholar]
- 33. van der Sman-de Beer F, Verhagen C, Rombach SM, Boorsma P, van Manen JG, Korevaar JC, et al. ACE I/D polymorphism is associated with mortality in a cohort study of patients starting with dialysis. Kidney Int 2005; 68:2237–43. [DOI] [PubMed] [Google Scholar]
- 34. Verduijn M, Marechal C, Coester AM, Sampimon DE, Boeschoten EW, Dekker FW, et al. The -174G/C variant of IL6 as risk factor for mortality and technique failure in a large cohort of peritoneal dialysis patients. Nephrol Dial Transplant 2012; 27:3516–23. [DOI] [PubMed] [Google Scholar]
- 35. Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between glutathione S-transferase M1 polymorphism and clinical outcomes in Chinese peritoneal dialysis patients. J Nephrol 2012; 25:310–6. [DOI] [PubMed] [Google Scholar]
- 36. Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between carnosinase gene CNDP1 leucine repeat polymorphism and the clinical outcome of Chinese PD patients. Clin Nephrol 2010; 74:343–5. [DOI] [PubMed] [Google Scholar]
- 37. Iliescu EA, Marcovina SM, Morton AR, Lam M, Koschinsky ML. Apolipoprotein(a) phenotype and lipoprotein(a) level predict peritoneal dialysis patient mortality. Perit Dial Int 2002; 22:492–9. [PubMed] [Google Scholar]
- 38. Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 2010; 78:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between CRP polymorphism and cardiovascular events in Chinese peritoneal dialysis patients. Clin J Am Soc Nephrol 2012; 7:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambie M, Chess J, Donovan KL, Kim YL, Do JY, Lee HB, et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 2013; 24:2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]