Abstract
Purpose
The purpose of this study was to examine the influence of a preferred-versus a prescribed-intensity exercise session on pain in women with fibromyalgia (FM).
Methods
Twenty-one women with FM (mean age = 44 yr) completed two randomly assigned exercise sessions consisting of 20 min of cycle ergometry at a self-selected intensity and a prescribed intensity. Experimental pain perception was assessed before and after aerobic exercise. During exercise, HR, watts, RPE, and muscle pain were assessed every 5 min. Clinical pain was assessed with the Short-Form McGill Pain Questionnaire (SF-MPQ) immediately and 24, 48, 72, and 96 h after exercise. Data were analyzed with repeated-measures ANOVA.
Results
Women with FM preferred a lower intensity of exercise than what was prescribed as indicated by significantly lower HR, watts, and RPE responses (P < 0.05). Muscle pain in the legs, however, was similar in the two conditions and significantly increased during exercise (P < 0.05). Pain thresholds and pain tolerances increased significantly after exercise, whereas peak pain ratings decreased after exercise (P < 0.05). Furthermore, pain (SF-MPQ) in the follow-up period was found to be lower than baseline (P < 0.05).
Conclusions
It is concluded that the women with FM who participated in this study experienced significant improvements in pain after exercise. The results from this study are novel and indicate that recommendations for exercise prescription for individuals with FM should consider the preferred-intensity exercise model as a strategy to reduce pain.
Keywords: PHYSICAL ACTIVITY, CHRONIC PAIN, RPE, EXERCISE PRESCRIPTION
Fibromyalgia (FM) is a common musculoskeletal pain condition associated with chronic widespread pain as well as stiffness, fatigue, mood disturbances, and other disabling symptoms (47). Currently, there is no cure for FM, and treatment usually requires a multidisciplinary perspective using both pharmacological and nonpharmacological approaches (3,30). Exercise is commonly recommended in the management of FM. The American Pain Society (1), for example, encourages individuals with FM to perform aerobic exercise for therapeutic benefits, and research indicates that exercise training has beneficial effects on global well-being, physical capacity, and physical function (6).
Adherence rates to exercise programs, however, are generally low in individuals with FM (6,33,38–40,45). Although not systematically studied, individuals with FM are typically deconditioned (9,28), and there are reports that women with FM experience increased symptoms such as pain and fatigue when they attempt to engage in an exercise session (27,38). Furthermore, pain has been shown to be predictive of poor activity tolerance in FM patients (12) and higher pain self-efficacy has been shown to be a significant discriminator for physical activity participation (11). One study in patients with chronic fatigue syndrome and FM demonstrated that physical activity levels were contingent on current pain and fatigue symptoms but that current activity levels were not predictive of subsequent physical symptoms (23). Thus, there is limited research examining changes in pain after an immediate bout of exercise in women with FM.
Normally, in healthy individuals changes in pain sensitivity occur after exercise indicative of an analgesic response (e.g., increases in pain thresholds and tolerances and lower pain ratings). This phenomenon has been termed exercise-induced analgesia (EIA) and has been demonstrated after exercise for several different experimental pain stimuli (for reviews, see Cook and Koltyn (8) and Koltyn (21)). In contrast, there are reports in the literature that FM patients do not experience EIA. Several investigators have reported an increase in pain as a result of exercise (25,32,41,46), whereas other investigators have reported decreases in pain after exercise (17,20,42). Currently, the optimal mode, duration, frequency, and intensity of exercise appropriate for individuals with FM to manage their symptoms are unknown. Also, it is unclear what intensity of exercise is preferred by women with FM to manage pain. Most exercise programs use a prescribed exercise protocol such as the one by the American Pain Society, which recommends exercising at a moderate intensity defined as between 60% and 75% of age-adjusted maximal HR two to three times per week. An alternate approach, however, would be the use of a preferred exertion model in which participants self-select their preferred intensity of exercise (36). It has been suggested that adherence to exercise programs may be increased if exercise prescriptions are based on preferred intensity of exercise because of the inverse association between exercise intensity and adherence rates (14). Furthermore, the incorporation of preferred intensity may also affect pain because self-selection of exercise intensity will allow individuals to change the intensity of exercise as necessary to remain comfortable and to minimize possible exacerbations in pain during the exercise bout. Therefore, the primary purpose of this study was to examine the influence of a preferred- versus a prescribed-intensity exercise session on pain in women with FM.
MATERIALS AND METHODS
Participants
Twenty-one women (age range = 18–59 yr) who had been diagnosed with FM according to the 1990 criteria set forth by the American College of Rheumatology (47) were recruited to participate in this study. A power analysis was conducted to estimate optimal sample size for detecting within groups differences among the two experimental conditions, with an α level of 0.05, power = 0.80, a correlation among repeated-measures = 0.70, and a moderate effect size. Effect size calculations were based on comparisons of sensitivity to experimental pain stimuli before and after aerobic exercise. Results from this analysis indicated that 21 participants would yield a power of 0.803 (15). Given the high percentage (80%–90%) of women versus men who are diagnosed as having FM, only women were recruited to participate in this study. Exclusion criteria included the following: 1) individuals diagnosed with major depressive disorder, 2) individuals with current substance abuse, and 3) individuals taking opioid or high-dosage antidepressant medications for major depressive disorder. Participants were further instructed to maintain and not change their current medication use throughout the course of the study. A trained interviewer screened all participants for exclusionary diagnoses using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Disorders (16).
Procedures
Informed consent was obtained from all participants, and all study procedures complied with guidelines of the Declaration of Helsinki. The University of Wisconsin Institutional Review Board approved the procedures for this study. Participants completed two exercise sessions on separate days consisting of 20 min of cycle ergometry at either a self-selected intensity or a prescribed intensity. The two sessions were randomized, counterbalanced (n = 11 received prescribed first; n = 10 received preferred first) and separated by 2 wk. Participants were instructed to go about their usual daily routines during the 4 d after exercise as well as between experimental sessions. Participants completed a packet of questionnaires consisting of: 1) a health/medical history, 2) a body pain diagram, 3) the Fibromyalgia Impact Questionnaire (5), 4) the Short-Form McGill Pain Questionnaire (SF-MPQ) (31), 5) the POMS (29), and 6) the Tampa Scale for Kinesiophobia (24). Before the self-selected intensity session, participants were instructed verbally on how to choose a preferred intensity (based on a script by Dishman et al. (14)) based on the following script:
Now you are going to cycle on the bike for 20 min. You will select an intensity of exercise that you prefer. This could be a level of exercise that you would choose for a normal daily exercise session. This exercise intensity should be appropriate for you and at a level that you feel in not too hard or too easy. This exercise should provide you with a good workout that you can tolerate. You will be given the opportunity to change the exercise level to a new level at any time during the exercise session by pushing the up and down arrows on the control panel.
The prescribed exercise session was chosen based on the recommendations and guidelines published by the American Pain Society (1) for the management of FM and consisted of exercising at moderate intensity defined as 60%–75% of age-adjusted maximal HR. A target HR zone was established for each participant using the Karvonen method and was maintained throughout the session. The wattage of the cycle ergometer was adjusted by the investigator to meet the prescribed intensity (target HR zone) throughout the exercise bout by pushing up or down arrows on the concealed control panel. Every 5 min during each exercise session, the following data were recorded: 1) power output (W), 2) HR via a HR monitor (Polar, Kempele, Finland), and 3) perceived exertion using Borg’s 6–20 RPE scale (4).
Multiple aspects of pain were assessed in this study and included the following: 1) muscle pain during exercise; 2) experimental pain perception including pain thresholds (i.e., point at which a stimulus is first perceived to be painful), pain tolerance (amount of noxious stimulation individual’s are willing to tolerate), and pain ratings (intensity and unpleasantness); and 3) pain in the postexercise period. Muscle pain in the legs was assessed every 5 min during the exercise sessions using a muscle pain intensity scale (10). The participants were asked to verbally rate the intensity of pain in their legs according to numerical responses on a 0–10 scale where 0 = no pain and 10 = extremely intense pain (almost unbearable) with an option to rate pain higher than 10. This scale has been shown to be a valid and reliable measure of leg muscle pain during exercise (10).
Experimental pain perception was assessed before and directly after each exercise session using the Forgione–Barber Pressure Stimulator. Approximately 3000 g of force was applied to the right forefinger for a maximum of 2 min. Previous research has demonstrated that this procedure produces a painful sensation but does not cause short-term or long-term tissue damage or injury (37). Further, our previous work in healthy men and women has shown that pain sensitivity consistently decreases after an immediate bout of exercise but changes little after quiet rest (22). Pain thresholds were determined by the elapsed time from the initial application of the pressure stimulus to the forefinger until the participant perceived the stimulus to be painful. Participants were instructed to press a button attached to a timer out of view of the participant indicating when the stimulus changed from no pain to slightly painful. Pain tolerances were assessed by recording the time the individual was willing to endure the pressure stimulus on their finger. In addition, peak pain intensity and pain unpleasantness ratings were assessed at tolerance time using the Gracely Box SL scales to assess both sensory and affective aspects of pain. The POMS was administered before and 15 min after each aerobic exercise session.
Symptoms of body pain were assessed in the postexercise period (i.e., immediately, 24, 48, 72, and 96 h) with the SF-MPQ and the body pain diagram to examine whether there were exacerbations in pain in the days after the exercise sessions. In addition, any medication taken within the last 24 h was recorded at the same time points. At the 96-h time point, an FM-specific patient global impression of change (PGIC) was assessed as recommended by the U.S. Food and Drug Administration Arthritis Advisory Committee (June 2003).
Statistical analyses
The data for pain thresholds, pain tolerances, peak pain ratings, and mood states were analyzed with two (conditions: preferred and prescribed exercise) × two (trials: before and after exercise) repeated-measures ANOVA. Watts, HR, muscle pain, and RPE were analyzed with two (conditions: preferred and prescribed exercise) × four (time: every 5 min during 20 min of exercise) repeated-measures ANOVA. In addition, the data for symptoms of pain (body pain diagram and SF-MPQ) were analyzed with two (conditions: preferred and prescribed exercise) × six (trials: before and immediately, 24, 48, 72, and 96 h after exercise) repeated-measures ANOVA. The level of statistical significance was set at α = 0.05 for all analyses. Effect sizes were computed to detect the magnitude of change using Cohen’s d (7).
RESULTS
Characteristics of the participants
The women who participated in this study had a mean age of 44 yr (SD = 12), and most of the participants were Caucasian (90%). The majority of the participants reported taking low-dose antidepressant medication (71%) and nonsteroidal anti-inflammatory drugs (43%), and medication usage did not change during the week between conditions. Average weekly physical activity levels were self-reported, and on average, the individuals performed 1–2 d of moderate-intensity exercise for 15–30 min per session. Vigorous activity was performed less frequently with the participants reporting on average doing <1 d of vigorous activity for ≤15 min. The average score for the Fibromyalgia Impact Questionnaire for this sample (mean = 48.3, SD = 14.0) was considered to be representative of an average FM patient (2).
Exercise conditions
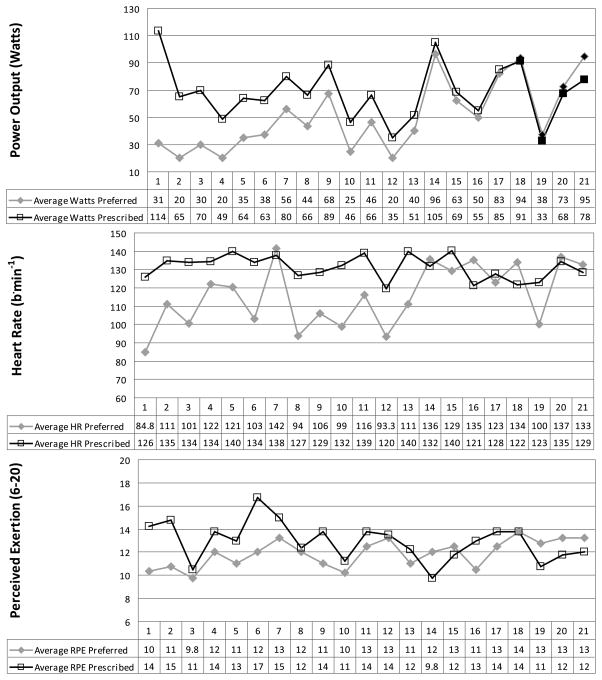
Participants completed two exercise conditions consisting of 20 min of cycling at a prescribed intensity and 20 min of cycling at a preferred intensity. All of the women completed the 20-min exercise sessions, with the exception of one woman who stopped cycling at 16 min during the prescribed-intensity exercise session. The results indicated that the women chose a significantly lower workload in the preferred exercise session in comparison with the prescribed exercise session. Watts, HR, and RPE responses were found to be significantly lower (P < 0.05) in the preferred exercise session versus the prescribed exercise session, and these results are summarized in Table 1. On average, HR during the prescribed exercise session corresponded to ~62% of age-adjusted maximum. HR during the preferred session averaged ~45% of age-adjusted maximum. Figure 1 details the prescribed and preferred watts, along with the associated HR and RPE responses for each individual. Four individuals chose a higher wattage for their preferred session than was prescribed. There was good agreement between the changes in watts between the preferred and prescribed sessions and changes in both HR (r = 0.69, P = 0.001) and RPE (r = 0.65, P = 0.001) responses. Further, responses were not dependent on whether the prescribed or preferred session occurred first. There were no significant differences in average watts (57.7 ± 29 vs 61.4 ± 19, P > 0.05), HR (120 ± 15 vs 126 ± 17, P > 0.05), or RPE (12.0 ± 1.5 vs 12.8 ± 1.5, P > 0.05) between sessions 1 and 2. Thus, the FM participants consistently chose lower exercise intensities for their preferred session, and this was associated with appropriate decreases in both physiological (HR) and perceptual (RPE) responses.
TABLE 1.
Means and SD for responses during exercise.
| Time
|
||||||||
|---|---|---|---|---|---|---|---|---|
| 5 min
|
10 min
|
15 min
|
20 min
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| HR | ||||||||
| Prescribed | 115.62 | 2.84 | 132.19 | 3.09 | 137.52 | 2.90 | 139.81 | 2.66 |
| Preferred | 108.33 | 2.89 | 114.24 | 3.89 | 119.76 | 4.67 | 120.71 | 4.43 |
| RPE | ||||||||
| Prescribed | 10.64 | 0.44 | 13.29 | 0.39 | 13.76 | 0.49 | 14.00 | 0.45 |
| Preferred | 10.19 | 0.33 | 11.64 | 0.34 | 12.71 | 0.39 | 12.95 | 0.42 |
| Watts | ||||||||
| Prescribed | 58.81 | 3.64 | 70.95 | 5.07 | 73.33 | 5.36 | 71.19 | 5.71 |
| Preferred | 44.76 | 3.90 | 52.86 | 6.58 | 54.76 | 7.49 | 50.00 | 5.95 |
FIGURE 1.
Individual responses for watts, HR, and RPE during the prescribed and preferred exercise conditions. Data plotted on the graph and in the table below each graph represent mean values for the 20-min exercise session. Seventeen of the 21 participants chose a lower wattage for their preferred session compared with the prescribed session. Participants 18 through 21 represent the four individuals who chose a higher wattage for their preferred session compared with the prescribed session. They are denoted on the watts graph (top) by black-filled data points.
Muscle pain in the legs, however, was not found to differ significantly (P > 0.05) between the preferred and the prescribed exercise sessions. Average muscle pain as assessed on a 0–10 scale was rated as mild in the preferred-intensity exercise session (mean = 2.0, SD = 0.8), whereas muscle pain was rated between mild and moderate in the prescribed-intensity exercise session (mean = 2.4, SD = 0.9).
Pain thresholds, pain tolerances, and peak pain ratings
The results for pain thresholds indicated a significant increase (F1,20 = 5.222, P = 0.033) in pain thresholds after exercise. Examination of the effect sizes for the two exercise sessions indicated that there was a large effect (d = 0.79) for threshold changes after the preferred-intensity exercise session, whereas the effect size for pain thresholds after the prescribed-intensity exercise session was small (d = 0.008). The results for pain thresholds are summarized in Figure 2.
FIGURE 2.
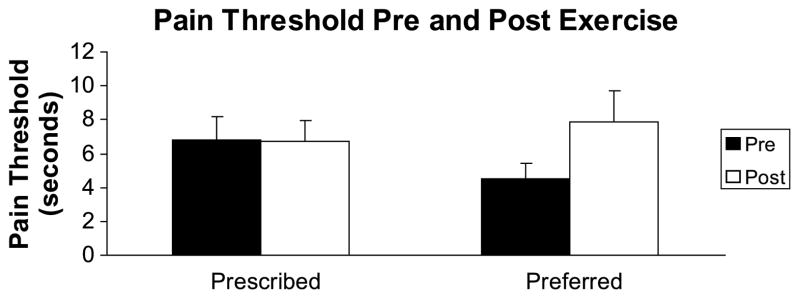
Means and SE for pain thresholds before and after exercise.
Pain tolerances were also found to increase significantly after exercise (F1,20 = 6.985, P = 0.016), indicating that the women were able to endure the noxious stimulation for a longer duration. The magnitude of change after the prescribed-intensity exercise session (10.0 ± 44 s, d = 0.20) was comparable to the preferred-intensity exercise session (12.3 ± 39 s, d = 0.28).
Peak pain ratings including pain intensity and pain unpleasantness were found to change after the exercise sessions. Peak pain intensity and peak pain unpleasantness ratings decreased significantly after the preferred and prescribed exercise sessions (F1,20 = 11.882, P = 0.003; and F1,20 = 16.980, P = 0.001, respectively), and the effect sizes ranged between 0.32 and 0.39. The results for peak pain intensity are summarized in Figure 3.
FIGURE 3.
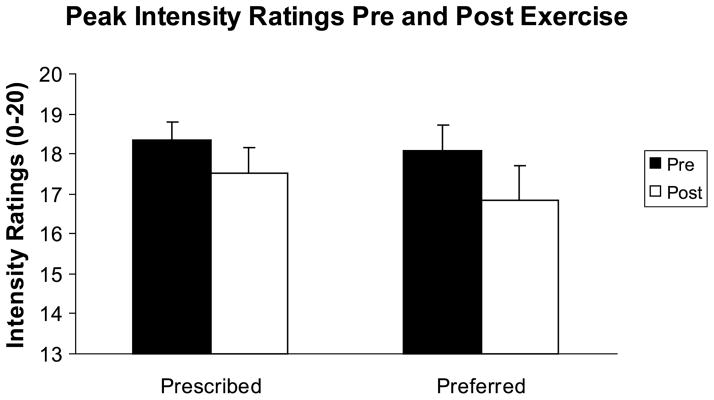
Means and SE for peak pain intensity ratings before and after exercise.
Pain after exercise
Pain was also assessed in the post-exercise period (i.e., immediately and at 24, 48, 72, and 96 h) to examine whether there were exacerbations in pain after exercise. The results indicated that pain was not exacerbated after exercise. The number and location of painful sites as assessed by the body pain diagram did not change significantly (P > 0.05) after either exercise session. In fact, pain assessed by the MPQ total score was found to be significantly lower (F5,95 = 3.506, P = 0.006) after the exercise sessions, and these results are summarized in Figure 4.
FIGURE 4.
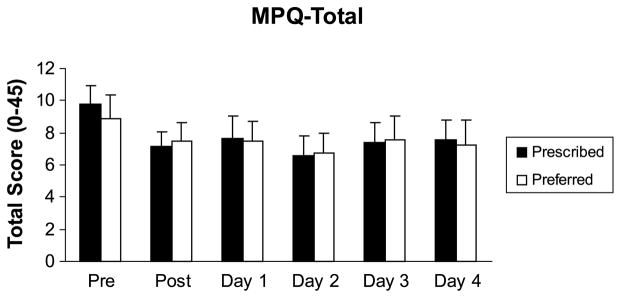
Means and SE for the MPQ total in the postexercise period.
Other results
Before each session, participants completed the Tampa Scale for Kinesiophobia to assess fear of movement and exercise. Results indicated that there was not a significant difference (P > 0.05) between the preferred exercise session (mean = 33.15, SD = 6.40) and the prescribed exercise session (mean = 34.90, SD = 6.75). In addition, participants completed the fibromyalgia-specific PGIC questionnaire on day 4 (i.e., 96 h) after each exercise session. The results indicated that there were no significant differences in the PGIC scores between the preferred exercise session (mean = 3.94, SD = 1.03) and the prescribed exercise session (mean = 4.06, SD = 0.94).
Mood states
Mood states were assessed with the POMS before and 15 min after each exercise bout. Results indicated that there were significant improvements in mood after exercise. Depression, anger, fatigue, and confusion decreased significantly (P < 0.05) after both exercise conditions. In addition, the total mood disturbance score was found to be significantly lower after both exercise conditions in comparison with before exercise (P < 0.05). Results for the POMS data are summarized in Table 2.
TABLE 2.
Means ± SD for POMS data before and after exercise.
| POMS | Prescribed
|
Preferred
|
||||
|---|---|---|---|---|---|---|
| Before | After | ES | Before | After | ES | |
| Tensiona | 7.38 ± 6.30 | 5.10 ± 3.59 | 0.46 | 4.57 ± 3.09 | 4.81 ± 3.16 | 0.08 |
| Depressionb | 5.86 ± 8.19 | 3.00 ± 5.45 | 0.42 | 3.33 ± 4.28 | 1.33 ± 2.35 | 0.61 |
| Angera,b | 4.38 ± 6.56 | 1.29 ± 3.26 | 0.63 | 1.86 ± 4.07 | 0.48 ± 1.08 | 0.54 |
| Vigorc | 8.95 ± 6.39 | 9.95 ± 7.19 | 0.15 | 10.81 ± 5.83 | 11.52 ± 7.13 | 0.11 |
| Fatigueb | 11.24 ± 7.41 | 8.48 ± 6.99 | 0.38 | 9.95 ± 8.41 | 6.48 ± 5.38 | 0.50 |
| Confusionb,c | 7.76 ± 4.89 | 6.81 ± 3.76 | 0.22 | 6.29 ± 4.51 | 4.9 ± 3.28 | 0.36 |
| TMSb,c | 127.67 ± 31.69 | 114.71 ± 21.26 | 0.49 | 115.19 ± 21.35 | 106.48 ± 14.10 | 0.49 |
Significant condition × trials interaction.
Significant preexercise to postexercise effect.
Significant condition effect.
ES, effect size; TMS, total mood score.
DISCUSSION
The purpose of this study was to examine the influence of a preferred- versus a prescribed-intensity exercise session on pain in women with FM. The results from this study indicated that the women with FM who participated in the study preferred to exercise at a lower intensity compared with the prescribed-intensity exercise session, which was based on recommendations from the American Pain Society (1). HR, watts, and RPE were found to be significantly lower during the preferred-intensity exercise session in comparison with the prescribed-intensity exercise session. There is an absence of published research examining the preferred exertion model of exercise in women with FM; therefore, it is currently unclear what intensity of exercise is preferred by women with FM to manage pain. The results from this study indicated that the women chose an intensity approximating ~45% of age-adjusted maximum and that was rated between light and somewhat hard on Borg’s perceived exertion scale (mean RPE = 12). These results provide support for the importance of an exercise prescription based on the individual using a preferred exertion model. If most women with FM prefer to exercise at an intensity of exercise that is lower than what is typically recommended, then prescriptions of exercise based on preferred intensity may have a positive effect on exercise adherence. In addition, using the preferred exertion model allows individuals with FM to choose the most appropriate intensity of exercise based on the severity of symptoms and pain experienced before exercise.
The results from this study also indicated that there were significant changes in experimental pain sensitivity after exercise. Pain thresholds and pain tolerances were found to increase, whereas pain intensity and pain unpleasantness ratings decreased after exercise. These improvements in pain were evident after both the preferred- and prescribed-intensity exercise sessions. Currently, the literature is equivocal regarding whether EIA occurs in women with FM. There are reports in the literature that FM patients do not experience EIA. In fact, they become more sensitive to experimental pain stimuli during and after exercise (i.e., a hyperalgesic response). Mengshoel et al. (32), for example, reported increases in muscle pain ratings after exercise, which remained elevated 24 h after exercise. Kosek et al. (25) and Staud et al. (41) also reported increases in pain during exercise in individuals with FM but opposite effects in healthy controls. In addition, Vierck et al. (46) found that temporal summation (i.e., wind up) of heat pain was enhanced during maximal exercise in FM patients in contrast to being significantly diminished in healthy controls. The findings from the present study are in contrast to these previous studies but are in agreement with two recent studies. Kadetoff and Kosek (20) reported that deltoid pain thresholds were elevated in women with FM during isometric exercise performed at a low intensity (i.e., 10% maximal voluntary contraction (MVC)). Staud et al. (42) reported that brief (~5 min) bouts of arm ergometry had a hypoalgesic effect for pressure pain thresholds. Moreover, they reported that clinical pain ratings decreased significantly during the rest periods immediately after exercise. In addition, there is one dissertation study conducted with women with FM in which a self-selected exercise session was incorporated along with prescribed exercise sessions (17). Results indicated that pain ratings were significantly lower 30 min after both preferred and prescribed exercises.
There are several potential explanations for the conflicting results across studies, including patient heterogeneity, type of pain stimulus used, and differences in exercise mode. However, it seems that studies involving higher exercise intensity and/or eccentric contractions consistently increase pain sensitivity in FM, whereas those involving low to moderate intensities without eccentric contractions result in a hypoalgesic response. Vierck et al. (46) reported increases in pain sensitivity to thermal pain testing in women with FM after a maximal exercise test. Mengshoel et al. (32) reported that FM patients had increases in pain after a combination of exercises that included eccentric contractions. In addition, Kosek et al. (25) and Staud et al. (41) found increases in pain sensitivity during exercise, but the stimulus involved isometric exercise at intensities higher than that used by Kadetoff and Kosek (20). The results from the present study indicated that EIA occurred after aerobic exercise performed at a preferred intensity that was of low intensity (~45% of age-adjusted max HR) as well as at a moderate intensity at the low end of the American Pain Society recommendations (~62% of age-adjusted max HR), potentially indicating that the intensity of exercise is an important consideration in terms of sensitivity of the nociceptive system and musculoskeletal pain.
Currently, the mechanisms responsible for EIA are poorly understood; however, results from the animal research indicate that there are multiple analgesia systems including opioid and nonopioid systems (35,43). The most commonly tested hypothesis for analgesia after exercise is that EIA is produced by exercise-induced release of endogenous opioids at peripheral, spinal, and/or central sites capable of pain modulation (19,44). Opioid antagonists have been found to attenuate the analgesic response after exercise of mild severity but have not had a consistent effect on analgesia after more severe exercise (34). However, analgesia that is insensitive to opioid antagonists can also occur, providing evidence for nonopioid analgesia. The specific neurochemistry of nonopioid analgesia is not fully understood, but several neurotransmitters, such as serotonin and norepinephrine, have been implicated. In addition, involvement of N-methyl-D-aspartic acid subtype of excitatory amino acid receptors have received some attention (26,35). It also has been suggested that the endocannabinoid hypothesis is a reasonable alternative to the endorphin hypothesis of EIA (13), but very little research has been conducted. Further research is needed, examining mechanisms of EIA and whether different analgesia systems are involved in EIA after high- versus low-intensity exercise and whether these mechanisms differ because of chronic widespread musculoskeletal pain.
In addition to the changes in experimental pain sensitivity, mood was also found to improve after both the preferred and the prescribed exercise sessions. This is a novel finding for short-term studies of exercise in FM and is consistent with the results from several exercise training studies (6). The overall magnitude of the change in mood was moderate, which is consistent with meta-analytic data showing that patients with various medical illnesses including cancer, cardiovascular disease, multiple sclerosis, anxiety, depression, low back pain, osteoarthritis, and FM become less anxious with exercise (18). When considering these short-term exercise results in conjunction with the pain sensitivity data, it seems that FM patients can reap the same benefits from low-intensity exercise as those achieved by healthy men and women after moderate- to high-intensity exercise.
Pain was also assessed in the postexercise period (i.e., immediately and 24, 48, 72, and 96 h after exercise) to examine whether there were exacerbations in pain after exercise. Collecting measures of pain in the days after an immediate bout of exercise is important for understanding how much recovery is needed between exercise sessions. There has only been a limited amount of research conducted examining pain responses in the postexercise period despite anecdotal reports from women with FM that pain is worse in the days after exercise. As mentioned earlier, Mengshoel et al. (32) indicated that FM patients reported increases in pain up to 24 h after exercise. In contrast, Vierck et al. (46) assessed pain at 10 min, 24 h, and 1 wk after exercise and found that pain was not exacerbated after exercise. The results from the present study also indicated that pain was not exacerbated after exercise. The number and location of painful sites did not change in the postexercise period. In fact, pain (assessed by the MPQ total score) was found to be improved up to 4 d after both the preferred- and prescribed-intensity exercise sessions.
In summary, women with FM who participated in this study preferred a lower intensity of exercise compared with a typically prescribed exercise intensity. A preferred exertion model seems to be as effective as a prescribed-intensity model in reducing pain. Therefore, the preferred exertion model could be used as an appropriate, and perhaps, a more generalizable strategy to reduce pain in women with FM, especially because pain and other debilitating symptoms of FM are highly variable among individuals. It should be acknowledged, however, that the results from this study may not be representative of all women with FM and should only be generalized to women with FM who have not been diagnosed with major depressive disorder. It is concluded that improvements in pain can occur after both a preferred-intensity exercise session and a prescribed-intensity exercise session (based on the American Pain Society recommendation (1)). The results from this study are novel and indicate that recommendations for exercise prescription for individuals with FM should consider the preferred exertion model of exercise as a strategy to reduce pain.
Acknowledgments
This study was supported by the Diane and Donald Masterson Gift Fund.
Footnotes
None of the authors has anything to disclose.
The results of this study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.American Pain Society. Guidelines for the management of Fibromyalgia syndrome pain in adults and children. APS Clinical Practice Guideline Series. 2005;4:109. [Google Scholar]
- 2.Bennett RM. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–62. [PubMed] [Google Scholar]
- 3.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana I. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg G, editor. Borg’s Perceived Exertion and Pain Scales. Champaign (IL): Human Kinetics; 1998. p. 104. [Google Scholar]
- 5.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–33. [PubMed] [Google Scholar]
- 6.Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KAR. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35:1130–44. [PubMed] [Google Scholar]
- 7.Cohen J. Statistical Power Analysis for Behavioral Sciences. 2. Hillsdale (NJ): Lawrence Earlbaum Associates; 1988. p. 567. [Google Scholar]
- 8.Cook DB, Koltyn KF. Pain and exercise. Int J Sport Psychol. 2000;31:256–77. [Google Scholar]
- 9.Cook DB, Nagelkirk PR, Poluri A, Mores J, Natelson BH. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. 2006;45(10):3351–62. doi: 10.1002/art.22124. [DOI] [PubMed] [Google Scholar]
- 10.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc. 1997;29(8):999–1012. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Culos-Reed SN, Brawley LR. Fibromyalgia, physical activity, and daily functioning: the importance of efficacy and health-related quality of life. Arthritis Care Res. 2000;13:343–51. doi: 10.1002/1529-0131(200012)13:6<343::aid-art3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.de Gier M, Peters ML, Vlaeyen WS. Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain. 2003;104:121–30. doi: 10.1016/s0304-3959(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38:2471–8. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dishman RK, Farquhar RP, Cureton KJ. Responses to preferred intensities of exertion in men differing in activity levels. Med Sci Sports Exerc. 1994;26(6):783–90. doi: 10.1249/00005768-199406000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. p. 140. [Google Scholar]
- 17.Gardiner RL, Howe CA. Psychological and physiological responses to prescribed versus preferred exercise in clients with fibromyalgia. Med Sci Sports Exerc. 1998;30(5):6. [Google Scholar]
- 18.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients. Arch Intern Med. 2010;170(4):321–31. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman P, Terenius L, Thoren P. Cerebrospinal fluid immunoreactive beta-endorphin concentration is increased by voluntary exercise in the spontaneous hypertensive rat. Regul Pept. 1990;28:233–9. doi: 10.1016/0167-0115(90)90021-n. [DOI] [PubMed] [Google Scholar]
- 20.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 22.Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Med Sci Sports Exerc. 1996;28(11):1418–21. doi: 10.1097/00005768-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52(1):296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 24.Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behavior. Pain Manage. 1990;4:35–43. [Google Scholar]
- 25.Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain. 1996;64:415–23. doi: 10.1016/0304-3959(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 26.Marek P, Mogil JS, Sternberg WF. N-methyl D-aspartic acid (NMDA) receptor antagonist MK-801 blocks non-opioid stress-induced analgesia II: comparison across three swim–stress paradigms in selectively bred mice. Brain Res. 1992;578:197–203. doi: 10.1016/0006-8993(92)90248-8. [DOI] [PubMed] [Google Scholar]
- 27.Martin L, Nutting A, MacIntosh BR, Edworthy SM, Butterwick D, Cook J. An exercise program in the treatment of fibromyalgia. J Reheumatol. 1996;23:1050–3. [PubMed] [Google Scholar]
- 28.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc. 2011;43(5):905–12. doi: 10.1249/MSS.0b013e3181fca1ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego (CA): Educational and Industrial Testing Service; 1992. p. 27. [Google Scholar]
- 30.Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol. 2005;32:6–21. [PubMed] [Google Scholar]
- 31.Melzack R. The Short-From McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 32.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin Exp Rheumatol. 1995;13:477–82. [PubMed] [Google Scholar]
- 33.Meyer BB, Lemley KJ. Utilizing exercise to affect the symptomatology of fibromyalgia: a pilot study. Med Sci Sports Exerc. 2000;32(10):1691–7. doi: 10.1097/00005768-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS, Belknap JK. Sex and genotype determine the selective activation of neurochemically-distinct mechanisms of swim stress–induced analgesia. Pharmacol Biochem Behav. 1997;56:61–6. doi: 10.1016/S0091-3057(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS, Sternberg WF, Balien H. Opioid and non-opioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996;59:123–32. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 36.Morgan WP. Prescription of physical activity: a paradigm shift. Quest. 2001;53:366–82. [Google Scholar]
- 37.Morgan WP, Horstman DH. Psychometric correlates of pain perception. Percept Mot Skills. 1978;47:27–39. doi: 10.2466/pms.1978.47.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Norregaard J, Lykkegaard J, Mehlsen J, Danneskiold-Samsoe B. Exercise training in treatment of fibromyalgia. J Musculoskeletal Pain. 1997;5:71–9. [Google Scholar]
- 39.Ramsay C, Moreland J, Ho M, Joyce S, Walker S, Pullar T. An observer-blinded comparison of supervised and unsupervised aerobic exercise regimens in fibromyalgia. Rheumatology (Oxford) 2000;39:501–5. doi: 10.1093/rheumatology/39.5.501. [DOI] [PubMed] [Google Scholar]
- 40.Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys Ther. 2003;83:340–58. [PubMed] [Google Scholar]
- 41.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–84. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Staud R, Robinson ME, Weyl EE, Price DD. Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J Pain. 2010;11:1376–83. doi: 10.1016/j.jpain.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sternberg WF, Liebeskind JC. The analgesic response to stress: genetic and gender considerations. Eur J Anaesthesiol. 1995;12:14–7. [PubMed] [Google Scholar]
- 44.Thoren P, Floras JS, Hoffman P. Endorphins and exercise: physiological mechanisms and clinical applications. Med Sci Sports Exerc. 1990;22(4):417–28. [PubMed] [Google Scholar]
- 45.van Santen M, Bolwijn P, Landewe R, et al. High or low intensity aerobic fitness training in fibromyalgia: does it matter? J Rheumatol. 2002;29:582–7. [PubMed] [Google Scholar]
- 46.Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited but brief contact of glaborous skin by a preheated probe. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]