Abstract
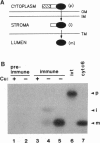
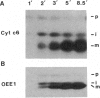
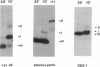
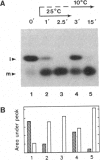
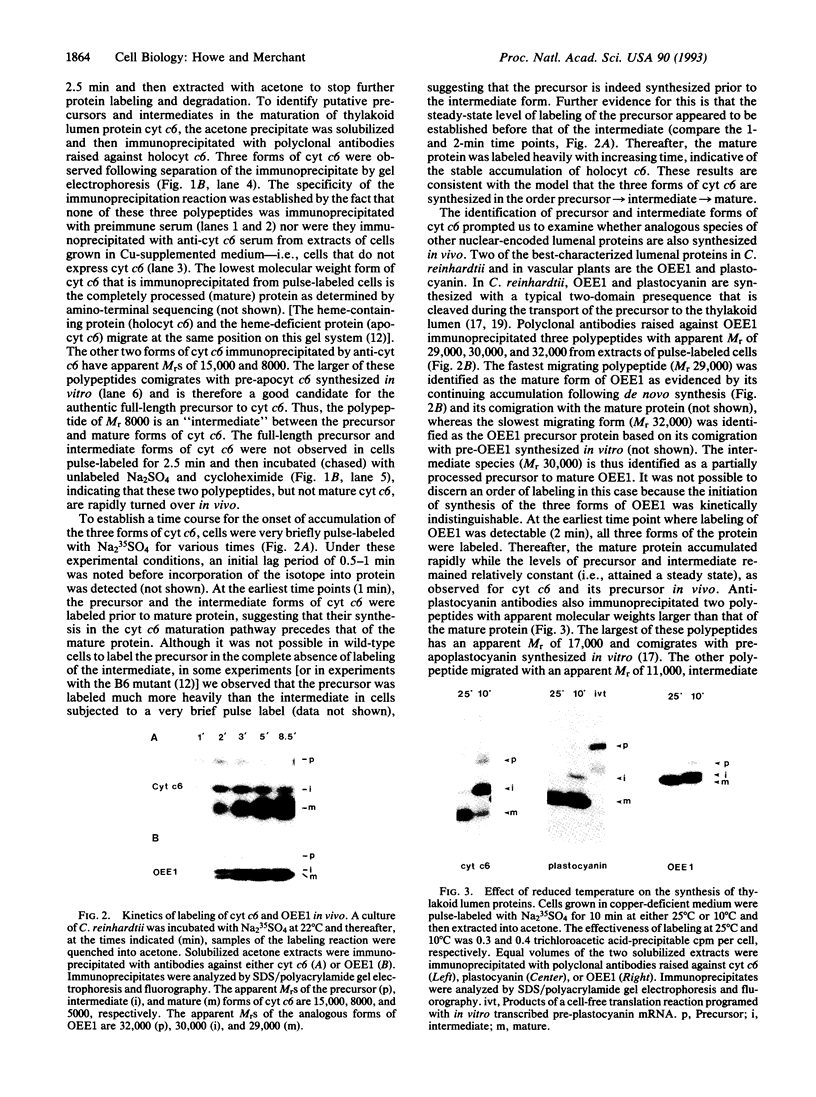
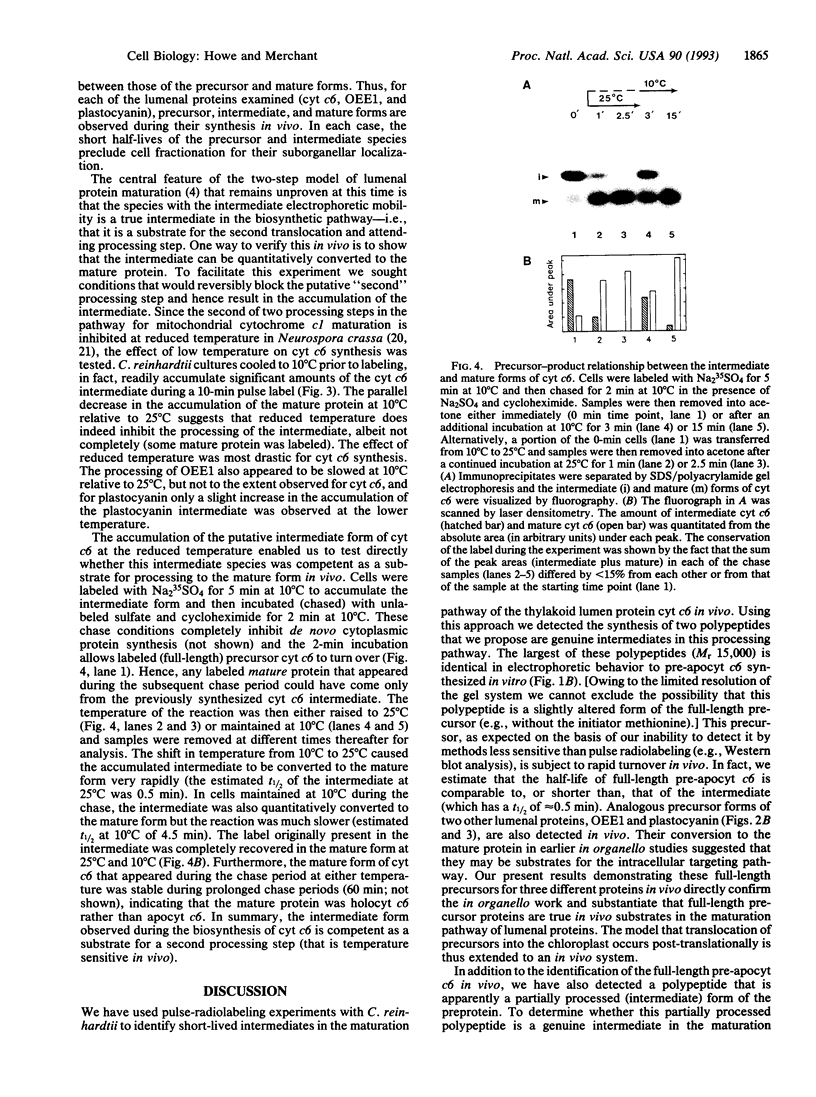
Many thylakoid lumen proteins are synthesized outside the chloroplast as larger molecular weight precursors and then processed to their mature size during transport to the lumenal space. We have examined the post-translational processing of thylakoid lumen proteins in vivo by pulse-radiolabeling experiments with Chlamydomonas reinhardtii. Antibodies against the lumenal protein cytochrome c6 specifically immunoprecipitated three polypeptides from extracts of briefly pulse-radiolabeled cells. The molecular weights and kinetics of synthesis and turnover indicate that these three polypeptides are (i) the full-length cytochrome c6 precursor, (ii) a partially processed precursor (intermediate), and (iii) the completely processed mature protein. Identification of analogous forms of two other lumenal proteins, plastocyanin and the oxygen evolving enhancer 1 protein, indicates that the maturation of thylakoid lumen proteins occurs post-translationally in vivo and that the partially processed intermediate is a general feature of the pathway. The intermediate form of cytochrome c6 accumulated to a greater extent in cells incubated at 10 degrees C, relative to cells incubated at 22 degrees C, concomitantly with a decrease in the accumulation of the mature protein. The intermediate accumulating at 10 degrees C is quantitatively converted to the mature protein upon incubation at higher temperature, thus demonstrating a precursor-product relationship between the intermediate and mature forms of cytochrome c6. Our results prove the model [Smeekens, S., Bauerle, C., Hageman, J., Keegstra, K. & Weisbeek, P. (1986) Cell 46, 365-375] that precursors of lumenal proteins are post-translationally converted to their mature forms in two steps through a distinct intermediate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauerle C., Dorl J., Keegstra K. Kinetic analysis of the transport of thylakoid lumenal proteins in experiments using intact chloroplasts. J Biol Chem. 1991 Mar 25;266(9):5884–5890. [PubMed] [Google Scholar]
- Bauerle C., Keegstra K. Full-length plastocyanin precursor is translocated across isolated thylakoid membranes. J Biol Chem. 1991 Mar 25;266(9):5876–5883. [PubMed] [Google Scholar]
- Cline K., Ettinger W. F., Theg S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992 Feb 5;267(4):2688–2696. [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J., Baecke C., Ebskamp M., Pilon R., Smeekens S., Weisbeek P. Protein Import into and Sorting inside the Chloroplast Are Independent Processes. Plant Cell. 1990 May;2(5):479–494. doi: 10.1105/tpc.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K. L., Merchant S. In Vivo Competition between Plastocyanin and a Copper-Dependent Regulator of the Chlamydomonas reinhardtii Cytochrome c(6) Gene. Plant Physiol. 1992 Sep;100(1):319–326. doi: 10.1104/pp.100.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G., Merchant S. The biosynthesis of membrane and soluble plastidic c-type cytochromes of Chlamydomonas reinhardtii is dependent on multiple common gene products. EMBO J. 1992 Aug;11(8):2789–2801. doi: 10.1002/j.1460-2075.1992.tb05346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James H. E., Bartling D., Musgrove J. E., Kirwin P. M., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Import and maturation of precursors to the 33-, 23-, and 16-kDa proteins of the photosynthetic oxygen-evolving complex. J Biol Chem. 1989 Nov 25;264(33):19573–19576. [PubMed] [Google Scholar]
- Kirwin P. M., Meadows J. W., Shackleton J. B., Musgrove J. E., Elderfield P. D., Mould R., Hay N. A., Robinson C. ATP-dependent import of a lumenal protein by isolated thylakoid vesicles. EMBO J. 1989 Aug;8(8):2251–2255. doi: 10.1002/j.1460-2075.1989.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klösgen R. B., Brock I. W., Herrmann R. G., Robinson C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992 Mar;18(5):1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Ko K., Cashmore A. R. Targeting of proteins to the thylakoid lumen by the bipartite transit peptide of the 33 kd oxygen-evolving protein. EMBO J. 1989 Nov;8(11):3187–3194. doi: 10.1002/j.1460-2075.1989.tb08477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Rapid degradation of apoplastocyanin in Cu(II)-deficient cells of Chlamydomonas reinhardtii. J Biol Chem. 1986 Dec 5;261(34):15850–15853. [PubMed] [Google Scholar]
- Merchant S., Bogorad L. The Cu(II)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J Biol Chem. 1987 Jul 5;262(19):9062–9067. [PubMed] [Google Scholar]
- Merchant S., Hill K., Kim J. H., Thompson J., Zaitlin D., Bogorad L. Isolation and characterization of a complementary DNA clone for an algal pre-apoplastocyanin. J Biol Chem. 1990 Jul 25;265(21):12372–12379. [PubMed] [Google Scholar]
- Mould R. M., Shackleton J. B., Robinson C. Transport of proteins into chloroplasts. Requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991 Sep 15;266(26):17286–17289. [PubMed] [Google Scholar]
- Nicholson D. W., Stuart R. A., Neupert W. Biogenesis of cytochrome c1. Role of cytochrome c1 heme lyase and of the two proteolytic processing steps during import into mitochondria. J Biol Chem. 1989 Jun 15;264(17):10156–10168. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Extramitochondrial pools and post-translational import of mitochondrial protein precursors in vivo. J Biol Chem. 1982 Nov 10;257(21):13062–13067. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J Biol Chem. 1982 Nov 10;257(21):13056–13061. [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Teintze M., Slaughter M., Weiss H., Neupert W. Biogenesis of mitochondrial ubiquinol:cytochrome c reductase (cytochrome bc1 complex). Precursor proteins and their transfer into mitochondria. J Biol Chem. 1982 Sep 10;257(17):10364–10371. [PubMed] [Google Scholar]
- Vorst O., Oosterhoff-Teertstra R., Vankan P., Smeekens S., Weisbeek P. Plastocyanin of Arabidopsis thaliana; isolation and characterization of the gene and chloroplast import of the precursor protein. Gene. 1988 May 15;65(1):59–69. doi: 10.1016/0378-1119(88)90417-9. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]