Abstract
♦ Background:
For patients with end-stage renal disease (ESRD), peritoneal dialysis (PD) serves as a possible renal replacement therapy. However, most PD patients, particularly those with ESRD and diabetes mellitus, reportedly discontinue PD early, resulting in shorter survival periods and poorer prognosis because of overhydration. Recently, the vasopressin-2 receptor antagonist tolvaptan was approved for volume control in patients with heart failure. The present study aimed to identify the effects of tolvaptan in diabetic PD patients.
♦ Methods:
In this pilot study, the tolvaptan group (n = 12) were treated with 15 mg/day of tolvaptan 2 weeks after PD initiation and were prospectively analyzed for 1 year, and patients in the control group (n = 12) did not receive tolvaptan and were retrospectively analyzed for 1 year. In addition to the biochemical tests, echocardiograms, serum atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels, peritoneal Kt/V, and creatinine clearance (CCr) were examined at baseline and at 6 and 12 months after PD initiation.
♦ Results:
In the control group, the urine volume, renal Kt/V, and renal CCr levels consistently decreased; however, these parameters were stably maintained during the study period in the tolvaptan group. Atrial natriuretic peptide, CRP levels and the left ventricular mass index of the tolvaptan-treated group were significantly lower than those in the control group, whereas total protein and albumin levels were significantly higher at 6 and 12 months in the tolvaptan group. There were no obvious adverse effects.
♦ Conclusions:
These data suggest that tolvaptan may preserve residual renal function and improve volume control in PD patients with diabetes mellitus.
Keywords: Peritoneal dialysis, end-stage renal disease, tolvaptan, vasopressin receptor, urine volume, ultrafiltration, Kt/V, creatinine clearance
For end-stage renal disease patients (ESRD), peritoneal dialysis (PD) is a renal replacement therapy that has an advantage in preserving residual renal function (1–3), stabilizing blood pressure (1,4,5), and maintaining a better quality of life (QOL) by alleviating visits to the hospital (3,6) or allowing patients to return to work easily (6,7). According to the latest data from the Japanese Society for Dialysis Therapy, at the end of 2012, 44.1% of ESRD patients who underwent renal replacement therapy had a comorbidity of diabetes mellitus (DM) and 36.6% (745 out of 2,034) of patients initiated PD because of diabetic nephropathy. However, most of these PD patients with DM reportedly dis continued PD at an early stage, achieved worse survival rates, and had poorer prognoses than PD patients without DM because of uncontrollable fluid management (8–10). Therefore, it is important to adequately control fluid levels in PD patients with DM to maintain the highest possible QOL.
Recently, a novel aquaretic, tolvaptan, which has been indicated for use for congestive heart failure, was approved in Japan. Tolvaptan, a vasopressin antagonist, binds to the vasopressin V2 receptor at the collecting ducts, resulting in an inhibition of water uptake via a decrease in aquaporin-2 translocation at the epithelial basement membrane (11–15). A randomized controlled trial with 319 congestive heart failure patients with left ventricular ejection fractions less than 40% showed that compared with the placebo group, the urine volume was increased and the body weight was well controlled in the tolvaptan-treated group (16). However, there are limited data regarding the use of tolvaptan in PD patients. Therefore, we investigated the efficacy of short-term tolvaptan use for diabetic PD patients with congestive heart failure.
Materials and Patients
Patients and Study Protocol
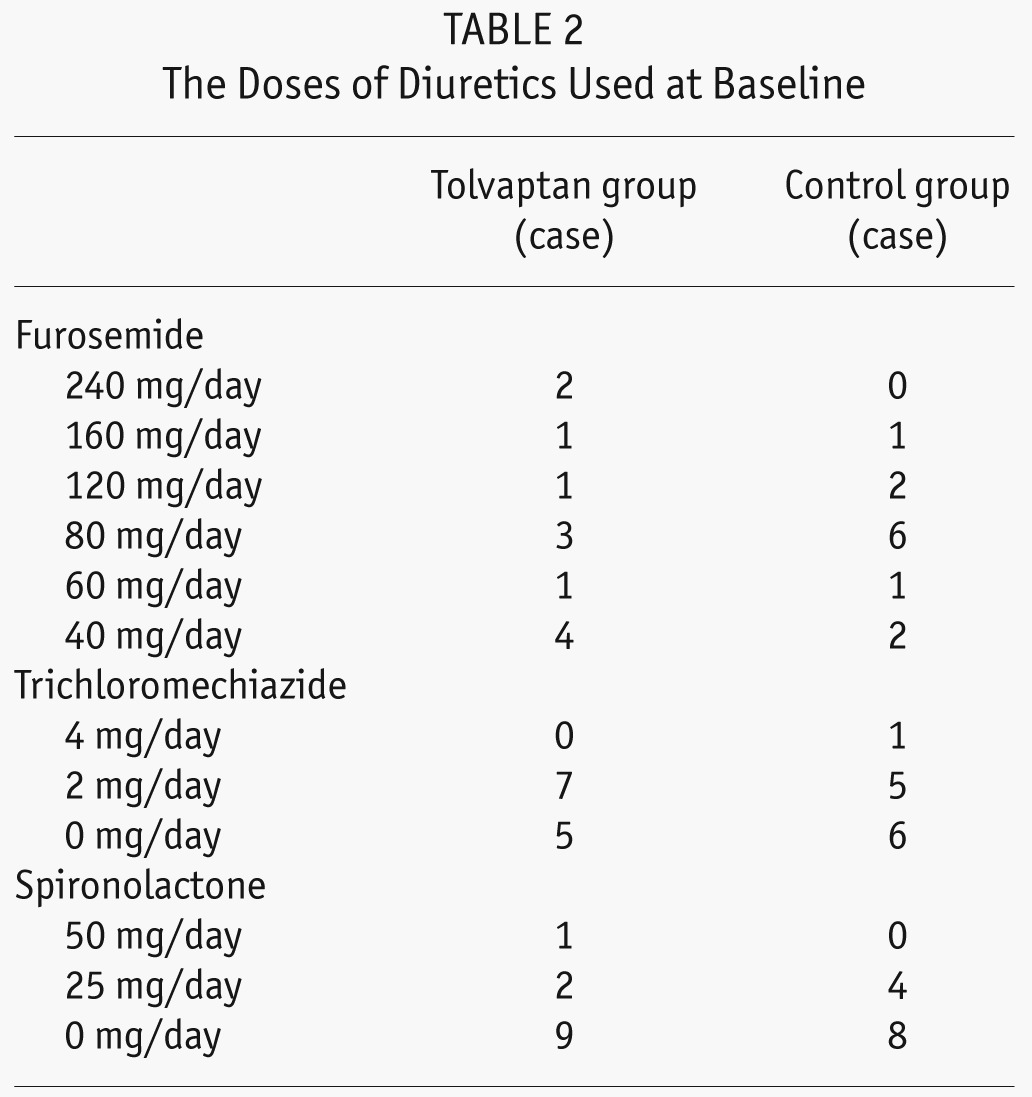
We analyzed 24 incident PD patients with ESRD and DM (mean age, 65 ± 15 years; mean DM duration, 15 ± 13 years), 6 of whom received continuous ambulatory PD and 18 automated PD (APD) (Table 1). For control and tolvaptan group, patients started PD during Jan. 1, 2010 to Oct. 31, 2011, and Nov. 1, 2011 to Nov. 31, 2012, respectively. The PD patients who had pulmonary congestion with a cardiothoracic ratio of more than 55% and whose heart functions were NYHA II, III, or IV regardless of the use of conventional diuretics were enrolled in the study. The dose of diuretics used is shown in Table 2. After regulatory approval of tolvaptan in Japan, patients who were introduced to PD and provided written consent on the use of tolvaptan were allocated to the tolvaptan group and were prospectively analyzed for a year. In contrast, patients who were introduced to PD before regulatory approval were allocated to the control group and were retrospectively analyzed for a year. All the patients in the present study demonstrated some level of pleural effusion, overt lower-limb edema, or dyspnea at PD initiation, and the group allocation was not based on the extent of patients' symptom progression. In the tolvaptan group (n = 12), the patients received 15 mg/day of tolvaptan 2 weeks after PD initiation, and in the control group (n = 12), the patients did not receive tolvaptan. Initially, all the patients were administered 4 bags of 1.36% glucose-based dialysates. Thereafter, 1 bag of icodextrin per day was prescribed to 10 patients in each group 7 – 10 days after PD initiation because of overt edema or insufficient ultrafiltration volume (UF) (< 300 mL/day). Each group included nine APD patients. The biochemical parameters, urine volume, Kt/V, and creatinine clearance (CCr) were evaluated at baseline and 3 days, 6 months, and 12 months after PD initiation. Laboratory parameters were measured at the start of PD and at 6 and 12 months after PD initiation. Examined data included age, DM duration, gender, body weight, body mass index, and blood pressure. Blood samples were examined for C-reactive protein (CRP), total protein, serum hemoglobin A1c, blood urea nitrogen, creatinine (Cr), β2 microglobulin, Na, K, Ca, P, albumin, i-PTH, blood sugar, atrial natriuretic peptide (ANP), and brain natriuretic peptide (BNP) levels. Patients also collected 24-h urine and effluent samples at their house and carried them to the hospital; the volume and urea nitrogen, creatinine, protein, blood sugar, Na, K, Ca, and P levels were measured at baseline and at 6 and 12 months after PD initiation.
TABLE 1.
Baseline Characteristics
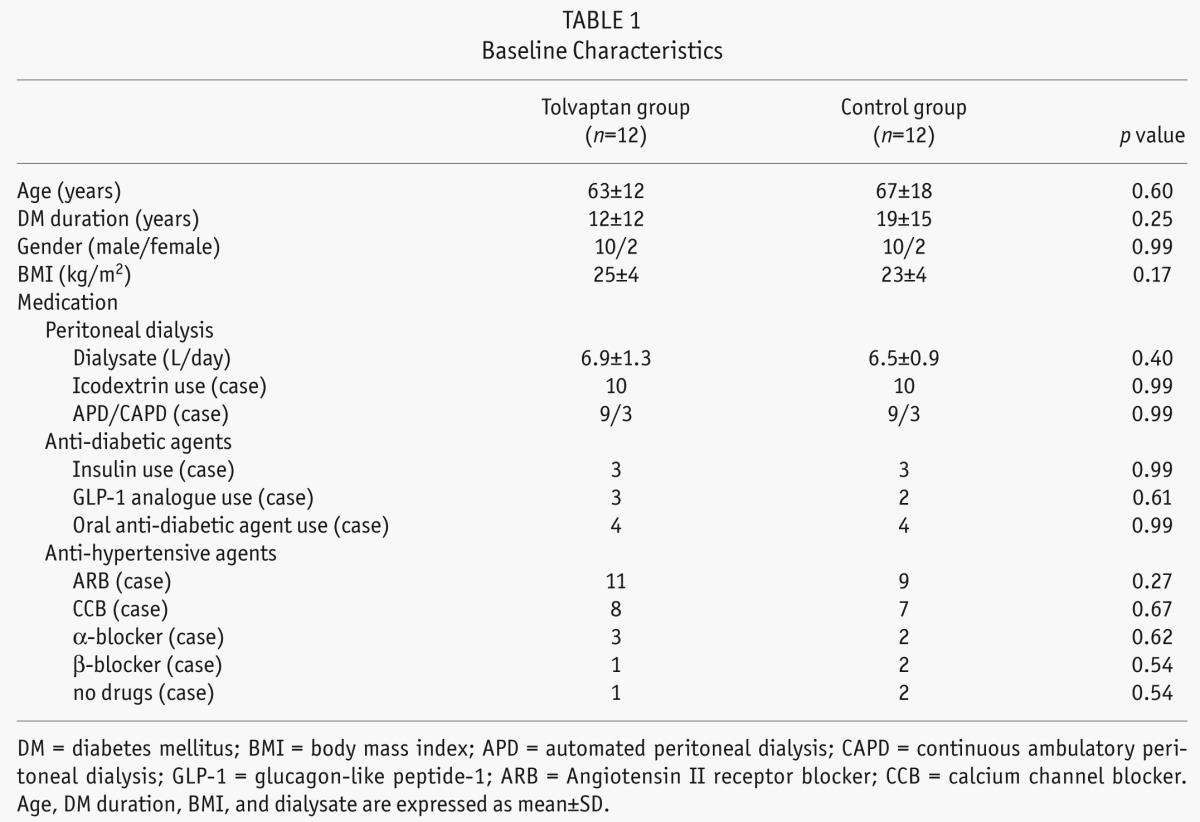
TABLE 2.
The Doses of Diuretics Used at Baseline
Echocardiographic Measurement
Echocardiographic measurements were performed at the start of PD and every 6 months for 1 year. Two-dimensional echocardiography was performed using a standard imaging transducer with a linear probe frequency of 5 MHz. The analyzed parameters included the left ventricular mass index (LVMI) and left ventricular eccentric hypertrophy, which was calculated using the Devereux and Reicheck formula (17):
 |
Statistical Analysis
Data were presented as means ± standard deviations. The baseline characteristics of patients were appropriately analyzed by Student-t, Mann-Whitney U-test, or chi-square test. For repeated measured variables, we included treatment and times as main effects and treatment*time as interaction, and analyzed by linear mixed model with compound symmetry covariance pattern. The p value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 19 statistical software for Windows (SPSS, Inc., Chicago, Ill., USA).
The study protocol was approved by the ethics committees at Konan Kosei Hospital. The study was performed in accordance with the ethical principles stated in the Declaration of Helsinki and Japanese Ministry of Health, Labour and Welfare.
Results
A total of 24 incident PD patients with DM were divided into 2 groups: those who received tolvaptan treatment (tolvaptan group; n = 12 patients) and those who did not (control group; n = 12 patients). No intergroup differences for age (63 ± 12 vs 67 ± 18) or gender (17% women in each group) were observed. At baseline, the analyzed laboratory data, medications used (diuretics, antihypertensive agents and antidiabetic agents), and PD prescription or modality were similar between both groups (Table 1 and 2).
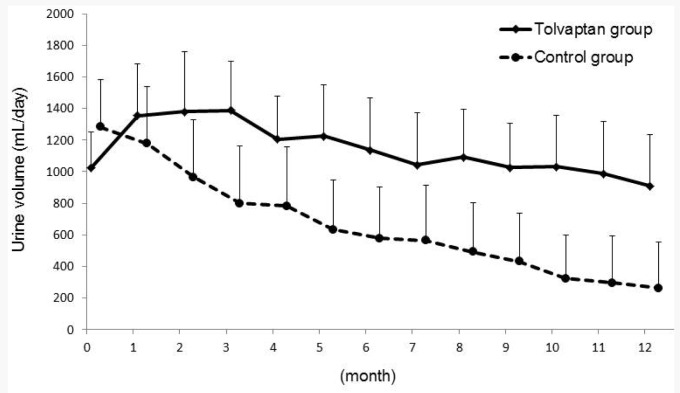
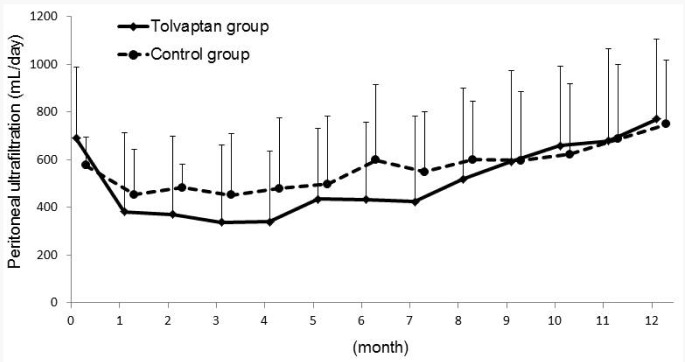
After 3 days of tolvaptan use, the urine volume, renal Kt/V (Kt/VRRF), and renal CCr (CCrRRF) values significantly increased over baseline (data not shown). During the study period, the urine volume consistently decreased after PD initiation in the control group, whereas it increased for 3 months and then gradually decreased in the tolvaptan group (Figure 1). The mixed model showed that the tolvaptan group had significantly higher urine volume during the study period (Figure 1). Peritoneal UF was stable in the control group (average peritoneal UF, 509 ± 195 mL/day). In contrast, UF markedly decreased and showed significant differences in comparison with UF at baseline during the first 4 months (average peritoneal UF, 340 ± 297 mL/day at 4 months, p < 0.05 vs baseline) in the tolvaptan group; it then gradually increased after 5 months, reaching the same level as UF in the control group resulting in no significant difference between groups (Figure 2).
Figure 1 —
Monthly urine volumes for 1 year. The p value for three factors: treatment, time and treatment*time were analyzed by linear mixed model as p<0.01, p<0.01, and p=0.185, respectively.
Figure 2 —
Monthly peritoneal ultrafiltration volumes for 1 year. The p value for three factors: treatment, time and treatment*time were analyzed by linear mixed model as p<0.4, p<0.01, and p=0.81, respectively.
Peritoneal Kt/V (Kt/VPM) and peritoneal CCr (CCrPM) values were stable in both the groups and showed no significant differences during the study period. Kt/VRRF and CCrRRF in the tolvaptan group were retained during the study period; however the control group showed gradual decrease over time.
CRP continued to increase from PD initiation in the control group, whereas CRP in the tolvaptan group constantly decreased. β2 microglobulin levels also increased in the control group, whereas they were stable in the tolvaptan group. Atrial natriuretic peptide and BNP levels, which indicate the body fluid status, were greatly decreased in the tolvaptan group with significant difference in ANP between groups. At baseline, LVMI was higher in the tolvaptan group than in the control group; however, it gradually decreased during the study period and showed significant difference between groups. There were no significant differences in body weight and diastolic blood pressure, but systolic blood pressure was significantly higher in the tolvaptan group during the study period (Table 3).
TABLE 3.
Changes in Laboratory Data Between the Tolvaptan and the Control Group Over Time
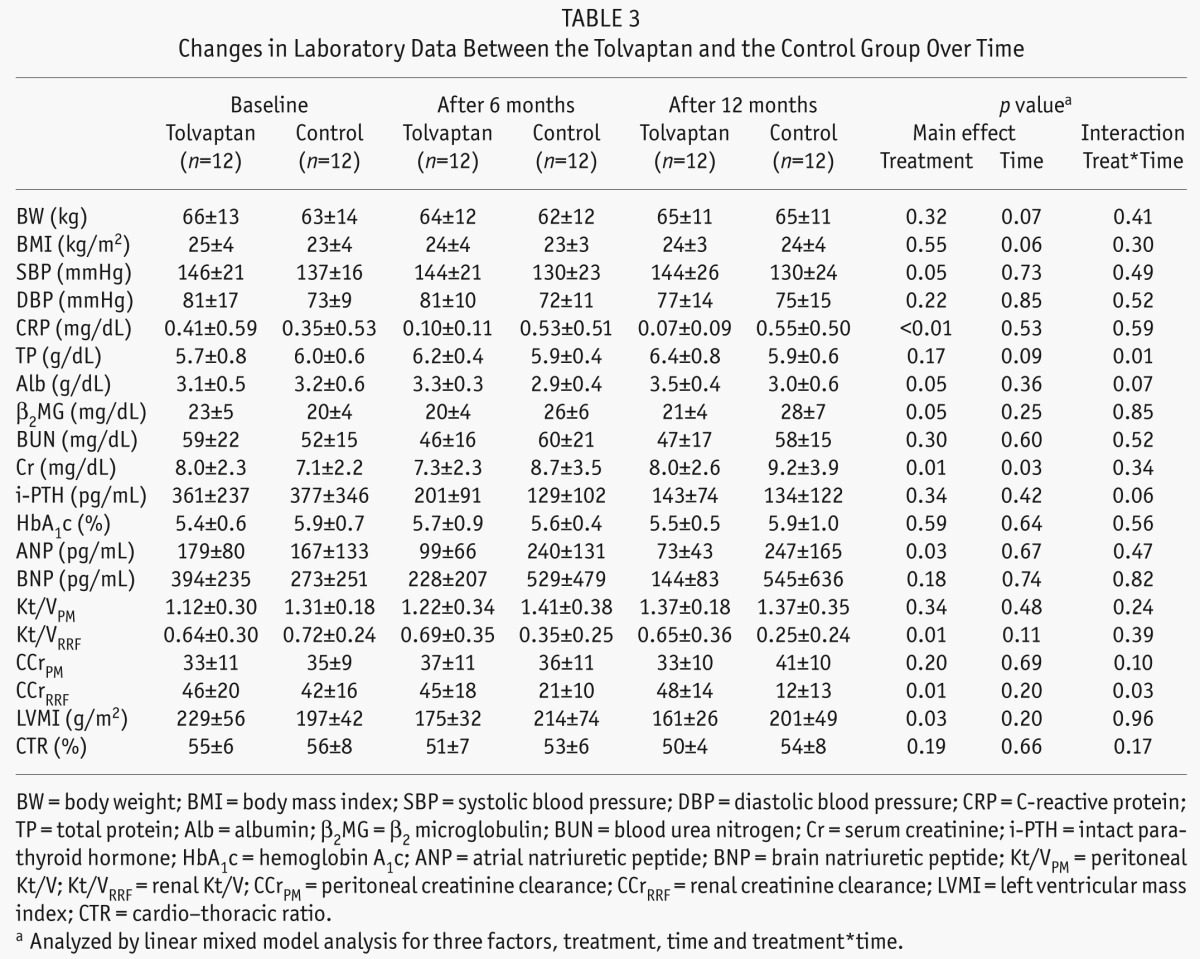
The dialysate prescription of 3 patients in the control group was switched from 1.36% to 2.27% glucose at 1, 2, and 6 months after PD initiation. One of these 3 PD patients was transferred to combined therapy, in which 1 day of hemodialysis per week was added to the PD therapy because of unmanageable volume overload, and the diuretics dose was increased in 7 patients in the control group. In contrast, in the tolvaptan group, the prescription of diuretics as well as the PD prescription were not changed during the study period.
There were no specific adverse events during the study period, including hypernatremia.
Discussion
It is very important to achieve good fluid control in patients with chronic kidney disease to preserve residual renal function (RRF) and improve patient survival. Although loop, thiazide, and aldosterone diuretics are often used to control fluid levels, the efficacy of the newly launched aquaretic tolvaptan, which acts as a vasopressin receptor inhibitor, has not yet been reported in PD patients. In the present study, tolvaptan was administered to incident PD patients with DM and was found to increase the urine volume and preserve renal function, in comparison with those in the control group that were not prescribed tolvaptan.
Reportedly, PD provides a high QOL level to patients because it provides increased daily activity time. In contrast, more than half of the patients reportedly discontinue PD because of uncontrollable body fluid levels (18,19). In addition, PD patients with DM reportedly exhibit shorter survival times than patients without DM (19). Tolvaptan, an oral selective arginine vasopressin receptor antagonist, has been approved to treat hyper-volemia in congestive heart failure patients (16,20–22). In the present study, diabetic PD patients with congestive heart failure who were refractory to diuretics were treated with 15 mg/day of tolvaptan 2 weeks after PD initiation for 12 months. Previous randomized, double-blind, placebo-controlled studies have shown that the 24-h urine volume and frequency increased in patients receiving tolvaptan (16,20–22). Consistent with these reports, in the present study, the urine volume in patients treated with tolvaptan increased at 1 month and continued to be higher than that in patients who were not treated with tolvaptan. The concentration of urine electrolytes such as Na, K, Ca, or P did not differ before and after the use of tolvaptan (data not shown), indicating that tolvaptan increased urine volume but not free water. In contrast, peritoneal UF in the tolvaptan group transiently decreased from 2 to 4 months, which may have been due to a compensation effect because of the increased urine by tolvaptan treatment during this period. Furthermore, tolvaptan decreased ANP,BNP and LVMI. These results indicated that tolvaptan was effective for fluid management in diabetic PD patients with congestive heart failure.
Previous prospective studies, such as the CANUSA (23,24), ADEMEX (25), Hong Kong (26), and NECOSAD-2 studies (27), demonstrated that RRF is a useful prog nostic factor for patient survival. A recent multivariate analysis of 383 stable PD patients found that in addition to age and log CRP and phosphate levels, RRF (≤ 4 mL/min/1.73 m2) is a useful prognostic factor (28). Perl and Bargman reported that RRF could be preserved by PD selection and the utilization of renin-angiotensin-aldosterone system blockers and by the avoidance of nephrotoxic or nonsteroidal anti-inflammatory drugs (29). In addition, body fluid control is an important factor for RRF. Previous studies demonstrated that dehydration led to a decrease in RRF (30,31). An important issue of this research was the change in renal function in the tolvaptan group, which showed a higher urine volume and UF compared to the control group. Regardless of the higher fluid control during the 12-month study period in the tolvaptan group, renal Kt/V and CCr levels were preserved, indicating that tolvaptan could preserve renal function. A recent report that the daily CCr was increased in the tolvaptan-responder group also supports this evidence (32).
Hypernatremia is reportedly an adverse effect of tolvaptan (33,34); however, no patient exhibited obvious adverse events such as hypernatremia during the study period. This may be partly explained by the fact that patients were allowed to drink water when thirsty and the urine volume had not markedly increased in the tolvaptan group because the maximum urine volume was 2.5 L/day. In contrast, some patients exhibited mild hyponatremia.
There were several limitations to the present study, including the small sample size, lack of randomization, and the fact that the control and tolvaptan groups were analyzed retrospectively and prospectively. Therefore, a larger and long-term prospective study is required to illustrate the beneficial effects of tolvaptan in fluid management, RRF preservation, and improvement of patient survival.
In conclusion, we retrospectively investigated the short-term effects of the novel aquaretic tolvaptan in PD patients with DM who exhibited uncontrollable body fluid loss. The tolvaptan group showed significantly increased urine volume at day 3 of treatment and maintained RRF (i.e., renal Kt/V and CCr) during the observation period. Finally, we found that tolvaptan preserves RRF, increases urinary volume in PD patients with DM.
Disclosures
The authors declare no financial conflicts of interest.
REFERENCES
- 1. Lang SM, Bergner A, Topfer M, Schiffl H. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Perit Dial Int 2001; 21(1):52–7. [PubMed] [Google Scholar]
- 2. Misra M, Vonesh E, Van Stone JC, Moore HL, Prowant B, Nolph KD. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int 2001; 59:754–63. [DOI] [PubMed] [Google Scholar]
- 3. Tokgoz Bulent. Clinical advantage of peritoneal dialysis. Perit Dial Int 2009; 29(Suppl 2):S59–61. [PubMed] [Google Scholar]
- 4. Rodby RA, Vonesh EF, Korbet SM. Blood pressure in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis 1994; 23(3):401–11. [DOI] [PubMed] [Google Scholar]
- 5. Cheigh JS, Kim H. Hypertension in continuous ambulatory peritoneal dialysis patients: What do we know and what can we do about it? Perit Dial Int 1999; 19(Suppl 2):S138–43. [PubMed] [Google Scholar]
- 6. Dimkovic N, Oreopoulos DG. Chronic peritoneal dialysis in the elderly: A review. Perit Dial Int 2000; 20:276–83. [PubMed] [Google Scholar]
- 7. Julius M, Kneidley JD, Carpentier-Alting P, Hawthorne VM, Wolfe RA, Port FK. A comparison of employment rates of patients treated with continuous ambulatory peritoneal dialysis vs in-center hemodialysis (Michigan End-Stage Renal Disease Study). Arch Intern Med 1989; 149:839–42. [PubMed] [Google Scholar]
- 8. Stenvinkel P, Chung SH, Heimbürger O, Lindholm B. Malnutrition, inflammation, and atherosclerosis in peritoneal dialysis patients. Perit Dial Int 2001; 21(Suppl 3):157–62. [PubMed] [Google Scholar]
- 9. Chung SH, Heimburger O, Stenvinkel P, Wang T, Lindholm B. Influence of peritoneal transport rate, inflammation, and fluid removal on nutritional status and clinical outcome in prevalent peritoneal dialysis patients. Perit Dial Int 2003; 23(2):174–83. [PubMed] [Google Scholar]
- 10. Wang AY. The “heart” of peritoneal dialysis. Perit Dial Int 2007; 27(Suppl 2):S228–32. [PubMed] [Google Scholar]
- 11. Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol 1997; 272:F817–22. [PubMed] [Google Scholar]
- 12. Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 1997; 272:14800–4. [DOI] [PubMed] [Google Scholar]
- 13. van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, et al. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 2002; 277:41473–9. [DOI] [PubMed] [Google Scholar]
- 14. Kuwahara M, Asai T, Terada Y, Sasaki S. The C-terminal tail of aquaporin-2 determines apical trafficking. Kidney Int 2005; 68:1999–2009. [DOI] [PubMed] [Google Scholar]
- 15. Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 1999; 274:4934–8. [DOI] [PubMed] [Google Scholar]
- 16. Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolcaptan, an oral vassopression antagonist in patients hospitalized for heart failure: the EVEREST Clinical Status Trial. JAMA 2007; 297:1332–43. [DOI] [PubMed] [Google Scholar]
- 17. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55:613–8. [DOI] [PubMed] [Google Scholar]
- 18. Kawaguchi Y, Ishizaki T, Imada A, Oohira S, Kuriyama S, Nakamoto H, et al. Searching for the reasons for drop-out from peritoneal dialysis: a nationwide survey in Japan. Perit Dial Int 2003; 23(Suppl 2):175–7. [PubMed] [Google Scholar]
- 19. Kawanishi H. Complementary dialysis for daily dialysis. Blood Purif 2004; 22(Suppl 2):30–3. [DOI] [PubMed] [Google Scholar]
- 20. Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 2006; 290:F273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe K, Dohi K, Sugimoto T, Yamada T, Sato Y, Ichikawa K, et al. Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J Cardiol 2012; 60:462–9. [DOI] [PubMed] [Google Scholar]
- 22. Pang PS, Konstam MA, Krasa HB, Swedberg K, Zannad F, Blair JE, et al. Effects of tolvaptan on dyspnoea relief from the EVEREST trials. Eur Heart J 2009; 2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CANADA-USA (CANUSA) Peritoneal Dialysis Study Group 2′3 Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 1996; 7:198–207. [DOI] [PubMed] [Google Scholar]
- 24. Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62. [DOI] [PubMed] [Google Scholar]
- 25. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13:1307–20. [DOI] [PubMed] [Google Scholar]
- 26. Lo WK, Ho YW, Li CS, Wong KS, Chan TM, Yu AW, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int 2003; 64:649–56. [DOI] [PubMed] [Google Scholar]
- 27. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT, et al. The relative impor tance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41:1293–302. [DOI] [PubMed] [Google Scholar]
- 28. Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Risk factors for mortality in stable peritoneal dialysis patients. Ren Fail 2012; 34:149–54. [DOI] [PubMed] [Google Scholar]
- 29. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009; 53:1068–81. [DOI] [PubMed] [Google Scholar]
- 30. Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int 2003; 63:1556–63. [DOI] [PubMed] [Google Scholar]
- 31. Konings CJ, Kooman JP, Gladziwa U, van der Sande FM, Leunissen KM. A decline in residual glomerular filtration during the use of icodextrin may be due to underhydration. Kidney Int 2005; 67:1190–1. [DOI] [PubMed] [Google Scholar]
- 32. Mori T, Oba I, Koizumi K, Kodama M, Shimanuki M, Tanno M, et al. Beneficial role of tolvaptan in the control of body fluids without reduction in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial 2013; 29:33–7. [PubMed] [Google Scholar]
- 33. Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T, Tolvaptan Investigators Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Cardiovasc Drugs Ther 2011; 25(Suppl 1):S19–31. [DOI] [PubMed] [Google Scholar]
- 34. Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y, et al. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res 2013; 41:835–47. [DOI] [PubMed] [Google Scholar]