Abstract
♦ Background:
The prognostic value of pulmonary hypertension at the start of peritoneal dialysis (PD) in patient survival is unclear.
♦ Methods:
We conducted a retrospective study of incident patients who initiated PD therapy from January 2007 to December 2011, and followed up through June 2013. Pulmonary hypertension was defined as an estimated systolic pulmonary artery pressure (PAP) of ≥ 35 mm Hg using echocardiography. Clinical parameters and laboratory findings were compared between patients with and without pulmonary hypertension and a logistic regression model was elaborated. Patient outcomes (all-cause and cardiovascular mortality) were recorded during follow-up. Survival curves were constructed by the Kaplan-Meier method, and the influences of pulmonary hypertension on outcomes were analyzed by Cox regression models.
♦ Results:
Pulmonary hypertension was prevalent in 99 (16.0%) of the 618 patients studied. The independent risk factors for pulmonary hypertension were female (odds ratio [OR] = 2.12; 95% confidence interval [CI]: 1.29 – 3.46), left atrial diameter (OR = 1.15; 95% CI: 1.10 – 1.20), left ventricular ejection fraction (OR = 0.97; 95% CI: 0.95 – 0.99), and serum sodium (OR = 0.94; 95% CI: 0.89 – 0.99). Over a median follow-up of 29.4 months, 93 patients (15.0%) died, 59.1% of them due to cardiovascular disease. Kaplan-Meier survival analysis showed that patients with pulmonary hypertension had worse overall rates of survival and cardiovascular death-free survival than those without pulmonary hypertension. After multivariate adjustment, pulmonary hypertension was independently associated with increased risk for both all-cause and cardiovascular mortality, with hazard ratios (HRs) of 2.10 (95% CI: 1.35 – 3.27) and 2.60 (95% CI: 1.48 – 4.56), respectively.
♦ Conclusions:
The prevalence of pulmonary hypertension at the start of PD was common and associated with increased risk of both all-cause and cardiovascular mortality in incident PD patients.
Keywords: End-stage renal disease, peritoneal dialysis, pulmonary hypertension, survival
Pulmonary hypertension is a devastating disorder, characterized by a progressive increase in pulmonary vascular resistance and pulmonary artery pressure (PAP), leading to right heart failure or even death (1,2). Recently, pulmonary hypertension has been increasingly recognized as a novel threat in patients with end-stage renal disease (ESRD), with an estimated prevalence range of 0 – 68.8% (3–7). The mechanisms involved in pulmonary hypertension development are still under investigation. However, it has been suggested that some factors responsible for this pathogenesis include pulmonary artery calcifications secondary to hyperparathyroidism, volume overload, severe anemia, hemodynamic modifications due to the size or the location of an arteriovenous fistula, and exposure to dialysis membranes. In contrast, pulmonary hypertension improves after successful kidney transplantation (8).
Previous studies have demonstrated that pulmonary hypertension, either pre-existing before initiating hemodialysis (HD) or developing incident after regular HD treatment, is related to increased mortality rates (5,7,9). Further, enhanced PAP has a poor survival rate extending to a period beyond dialysis. In a study regarding renal transplant recipients, severe pulmonary hypertension, defined as mean PAP > 50 mmHg, conferred a hazard ratio (HR) of 3.75 (95% confidence interval [CI]: 1.17 – 11.97) of death compared to patients with lower PAP (9). However, the association between pulmonary hypertension at the beginning of peritoneal dialysis (PD) therapy and the prognosis remains unclear in such patients.
In the current study, we hypothesized that the pres ence of pulmonary hypertension at baseline echocardiography may be related to an elevated risk of cardiovascular and all-cause mortality in PD patients. Moreover, we attempted to identify the risk factors associated with the development of pulmonary hypertension among these patients.
Methods
Ethics Statement
This study was approved by the First Affiliated Hospital of Sun Yat-sen University Institutional Review Boards. All participants provided their written informed consent before inclusion.
Patients
A total of 1,378 patients who initiated PD therapy in our center from 2007 to 2011 were retrieved from our database. Patients older than 18 years undergoing PD for more than 3 months were included (n = 1,272). We excluded patients according to the following criteria: acute cardiovascular, infectious, or major bleeding events within 1 month of recruitment (n = 156), history of chronic HD (n = 31) or renal transplant (n = 15); comorbidities with chronic obstructive pulmonary disease, pulmonary embolism, rheumatic heart disease, congenital heart disease, or portal hypertension (n = 20); 376 patients had no echocardiographic data available and 56 patients did not have their PAP measured by echocardiography. Ultimately, a total of 618 patients were included in this study.
Demographic and Clinical Data
Variables included demographic characteristics (age and sex), body mass index, etiology of ESRD, presence of diabetes and cardiovascular disease (CVD), mean arterial pressure, and laboratory parameters were obtained within the first month of PD initiation. Presence of diabetes was defined as a self-reported history of physician diagnosis, the use of drug or dietary therapy, or a fasting glucose level of 126 mg/dL or greater. Cardiovascular disease included ischemic heart disease, congestive heart failure, cerebrovascular disease, and peripheral vascular disease (10). All biochemical and hematological tests were measured in the biochemical laboratory of the First Affiliated Hospital of Sun Yat-sen University.
Echocardiography
The echocardiographic examination was performed within the first month of PD initiation by a skilled echocardiographist according to American Society of Echocardiography recommendations (11). The echocardiographist was blind to the participants' other data. Two-dimensional echocardiographically guided M-mode images were recorded from standardized views. Cardiac morphological variables including end-diastolic interventricular septum thickness, left ventricular internal diameter in diastole, end-diastolic posterior wall thickness, left ventricular ejection fraction (LVEF), left ventricular diastolic dysfunction, and left atrial diameter were measured by conventional echocardiography.
Systolic right ventricular (or pulmonary artery) pressure was calculated using the modified Bernoulli equation: PAP = 4 × (tricuspid systolic jet)2 + 10 mm Hg (estimated right atrial pressure, RAP) (12,13). Pulmonary hypertension was defined as a systolic PAP ≥ 35 mm Hg (14).
Left ventricular mass was calculated using the Devereux formula (15). Left ventricular mass index was calculated by dividing left ventricular mass by body surface area. Left ventricular hypertrophy (LVH) was defined as left ventricular mass index more than 131 g/m2 in men and more than 110 g/m2 in women (16).
Left ventricular systolic function was assessed by LVEF. The ratio of early transmitral flow velocity (E) and early diastolic mitral annular velocity (e') was used to estimate left ventricular diastolic function. An E/e' ratio > 15 was considered as an elevated left ventricular filling pressure and a ratio < 8 indicated low left ventricular filling pressure (17).
Outcomes
Our primary outcomes were all-cause and cardiovascular mortality. Cardiovascular mortality was defined as death associated with either a definite myocardial ischemic event, heart failure, cerebrovascular accident, arrhythmia, or peripheral vascular accident, all of which were defined according to standard clinical criteria and sudden death. Sudden death was diagnosed as unexpected natural death occurring within 1 hour of the onset of symptoms and without any prior condition that would appear fatal (18). Survival time was defined as the time from enrollment to death or administrative censoring (i.e. transfer to HD, renal transplantation, transfer to other dialysis centers, loss to follow-up, or end of the study period) until June 30, 2013.
Statistical Analyses
The data are expressed as mean ± standard deviation (SD), medians (interquartile ranges) and frequencies (%) where appropriate. The differences between pulmonary hypertension and non-pulmonary hypertension groups were assessed using t-test or Mann-Whitney U test for normally and non-normally distributed continuous variables and the chi-square test for categorical variables, as appropriate. Odds ratios (ORs) for pulmonary hypertension based on logistic regression for each covariate were computed. Those covariates with a p value < 0.1 in univariate analysis were considered for the multivariate analysis by backward stepwise selection logistic regression. Survival curves of patients were generated by the Kaplan-Meier method and compared by the log-rank test. Univariate Cox regression analysis was performed for all-cause and cardiovascular mortality. Factors with p < 0.1 in univariate analysis were further included in the multivariate Cox regression analysis and the backward stepwise method was used to identify independent predictors for death. The C-index was calculated to assess the ability of pulmonary hypertension incorporated with the established risk factors in predicting both the allcause and cardiovascular mortality, and the multivariable linear model was used to test the multicollinearity of the variables regarding cardiac measures (Table S1).
All statistical analyses were performed using SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA) and R (version 2.15.3; Free Software Foundation Inc., www.r-project.org). A p value < 0.05 was considered statistically significant.
Results
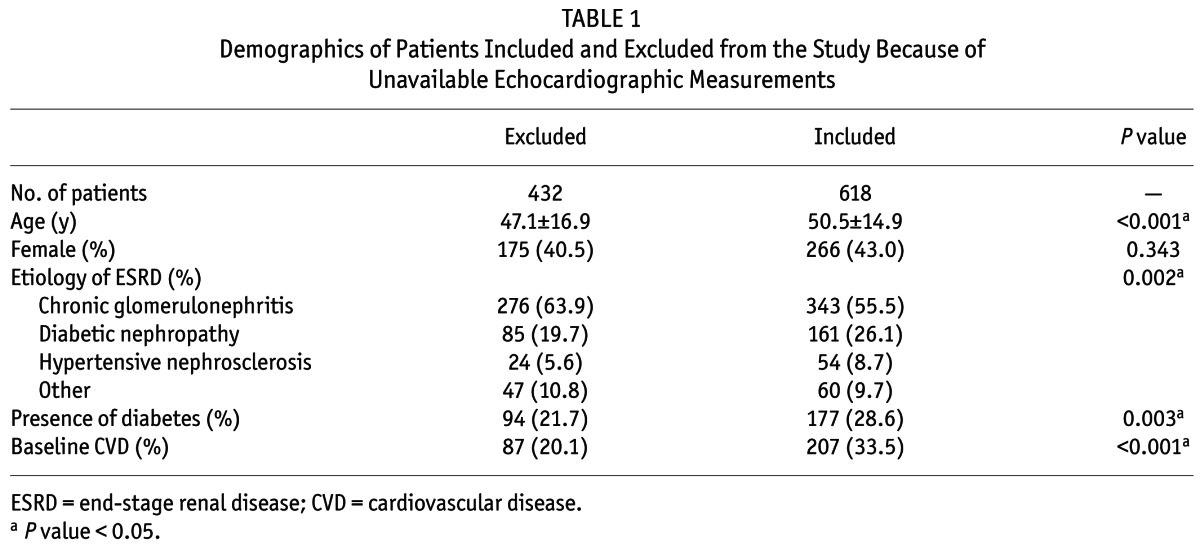
A total of 618 patients who began PD treatment in our center between January 1, 2007, and December 31, 2011, were eligible for enrollment in our study. Compared with 432 patients excluded because of unavailable PAP measurements by echocardiography, those included were significantly older and were more likely to have diabetes and CVD, whereas there were no differences in sex distribution between patients included and excluded (Table 1).
TABLE 1.
Demographics of Patients Included and Excluded from the Study Because of Unavailable Echocardiographic Measurements
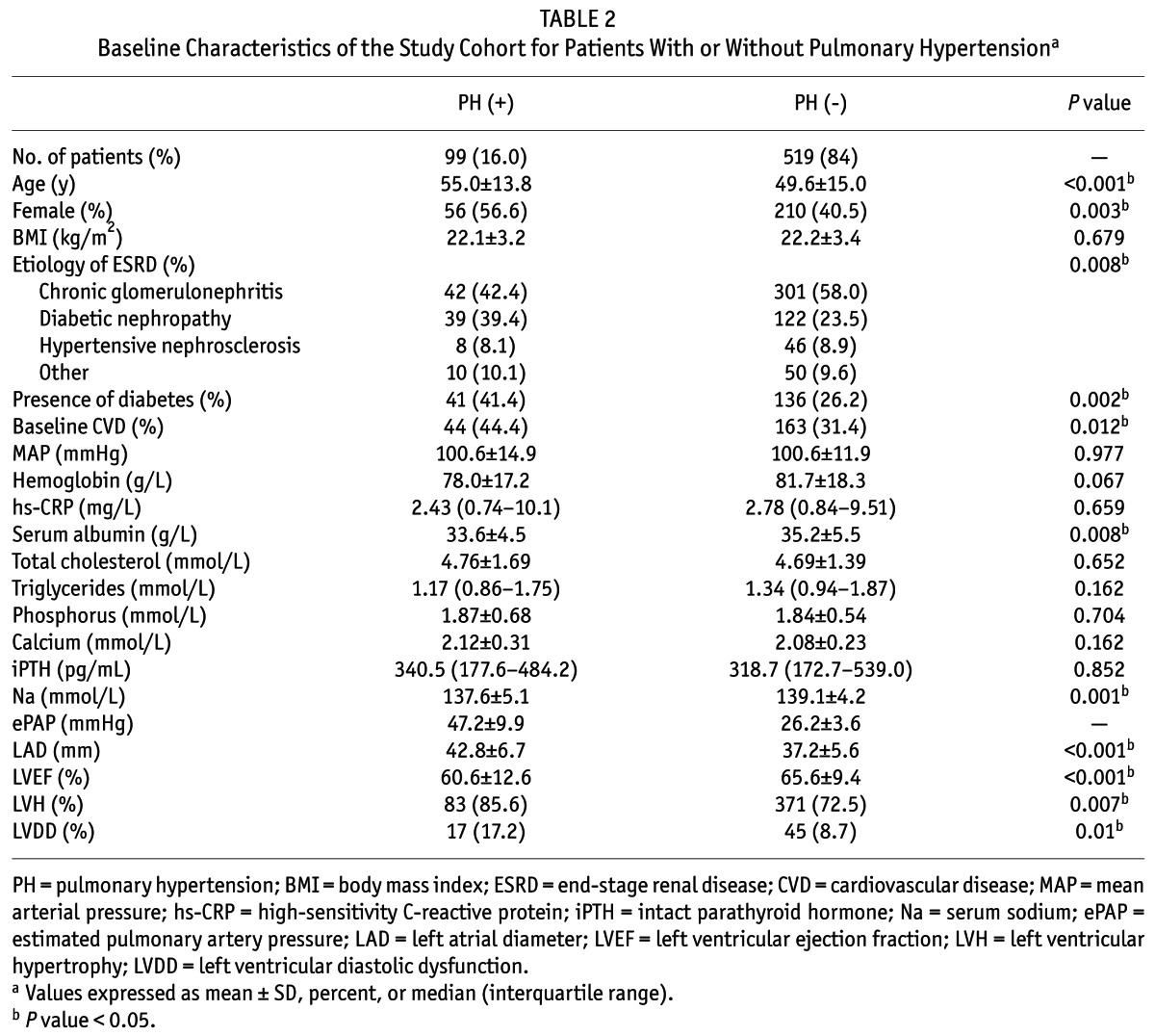
The main baseline demographic and clinical characteristics of the study population are presented in Table 2. Pulmonary hypertension was found in 99 patients (16.0%). Compared with patients without pulmonary hypertension, those with pulmonary hypertension were more likely to be older and female, had a history of diabetes and pre-existing CVD, had lower levels of serum albumin and serum sodium. In terms of echocardiographic data, patients with pulmonary hypertension had a significantly higher level of left atrial diameter (LAD) and lower level of LVEF, as well as a higher prevalence of LVH and left ventricular diastolic dysfunction.
TABLE 2.
Baseline Characteristics of the Study Cohort for Patients With or Without Pulmonary Hypertensiona
We performed logistic regression analysis to assess the association of clinical characteristics and other echocardiographic variables with pulmonary hypertension. The results showed that female (OR, 2.12; 95% CI: 1.29 – 3.46), serum sodium (OR, 0.94; 95% CI: 0.89 – 0.99, per 1 mmol/L higher), LAD (OR, 1.15; 95% CI: 1.10 – 1.20, per 1 mm higher) and LVEF (OR, 0.97; 95% CI: 0.95 – 0.99, per 1% higher) were independently associated with pulmonary hypertension (Table 3).
TABLE 3.
Multivariate Odds Ratios for Prevalence of Pulmonary Hypertensiona

During a median follow-up of 29.4 months (interquartile range: 20.4 – 38.9 months), 93 patients (15.0%) died. As shown in Table 4, the major causes of death were cardiovascular disease (59.1%), followed by sepsis including peritonitis (7.5%) and other infections (12.9%). Of all deaths, 32 patients (34%) with pulmonary hypertension (crude mortality rate [CMR] 118.9/1000 patient-years) vs 61 patients (11%) without pulmonary hypertension (CMR 46.8/1000 patient-years) died during this period. Notably, 23.2% of patients with pulmonary hypertension vs 6.2% of patients without pulmonary hypertension died from cardiovascular causes. Kaplan-Meier survival analysis showed that patients with pulmonary hypertension were likely to have worse overall rates of survival and cardiovascular death-free survival. The overall survival rates at 1, 3, and 5 years were 79%, 63%, and 30% in patients with pulmonary hypertension, and 92%, 79%, and 71% in those without pulmonary hypertension, respectively (p < 0.001; Figure 1A). Further, the 1-, 3-, 5-year cardiovascular mortality rates of patients with and without pulmonary hypertension were 17% vs 5%, 30% vs 10%, and 40% vs 10%, respectively (p < 0.001; Figure 1B).
TABLE 4.
Causes of 93 Deaths During Follow-Up Perioda
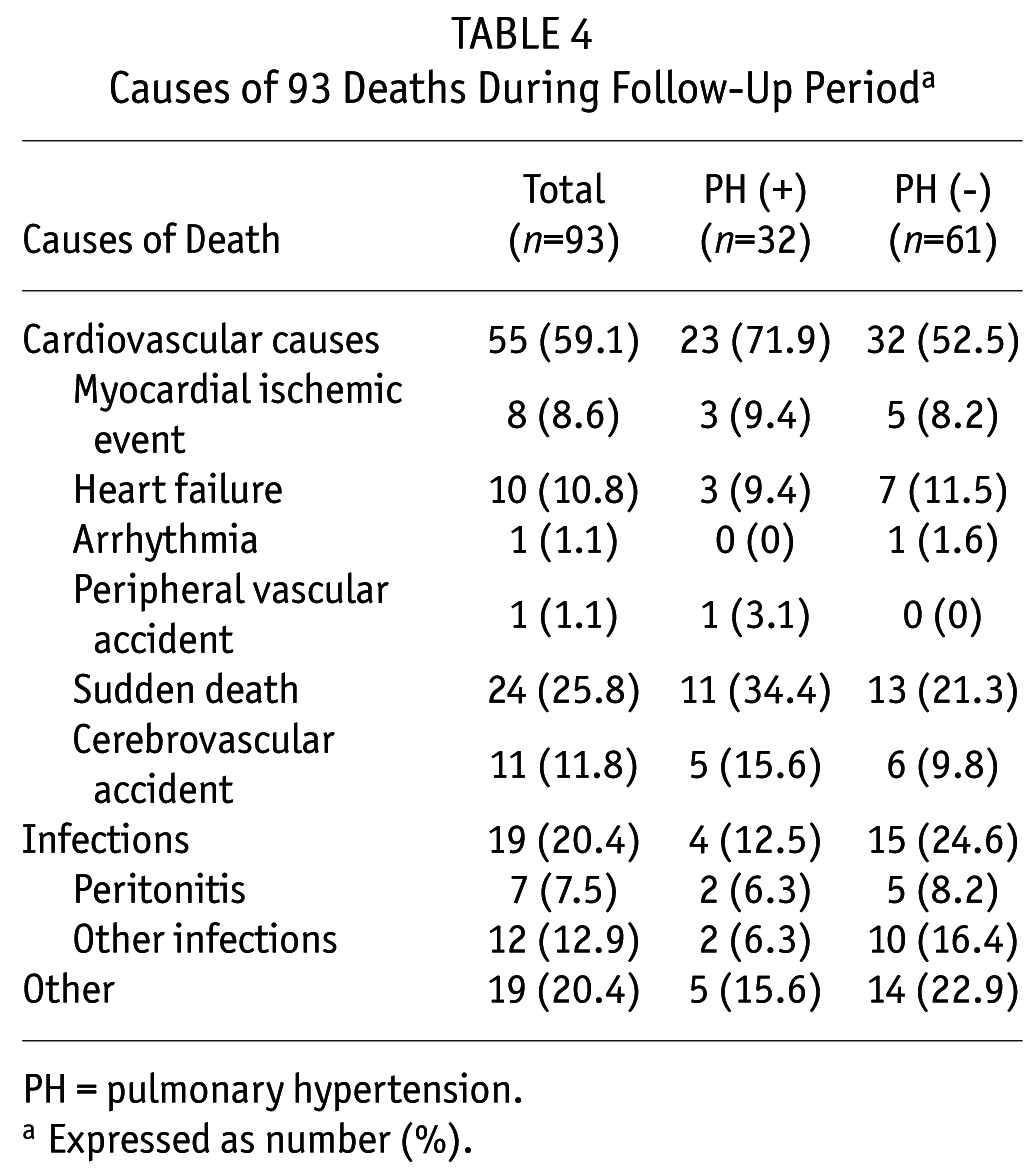
Figure 1 —
Kaplan-Meier survival curves analysis for all-cause mortality (A) and cardiovascular mortality (B) in patients with and without pulmonary hypertension. PH = pulmonary hypertension.
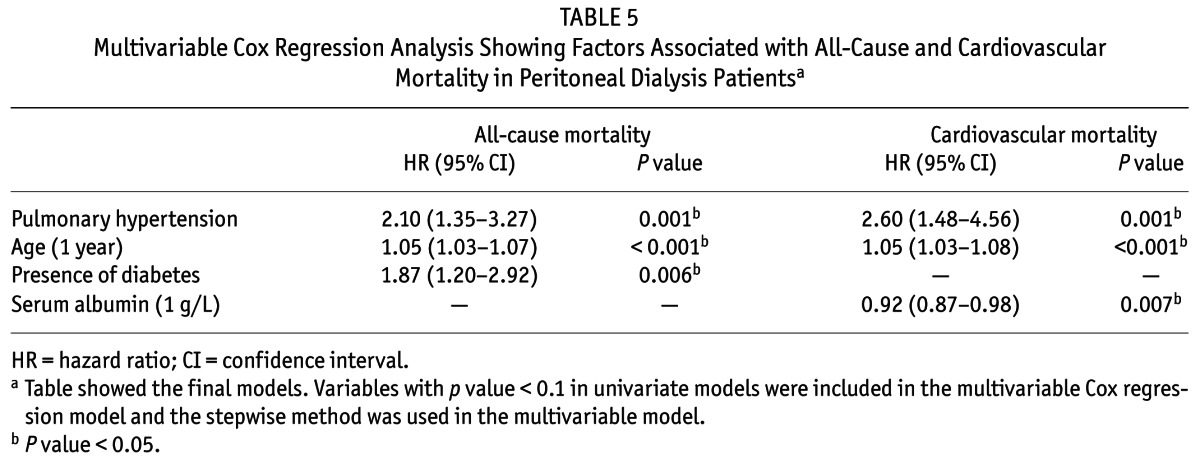
Univariate analysis revealed that presence of pulmonary hypertension, diabetes and CVD, older age, a higher high-sensitivity C-reactive protein level, an enlarged LAD and reduced LVEF were associated with an increased risk of all-cause and cardiovascular mortality. In addition, lower levels of serum sodium and intact parathyroid hormone were significant risk factors for all-cause mortality, whereas a lower level of serum albumin was a risk factor for cardiovascular mortality (Table S2). Controlling for confounding covariates as shown in the multivariate Cox regression models in Table 5, pulmonary hypertension conferred increased risk on both all-cause mortality and cardiovascular mortality, with an adjusted HR of 2.01 (95% CI: 1.35 – 3.27; p = 0.001) and 2.60 (95% CI: 1.48 – 4.56; p = 0.001), respectively. We also found that the C-index significantly increased after pulmonary hypertension was added into the model with established risk factors in prediction of both all-cause and cardiovascular mortality (Table S3). For all-cause mortality, the age (HR 1.05; 95% CI: 1.03 – 1.07 per 1 year increase; p < 0.001) and diabetes (HR 1.87; 95% CI: 1.20 – 2.92; p = 0.006) were also independent predictors. Regarding cardiovascular mortality, other independent risk factors included age (HR 1.05; 95% CI: 1.03 – 1.08 per 1 year increase; p < 0.001) and serum albumin levels (HR 0.92; 95% CI: 0.87 – 0.98 per 1 g/L increase; p = 0.007).
TABLE 5.
Multivariable Cox Regression Analysis Showing Factors Associated with All-Cause and Cardiovascular Mortality in Peritoneal Dialysis Patientsa
Discussion
In the present study, we demonstrated that pulmonary hypertension was present in 16.0% of patients at PD initiation and independently associated with increased risk of both all-cause and cardiovascular mortality. Being female, having an enlarged left atrium, a reduced LVEF, and lower level of serum sodium were risk factors for developing pulmonary hypertension in this population.
Recently, pulmonary hypertension has been reported as a new entity and an unrecognized threat in patients with ESRD (19). Based on echocardiography, the prevalence of pulmonary hypertension ranges from 9 – 39% in individuals with stage 5 chronic kidney disease, and 18.8 – 68.8% in HD patients. The estimated rates of pulmonary hypertension were lower in patients treated with PD (0 – 42%) than HD (20), although limited information is available regarding pulmonary hypertension in PD populations. In the present study, we found 16% of patients had pulmonary hypertension, which was similar to an observation in a cohort of 135 PD patients, 12.6% of whom had pulmonary hypertension (4). Nevertheless, Kumbar et al. reported that 42% of 36 PD patients showed a systolic PAP greater than 35 mm Hg (21), which was much higher than ours. Prevalence estimates vary widely among studies, probably due to patients studied. Most ESRD patients are affected by 1 or more comorbid conditions such as congestive heart failure, hypertension, and diabetes mellitus, which are the common causes of pulmonary hypertension. As noted by the study of Kumbar et al. (21), patients with congestive heart failure were not excluded and the mean ejection fraction of the patients with pulmonary hypertension was 46.3%, which was much lower than the patients in our study. Consistently, we found that an enlarged left atrium and a lower LVEF were strongly associated with developing pulmonary hypertension, suggesting a very important determinate effect of left ventricular disorders in the pathogenesis of pulmonary hypertension. Likewise, patients with pulmonary hypertension showed a higher prevalence of LVH and left ventricular diastolic dysfunction, indicating myocardial dysfunction or a certain extent of chronic volume overload in our PD patients, which was consistent with another study (4). We also found that pulmonary hypertension was predominant in females in incident PD patients, which has been reported in the general population (22,23). The mechanisms underlying this gender difference remains unclear. In addition, there is evidence that time on HD correlates with elevated prevalence of pulmonary hypertension. Because we enrolled incident rather than prevalent patients, the relationship between PD vintage and the incidence of pulmonary hypertension needs further study.
Many studies have attempted to investigate the impact of pulmonary hypertension on clinical outcomes in ESRD patients, which consistently reveals an association with enhanced mortality in patients with pulmonary hypertension. A larger retrospective analysis of 127 HD patients showed that presence of pulmonary hypertension was an independent risk factor for death, regardless of pulmonary hypertension established before initiation of HD (HR 3.6; 95% CI: 1.8 – 7.0, p = 0.0002) or developed after regular HD treatment (HR 2.1, 95% CI: 1.1 – 4.1, p = 0.02) (5). Of note, in a study of 215 patients on maintenance dialysis therapy, the pre-existing pulmonary hypertension before kidney transplantation also portended a 4-fold greater risk of death after transplantation (9), suggesting that transplantation may not reverse the excess mortality risk of established pulmonary hypertension. Few reports have examined the effects of pulmonary hypertension on survival in PD patients. As mentioned above, Kumbar et al. showed that 60% of pulmonary hypertension patients and 38% of patients without pulmonary hypertension died (p = 0.19) during the study period, reflecting a tendency that pulmonary hypertension might be associated with a higher mortality risk (21). In the present study, we demonstrated at first that the presence of pulmonary hypertension at the beginning of PD was associated with both all-cause and cardiovascular mortality. Because predictors for future adverse outcomes are clinically relevant, our findings might have great significance from the viewpoint of risk stratification in ESRD patients at the beginning of PD therapy.
Although our study is the largest study on pulmonary hypertension in PD patients to date, it has certain limitations. First, we excluded a segment of patients due to unavailable PAP measurements by echocardiography, which may carry a risk for bias. Second, despite efforts to exclude patients with obvious factors which may result in hemodynamic modifications and lead to elevated pulmonary hypertension, we could not completely rule out the subclinical impact of comorbidities on the development of pulmonary hypertension. We also did not measure residual renal function within the first month of PD initiation. Third, pulmonary hypertension was not measured by the reference-standard of right-heart catheterization because this is invasive and Doppler echocardiography has shown a reasonable correlation (12,13,24). Also, we used the modified Bernoulli equation that includes a fixed 10 mm Hg estimate for the RAP. Assuming a fixed RAP of 10 mm Hg had been shown to be less reproducible than alternative methods of estimating the RAP by echocardiography such as using the diameter and collapse of the inferior vena cava during spontaneous respiration (25,26). Finally, since the competing risk of death, treatment failure, or renal transplantation were not incorporated into the Cox proportional hazards regression model, use of a competing-risks (Fine-Gray) survival model to estimate mortality may lead to less bias.
In conclusion, pulmonary hypertension is common in ESRD patients at inception of PD and associated with increased risk of both all-cause and cardiovascular mortality in incident PD patients. As left ventricular dysfunction and excess volume load may contribute to develop pulmonary hypertension in PD patients, it is worthwhile to explore whether improving left ventricular dysfunction or reducing excess volume attenuates pulmonary hypertension, and therefore decrease mortality.
Disclosures
The authors have no financial conflicts of interest to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2011BAI10B05), the National Basic Research Program of China (2012CB517700-2012CB517706), the Guangzhou Committee of Science and Technology, China (2010U1-E00831), and 5010 Clinical Program of Sun Yat-sen University (2007007).
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The task force on diagnosis and treatment of pulmonary arterial hypertension of the European society of cardiology. Eur Heart J 2004; 25:2243–78. [DOI] [PubMed] [Google Scholar]
- 2. Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007; 132:373–9. [DOI] [PubMed] [Google Scholar]
- 3. Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest 2003; 123:1577–82. [DOI] [PubMed] [Google Scholar]
- 4. Unal A, Sipahioglu M, Oguz F, Kaya M, Kucuk H, Tokgoz B, et al. Pulmonary hypertension in peritoneal dialysis patients: prevalence and risk factors. Perit Dial Int 2009; 29:191–8. [PubMed] [Google Scholar]
- 5. Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int 2009; 75:969–75. [DOI] [PubMed] [Google Scholar]
- 6. Domenici A, Luciani R, Principe F. Pulmonary hypertension in dialysis patients. Perit Dial Int 2010; 30:251–2. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 2012; 27:3908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sise ME, Courtwright AM, Channick RN. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 2013; 84:682–92. [DOI] [PubMed] [Google Scholar]
- 9. Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation 2008; 86:1384–8. [DOI] [PubMed] [Google Scholar]
- 10. Weiner DE, Tighiouart H, Vlagopoulos PT, Griffith JL, Salem DN, Levey AS, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol 2005; 16:1803–10. [DOI] [PubMed] [Google Scholar]
- 11. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in m-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978; 58:1072–83. [DOI] [PubMed] [Google Scholar]
- 12. Hatle L, Angelsen BA, Tromsdal A. Non-invasive estimation of pulmonary artery systolic pressure with Doppler ultrasound. Br Heart J 1981; 45:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 1984; 70:657–62. [DOI] [PubMed] [Google Scholar]
- 14. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54:S43–54. [DOI] [PubMed] [Google Scholar]
- 15. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–8. [DOI] [PubMed] [Google Scholar]
- 16. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–87. [DOI] [PubMed] [Google Scholar]
- 17. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European Society of Cardiology. Eur Heart J 2007; 28:2539–50. [DOI] [PubMed] [Google Scholar]
- 18. Wang AY, Wang M, Woo J, Lam CW, Li PK, Lui SF, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol 2003; 14:159–68. [DOI] [PubMed] [Google Scholar]
- 19. Yigla M, Abassi Z, Reisner SA, Nakhoul F. Pulmonary hypertension in hemodialysis patients: an unrecognized threat. Semin Dial 2006; 19:353–7. [DOI] [PubMed] [Google Scholar]
- 20. Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, et al. Pulmonary hypertension in CKD. Am J Kidney Dis 2013; 61:612–22. [DOI] [PubMed] [Google Scholar]
- 21. Kumbar L, Fein PA, Rafiq MA, Borawski C, Chattopadhyay J, Avram MM. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial 2007; 23:127–31. [PubMed] [Google Scholar]
- 22. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173:1023–30. [DOI] [PubMed] [Google Scholar]
- 23. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137:376–87. [DOI] [PubMed] [Google Scholar]
- 24. Taleb M, Khuder S, Tinkel J, Khouri SJ. The diagnostic accuracy of Doppler echocardiography in assessment of pulmonary artery systolic pressure: a meta-analysis. Echocardiography 2013; 30:258–65. [DOI] [PubMed] [Google Scholar]
- 25. Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 2011; 97:612–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



