Highlights
* Heightened VS activation to prosocial behaviors relates to longitudinal declines in risk taking. * The VS predicted changes in risk taking behaviors above and beyond self-reported intentions. * The same brain regions that confer vulnerability for risk taking are also protective. * Heightened reward sensitivity can be an asset for adolescents, reducing risk taking over time.
Keywords: Adolescence, Reward, Risk taking, fMRI, Family
Abstract
Adolescence is a period of intensified emotions and an increase in motivated behaviors and passions. Evidence from developmental neuroscience suggests that this heightened emotionality occurs, in part, due to a peak in functional reactivity to rewarding stimuli, which renders adolescents more oriented toward reward-seeking behaviors. Most prior work has focused on how reward sensitivity may create vulnerabilities, leading to increases in risk taking. Here, we test whether heightened reward sensitivity may potentially be an asset for adolescents when engaged in prosocial activities. Thirty-two adolescents were followed over a one-year period to examine whether ventral striatum activation to prosocial rewards predicts decreases in risk taking over a year. Results show that heightened ventral striatum activation to prosocial stimuli relates to longitudinal declines in risk taking. Therefore, the very same neural region that has conferred vulnerability for adolescent risk taking may also be protective against risk taking.
Adolescence is a time of heightened reward sensitivity, an orientation toward excitement and arousal, and the development of motivated behaviors and passions (Dahl, 2004, Ernst et al., 2009). These emotions can be both positive and negative for adolescents’ health, creating vulnerabilities as well as opportunities to transform these emotions into positive goals (Dahl, 2004). For instance, adolescents may direct these emotions toward problematic activities, such as drug experimentation, engagement with deviant peers, risky sexual behaviors, school truancy, and reckless driving. On the other hand, adolescents may direct these emotions toward positive, goal-directed behaviors, such as after-school sports, religious participation, prosocial behaviors, hobbies, and healthy peer and romantic relationships.
Several recent models of brain development concur that neural systems important in detecting motivationally and emotionally relevant cues in the environment undergo massive remodeling during adolescence (Casey et al., 2011, Nelson et al., 2005, Ernst et al., 2009, Steinberg, 2008). The ventral striatum (VS), a neural region involved in the evaluation of rewards, shows nonlinear developmental patterns, peaking in functional reactivity in mid-adolescence (Galvan et al., 2006). This heightened VS reactivity is thought to lead to increased reward seeking during adolescence (Somerville et al., 2010, Steinberg, 2008). Neurobiological evidence from both rodent and human studies indicates that the remodeling of the VS around the time of adolescence is associated with increased sensitivity to rewarding stimuli, such that adolescents exhibit exaggerated activation in the VS to rewards (Andersen et al., 2000, Brenhouse et al., 2008, Douglas et al., 2004, Doremus-Fitzwater et al., 2010, Teicher et al., 1995, Ernst et al., 2005, Galvan et al., 2006, Van Leijenhorst et al., 2010).
This peak in reward sensitivity has largely been suggested to create vulnerabilities, contributing to the high rate of problem behaviors during adolescence. Significant work has examined how heightened reward sensitivity may underlie adolescent risk taking. For example, Galvan et al. (2006) found that adolescents show heightened VS activation to rewards relative to both children and adults. Moreover, VS activation to reward anticipation was associated with increased likelihood of engaging in risky behavior such as illicit drug use, heavy drinking, and illegal behaviors (Galvan et al., 2007). Together, these results suggest that adolescents are more behaviorally and neurobiologically sensitive to rewarding stimuli, and this sensitivity is associated with real-life risk taking behaviors. These studies, among others (e.g., Steinberg, 2010, Chein et al., 2010, Van Leijenhorst et al., 2010), support the notion that heightened reward sensitivity during adolescence may contribute to risk taking during this developmental period.
In contrast, relatively little work has examined how heightened reward sensitivity can create opportunities for adolescents. If adolescents direct their emotions and motivations toward positive, goal-directed behaviors, such as prosocial activities, heightened reward sensitivity may potentially be an asset. Efforts to achieve a goal can activate high intensity, rewarding feelings that also engage the reward system but may not lead to bad outcomes (Dahl, 2004). Therefore, the very same neural regions that create vulnerabilities for adolescents may also be protective against risk taking if engaged in a positive way. For example, neuroimaging research in adults has found that prosocial behaviors engage the VS even more so than do personal rewards, suggesting that helping others is a meaningful and rewarding experience (Harbaugh et al., 2007, Izuma et al., 2009, Moll et al., 2006). This heightened reward sensitivity to others’ gains may be one way that VS activation could be positive and lead to healthy outcomes in adolescence.
Helping the family is a salient and frequent type of prosocial behavior among adolescents, often occurring on a daily basis. For instance, 98% of adolescents from diverse cultural and economic backgrounds report helping their family on a weekly basis (Telzer and Fuligni, 2009). Families from Latin American backgrounds place particular emphasis on the importance of high family unity, family social support, and interdependence for daily activities (Cuellar et al., 1995). Because of these cultural values, adolescents from Mexican backgrounds are often motivated to help their family, spending more than twice as much time helping their family each day than their peers from European backgrounds (Telzer and Fuligni, 2009).
Participating in a daily routine, such as family assistance, that is meaningful with respect to group goals and values builds confidence and leads to enhanced well being (Weisner et al., 2005). Indeed, we have found that adolescents who assist their family and feel that they are fulfilling important roles within their family, such as that of a good family member, have better physical and psychological well being (Fuligni et al., 2009, Telzer and Fuligni, 2009). Moreover, at the neural level, decisions to help the family engage brain regions involved in reward processing. For example, when making personal sacrifices for one's family, adolescents who report a greater sense of meaning and fulfillment from helping their family show greater activation in the ventral striatum (Telzer et al., 2010). Thus, family relationships that are personally meaningful provide adolescents with a sense of reward, and this reward may be protective and lead to positive, healthy outcomes.
The increase in intense motivations and passions in adolescence can be channeled into a range of behaviors (Dahl, 2004). On the one hand, if directed toward problematic activities, such as drug experimentation and engagement with deviant peers, this heightened reward sensitivity may be a vulnerability. On the other hand, if directed toward meaningful activities, such as providing assistance to one's family, this heightened reward sensitivity may be a source of protection, reducing susceptibility to risky behavior. In the current study, our first goal was to examine how neural activation to prosocial rewards relates to adolescent risk taking behavior. Adolescents were followed over a one-year period to examine whether VS activation to prosocial rewards predicted longitudinal declines in risk taking behavior over the following year.
Our second goal was to examine whether neural activation to prosocial rewards predicts longitudinal changes in risk taking behavior above and beyond adolescents’ self-reports of their likelihood of engaging in risky behavior over the next year. Although self-reported intentions predict some variability in future risk-taking behavior (Wolford and Swisher, 1986), evidence also suggests that self-reports are not sufficient to capture the multidimensional nature of risk taking (Aklin et al., 2005). Perhaps this is because adolescents may lack the insight or cognitive ability to provide an accurate report of their own intentions (Aklin et al., 2005). Thus, implicit processes may explain variability in behavior change that is not explained by self-reported measures such as attitudes and intentions (Falk et al., 2010).
Finally, we examined whether neural activation to prosocial rewards predicts changes in risk taking behavior above and beyond adolescents’ self-reported values to assist their family. Prior research suggests that having a strong sense of family obligation is associated with lower rates of risk taking (German et al., 2009, Gil et al., 2000, Kaplan et al., 2001, Romero and Ruiz, 2007, Unger et al., 2002, Vega et al., 1993). Therefore, in the current study, we measured neural activation to prosocial rewards as well as adolescents’ intentions to engage in risky behavior in the following year, and their family obligation values to examine whether VS activation predicts longitudinal changes in risk taking above and beyond adolescents’ self-reports. Examining neural activity in conjunction with self-reported intentions and values will help us to gain a deeper understanding of brain–behavior relationships over time.
1. Methods
1.1. Participants
At the first time point, forty-eight adolescents from Mexican-American backgrounds participated in an fMRI scan during which they completed a family contribution task. Participants completed self-report measures of their risk taking behaviors, the likelihood of engaging in risky behaviors in the next year, and their family obligation values (see below). Of the 48 participants who were scanned, 8 were excluded due to incomplete data (i.e., did not accept enough trials during the fMRI task for statistical analysis (N=7); did not complete the self report measures (N=1)). Approximately one year following the scan (M=10.08 months, SD=1.07), participants completed the self-report measure of their risky behaviors again. Our final sample consisted of 32 participants who provided self-report ratings again at Time 2.
At Time 1, participants were in the 10th or 11th grades and ranged in age from 15 to 17 years (Mage=16.3; 14 males, 18 females). At Time 2, participants were in the 11th or 12th grades and ranged in age from 16 to 18 (Mage=17.1). All but one participant spoke and read English fluently. For the Spanish-speaking participant, all tasks and questionnaire measures were described and administered in Spanish. Participants completed written consent and assent in accordance with UCLA's Institutional Review Board.
1.2. Questionnaire measures
1.2.1. Risky behavior
Risky behavior was assessed with the Rule-Breaking subscale of the Youth Self-Report form of the Child Behavior Checklist (Achenbach, 1991). At both time points, adolescents rated 111 items on a 3-point scale (0=not true of me, 1=somewhat or sometimes true of me, 2=true or often true of me). The Rule-Breaking subscale includes 16 items that capture a range of risky behaviors, such as associating with deviant peers, lying, stealing, drinking alcohol without parental approval, using drugs, and skipping school.
Participants’ scores at Time 1 reflect their concurrent risky behavior at the time of the fMRI scan. Scores at Time 2, after controlling for Time 1, reflect changes (increases or decreases) in participants’ risky behavior during the one year following the scan. To control for scores at Time 1, residualized scores for Time 2 were calculated, whereby the group-level variance in Time 2 scores that was explained by Time 1 scores was removed.
1.2.2. Risky behavior likelihood
At Time 1, adolescents completed the Cognitive Appraisal of Risky Events (CARE) Questionnaire (Fromme et al., 1997). Participants answered 30 questions on a 7-point scale (1=not at all likely to 7=extremely likely) indicating the likelihood that they will engage in risky behaviors in the next year. The CARE asks about risky behavior in the following domains: illicit drug use (e.g., smoking marijuana), aggressive and illegal behaviors (e.g., driving after drinking alcohol, making a scene in public), risky sexual behaviors (e.g., sex without protection against pregnancy or sexually transmitted diseases), heavy drinking (e.g., drinking alcohol too quickly), academic/work behaviors (e.g., missing class or work), and high risk sports (e.g., rock or mountain climbing). An index of risky behavior likelihood was calculated for each participant by taking the mean of all items except those regarding high risk sports, as these behaviors are not represented in the CBCL.
1.2.3. Family obligation values
At Time 1, adolescents completed 12 items that assessed their values regarding providing assistance to their family (Fuligni et al., 1999). Using a 5-point scale (1=almost never to 5=almost always) adolescents indicated how often they felt they should assist with household tasks and spend time with their family, such as “help take care of your brothers and sisters,” “eat meals with your family,” and “spend time with your family on weekends.”
1.3. fMRI paradigm
We created a family assistance task modeled after the work of Moll et al. (2006) on charitable giving. Prior to the scan, participants were trained on the task. Participants could earn money for themselves and their families by responding to a series of financial offers. Using a handheld buttonbox, participants accepted or rejected offers that varied in terms of whether they represented gains or losses for the participants and their families (see Fig. 1). Specifically, there were 4 types of offers that were presented to participants: (1) Noncostly-Rewards, in which participants earned money without a cost to the family (e.g., YOU +$3.00 FAM −$0.00); (2) Noncostly-Donations, in which the family earned money without a cost to the participant (e.g., YOU −$0.00 FAM +$3.00); (3) Costly-Rewards, in which the participant earned money at a cost to the family (e.g., YOU +$3.00 FAM −$1.00); and (4) Costly-Donations, in which the family earned money at a cost to the participant (e.g., YOU −$1.00 FAM +$3.00). The financial values of the offers ranged from −$3.00 to +$7.00 to reduce heuristic responding and fatigue (Andreoni and Miller, 2002, Harbaugh et al., 2007). The costly trials varied in terms of the ratio of the amount of gain to the amount of loss in order to vary the difficulty of the decisions and obtain a wider range of individual differences in responses. The gain, however, was always greater than the loss.
Fig. 1.
Family assistance task.
Participants completed 56 unique payment trials, each presented once per run, totaling 112 payment trials. The Costly-Donation trials were presented 40 times total, and the other conditions were presented 24 times total. In addition there were 24 trials to control for the visual and motor aspects of the task, in which YOU and FAM were presented without a financial gain or loss. For these control trials, participants were instructed to press either button, and it would not affect their payments. Trial order was randomized for each participant. Each payment offer was presented for 3s, followed by a fixation for an inter-trial period that was jittered lasting 3s on average (range=.5–8s). Participants were not shown the running total of their own or their family's earnings. At the end of the task, participants and their family were paid their earnings in cash. Participants who accepted fewer than 7 trials for any condition were excluded from the analyses (N=7).
In the current study, we focused on the contrast between the Costly-Donation and Noncostly-Reward trials. Doing so allowed us to focus on neural activation when making a donation to the family that involves self-sacrifice, a behavior that most closely approximates prosocial behavior and generosity. Costly-Donation trials were contrasted to pure cash gains for oneself, which have been shown to be a hedonistically rewarding experience that is associated with activation in the mesolimbic reward system (Moll et al., 2006).
1.4. fMRI data acquisition and analysis
1.4.1. fMRI data acquisition
Imaging data were collected using a 3T Siemens Trio MRI scanner. The task was presented on a computer screen, which was projected through scanner-compatible goggles. The Family Contribution task consisted of 342 functional T2*-weighted echoplanar images (EPI) [slice thickness, 4mm; 34 slices; TR=2s; TE=30ms; flip angle=90°; matrix=64×64; FOV=200mm; voxel size 3mm×3mm×4mm]. A T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 2.3; TE: 2.1; FOV: 256; matrix: 192×192; sagittal plane; slice thickness: 1mm; 160 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
1.4.2. fMRI data preprocessing and analysis
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant's images included slice-timing to adjust for temporal differences in slice acquisition within each volume and spatial realignment to correct for head motion (no participant exceeded 2mm of maximum image-to-image motion in any direction). The realigned and slice-timing-corrected functional data were coregistered to the high resolution MPRAGE, which was then segmented into cerebrospinal fluid, gray matter, and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8mm Gaussian kernel, full width at half maximum, to increase the signal-to-noise ratio.
Whole brain statistical analyses were performed using the general linear model in SPM8. Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128s was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1. The task was modeled as an event-related design. Linear contrasts comparing Costly-Donations (CD) to Noncostly-Rewards (NCR) were calculated for each participant. Events were modeled with a 3s duration beginning with the appearance of the payment screen.
The individual subject contrasts were submitted to random-effects, group-level analyses. The following analyses were run at each voxel across the entire brain volume: (1) regression analyses examining how neural activation during costly contributions (CD>NCR) relates to concurrent risky behaviors at Time 1; (2) regression analyses examining how neural activation during costly contributions (CD>NCR) is associated with longitudinal changes in risky behaviors at Time 2, controlling for Time 1 scores, and (3) regression analyses examining how neural activation during costly contributions (CD>NCR) is associated with longitudinal changes in risky behaviors at Time 2, controlling for Time 1 scores, Time 1 risky behavior likelihood, and family obligation values. This final analysis examines whether neural activation to family contributions predicts changes in risky behavior over the next year, above and beyond the effects of adolescents’ self-reported intentions of engaging in risky behavior over the next year and their family obligation values.
To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (Ward, 2000). Results of 3dClustSim indicated a voxel-wise threshold of p<.005 combined with a minimum cluster size of 35 voxels for the whole brain, corresponding to p<.05, false discovery rate (FDR) corrected.
2. Results
2.1. Behavioral results
We did not find evidence of normative changes in risk taking behavior from Time 1 (M=5.53, SD=3.42) to Time 2 (M=5.69, SD=3.89), t(31)=.28, ns. Risky behaviors at Time 1 were moderately correlated with risky behaviors at Time 2, r(31)=.64, p<.001. However, there was variability in this association, such that some adolescents’ risk taking declined whereas others’ increased. The residualized scores for Time 2 risk taking show values that range from −5.12 (decline in risk taking) to 6.94 (increase in risk taking). Risky behavior likelihood (measured using the CARE) was correlated with risky behaviors at Time 1 (r(31)=.61, p<.001) and risky behaviors at Time 2 (r(31)=.54, p<.005). Thus, adolescents who report a greater likelihood of engaging in risk taking are more likely to report higher levels of risk taking concurrently and one year later. Adolescents’ family obligation values were marginally associated with lower risk taking at Time 2 (r(31)=−.34, p=.058), but were not associated with risk taking at Time 1 or with risky behavior likelihood.
On the family assistance task, participants accepted significantly more Noncostly-Rewards (M=97.13% of offers, SD=4.90) than Costly-Donations (M=61.88% of offers, SD=23.14), t(31)=8.40, p<.001, suggesting that participants were sensitive to the different conditions. These acceptance rates are similar to those found among older adolescents with a modified version of the same task (Telzer et al., 2010). Participants took longer to make decisions to accept Costly-Donations (Mrt=1.49s, SD=.39) than Noncostly-Rewards (Mrt=1.18s, SD=.25), t(31)=5.81, p<.001. On average across all trials in the task (i.e., 112 payment trials for all 4 conditions), participants earned $131.59 for themselves and $162.47 for their family, which represents 66.1% and 58.4% of the total earning possible, respectively. Self reported values and behaviors, including risky behavior at Time 1 and Time 2, risky behavior likelihood, and adolescents’ family obligation values, were not related to adolescents’ behavior on the task.
2.2. fMRI results
Our first analyses examined the main effects of CD and NCR trials. Whole-brain analyses comparing each condition to control trials show that both CD and NCR trials activate the VS (see Table 1). Significant differences emerged in the dACC, ventral midbrain, anterior insula, and cuneus for CD>NCR, and in the inferior insula and fusiform gyrus for NCR>CD.
Table 1.
Neural regions activated during Costly Donation and Noncostly Reward trials.
| Contrast | Anatomical region | x | y | z | t | k |
|---|---|---|---|---|---|---|
| CD>control | R VS | 9 | 14 | 1 | 4.15 | 181a |
| R DS | 9 | 14 | 7 | 5.49 | 181a | |
| L VS | −12 | 20 | −2 | 4.16 | 75 | |
| dACC | 6 | 38 | 25 | 6.38 | 223 | |
| R anterior insula | 36 | 20 | −2 | 6.54 | 35 | |
| L anterior insula | −30 | 20 | −2 | 6.37 | 68 | |
| Ventral midbrain | 3 | −19 | −14 | 4.27 | 214 | |
| Cuneus | −3 | −85 | −5 | 12.72 | 2814 | |
| L precentral gyrus | −42 | 5 | 31 | 4.29 | 82 | |
| NCR>control | R VS | 15 | 20 | 1 | 4.75 | 170b |
| R DS | 3 | 11 | 10 | 5.90 | 170b | |
| L VS | −12 | 17 | 1 | 3.32 | 99 | |
| dACC | 6 | 41 | 19 | 6.61 | 179 | |
| Cuneus | −6 | −85 | −5 | 15.71 | 3220 | |
| CD>NCR | dACC | 12 | 26 | 28 | 5.02 | 204 |
| Ventral midbrain | 6 | −16 | −8 | 4.16 | 101 | |
| L anterior insula | −30 | 17 | 7 | 4.14 | 173 | |
| Cuneus | 24 | −73 | −5 | 6.45 | 610 | |
| NCR>CD | R inferior insula | 39 | −4 | −2 | 4.38 | 173 |
| L inferior insula | −39 | −7 | −2 | 3.77 | 90 | |
| L Fusiform gyrus | −30 | −40 | −11 | 3.54 | 52 | |
Note: CD>control refers to the contrast comparing Costly Donations trials to the control trials. NCR>control refers to the contrast comparing Noncostly Reward trials to the control trials. L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. Anatomical regions that share functional clusters are denoted with the same superscript letter. All regions are listed at cluster-forming threshold of p<.05 corrected for multiple comparison. The following abbreviations were used for the specific brain regions: VS, ventral striatum; DS, dorsal striatum; dACC, dorsal anterior cingulate cortex.
Next, we examined whether variability in neural activation during costly contributions to the family (CD>NCR) relates to concurrent risky behaviors at Time 1. Time 1 risky behaviors were entered as a regressor in whole brain regression analyses. No brain regions were significantly associated with Time 1 risky behaviors.
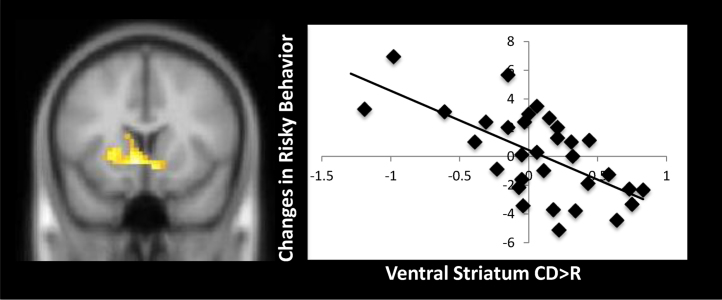
Next we examined whether variability in neural activation during costly contributions to the family relates to longitudinal changes in risky behaviors. The residualized scores for Time 2 risky behavior, controlling for Time 1 risky behavior, were entered in whole brain regression analyses. Results indicate that increased BOLD response in the ventral striatum during CD>NCR was significantly associated with longitudinal decreases in risky behaviors (see Fig. 2). No other brain regions were associated with changes in risk taking to CD>NCR. Next, we examined each of the conditions separately (CD and NCR) versus the implicit baseline to determine whether either contrast was driving the effect. Changes in risk taking behavior were not associated with neural activation during CD or NCR trials, suggesting that it is the relative difference between the two contrasts that is associated with risk taking behavior. In other words, the extent to which adolescents show heightened activation in the VS to family donations compared to personal gains predicts changes in risk taking.
Fig. 2.
Ventral striatum activation when making financial sacrifices to the family is associated with longitudinal declines in risk taking behavior. Note: x, y, z=−6, 14, −5, t(31)=3.71, p<.005, corrected, 109 contiguous voxels. For the scatterplot, parameter estimates of signal intensity were extracted for each individual from the entire, group-level cluster of activation.
Finally, we entered Time 1 risk behavior likelihood and family obligation values as covariates in whole brain regression analyses to examine whether ventral striatum activation to family contributions predicts declines in adolescents’ risk taking above and beyond their own intentions and values. Results show that the ventral striatum continues to predict decreases in risk taking over time (x, y, z=−9, 14, −5, t(29)=4.33, p<.005, corrected, 65 contiguous voxels). When examining each of the covariates, results show that adolescents with higher family obligation values show heightened activation in the bilateral VS when contributing to their family (right VS: x, y, z=21, 17, −2, t(28)=4.42, p<.005, 58 contiguous voxels; left VS: x, y, z=−15, −1, −2, t(28)=4.52, p<.005, 125 contiguous voxels). In other words, adolescents who reported valuing helping their family more showed greater reward-related activation when providing contributions to their family during the fMRI task. Risk taking likelihood was not associated with neural activation to family contributions.
3. Discussion
Adolescence is a period of intensified emotions and an increase in motivated behaviors and passions (Dahl, 2004). Evidence from developmental neuroscience suggests that this heightened emotionality occurs because of changes in the brain's neural circuitry. A peak in functional reactivity in the ventral striatum to emotionally relevant and rewarding stimuli around the time of puberty renders adolescents more oriented toward reward-seeking behaviors (Casey et al., 2011, Steinberg, 2008). This orientation to reward can be directed toward adaptive, positive behaviors such as prosocial behaviors, or toward maladaptive, health compromising behaviors, such as sensation seeking and risk taking. Most prior work has focused on how these neural changes may create vulnerabilities for adolescents, leading to increases in risk taking during this developmental period. In the current study, we show that heightened reward sensitivity in the context of meaningful, prosocial behaviors relates to longitudinal declines in adolescent risk taking. Therefore, the very same neural regions that have conferred vulnerability for adolescent risk taking may also be protective for this same behavior.
Our findings suggest that VS activation may represent an individual difference in the importance and rewarding nature of family assistance. The more reward individuals gain from providing assistance to their family the more their risk taking behaviors decrease over the high school years. Although our data do not speak to the direct mechanisms by which this reward sensitivity is protective, it is possible that adolescents who attain more reward from prosocial behaviors find risk taking contexts to be comparatively less rewarding. Indeed, it was only for the contrast comparing CD to NCR trials that we found evidence for declines in risky behavior; this association was not observed for CD or NCR trials alone. Therefore, adolescents who show the greatest difference in the VS to family contributions relative to personal rewards exhibit declines in risk taking over time. Behavioral work has shown that adolescents who are more prosocial and altruistic and who have more prosocial peers are less likely to engage in risky behaviors (Oman et al., 2004, Machin and Sankey, 2008). Perhaps youth who attain greater reward from helping others find risk taking to be inconsistent with their values and therefore not a rewarding experience. Future studies should examine whether heightened reward activation in the context of positive behaviors (e.g., prosocial behaviors) relates to decreased reward activation in the context of negative behaviors (e.g., risk taking). If this were the case, it would suggest that redirecting adolescents’ emotions toward meaningful activities, such as providing assistance to one's family, could greatly reduce susceptibility to risky behavior.
Our findings are consistent with other developmental neuroimaging research that shows that heightened ventral striatum activation can be adaptive. For example, in a longitudinal study examining changes in neural responses to emotional facial expressions, Pfeifer et al. (2011) found that increases in VS activation were associated with decreases in risky behavior, suggesting that the VS may also be involved in emotion regulation during adolescence. Thus, depending on the context, heightened VS activation may be both a vulnerability as well as a protective factor. Perhaps only when involved in risky behaviors (e.g., Chein et al., 2010) or personal rewards (e.g., Galvan et al., 2007) is VS activation maladaptive. In contrast, when directed toward positive, prosocial rewards (e.g., current study) or opportunities to engage in emotion regulation (e.g., Pfeifer et al., 2011), heightened VS activation is adaptive.
Interestingly, VS activation to prosocial behaviors did not predict risk taking behavior at Time 1 even though Time 1 and Time 2 risky behavior were correlated. We did not find a normative increase or decrease in risk taking from T1 to T2; some adolescents’ risk taking increased whereas others’ decreased. Together, our findings suggest that adolescents’ risky behavior is changing in meaningful, predictable ways: only for adolescents who showed greater activation in the VS when contributing to their family compared to gaining personal rewards showed declines in risk taking behavior over time. The ability to prospectively predict future engagement in risk-taking behaviors based on adolescents’ current neural sensitivity to rewarding behaviors can have profound effects on our ability to develop and implement individualized prevention programs, which have been shown to produce greater behavior change than general, one-size-fits-all programs (McLeod and Shantz, 2002).
This study is significant in light of a growing trend in neuroimaging research to move beyond brain mapping and statistical association to actual prediction of behavior (Falk et al., 2010). Traditional neuroimaging research has typically used behavioral measures as regressors to predict responses in different brain regions. In other words, are neural responses modulated by individual traits? Advances in neuroimaging have begun to use neural activation to predict behavior either concurrently (Haxby et al., 2001) or in the future (Soon et al., 2008, Falk et al., 2010, Falk et al., 2011, Masten et al., 2011), allowing researchers to examine whether there are neural markers or precursors of future behaviors or feelings. For instance, Masten et al. (2011) found that increased activation in the subgenual anterior cingulate cortex (subACC) during experiences of peer exclusion predicted longitudinal increases in depressive symptoms among adolescents. Thus, responsivity of the subACC may be a neural risk factor for depression. Similarly, Falk et al. (2010) found that activation in the medial PFC in response to ads designed to help smokers quick smoking predicts reductions in smoking behavior above and beyond adults’ self-reported intentions to quit smoking. We build upon this research and show that neural activation can predict changes in risk taking behavior over the course of a year, and this neural activation is even more predictive than self-reported values and intentions to engage in that behavior. By measuring VS activity in the moment as participants engaged in prosocial behaviors to their family, we were able to predict changes in participants’ risk taking behaviors above and beyond their own self-reported values and intentions.
Our results suggest that finding ways for adolescents to direct motivations and passions toward positive behaviors can have lasting implications for their health. Thus, parents, teachers, and practitioners should help adolescents channel their emotions into positive behaviors, such as prosocialality. In addition, future research should examine how other meaningful activities in adolescents’ lives, such as participating in community service, engaging in positive peer relationships such as academic clubs, and religious engagement can similarly reduce risk taking among diverse adolescents. Identifying the behaviors that are the most meaningful and rewarding for each individual adolescent will have the greatest impact on their health.
In conclusion, adolescents are inclined toward novelty and excitement, and passions are ignited (Dahl, 2004). Indeed, much research has documented how these new and intense emotions can create vulnerabilities for adolescents, leading to maladaptive behaviors. In contrast, little research has examined how these passions can create opportunities for adolescents to channel their emotions into positive goals and behaviors. Our findings are among the first to suggest that heightened reward sensitivity can be positive for adolescents, reducing risk taking behaviors over time. If adolescents direct their emotions and motivations toward positive, goal-directed behaviors, such as prosocial activities, reward sensitivity can be an asset.
Conflict of interest
We have no biomedical, financial, or potential conflicts of interest.
Acknowledgements
Support for this study was provided by the NICHD (R01HD057164-S and R01HD057164; Fuligni), the Center for Culture, Brain and Development Research Grant (Fuligni and Galvan), an NSF Doctoral Dissertation Improvement Grant (Telzer), an SRCD Dissertation Fund Award (Telzer), an APF and COGDOP Graduate Research Grant (Telzer), and a University of California Institute for Mexico and the United States Dissertation Research Grant (Telzer). Preparation of this manuscript was supported in part by a National Research Service Award Graduate Fellowship (Telzer). We would like to thank Lauren Burakowski for help with the fMRI data collection, and Michelle Pasco, Blanche Wright, Reina Amiling, and Nilufer Rustomji for help with the questionnaire data collection.
References
- Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- Aklin W.M., Lejuez C.W., Zvolensky M.J., Kahler C.W., Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behavior Research and Therapy. 2005;43:215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Thompson A.T., Rutstein M., Hostetter J.C., Teicher M.H. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andreoni J., Miller J.H. Giving according to GARP: an experimental test of the consistency of preferences for altruism. Econometrica. 2002;70:737–753. [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. Journal of Neuroscience. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Somerville L.H. Breaking and accelerating of the adolescent brain. Journal of Research on Adolescence. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albertm D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science. 2010;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar I., Arnold B., Gonzalez G. Cognitive referents of acculturation: assessment of cultural constructs in Mexican Americans. Journal of Community Psychology. 1995;23:339–356. [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Varlinskaya E.I., Spear L.P. Motivation systems in adolescence: possible implications for age differences in substance above and other risk taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L.A., Varlinskaya E.I., Spear L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacology Biochemistry and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Berkman Whalen D., Lieberman M.D. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Mann T., Harrison B., Lieberman M.D. Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience. 2010;30:8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme K., Katz E., Rivet K. Outcome expectancies and risk-taking behavior. Cognitive Therapy Research. 1997;21:421–442. [Google Scholar]
- Fuligni A.J., Telzer E.H., Bower J., Irwin M.R., Kiang L., Cole S.W. Daily family assistance and inflammation among adolescents from Latin American and European backgrounds. Brain, Behavior, and Immunity. 2009;23:803–809. doi: 10.1016/j.bbi.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A.J., Tseng V., Lam M. Attitudes toward family obligations among American adolescents from Asian, Latin American, and European backgrounds. Child Development. 1999;70(4):1030–1040. [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- German M., Gonzales N.A., Dumka L. Familism values as a protective factor for Mexican-origin Adolescents exposed to deviant peers. The Journal of Early Adolescence. 2009;29:16–42. doi: 10.1177/0272431608324475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A.G., Wagner E.F., Vega W.A. Acculturation, familism, and alcohol use among Latino adolescent males: longitudinal relations. Journal of Community Psychology. 2000;28:443–458. [Google Scholar]
- Harbaugh W.T., Mayr U., Burghart D.R. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Gobbini M.I., Furey M.L., Ishai A., Schouten J.L., Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2009;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Kaplan C.P., Napoles-Springer A., Stewart S.L., Perez-Stable E.J. Smoking acquisition among adolescents and young Latinas: the role of socioenvironmental and personal factors. Addictive Behaviors. 2001;26:531–550. doi: 10.1016/s0306-4603(00)00143-x. [DOI] [PubMed] [Google Scholar]
- Machin M.A., Sankey K.S. Relationships between young drivers’ personality characteristics, risk perceptions, and driving behaviour. Accident Analysis and Prevention. 2008;40:541–547. doi: 10.1016/j.aap.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., McNealy K., Pfeifer J.H., Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J.H., Shantz C. A short-term outcome evaluation of the I'm Special drug abuse prevention program: a revisit using SCAT Inventory. Journal of Drug Education. 2002;20:127–138. doi: 10.2190/EP82-L54M-U2J8-6PBJ. [DOI] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. Human fronto-mesolimbic networks guide decisions about chartiable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Liebenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Oman R.F., Vesely S., Aspy C.B., McLeroy K.R., Rodine S., Marshall L. The potential protective effect of youth assets on adolescent alcohol and drug use. American Journal of Public Health. 2004;94:1425–1430. doi: 10.2105/ajph.94.8.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A.J., Ruiz M. Does familism lead to increased parental monitoring?: Protective factors for coping with risky behaviors. Journal of Child and Family Studies. 2007;16:143–154. [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon C.S., Brass M., Heinze H.J., Haynes J.D. Unconscious determinants of free decisions in the human brain. Nature Neuroscience. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Teicher M., Andersen S., Hostetter J., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J. Daily family assistance and the psychological well being of adolescents from Latin American, Asian, and European backgrounds. Developmental Psychology. 2009;45:1177–1189. doi: 10.1037/a0014728. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E., Lieberman M.D., Fuligni A.J. Gaining while giving: an fMRI investigation of the rewards of family assistance among White and Latino adolescents. Social Neuroscience. 2010;5:508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J.B., Ritt-Olson A., Teran L., Huang T., Hoffman B.R., Palmer P. Cultural values and substance use in a multiethnic sample of California adolescents. Addiction Research and Theory. 2002;10:257–279. [Google Scholar]
- Van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Vega W.A., Zimmerman R.S., Warheit G.J., Apospori E., Gil A.G. Risk factors for early adolescent drug use in four ethnic and racial groups. American Journal of Public Health. 1993;83:185–189. doi: 10.2105/ajph.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.D. 2000. Simultaneous Inference for fMRI Data.http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf [Google Scholar]
- Weisner T.S., Matheson C., Coots J., Bernheimer L.P. Sustainability of daily routines as a family outcome. In: Maynard A., Martini M., editors. Learning in Cultural Context: Family, Peers and School. Kluwer/Plenum; New York: 2005. pp. 47–74. [Google Scholar]
- Wolford C., Swisher J. Behavioral intention as an indicator of drug and alcohol us. Journal of Drug Education. 1986;16:305–326. doi: 10.2190/J2AB-GAH8-PHEG-0FQU. [DOI] [PubMed] [Google Scholar]