Abstract
Noonan syndrome with multiple lentigines (NSML) frequently manifests with hypertrophic cardiomyopathy (HCM). Recently, it was demonstrated that mTOR inhibition reverses HCM in NSML mice. We report for the first time on the effects of treatment with a rapamycin analog in an infant with LS and a malignant form of HCM. In the boy, progressive HCM was diagnosed during the first week of life and diagnosis of NSML was established at age 20 weeks by showing a heterozygous Q510E mutation in the PTPN11 gene. Immunoblotting with antibodies against pERK, pAkt, and pS6RP in fibroblasts demonstrated reduced RAS/MAPK and enhanced Akt/mTOR pathway activities. Because of the patient’s critical condition, everolimus therapy was started at age 24 weeks and continued until heart transplantation at age 36 weeks. Prior to surgery, heart failure improved from NYHA stage IV to II and brain natriuretic peptide values decreased from 9600 to <1000 pg/ml, but no reversal of cardiac hypertrophy was observed. Examination of the explanted heart revealed severe hypertrophy and myofiber disarray with extensive perivascular fibrosis. These findings provide evidence that Akt/mTOR activity is enhanced in NSML with HCM and suggest that rapamycin treatment could be principally feasible for infantile NSML. But the preliminary experiences made in this single patient indicate that therapy should start early to prevent irreversible cardiac remodelling.
Keywords: oonan syndrome with multiple lentigines, NSML, PTPN11, mTOR, hypertrophic cardiomyopathy, RASopathy
INTRODUCTION
Noonan syndrome with multiple lentigines (NSML), formerly known as LEOPARD syndrome, is a rare congenital multisystem disorder [Sarkozy et al., 2008]. About 90% of patients harbor heterozygous missense mutations in the protein tyrosine phosphatase, non-receptor type 11 (PTPN11) gene, which encodes the SH2 domain-containing protein tyrosine phosphatase 2 (SHP2), an upstream and/or parallel regulator of the RAS/MAPK signalling cascade [Sarkozy et al., 2008; Marin et al., 2001]. Progressive cardiac conduction abnormalities occur in about 75% of subjects, while HCM is observed in greater than 80% of patients with cardiac involvement. HCM can manifest during infancy, and may be associated with severe left ventricular outflow tract (LVOT) obstruction. Both conduction anomalies and HCM can result in sudden death or ultimately heart failure [Sarkozy et al., 2008].
PTPN11 mutations also cause approximately 50% of the allelic variant disorder Noonan syndrome (NS) [Tartaglia et al., 2001]. NS is more common (~1:2500) and is characterized by variably penetrant defects, including HCM, similar to those in NSML [Noonan, 1968; Sznajer et al., 2007]. However, point mutations identified in PTPN11 associated with NS are distinct from those related to NSML [Kontaridis et al., 2006; Gelb and Tartaglia, 2011]. While NS mutations behave as gain-of-function alleles with increased basal phosphatase activity [Keilhack et al., 2005), NSML mutations are catalytically impaired [Lauriol and Kontaridis, 2011]. Recent research has shown that NSML mice with mutations in PTPN11 evoke HCM through hyperactivation of the AKT/mTOR pathway [Marin et al., 2011; Gelb and Tartaglia, 2011]. Moreover, Marin et al. showed that treatment of NSML mice with rapamycin could prevent the onset of disease when administered early as well as reverse HCM once established [Marin et al., 2011]. Based on the latter finding, it has been concluded that mTOR inhibitors such as rapamycin can be considered for HCM treatment in NSML patients [Marin et al., 2011].
The aim of this study was to evaluate possible therapeutic effects of rapamycin treatment in an infant with NSML and rapidly progressive HCM due to a PTPN11 Q510E mutation. In addition, we sought to analyze the effects of this specific severe mutation in human cells, as the germ-line mutation is embryonically lethal in mice [Schramm et al., 2012]. To accomplish this, we assessed adverse events and monitored cardiac function during rapamycin analog treatment, investigated RAS/MAPK and AKT/mTOR signalling in mutant fibroblasts, and performed pathological examinations of the explanted heart.
CLINICAL REPORT
The patient is the first child of healthy unrelated German parents. Pregnancy was normal, and no signs of HCM were noticed during routine ultrasound examinations at 30 weeks gestational age and immediately prior to birth. The boy was born spontaneously at term without complication. Weight, length, and head circumference were all between the 10th and 50th percentiles. A heart murmur prompted echocardiography on postnatal day 4, revealing marked HCM. Over the course of the next few weeks, cardiac hypertrophy worsened, and couplets and non-sustained runs of ventricular extrasystoles became apparent at 6 weeks of age. By 10 weeks, the patient was in New York Heart Association (NYHA) stage IV, with left-ventricular wall thickness during diastole (LVPWT) at 8.5 millimeters (mm) (normal <5 mm, z-score 5.5) (Fig. 1A) and an interventricular septum thickness at end-diastole (IVST) at 9 mm (normal <6 mm, z-score 4.5). In addition, echocardiographic analysis displayed mitral valve insufficiency grade I, with fractional shortening reduced to 45%. The LVOT and right ventricular outflow tract (RVOT) gradients were 70 and 50 mm Hg, respectively.
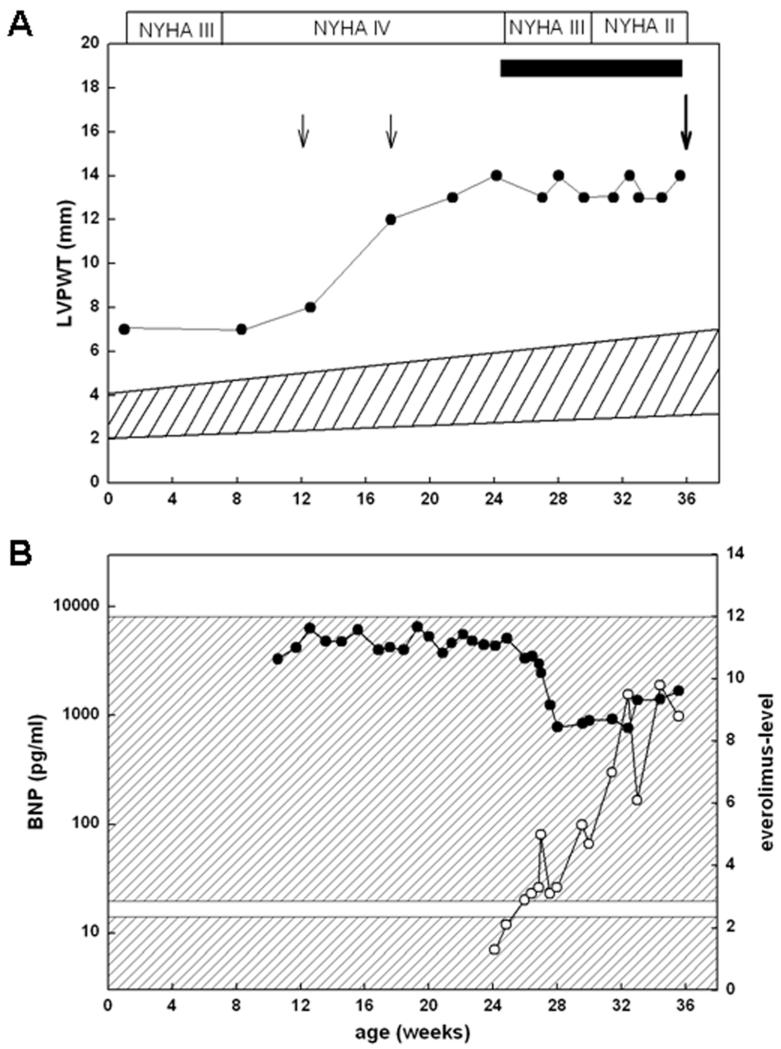
Figure 1.
A: Left ventricular posterior wall thickness (LVPWT) and NYHA stages in relation to age. The black bar indicates the everolimus treatment period, black filled cycles correspond to single LVPWT values, and the hatched area reflects the normal range of LVPWT. Small arrows indicate transcatheter ablations and the large arrow marks the time point when heart transplantion was performed. B: Everolimus (open cycles) and BNP levels (closed cycles) in relation to age. The lower hatched area corresponds to the normal BNP range (<20 pg/ml) and the upper hatched area marks the therapeutic everolimus level range (3-12 μg/ml).
Moreover, brain natriuretic peptide (BNP) levels, a fetal gene marker that becomes up-regulated as a consequence of HCM, was significantly elevated at 9600 pg/ml (normal <50 pg/ml) (Fig. 1B). Despite intensive anti-congestive treatment with propanolol, verapamil, furosemide, and spironolactone, cardiac hypertrophy in the patient exacerbated, and LVOT and RVOT obstruction increased to 100 and 70 mm Hg, respectively. Despite the presence of biventricular hypertrophy, unsuccessful transcatheter ablations of septal hypertrophy through instillation of 3 × 0.2 ml ethanol were performed in an attempt to improve left-ventricular dysfunction, at 12 weeks and at 16 weeks of age, respectively.
Clinical examination at age 20 weeks disclosed mild facial dysmorphism (Fig. 2A), with absent acoustically evoked potentials, demonstrating a bilateral deafness. A RASopathy was suspected and genetic analysis confirmed a heterozygous missense mutation in exon 13 of the PTPN11 gene (c.1528C>G; p.Q510E) (Fig. 2B). The boy was listed for heart transplantation, but because of his highly unstable condition, an alternative potential therapeutic approach was sought. Since it was recently demonstrated that rapamycin could reverse HCM in a mouse model of NSML, we initiated a treatment strategy utilizing the rapamycin analog everolimus at a dose of 2mg/kg/day beginning at 24 weeks of age. Approval by the local ethics committee (AZ 23-05-11) and written informed consent from the parents were obtained prior to this therapy. Treatment with the rapamycin analog was added to the ongoing medication regimen of the patient, consisting of furosemide, etacrynic acid, spironolactone, propanolol, verapamil, phenobarbitone, esomeprazole, and acetylsalicylic acid. Serum levels for everolimus were monitored throughout the time course and varied between 2.0-9.9 μg/l (therapeutic range 3-12 μg/l) (Fig. 1B). White blood cell count, creatinine, and glomerular filtration rate were within normal range over the whole treatment period. No respiratory tract or oral infection occurred. Immediately prior to start of everolimus treatment, echocardiography displayed a LVPWT of 14 mm (normal 6.0 mm, z-score 8.5) an IVST of 12 mm (normal < 6.3 mm, z-score 7.5), LVOT obstruction (gradient 100 mm Hg), and a thickened and deformed mitral valve, while the ejection fraction was mildly reduced (47%). Cardiac catheter examination revealed an elevated left atrial pressure (17 mmHg, normal < 10 mmHg), mitral valve insufficiency grade III, and an increased left-ventricular enddiastolic pressure (22 mmHg, normal < 12mmHg). Continuous electrocardiogram monitoring revealed frequent runs of non-sustained ventricular extrasystoles and self-limiting episodes of supraventricular tachycardia (SVT). During treatment, we observed that heart failure improved to NYHA stage II and BNP values decreased to 950-1000 pg/ml within 3 weeks (Fig. 1B). Echocardiographically, mitral valve insufficiency improved to grade I-II, while the LVOT gradient decreased minimally to 90 mm Hg. In addition, no runs of non-sustained ventricular extrasystoles were noticed and episodes of SVT had decreased distinctly after 3-4 weeks, although frequent multiple single ventricular extrasystoles were still recorded. Because of this functional improvement, one potential donor heart was rejected after 5 weeks of everolimus therapy. However, although LVPWT did not increase further during everolimus treatment, no reversal of cardiac hypertrophy, as measured by this parameter, was observed during a treatment period of 12 weeks (Fig. 1A). Therefore, we decided to accept a further organ offered to the patient at that time. Following surgery at age 36 weeks, the boy’s cardiac function normalized. He developed temporary renal insufficiency due to cyclosporine A treatment following heart transplantation. Moreover, feeding difficulties not related to his cardiac status necessitated gastrostomy. Cochlear implant was performed at age 26 months. At last follow-up, at 36 months of age, the boy was walking, but did not speak and was still being fed through a gastrostomy tube.
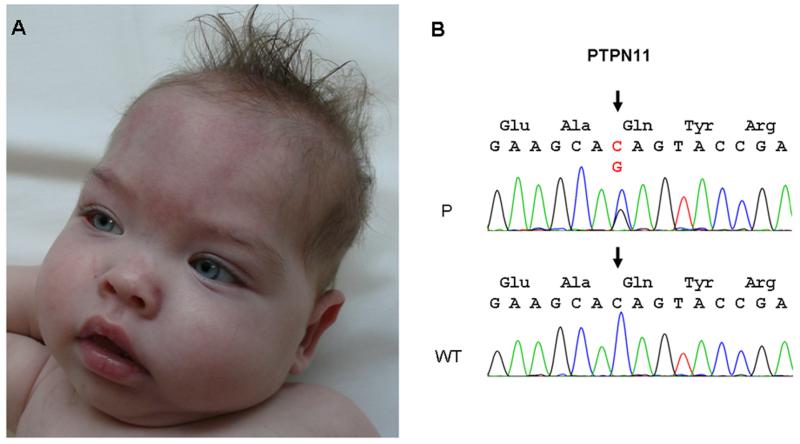
Figure 2.
A: Facial appearance of the patient included hypertelorism, flat nasal bridge, thick lips, and dysplastic low set ears. B: Electropherogram demonstrating a heterozygous C-to-G transition at position 1526 (arrow) in exon 13 of the PTPN11 gene in the patient (P) compared to the wild type (WT) sequence.
METHODS AND MATERIALS
Cardiac assessment
Severity of heart failure was expressed in NYHA classes (I = no signs, II = mild, III = moderate, and IV = severe) according to the classification for infants proposed by Ross and colleagues [Ross et al., 1992], and assessed by quantitative levels of BNP obtained from the patient’s serum at various time intervals. Cardiac hypertrophy was assessed by echocardiography using 2D-echocardiography and M-mode on a Toshiba Aplio SSA-770A with a 5-MHz transducer. Standard apical two- and four-chamber views were used for determination of LVPWT and other parameters of cardiac function. Data were related to normal values [Kampmann et al., 2011].
Genetic analysis
Molecular genetic testing included the following sections of genes encoding proteins of the RAS/MAPK pathway, and known to be particularly associated with neonatal HCM: exons 7, 12, and 13 of PTPN11, exons 2-5 of HRAS, and exons 7, 12, 14, and 17 of RAF1. Mutation analysis was carried out by PCR followed by bidirectional direct sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and a 3500xL Genetic Analyzer (Life Technologies).
Histopathological examinations
Tissue samples were processed using standard protocols. Five-micrometer paraffin sections were stained with hematoxylin and eosin (H&E), Giemsa and Masson trichrome. Immunohistochemistry was performed with anti-desmin (Dako, M760) and anti-SMA (Dako, M0851) antibodies. For electron microscopy, tissue samples were fixed in 3.9% glutaraldehyde, processed with a Leica EM TP tissue processor, and contrasted with a Leica EM AC20 instrument (Ultrastain kit). 1-2 μm thick sections of plastic-embedded tissue were stained with methylene blue (MB). Ultrathin sections were analyzed with an electron microscope (Zeiss EM 109).
Biochemical analyses
Skin fibroblasts isolated from the NSML patient and a normal (wild type) donor of the same age were cultured in AmnioMax C-100 medium (Invitrogen) with AmnioMax C-100 supplement (Invitrogen). Cells were starved overnight in medium without supplement and then were either left unstimulated or were stimulated with 10 nmol/l insulin-like growth factor (IGF) or 25 ng/ml epidermal growth factor (EGF) for the indicated times. After protein extraction, immunoblots were performed, following the manufacturer’s directions, with anti-phospho-AKT, anti-phospho-ERK1/2, anti-phospho-S6RP, and their respective total proteins (Cell Signaling). Bands were visualized with enhanced chemiluminescence and quantified by densitometry using ImageJ 1.41 software (Wayne Rasband, http://rsbweb.nih.gov/ij/). Protein expression was referred to the total amount of respective proteins. All analyses were performed 4-5 times. Data are presented as mean ± SEM. Statistical significance was determined using 1-way ANOVA or 2-way repeated measure ANOVA, as appropriate. If ANOVA was significant, individual differences were evaluated using the Bonferroni post-test. Values of P < 0.05 were considered statistically significant.
RESULTS
Pathological examination
Right ventricular endomyocardial biopsy was performed at 12 weeks of age, demonstrating mild interstitial myocardial fibrosis and distinct perivascular fibrosis with thickened arteriolar walls (Supplemental figure 1A+B). There was also mild disarray of hypertrophic cardiomyocytes, but no significant decrease of desmin filaments was observed (Supplemental figure 1C).
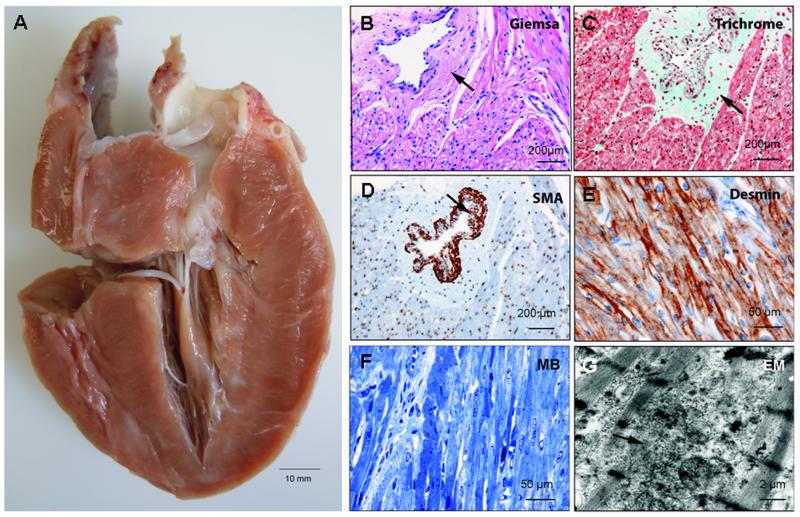
At explant when the patient was 36 weeks of age, the heart weighed 101 grams, approximately 2.5 times more than normal. Macroscopically, there was marked biventricular hypertrophy (Fig. 3A). The cardiac cavities were not enlarged but the endocardium was somewhat thickened. During cutting, the myocardium appeared firm and had a whitish-streaked appearance. Microscopic examination revealed perivascular fibrosis and foci of mild interstitial fibrosis. Cardiomyocyte size was increased and myocyte nuclei were enlarged. Perivascular fibrosis was associated with a marked increase in arteriolar wall thickness, likely due to media hyperplasia (Fig. 3B-D). In addition, desmin antibody staining disclosed myofiber disarray (Fig. 3E). This was corroborated by methylene blue stained semi-thin sections (Fig. 3F) and ultrastructural analysis (Fig. 3G), demonstrating disarray of myofibrils and Z-bands.
Figure 3.
A: Pathological examination of the explanted heart at age 36 weeks showing massive biventricular hypertrophy, thickened endocardium, and small ventricular cavities. B-D: Microscopic analysis depicting marked perivascular fibrosis and profound wall thickening of an arteriole (arrows). (e, g) Desmin staining, methylene blue stained semi-thin section (MB), and electron microscopy demonstrating disarray of myofibers and Z-bands.
Biochemical analyses
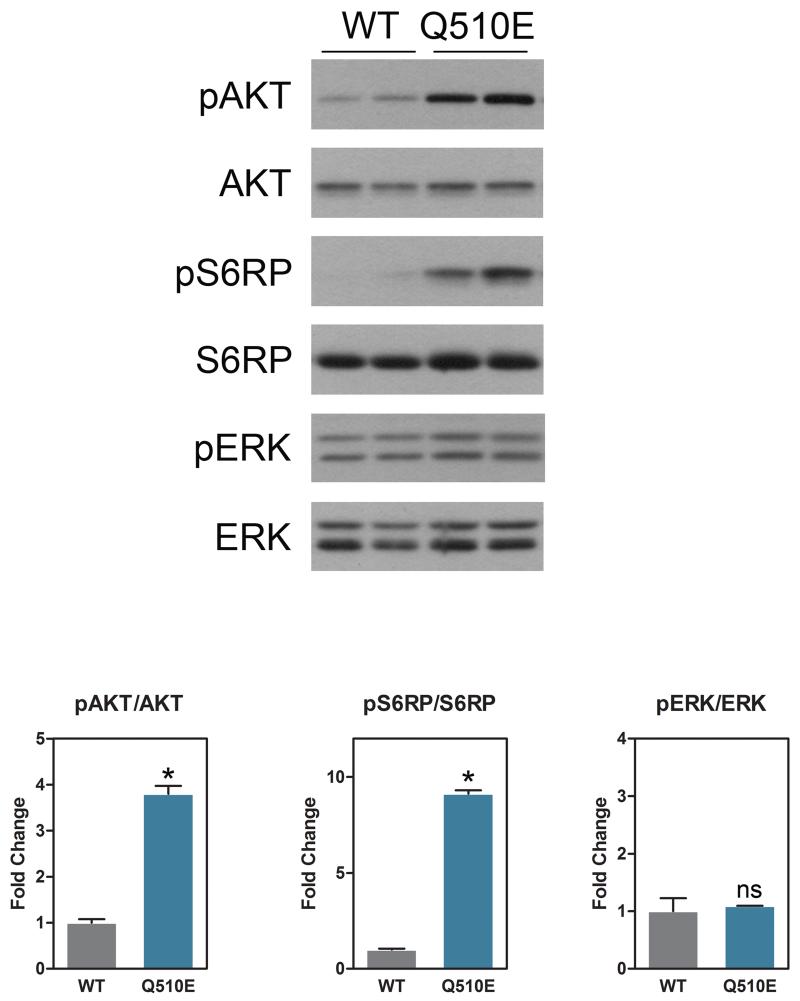
Figure 4 summarizes the results of immunoblotting and corresponding quantification of fibroblast protein lysates. Baseline levels of pAKT and its downstream effector pS6RP were both significantly increased in Q510E cells as compared to WT. No significant differences in baseline, however, were observed in levels of pERK, a key downstream effector in the RAS/MAPK pathway, between WT and Q510E. In response to EGF stimulation, S6RP also showed significantly increased phosphorylation at 2 minutes of stimulation, whereas pAKT, pERK and the other time points in S6 were not significantly different between Q510E and WT cells (Supplemental Fig. 2). Similarly, with IGF stimulation, pS6RP was significantly increased at baseline and at 10 minutes following stimulation, although the data showed a trend towards all time points being increased in the Q510E as compared to WT in response to growth factor stimulation. No significance in response to stimulation with IGF was observed in pAKT or pERK between Q510E and WT cells (Supplemental Fig. 2).
Figure 4.
Immunoblotting and corresponding quantification of fibroblast protein lysates with antibodies against pERK, pAKT and pS6RP and their respective total proteins, derived from Q510E mutant and wild type (WT) fibroblasts under baseline conditions, demonstrating that Q510E mutant cells have elevated AKT/mTOR signaling at baseline. n=4-5 independent experiments. Data represent the mean ± SEM; p values were derived using 2-tailed Student’s t test. * p<0.05 ; ns=non-signifiant.
DISCUSSION
In this report, we comprehensively assessed the clinical, genetic, histopathological, and functional characteristics of an infant with NSML and a malignant form of HCM caused by the PTPN11 Q510E mutation that was treated with a rapamycin analog for 12 weeks. Until now, only four subjects with this specific mutation have been reported. As in our patient, all four showed a severe biventricular, rapidly progressive HCM with neonatal or prenatal onset resulting in heart failure during infancy [Takahashi et al., 2005; Digilio et al. 2006; Faienza et al., 2009].
Mutations in several genes encoding components of the RAS/MAPK signalling pathway have been linked to RASopathies, disorders that include NS and NSML, but also cardio-facio-cutaneous, neurofibromatosis and Costello syndromes [Digilio et al., 2011]. Most subjects with RASopathy and HCM bear mutations at specific locations in distinct genes (PTPN11, HRAS, RAF1), usually resulting in excess signalling through the RAS/MAPK cascade [Digilio et al. 2011; Gelb and Tartaglia, 2011]. Recently, however, it was shown that SHP2 is not only important for proper function of the RAS/MAPK cascade, but also for correct signal transduction via the AKT/mTOR pathway [Gelb and Tartaglia, 2011]. Marin et al. demonstrated that HCM in mice harboring the heterozygous PTPN11 Y279C NSML mutation is secondary to AKT/mTOR hyperactivity. They also found that treatment with rapamycin at the initial stages of HCM can completely reverse hypertrophy [Marin et al., 2011]. Similarly, Schramm et al. showed that HCM reversed in transgenic NSML mice overexpressing cardiomyocyte-specific Q510E mutant PTPN11, when treated with rapamycin immediately after birth [Schramm et al., 2012]. Importantly, while these data provided the first evidence for increased AKT/mTOR signalling in NSML, all experiments were performed in mice and the precise mechanistic role of the NSML mutations in humans remained unknown. Therefore, we conducted biochemical analyses in human Q510E mutant fibroblasts, which validated that NSML mutations in human patients also result in reduced RAS/MAPK phosphorylation and increased AKT/mTOR activity. Collectively, our data provide the first evidence that hyperactivation of the AKT/mTOR signalling pathway is the major mechanism leading to HCM in human NSML.
In our patient, the severity of heart failure assessed by NYHA stages and BNP levels improved markedly during everolimus therapy, suggesting that HCM in NSML patients can principally be treated with mTOR antagonists. However, although anticongestive medicaments remained unchanged during everolimus treatment, minor dosage modifications were performed. Therefore, it can not be excluded with certainty that improvements of hemodynamic factors secondary to these adaptations added to the functional amelioration of the cardiac status. Moreover, safety of everolimus therapy should be properly evaluated, since some reports indicated that loss of mTOR expression may also promote pathological hypertrophy and heart failure [Song et al., 2010; Shende et al., 2011]. In this single patient treated for a relatively short period of time, careful monitoring revealed no side effects. Overall, based on this preliminary experience we believe that short-term and/or low-dose administration of rapamycin could be beneficial in NSML patients with severe or rapidly progressive forms of HCM. As such, clinical trials of rapamycin and/or analogs should be considered for the treatment of severe NSML-associated HCM.
It is important to note, however, that despite the functional cardiac improvement after 12 weeks of treatment, we did not observe reversal of the severe hypertrophy of this patient. Pathological examination of the explanted heart demonstrated irreversible cardiac remodelling, with myofiber disarray and marked perivascular fibrosis with arteriolar wall thickening. Less severe but similar signs of hypertrophic remodelling were observed in the initial endomyocardial biopsy at 12 weeks of age, suggesting that structural alterations in our patient occurred early, just after birth and before treatment had begun. Therefore, it is possible that everolimus therapy in this subject, who had advanced hypertrophic disease, was initiated too late to reverse pathological remodelling, and that earlier treatment may have been more successful. Moreover, the duration of treatment in this patient was relatively short, in comparison to the duration in NSML mouse studies (equivalent to one year in humans) [Marin et al., 2011; Schramm et al., 2012]. Thus, we cannot exclude the possibility that extended therapy beyond 12 weeks may have been more effective.
If HCM in infants with NSML is to be regarded as potentially treatable, it is important to include NSML in the differential diagnosis of infantile HCM. In some patients with PTPN11 mutations, HCM has been diagnosed prenatally by ultrasound [Digilio et al. 2006]. Today, knowledge about the genotype/phenotype correlation in subjects with PTPN11 and other RAS/MAPK pathway gene defects allows rapid and cost-efficient screening for mutations associated with HCM [Zenker, 2011]. Since early detection is key in therapy, this should enable identification of patients diagnosed prenatally or during early infancy with HCM who would benefit from immediate rapamycin treatment. Furthermore, to our knowledge, the subject described in this paper is the first with NSML to undergo successful heart transplantation, thereby exemplifying that this procedure represents a therapeutic option for NSML patients with terminal heart failure.
In conclusion, our observations provide further evidence that elevated baseline AKT/mTOR activity is likely to be the main mechanism underlying HCM in infantile NSML, suggest that rapamycin or rapamycin analog treatment is, in principle, feasible for improving cardiac function, and indicate that early treatment, prior to irreversible cardiac remodelling, may be the key to gaining the greatest benefit from this therapeutic strategy. Our experiments also show the feasibility of individualized therapies for patients with PTPN11 mutations and other defects in genes coding components of the RAS/MAPK pathway, as well as the potential utility of patient-derived fibroblasts for the evaluation of treatment options that can be most beneficial for the individual patient.
Supplementary Material
ACKNOLEDGMENTS
The authors thank the patient’s family for their cooperation and permission to publish the data. The authors also thank Cornelia Dambmann and Gudrun Schmidt for their excellent technical assistance.
The work of M.I.K. is funded by National Institutes of Health Grants R01-HL102368 and R01-HL114775.
REFERENCES
- Gelb BD, Tartaglia M. RAS signalling pathway mutations and hypertrophic cardiomyopathy: getting into and out of the thick of it. J Clin Invest. 2011;121:844–847. doi: 10.1172/JCI46399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Sarkozy A, Pacileo G, Limongelli G, Marino B, Dallapiccola B. PTPN11 gene mutations: linking the Gln510Glu mutation to the “LEOPARD syndrome phenotype”. Eur J Pediatr. 2006;165:803–805. doi: 10.1007/s00431-006-0163-7. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Lepri F, Baban A, Dentici ML, Versacci P, Capolino R, Ferese R, De Luca A, Tartaglia M, Marino B, Dallapiccola B. RASopathies: Clinical diagnosis in the first year of life. Mol Syndromol. 2011;1:282–289. doi: 10.1159/000331266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faienza MF, Giordani L, Ferraris M, Bona G, Cavallo L. PTPN11 gene mutation and severe neonatal hypertrophic cardiomyopathy: what is the link? Pediatr Cardiol. 2009;30:1012–1015. doi: 10.1007/s00246-009-9473-7. [DOI] [PubMed] [Google Scholar]
- Kampmann C, Wiethoff CM, Wenzel A, Stolz G, Betancor M, Wippermann CF, Huth RG, Habermehl P, Knuf M, Emschermann T, Stopfkuchen H. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart. 2000;83:667–672. doi: 10.1136/heart.83.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhack H, David FS, McGregor M, Cantley LC, Neel BG. Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J Biol Chem. 2005;280:30984–30993. doi: 10.1074/jbc.M504699200. [DOI] [PubMed] [Google Scholar]
- Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- Lauriol J, Kontaridis MI. PTPN11-associated mutations in the heart: has LEOPARD changed Its RASpots? Trends Cardiovasc Med. 2011;21:97–104. doi: 10.1016/j.tcm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, Kontaridis MI. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121:1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan JA. Hypertelorism with Turner phenotype. 1968. A new syndrome with associated congenital heart disease. Am J Dis Child. 116:373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- Ross RD, Bollinger RO, Pinsky VW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–75. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- Sarkozy A, Digilio MC, Dallapiccola B. Leopard syndrome. Orphanet J Rare Dis. 2008:3. doi: 10.1186/1750-1172-3-13. doi: 10.1186/1750-1172-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm C, Fine DM, Edwards, Reeb AN, Krenz M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am J Physiol Heart Circ Physiol. 2012;302:H231–H243. doi: 10.1152/ajpheart.00665.2011. [DOI] [PubMed] [Google Scholar]
- Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Rüegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznajer Y, Keren B, Baumann C, Pereira S, Alberti C, Elion J, Cavé H, Verloes A. The spectrum of cardiac anomalies in Noonan syndrome as a result of mutations in the PTPN11 gene. Pediatrics. 2007;119:e1325–1331. doi: 10.1542/peds.2006-0211. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kogaki S, Kurotobi S, Nasuno S, Ohta M, Okabe H, Wada K, Sakai N, Taniike M, Ozono K. A novel mutation in the PTPN11 gene in a patient with Noonan syndrome and rapidly progressive hypertrophic cardiomyopathy. Eur J Pediatr. 2005;164:497–500. doi: 10.1007/s00431-005-1679-y. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Zenker M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr Opin Pediatr. 2011;23:443–451. doi: 10.1097/MOP.0b013e32834881dd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.