Abstract
Despite being considered a model organism in toxicity studies, particularly in assessing the environmental impact of endocrine disrupting compounds (EDCs) and other chemicals, the molecular basis of development is largely unknown in Chironomus riparius. We have characterized the expression patterns of important genes involved in the ecdysone pathway from embryos to pupa, but specially during the different phases of C. riparius fourth larval instar, according to the development of genital and thoracic imaginal discs. Real-Time PCR was used to analyze: EcR and usp, two genes encoding the two dimerizing partners of the functional ecdysone receptor; E74, an early response gene induced by ecdysteroids; vg (vitellogenin), an effector gene; hsp70 and hsc70, two heat-shock genes involved in the correct folding of the ecdysone receptor; and rpL13, as a part of the ribosomal machinery. Our results show for the first time stage and sex-dependent variations in ecdysone-responsive genes, specially during the late larval stage of C. riparius. The induction in the expression of EcR and usp during the VII-VIII phase of the fourth instar is concomitant with a coordinated response in the activity of the other genes analyzed, suggesting the moment where larvae prepare for pupation. This work is particularly relevant given that most of the analyzed genes have been proposed previously in this species as sensitive biomarkers for the toxicological evaluation of aquatic ecosystems. Identifying the natural regulation of these molecular endpoints throughout the Chironomus development will contribute to a more in-depth and accurate evaluation of the disrupting effects of EDCs in ecotoxicological studies.
Introduction
Metamorphosis, the transition from the larval stage to the adult, involves important molecular and cellular alterations and results in dramatic morphological and physiological changes in holometabolous insects. These variations demand the loss of embryo tissues and the differentiation or the novo formation of other ones. The harmonized action of two hormones, 20-hydroxyecdysone (20E) and juvenile hormone (JH), is responsible for coordinating insect growth and development, and the balance between them defines the outcome of each developmental transition. In a larva-to-larva molt a JH titer is needed, while metamorphosis takes place when the JH level drops and a 20E titer occurs during the final larval instar [1].
The wide variety of insect groups within Holometabola limits the ability to generalize about development control. The effects of hormones on a group may not be the same in others because of different growth patterns and cell specificities. These differences in responses to hormones add complexity to the interpretation of many findings, and generalizing about common mechanisms in evolutionarily distant and well-described groups should be done cautiously.
The steroid signaling mechanisms have been extensively studied in Drosophila melanogaster and Manduca sexta, model insects in developmental studies and neurobiology, and more recently in Tribolium castaneum and Aedes aegypti, an important pest and vector for several diseases, respectively [2]. In contrast, there is little information on the stage-specific expression of hormonal-related genes during the developmental process in the midge Chironomus riparius, which is considered by US EPA and OECD a model organism for ecotoxicity testing and has several internationally validated guidelines [3–5] used for regulatory purposes in environmental toxicology. This species has also been selected as a reference organism in the study of the potential adverse effects of endocrine disrupting chemicals [6] and as a model to evaluate endocrine disrupting effects in aquatic invertebrates for the European IDEA project [7]. Although some changes could somehow be explained in Chironomus from known patterns of closely related species, such as Drosophila or Aedes, physiological differences regarding their life cycle or feeding behavior make interesting a more in-depth study of Chironomus development.
Transcriptional response to ecdysteroids in insects requires the action of two nuclear receptor superfamily members, the ecdysone receptor (EcR) and the ultraspiracle (USP) [8]. The activation of the EcR/USP heterodimer initiates the cascade expression of ecdysone-responsive genes that leads to drastic changes in cell proliferation, apoptosis and the disappearance of larval organs, as well as the differentiation of adult tissues.
The expression of EcR and usp has been well described during D. melanogaster development, from embryos to adults [9,10]. Different isoforms of these genes have been also characterized in many insects other than Drosophila and dissimilar time-space expression profiles have been reported ([11] and references therein). For example, multiple forms of EcR and usp and their complex regulation have been observed in M. sexta [12], and cDNAs from different isoforms of both genes have been characterized in Chironomus tentans [13,14]. Moreover, the ecdysone receptor gene has been described recently in C. riparius [15], although little is known about the developmental expression profile of EcR and usp in this species, specially during the fourth larval instar, which is the most critical stage for individuals undergoing metamorphosis.
Deep into the ecdysone-responsive genes cascade, E74 is one of the early genes induced by ecdysteroids. It has been described as a transcription factor that plays a critical role at the time of metamorphosis in D. melanogaster [16,17] and M. sexta [18], where expression patterns of this gene correlate with pupal commitments.
As a part of their development, insects supply their eggs with protein, lipids, carbohydrates and other resources for feeding the growing embryos. Several types of YPP (Yolk Protein Precursor) are accumulated by insect oocytes in response to endogenous estrogens, but vitellogenin (Vg) is the most abundant. Multiple vg genes/cDNAs have been sequenced in many insects [19]. The synthesis of Vg is regulated at transcriptional level and vg gene is normally silenced in males and immature females probably due to low levels of estrogens in plasma, although may be activated by (xeno-)estrogens. Therefore, Vg has been proposed as a useful biomarker in the evaluation of the estrogenic effects of pollutants in vertebrates and invertebrates, including C. riparius [20,21]. Nevertheless, there is a lack of information about expression patterns throughout the development of this midge.
It is worth highlighting the importance of heat-shock proteins (HSPs) in the folding and maturation of steroid hormone receptors. Among all HSPs, the HSP70 family represents one of the most highly conserved proteins identified to date in all organisms in which they have been described. The family includes constitutive members such as cognate proteins (HSC70), highly abundant in normal cellular conditions, and inducible proteins (HSP70) under a broad spectrum of physical and chemical stress conditions [22]. A functional relationship between steroid hormones and HSPs has been reported and it is known that HSP70, HSC70 and HP90 play an important role in the folding and maturation of steroid hormone receptors and different transcription factors [23]. There have been recent advances in the molecular description of hsp70 genes in a variety of insects, including C. riparius, as well as in their evaluation in response to different environmental stressful conditions [24–27]. It also has been suggested that hsc70/hsp70 ratio may be a potential indicator of polluted environments [28]. On the contrary, the information about the response of these genes across developmental stages in C. riparius in relation to their chaperone activity and their role in the folding of the ecdysone receptor is still scarce.
Finally, ribosomal protein genes are essential for cellular growth and development, since they code for the necessary machinery for protein synthesis, together with the four rRNAs (28S, 18S, 5.8S, 5S). They are considered housekeeping genes and they are constitutively expressed. Furthermore, additional ribosomal functions have been described for some ribosomal proteins, included L13, such as the control of transcriptional regulation, specially in the development and metamorphosis of insects. To date, more than 80 different types of ribosomal proteins have been identified in eukaryotes, but only the genes encoding for six ribosomal proteins have been characterized in C. riparius. Despite their constitutive expression, alterations in the levels of some ribosomal proteins under exposures to different xenobiotics have been reported in this species [29–32]. Due to the recent description of ribosomal genes as potential biomarkers of toxicity and given the lack of information on their potential role during development, evaluating possible ontogenetic-dependent changes is of particular interest.
In the present work we characterize the expression patterns of important genes in the ecdysone pathway in C. riparius: EcR, usp, E74, vg, hsp70, hsc70 as well as the housekeeping gene L13. They have been analyzed from embryo to pupa, but specially throughout the fourth larval instar, which is usually the selected stage for ecotoxicity testing. We report for the first time in this organism a developmental stage and sex-dependent expression of all the analyzed genes and describe the coordinated response that triggers the mechanisms to start the metamorphosis.
Material and Methods
Ethics statement
Sampling and protocols used in this study conform to the administrative and ethical laws of the regional government (Xunta de Galicia) and did not involve endangered or protected species.
Test animals and culture conditions
The experimental animals were the aquatic embryos, larvae and pupae of the midge Chironomus riparius. They were reared in the laboratory in strict accordance with the recommendations given in standardized international guidelines [3–5]. They were grown from egg masses in aqueous culture medium (0.5 mM CaCl2, 1 mM NaCl, 1 mM MgSO4, 0.1 mM NaHCO3, 0.025 mM KH2PO4, 0.01 mM FeCl3) supplemented with nettle leaves, commercial fish food, and cellulose tissue in polyethylene tanks (500 ml). Cultures were maintained under constant aeration at 20°C and standard light-dark periods 16:8. Additionally, a brief approach was conducted using larvae from natural populations of the midge collected in the Sar river (Galicia, NW Spain).
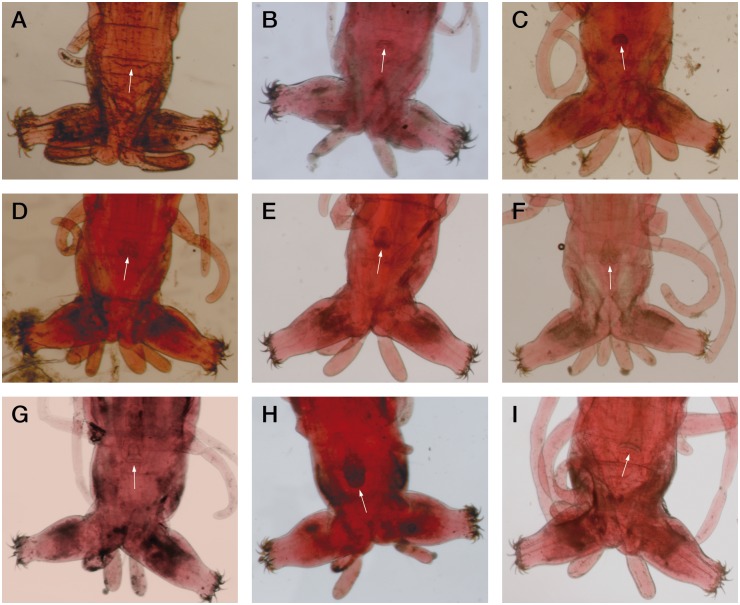
Embryos were selected 48 h after ovoposition. First to fourth instar larvae were identified by measuring head capsule width, while the phases within the fourth instar were established from the development of genital and thoracic imaginal discs according to [33], where the 4th instar was divided into nine phases (phase I to phase IX). For the sake of promptness, and in order to reduce stressful conditions to larvae, fourth instar individuals were grouped into five broader categories that could be easily established under the microscope: 1) I-II phase; 2) III-IV phase; 3) V-VI phase; 4) VII-VIII phase; and 5) IX phase. Additionally, the sex of the larvae was established where possible. Larval individuals were sexed and aged under a binocular microscope (x40 magnification) (Fig 1), and four different samples of each category were prepared for analysis of expression profile during embryo stage, larval development and pupation.
Fig 1. Representative images of the separation of larvae carried out in this work.
Fourth instar larva were aged and sexed based on the development of genital and thoracic imaginal discs, according to [33], who divided the fourth instar into nine phases (phase I to phase IX). In order to reduce stressful conditions to larvae, phases were grouped into five broader categories (I-II; III-IV; V-VI; VII-VIII and IX, respectively). A. (I-II); B. (III-male); C. (IV-female); D. (V-male); E. (VI-female); F. (VII-male); G. (VIII-female); H. (IX-male) and I. (IX-female).
RNA extraction
RNA was extracted from a total of 4 egg masses and 20 frozen individuals (larvae or pupa, depending on the developmental stage or phase), divided into groups of five, using a guanidine isothiocyanate based method, performed with a commercial kit (TRIzol, Invitrogen). To isolate embryo RNA, a prior treatment was performed to remove the gelatinous cover of the egg mass. The egg mass was treated with 1xPBS (137 mM ClNa, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4) and 0.2% sodium hypochlorite until the gelatinous cover disappeared. Subsequently, the eggs were washed several times with 1xPBS until the sodium hypochlorite was completely eliminated. Briefly, eggs or frozen material were homogenated in one volume of TRIzol and left for 5 min at room temperature. Then, 0.2 volumes of chloroform were added to each sample, mixed and left for 5 min at room temperature. Subsequently, the samples were centrifuged for 15 min at 4°C and 15000 g. Following transfer of the aqueous phase, the RNA was finally recovered by isopropyl alcohol precipitation (0.5 v/v), washed with 70% ethanol, and resuspended in DEPC water. The RNA was then treated with RNase-free DNase (Roche) followed by phenolization. The quality and quantity of total RNA were determined by agarose electrophoresis and absorbance spectrophotometry (Nanodrop1000, Thermo), and the purified RNA was finally stored at -80°C. RNA samples were sent to the Biology and Environmental Toxicology Group (UNED), where expression assays were carried out.
Reverse transcription and Real-Time PCR
After checking RNA integrity in 1.5% agarose gels, reverse transcription was performed with 0.5 μg of the isolated RNA, and 0.5 μg oligo dT20 primer (Sigma) was used with M-MLV enzyme (Invitrogen). The cDNA obtained was used as template for the Polymerase Chain Reaction (PCR).
Quantitative Real-Time PCR (qRT-PCR) was used to evaluate the mRNA expression profile of EcR, usp, E74, vg, hsp70, hsc70 and L13 genes during different developmental stages (embryo, 1st, 2nd, 3rd and 4th instar larvae, and pupa). The q-PCR was performed using CFX96 thermocycler (BioRad) and SsoFast EvaGreen Supermix (BioRad), with 25 ng of cDNA and 300nM of forward and reverse primers per reaction. Ribosomal gene 26S, actin and GAPDH were employed as endogenous reference genes [34,35]. EcR, usp, E74, hsc70 and hsp70 primers are described in [36,37]. The vg primers were designed from C. riparius sequences present in the database: # HQ260608 [38]. The L13 primers are described in [39].
To accurately determine the efficiencies of the PCR reactions, reaction mixtures with template dilutions 1:2 in five steps were also run in the same PCR conditions, and the slopes of the regression curves were calculated (R2>0.98). The Real-Time PCR primers sequences used in this study, efficiencies and fragment size of each gene-specific pair of primers are listed in Table 1. The acceptable range for PCR efficiencies calculated using standard curve serial dilution experiments is 90–110% [40].
Table 1. Primers used for Real-Time PCR amplification of the genes studied in C. riparius.
| Gene | Name | DNA sequence (5′-3′) | Fragment size (bp) | Efficiency (%) |
|---|---|---|---|---|
| actin | Forward | GATGAAGATCCTCACCGAACG | 201 | 104 |
| Reverse | CGGAAACGTTCATTACCG | |||
| GAPDH | Forward | GGTATTTCATTGAATGATCACTTTG | 110 | 96.6 |
| Reverse | TAATCCTTGGATTGCATGTACTTG | |||
| 26S | Forward | TTCGCGACCTCAACTCATGT | 220 | 90.4 |
| Reverse | CCGCATTCAAGCTGGACTTA | |||
| EcR | Forward | CCATCGTCATCTTCTCAG | 180 | 106.6 |
| Reverse | TGCCCATTGTTCGTAG | |||
| usp | Forward | GCCCAATCATCCGTTAAGTGG | 114 | 108.1 |
| Reverse | CGTTTGAAGAATCCTTTACATCC | |||
| E74 | Forward | TCTTACTGAAACTTCTTCAAGATCG | 111 | 103.2 |
| Reverse | GCTTTGAGACAGCTTTGGAATCG | |||
| vg | Forward | GATTGTTCCATGTGCAG | 215 | 112.3 |
| Reverse | TTTGAGTATGGTGGAGAATC | |||
| hsp70 | Forward | ACTTGAACCAGTTGAGCGT | 132 | 103.8 |
| Reverse | TTGCCACAGAAGAAATCTTG | |||
| hsc70 | Forward | CGTGCTATGACTAAGGACAA | 239 | 99.3 |
| Reverse | GCTTCATTGACCATACGTTC | |||
| rpL13 | Forward | AAGCTGCTTTCCCAAGAC | 351 | 109.3 |
| Reverse | TTGGCATAATTGGTCCAG |
Real-Time PCR was run in the following cycling conditions: initial denaturation at 95°C for 3 min, 35 cycles of 95°C denaturation for 5 s, 58°C annealing for 15 s and 65°C elongation for 10 s. To verify the accuracy of each amplicon, a melting curve analysis was carried out after amplification BioRad CFX Manager 2.1 software was used to calculate the mRNA levels by the normalized gene expression (2-ΔΔCT) against three endogenous reference genes (26S, actin and GAPDH). Additionally, several statistical analyses were carried out to deeply interpret the results. A total of four egg masses and 20 individuals of each developmental category (1st, 2nd and 3rd instar larvae, five phases of the 4th instar, and pupa, respectively) were used in this study. For each category, samples were divided into four groups containing one egg mass, five larvae or five pupae, as appropriate. To avoid variations caused by experimental procedures each group was analyzed three times (three PCR amplification replicates) and each q-PCR replicate was run in duplicate wells.
Determination of ecdysteroids levels during development
Ecdysteroids levels were measured via competitive Enzyme Immunoassay (EIA) 20-Hydroxyecdysone EIA kit (A05120) SPI-Bio kit (Bertin Pharma), using 20E and 20E acetylcholinesterase as the standard and enzymatic tracer, respectively. For sample preparation, individual larvae were weighed and homogenized in 250 μl of iced-cold 75% aqueous methanol and centrifuged at 13000 g at 4°C for 15 min. Precipitates were resuspended in an additional 100 μl of aqueous methanol and kept on ice for 30 min. After a new centrifugation as above, the supernatant was combined with the previous one. Samples were vacuum dried and resuspended in 100ul of enzyme immunoassay (EIA) buffer. Ellmann reagent was used for the chromogenic reaction and absorbance was read at 415 nm. All assays were performed in triplicate.
Statistical analysis
Normality and homoscedasticity of data were tested using the Shapiro-Wilk and Levene tests respectively. Datasets were, if necessary, normalized using natural log (ln) or square root transformations. The levels of the specific gene transcripts were analyzed with ANOVA, followed by Games Howell’s or Tukey’s post Hoc tests when appropriate. If transformed data were not homogeneous or normally distributed the Kruskal-Wallis test was used, and the differences between pairs were analyzed using the multiple comparisons of mean ranks for all groups test of [41]. Differences were considered significant at p<0.05.
A cluster analysis method for grouping the different developmental stages was applied using the mean expression values of each gene. All the analyses were performed using SPSS 21 (IBM).
Results
Expression patterns of hormonal receptors and ecdysone-inducible genes
At the molecular level, the first step in the action of 20E consists in its binding to a complex of two nuclear receptors: the ecdysone receptor (EcR) and its heterodimerization partner ultraspiracle (USP). The induction of EcR by the 20E controls the transcription of a set of early response genes, as E74 among others.
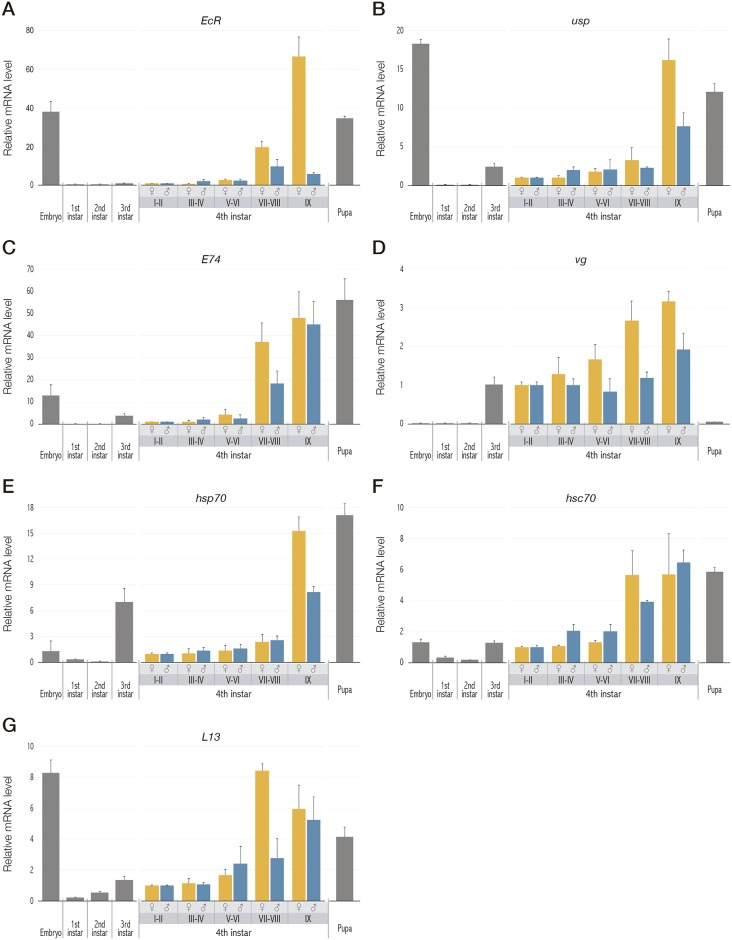
Our results show a coordinated expression pattern with a stage dependent variation of all these genes, especially when comparing early larval stages with late larval stages or even pupae. We found similar responses for EcR, usp and E74 genes, which consisted of a high transcriptional activity in the embryo stage that dropped drastically at the 1st larval instar. However, it is noteworthy that although transcript levels remained low throughout early phases of the 4th instar, a strong induction was clearly measured for late phases and pupa stages. As shown in Figs 2A and 3A, EcR was strongly and significantly induced at VII-VIII phase and reached maximum levels in the IX phase (prepupa) (respectively, 60-fold and up to 260-fold when compared to the lowest level measured in this study). Interestingly, usp showed a similar trend but with lower transcriptional rates than those of EcR, with maximal inductions of 30-fold and 130-fold respectively in VII-VIII and IX phases (Figs 2B and 3B). Apart from these developmental changes, the expression pattern of both EcR and usp showed a sex-dependent response during the 4th larval stage (Fig 2A and 2B), with significant differences between males and females, particularly in the onset of metamorphosis (VII-VIII and IX phases).
Fig 2. Ontogenetic variations in the expression pattern of the genes analyzed.
Transcriptional levels of genes from C. riparius involved in the ecdysone-related pathway (EcR, usp, E74 and vg), the folding and maturation of steroid hormone receptors (hsp70 and hsc70) and the synthesis of the ribosomal protein L13. Gene expression was measured from embryo to pupa stages of development. The mRNA values were calculated relative to actin, GAPDH and 26s as reference genes. Each bar is the mean ± SE obtained from four independent samples, each with three experimental replicates. A total of 4 egg masses and 20 larvae of each stage or phase were used. All the analyzed genes showed significant differences among the different stages (Fig 3).
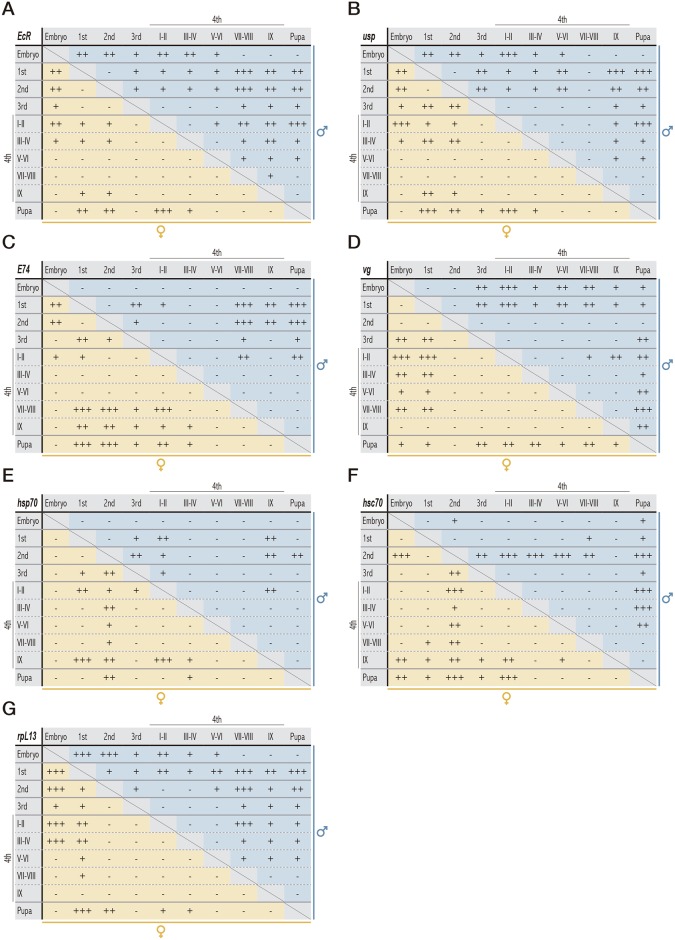
Fig 3. Significant differences in the expression pattern of the genes analyzed.
All the analyzed genes showed significant differences among the different stages according to Kruskal-Wallis Post hoc test. Three p values were tested in all cases.—(no differences), + (p < 0.05), ++ (p < 0.01), +++ (p < 0.001).
The expression pattern of E74 (Figs 2C and 3C) showed an induction in late larvae (VII-VIII and IX phases) in unison with maximal values of EcR, and reached its highest values in pupation (50-fold when compared to the lowest level measured in this study) concomitantly with a slight drop in the expression level of the ecdysone receptor gene. This result suggests that this up-regulation might be part of the early gene response to ecdysteroids that occurs specifically at the point of pupa commitment. Furthermore, as an example of effector gene in the ecdysone pathway, we focused on the study of vg, which is considered as a reproductive biomarker. Interestingly, vg gene showed a tendency to increase throughout the fourth stage concomitantly with the induction detected in EcR levels, and followed by a sharp and significant decline in pupal stage (Figs 2D and 3D).
Finally, a brief study focused in late development (from third instar larvae to pupa) was carried out using larvae from natural populations of the midge. Similar transcriptional response of these genes was observed when compared to laboratory cultures, with low levels detected in early developmental stages and strong inductions at late larval and pupal stages (S1A–S1D Fig).
Changes in the expression of the 70-kDa heat-shock inducible and cognate genes
Considering the role of the HSP70 and HSC70 as chaperones involved in EcR folding, the basal expression of hsp70 and hsc70 genes was evaluated throughout the C. riparius development, with special interest in the different phases described during the fourth instar larval stage. Both genes showed significant differences among the different stages (Fig 3E and 3F). Similarly to the expression profiles observed for ecdysone-responsive genes, notable differences between the early and late stages were observed for heat-shock genes. Thus, hsp70 transcript levels were significantly high at IX phase (prepupa) and remained high in the pupal stage (15-fold when compared to the lowest level measured in this study), while a clear increase was also detected for 3rd instar larvae (Fig 2E). Similarly, relative low levels of hsc70 mRNA were found at early development and a strong and significant activation was detected in late larval stages (VII-VIII and IX phases) and continued in pupae (about 6-fold more than in earlier phases) (Fig 2F).
It is worth pointing out that the responses of both hsp70 and EcR genes occurred concomitantly, with low levels at early 4th instar larvae and a maximum transcriptional activity at late 4th instar larval phases and pupae.
The study of larvae in natural populations revealed a similar response of these genes throughout the fourth larval instar, compared with laboratory cultures (S1E and S1F Fig).
Variations in the expression levels of the ribosomal housekeeping gene L13
Real-Time PCR experiments were carried out to evaluate the expression patterns of L13 gene, and changes in L13 transcript levels were detected throughout the different stages analyzed. Fig 2G shows a coordinated response of L13 and the remaining studied genes, which were strongly upregulated at the end of larval development (from VII-VIII phase onwards). Deep into the 4th larval stage, up to an 8-fold induction was observed when compared to early fourth instar phases. It is also remarkable the high expression level of this gene at embryo stage.
Similarly to the expression profiles observed in larvae from laboratory, notable differences between early and late stages were observed in natural populations (S1G Fig).
Analysis of sex-dependent variations
Taking into account the role of ecdysone-responsive genes in sexual differentiation and also in the production of energy reserves in mature eggs that will be deposited by adult females, we consider that sex-dependent changes could determine transcriptional processes. As a first approach to confirm this assumption, data of 4th instar larva were preliminarily analyzed taking into account that, except for the I-II phase, samples of each group into the 4th instar were separated by sex when collected.
Apart from the developmental changes described previously, the expression pattern of both EcR and usp showed a sex-dependent response during the 4th larval stage (Fig 2A and 2B), with significant differences between males and females, particularly in the onset of metamorphosis (VII-VIII and IX phases). Although differences between the expression levels of males and females were also detected for E74 and vg (Fig 2C and 2D), these variations were not as remarkable as those found in the case of EcR and usp. Furthermore, except for the prepupa values of hsp70, heat-shock genes behaved similarly in males and females (Fig 2E and 2F). Finally, the transcriptional response of L13 was quite similar between sexes, except for a higher response of females from phase VII-VIII.
Results obtained in larvae from field populations also showed a sex-dependent variation in the transcriptional response of the analyzed genes, with a transcriptional tendency similar to that described for laboratory cultures (S1 Fig).
Analysis of the expression profile dataset
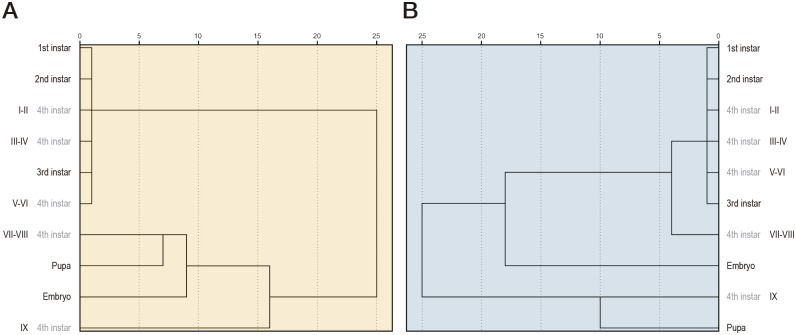
Taking into account the mean value for each gene in each larval stage/phase, an analysis of the entire dataset based on the sex of the samples was finally carried out. Clustering is an exploratory tool for looking at associations within gene expression data that allowed us to hypothesize about relationships between gene response and class/group of larvae. According to the response of the analyzed genes, the hierarchical clustering dendrograms obtained (Fig 4) found two main groups of biological samples in our data: 1) a group containing 1st, 2nd, 3rd instar larvae and early 4th instar phases; and 2) a group including the late phases of the 4th stage (VII-VIII and IX) and the pupa, as well as the embryo, which showed extreme values depending on the gene.
Fig 4. Hierarchical clustering dendrogram of the groups established for the expression analysis of ecdysone related genes.
Cluster analysis arranges biological samples into groups based on the expression levels measured in female (A) and male (B) larvae. Relationships among samples are represented by a dendrogram whose branch lengths reflect the degree of similarity between them as assessed by pairwise comparisons of gene expression profiles. Two groups of developmental stages or phases are clearly separated with slight differences between sexes: 1) 1st, 2nd, 3rd instar larvae and early 4th instar phases; and 2) late 4th instar larvae, pupa and embryo. The root represents the whole data set and a leaf corresponds to a single object in it. An internal node represents the union of all objects in its sub-tree. The weight of an internal node represents the distance between its two child nodes.
In the case of larvae from natural populations, the cluster analysis based on the expression levels found two groups of biological samples in our data. On the one hand, the 3rd instar larvae and early 4th instar phases; and on the other hand, the late phases of the 4th stage and pupa (S2 Fig).
Variations in ecdysteroid levels during larval development
Ecdysteroid titers (mainly ecdysone and 20E) have been quantified by several investigators in different model insects, such as D. melanogaster or M. sexta [1,42–46]. In contrast, little is known about ecdysteroid variations in C. riparius. As a first approach, levels of total body ecdysteroids were measured using an antiserum with a maximum and similar affinity for 20E and ecdysone, and the results were expressed in 20E equivalents (Fig 5). However, according to the antiserum specifications, it also presented a high cross-reactivity percentage with 2-deoxy-20-hydroxy-ecdysone and 2-deoxy-ecdysone (88% and 63%, respectively), given that both molecules are precursors in the hormone pathway (just before ecdysone synthesis).
Fig 5. Ecdysteroid titers determination throughout C. riparius development.
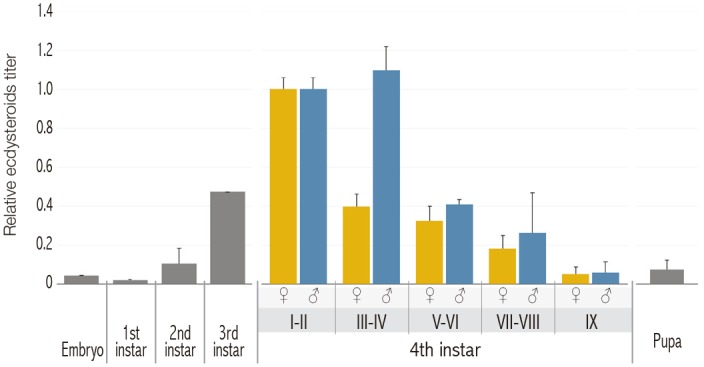
Ecdysteroid levels in the whole organism were analysed from embryo to pupa. Two EIA assays were performed and results are expressed as mean ± SE. A total of six egg masses and eight individuals of each developmental category were used in this study. Data corresponding the 4th instar larvae are shown separately by sex. Results are shown in 20E equivalents (relative levels; pg/larva).
In contrast to what was expected according to previous studies, in which 20E titers reach their maximum at the end of the last larval stage, the highest levels of ecdysteroids in our samples were measured at the beginning of the 4th instar, just after the third to fourth instar molt. High percentages of cross-reactivity could be responsible for a high level of ecdysteroids immediately after larvae molting, where the EIA kit would be detecting not only 20E but also ecdysone and its precursors. To avoid that antiserum unspecificity and seeking to clarify our ELISA results, we tried to carry out a specific determination of 20E by chromatography. However, none of the methodological approaches (different columns, different number of larvae in samples, etc.) to the analysis by HPLC / MS / MS gave a positive result, since 20E levels were below the detection limit in all cases, according to the Chromatography Service (SIDI, Universidad Autónoma de Madrid). Thus, unfortunately, the data were inconclusive and further experiments will be necessary to determine the appropriate conditions for the extraction and analysis of 20E in C. riparius samples.
Discussion
Although the ecdysone mode of action has been well described at the molecular level in Drosophila and other insects [1,2,47] there is a lack of information in another model insect such as C. riparius, a widely used test organism for ecotoxicology studies [4,5]. Therefore, a major concern in developmental studies in C. riparius is to know whether ecdysone-inducible genes change their expression patterns in a stage-dependent manner or not, given that they are commonly used as molecular biomarkers [15,27,35–37,48–51].
In this work, a deep study of the ontogenetic and sex-dependent variation of such genes was carried out in Chironomus riparius laboratory specimens, complemented with the study of late development in natural populations.
Results obtained in this work describe for the first time a stage-dependent variation in all the ecdysone-responsive genes analyzed throughout the C. riparius development, with high inductions in the late 4th instar phases, pupae and, for some genes, also in the embryo stage. The induction of both EcR and usp genes correlates well with a high activity in the embryo phase, but also with the endogenous 20E peak at the end of development, previously described in other holometabolous insects ([52] and references therein). Although data obtained in our ELISA contrast with the highest levels described in other insects for 20E at the end of larval development, our results are consistent with studies where differences in the profile of both ecdysone and 20E are demonstrated. For example, it has been described that Gryllus bimaculatus reaches the highest levels of ecdysone in haemolymph in earlier phases of the last larval stage, while the 20E curve shows a different trend, with the maximum peaks in males and females displaced to the late phases [53].
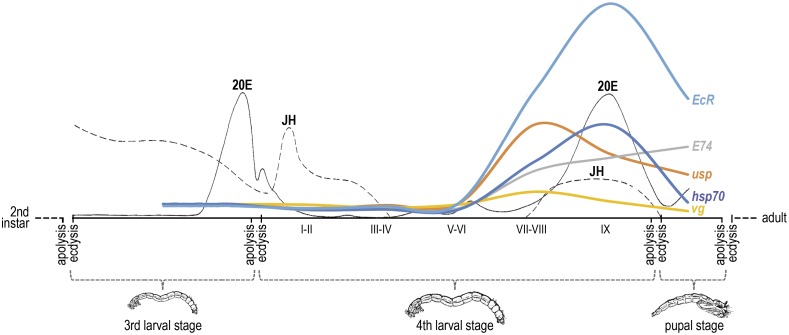
In response to this hormone titer more transcripts are expected to produce the proteins that finally form the heterodimer, which acts as an ecdysone receptor. Consequently, the coordinated response measured at the end of the 4th larval stage for the other analyzed genes suggests that it is in this phase where the 20E levels might increase to trigger the mechanisms that lead to metamorphosis (Fig 6).
Fig 6. Profiles for EcR, usp, E74, vg and hsp70 in late developmental stages in C. riparius.
Schematic drawing of the life cycle of a non-biting midge (Diptera: Chironomidae, Chironomus) based on the results obtained in this work. Larval instars were identified by measuring head capsule widths. Age and sex of fourth instar larvae were established from the development of genital and thoracic imaginal discs according to [33]. Lines corresponding to hormone titers (20E and JH) are based on previous works [1,43–45].
Our results are in consonance with previous studies in Drosophila that describe changes in both EcR and usp genes at the pre-metamorphic stage. These studies show that usp fluctuations are lighter than those of EcR, suggesting that EcR is the main responsible for the response to 20E while usp transcribes in a constitutive manner [9,54]. Consistent with that, our results indicate a coordinated expression of both genes at the end of the 4th instar with a clear higher induction of EcR when compared to usp. In addition, the transcriptional activity observed in usp at early 4th instar larvae may be due to its constitutive expression.
EcR and usp genes have been characterized in A. aegypti, showing stage and tissue-specific responses. Whereas usp mRNA titer remains constant in fat body during development, both usp and EcR transcript levels fluctuate in ovaries during vitellogenesis. The kinetics of ovarian usp mRNA coincides with that of the ecdysteroid receptor, with high levels during the previtellogenic period and shortly after the onset of vitellogenesis [55,56]. In contrast, USP levels in the fat body remain relatively constant throughout most of the vitellogenic cycle [57,58]. The ecdysone receptor and the ultraspiracle proteins have also been described in C. tentans, where a tissue-specific regulation of the usp gene also occurs [59]. Data obtained in our study from total male and female larvae mRNAs support the idea that specific physiological processes in females, such as vitellogenesis, could be the reason for the different transcriptional activities of EcR, usp, and vg, among others, when compared to males. Interestingly, this sex and tissue-dependent fluctuations are high enough to be detected when the whole sample (including both males and females) is analyzed.
The expression pattern reported in this work suggests that the E74 gene has a similar role at the onset of the metamorphosis in C. riparius as it does in other insects such as D. melanogaster or M. sexta. Measured levels of E74 mRNA correlate well with timing and induction parameters necessary to reach pupation. At the time of pupal commitment, E74 induction in C. riparius is similar to that observed at the mid-3rd and the mid-5th instar transitions in D. melanogaster and M. sexta, respectively, representing a classical early gene response to 20E. In M. sexta, E74B mRNA is detected in late larvae, when cells are becoming committed to their pupal fate [18]. In culture tissues of M. sexta, induction occurs as a direct response to a 20E peak in absence of JH, and it has been described that the exogenous addition of 20E induces a more robust response, indicating that 20E acts directly on the MsE74 gene. A similar conclusion was deduced in D. melanogaster, where E74A and E74B mRNAs were detected during the final larval instar at the peak of ecdysteroids needed for pupation, induced in vitro by high concentrations of 20E [60]. Our work suggests a role of CrE74 gene in the process of pupation, although genomic information in C. riparius is still scarce and further studies are needed to establish if different isoforms of this gene are present in this species and, in that case, the role that each one plays in the development and metamorphosis.
Vg cDNAs have been isolated from several insect species ([61] and references therein). The synthesis of Vg is regulated at transcriptional level and the JH and 20E hormones, and also nutrients or insulin-like peptides, have been described as yolk protein precursor regulators [62]. For example, transition from previtellogenesis to vitellogenesis corresponds to a late response of 20E in some insects, such as D. melanogaster and Bombyx mori [63]. Vg transcription is also triggered by ecdysteroids in A. aegypti [56]. However, a recent work in Blatella germanica has shown that JH also operates in vitellogenin expression through a cascade of genes similar to that described in the ecdysteroid hierarchy [64]. Recent studies in T. castaneum have shown that both JH and 20E are required for vg gene expression and, furthermore, JH regulates Vg synthesis in the fat body and 20E influences Vg synthesis through its action on oocyte maturation [65,66]. Our data support the idea that ecdysteroids induce vitellogenin expression in C. riparius 4th instar larvae, suggesting that most of the vitellogenesis process takes place in this larval instar.
Although expression of vg gene occurs throughout all the phases described at the 4th instar, the induction observed during the final stretch (VII-VIII and prepupa phases) seems to be part of the molecular hormone-related response to metamorphosis, due to the endogenous increase of 20E and the down-regulation of JH as a start signal for pupation. Since C. riparius is a non-biting species and adults do not feed, this seems to be the development stage in which vitellogenin production is needed in order to ensure adequate levels of energy reserves in the next generation of oocytes and embryos.
Wülter & Götz [33] described the formation of oocytes in C. riparius, which occurs during the 4th instar of the larval stage, and gave morphological details of these changes. However, no information is available about the molecular changes that lead to oocytes production and the synthesis of energy reserves in eggs. This work constitutes the first molecular approach to vitellogenesis in C. riparius, and describes variations in the expression of a gene directly involved in the formation of the egg yolk. Our results report for the first time developmental stage and sex-related changes in the vitellogenin gene expression throughout the 4th larval instar in C. riparius, which correlate in time with morphological changes described decades ago in the development of the vitello during the oocyte formation [33] and also with the sex-dependent glycogen reserves variations previously described in this organism [67]. Although further studies are needed to understand the mechanisms that underlie vitellogenesis in C. riparius, these data suggest that the final overexpression of vg gene could be necessary in the synthesis of egg vitellogenin in females before pupation.
Besides the physiological relevance of molecular variations of ecdysone-responsive genes related to the pupation and metamorphosis processes, it is worth pointing out that C. riparius is considered a relevant model organism for ecotoxicological assessments and therefore studies focusing on the variation of genes used as molecular biomarkers of toxicity throughout the development are particularly important. For example, different xenobiotics analogues to 20E seem to mimic the natural hormone actions, modulating the activity of the ecdysone nuclear receptor and affecting different regulatory genes directly connected with the cascade of genetic signals switched on by the ecdysone hormone (E74 and vg among others) [15,27,35–37,48–51], which brings significant implications in different developmental stages in C. riparius. Regarding this, the knowledge of a stage-dependent regulation of EcR and the ecdysone-responsive genes in C. riparius may contribute to a more precise evaluation of the disrupting effects of EDCs in this organism.
Heat-shock proteins interact with multiple key components of signaling pathways that regulate growth and development. Certain HSPs function as chaperones by mediating intramolecular or intermolecular interactions, such as folding events or intracellular signaling and protein degradation/refolding, respectively [68,69]. This work reports for the first time in C. riparius a concomitant stage-dependent induction of the hsp70 and hsc70 genes and the gene encoding the ecdysone receptor, suggesting that this up-regulation is part of the early gene response to ecdysteroids due to their role in the folding and maturation of steroid hormone receptors [23]. Moreover, sex-dependent changes in the expression of both heat-shock genes correlate with the sex-dependent activity of EcR. As in many other organisms, the functions of HSPs in C. riparius are typically associated with stress response and tolerance [24–28], while their role during development remains unclear. Our data suggest the involvement of both genes in metabolic processes not associated with insect cellular stress, such as the ecdysone-mediated pathway that controls larval development and insect metamorphosis.
Some recent studies have shown that differential expression profiles of some ribosomal proteins take place during C. riparius life cycle, with a higher activity of these genes in embryos and pupae when compared to larvae [31,32,39]. However, these studies normally show a global expression level at larval stage, without distinguishing among the different larval instars or the phases included within the fourth instar. In this study we report for the first time a differential expression profile of L13 gene during the 4th larval instar, with a strong induction from late phases of this stage to pupa. The detection of L13 in every developmental category studied for this research, which was expected given its role in housekeeping functions, as well as the concomitant induction of this gene together with the other of genes analyzed, are in concordance with a previous study in D. melanogaster that describes the existence of stages with high transcription and translation rates [70]. Considering the significant increase measured at the end of the juvenile stage in all the genes studied, high levels of L13 transcript at that time could be related to the need for increased translational activity and, consequently, ribosome synthesis.
The fact that genes involved directly or indirectly in the ecdysone cascade are used as potential endpoints in the evaluation of EDCs effects reinforces the need for a more in-depth knowledge of their expression patterns during larval development in this species. Knowing the ontogenetic variations that take place in this model organism commonly used in ecotoxicity studies, it will be possible to design more accurate toxicity tests that minimize natural variations in the observed responses of such biomarkers and assess more thoroughly the effect of EDCs. These tests could be based on the use of early 4th instar larva or even 3rd instar larva, when intrinsic variations of such genes are scarce.
Conclusions (and Significance)
This is the first study in C. riparius about stage-dependent variations of genes related to ecdysone response from embryo to pupa, specially during the different phases of the fourth instar larval stage. Our analyses reveal the point where EcR and usp are significantly upregulated into the 4th instar. Furthermore, we have detected a coordinated overexpression of both genes with the remaining analyzed genes, suggesting that this is the moment when larvae undergo metamorphosis. This work also reveals a sex-dependent transcriptional activity of the analyzed genes during late development, some of which are associated with important physiological processes such as vitellogenesis. However, further studies are needed to establish the specific role that each gene plays in the development and metamorphosis of C. riparius males and females.
Given that C. riparius is considered a model species in ecotoxicology studies and is widely used to evaluate the impact of contaminants at the molecular level, specially the 4th larval instar, our results should be taken into account in ecotoxicity testing. Indeed, most of the genes used in this work have been proposed as sensitive biomarkers of environmental stress. Thus, understanding the molecular basis of metamorphosis as well as the stage and sex-specific regulation of these ecdysone-related genes will allow us to construct more accurate models for the prediction of toxic effects, assessing more precisely what effects are due to a toxin and which are conditioned by natural physiological processes and also contributing to a more in-depth evaluation of the disrupting effects of EDCs in this organism.
Supporting Information
Transcriptional levels of genes, from natural population of C. riparius, involved in the ecdysone-related pathway (EcR, usp, E74 and vg), the folding and maturation of steroid hormone receptors (hsp70 and hsc70), and the synthesis of the ribosomal protein L13. Gene expression was measured during 3rd, 4th and pupa stages of development. The mRNA values were calculated relative to actin, GAPDH and 26s as reference genes. Each bar is the mean ± SE obtained from four independent samples, each with three experimental replicates (a total of 20 larvae of each stage or phase, and separate sex were used). Significant differences among groups: *p≤ 0.05; **p≤ 0.005. Different letters indicate significant differences across groups (p≤ 0.05; p≤ 0.005).
(TIF)
Cluster analysis arranges biological samples into groups based on the expression levels. Relationships among samples are represented by a dendrogram whose branch lengths reflect the degree of similarity between them as assessed by a pairwise similarity genes response. Two groups of developmental stages or phases are clearly separated, corresponding to 3rd and early 4th instar larva (groups 1–4), and to late 4th instar larva and pupa, respectively (groups 5–7). The root represents the whole data set. A leaf represents a single object in the data set. An internal node represents the union of all objects in its sub-tree. The weight of an internal node represents the distance between its two child nodes.
(TIF)
Acknowledgments
Authors would like to thank Enrique Rego for his helpful comments and statistical processing; Dr. P. Fernández and Dr. M.J. Hazen (Universidad Autónoma de Madrid, Spain) for their assistance with spectrophotometry equipment used for immunoassays; and the Faculty of Sciences of the National Distance Education University (UNED). We also thank C. González (Vitro S.A., Spain) and Berthin Pharma (France) for providing the 20E EIA detection kits for their validation in this work.
Data Availability
All relevant data are within the paper.
Funding Statement
IO's research was funded by an FPI grant from Ministerio de Ciencia e Innovación (http://www.idi.mineco.gob.es; CTM2009-07189). Research funding was provided by Ministerio de Economía y Competitividad (grant number CTM-2012-37547) and Ministerio de Ciencia e Innovación (grant number CGL2009-10868), Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dubrovsky EB. Hormonal cross talk in insect development. Trends Endocrinol Metab. 2005;16: 6–11. 10.1016/j.tem.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179: 477–84. 10.1016/j.ygcen.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 3. EPA. Methods for Measuring the Toxicity and Bioaccumulation of Sediment-associated Contaminants with Freshwater Invertebrates. Second Edition Washington: United States Environmental Protection Agency; 2000. p. 212. [Google Scholar]
- 4. OECD. Test No. 218: Sediment-Water Chironomid Toxicity Test Using Spiked Sediment. Paris: Organisation for Economic Co-operation and Development; 2004. p. 21. [Google Scholar]
- 5. OECD. Test No. 219: Sediment-Water Chironomid Toxicity Test Using Spiked Water. Paris: Organisation for Economic Co-operation and Development; 2004. p. 21. [Google Scholar]
- 6.Chironomids: suitable test organisms for risk assessment investigations on the potential endocrine disrupting properties of pesticides [Internet]. [cited 9 Sep 2014]. Available: http://download.springer.com/static/pdf/667/art%253A10.1007%252Fs10646-006-0117-x.pdf?auth66=1410428279_90c5297641ca55e72f530e84ccb5e8fd&ext=.pdf [DOI] [PubMed]
- 7. Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, et al. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicol Environ Saf. 2003;54: 302–14. [DOI] [PubMed] [Google Scholar]
- 8. Thomas HE, Stunnenberg HG, Stewart AF. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993;362: 471–5. 10.1038/362471a0 [DOI] [PubMed] [Google Scholar]
- 9. Henrich VC, Szekely AA, Kim SJ, Brown NE, Antoniewski C, Hayden MA, et al. Expression and function of the ultraspiracle (usp) gene during development of Drosophila melanogaster . Dev Biol. 1994;165: 38–52. 10.1006/dbio.1994.1232 [DOI] [PubMed] [Google Scholar]
- 10. Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster . J Insect Physiol. Elsevier Ltd; 2012;58: 293–302. 10.1016/j.jinsphys.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 11. Fahrbach SE, Smagghe G, Velarde RA. Insect nuclear receptors. Annu Rev Entomol. Annual Reviews; 2012;57: 83–106. 10.1146/annurev-ento-120710-100607 [DOI] [PubMed] [Google Scholar]
- 12. Hiruma K, Riddiford LM. Developmental expression of mRNAs for epidermal and fat body proteins and hormonally regulated transcription factors in the tobacco hornworm, Manduca sexta . J Insect Physiol. 2010;56: 1390–5. 10.1016/j.jinsphys.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 13. Imhof MO, Rusconi S, Lezzi M. Cloning of a Chironomus tentans cDNA encoding a protein (cEcRH) homologous to the Drosophila melanogaster ecdysteroid receptor (dEcR). Insect Biochem Mol Biol. 1993;23: 115–124. 10.1016/0965-1748(93)90089-B [DOI] [PubMed] [Google Scholar]
- 14. Vögtli M. Functional characterization of two Ultraspiracle forms (CtUSP-1 and CtUSP-2) from Chironomus tentans . Insect Biochem Mol Biol. 1999;29: 931–942. 10.1016/S0965-1748(99)00068-5 [DOI] [PubMed] [Google Scholar]
- 15. Nair PMG, Choi J. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environ Toxicol Pharmacol. 2012;33: 98–106. 10.1016/j.etap.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 16. Thummel CS, Burtis KC, Hogness DS. Spatial and temporal patterns of E74 transcription during Drosophila development. Cell. 1990;61: 101–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/1690603 [DOI] [PubMed] [Google Scholar]
- 17. Fletcher JC, Burtis KC, Hogness DS, Thummel CS. The Drosophila E74 gene is required for metamorphosis and plays a role in the polytene chromosome puffing response to ecdysone. Development. 1995;121: 1455–65. Available: http://www.ncbi.nlm.nih.gov/pubmed/7789275 [DOI] [PubMed] [Google Scholar]
- 18. Stilwell GE, Nelson CA, Weller J, Cui H, Hiruma K, Truman JW, et al. E74 exhibits stage-specific hormonal regulation in the epidermis of the tobacco hornworm, Manduca sexta . Dev Biol. 2003;258: 76–90. 10.1016/S0012-1606(03)00105-2 [DOI] [PubMed] [Google Scholar]
- 19. Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J Insect Physiol. 2008;54: 1447–58. 10.1016/j.jinsphys.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 20. Hahn T, Schenk K, Schulz R. Environmental chemicals with known endocrine potential affect yolk protein content in the aquatic insect Chironomus riparius . Environ Pollut. 2002;120: 525–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/12442778 [DOI] [PubMed] [Google Scholar]
- 21. Porte C, Janer G, Lorusso LC, Ortiz-Zarragoitia M, Cajaraville MP, Fossi MC, et al. Endocrine disruptors in marine organisms: approaches and perspectives. Comp Biochem Physiol Part C, Toxicol Pharmacol. 2006;143: 303–15. 10.1016/j.cbpc.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 22. Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62: 670–84. 10.1007/s00018-004-4464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wegele H, Müller L, Buchner J. Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151: 1–44. 10.1007/s10254-003-0021-1 [DOI] [PubMed] [Google Scholar]
- 24. Karouna-Renier NK, Rao KR. An inducible HSP70 gene from the midge Chironomus dilutus: characterization and transcription profile under environmental stress. Insect Mol Biol. 2009;18: 87–96. 10.1111/j.1365-2583.2008.00853.x [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q, Denlinger DL. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect Physiol. 2010;56: 138–50. 10.1016/j.jinsphys.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 26. Morales M, Planelló R, Martínez-Paz P, Herrero O, Cortés E, Martínez-Guitarte JL, et al. Characterization of Hsp70 gene in Chironomus riparius: expression in response to endocrine disrupting pollutants as a marker of ecotoxicological stress. Comp Biochem Physiol C Toxicol Pharmacol. Elsevier Inc.; 2011;153: 150–8. 10.1016/j.cbpc.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 27. Herrero O, Planelló R, Morcillo G. The plasticizer benzyl butyl phthalate (BBP) alters the ecdysone hormone pathway, the cellular response to stress, the energy metabolism, and several detoxication mechanisms in Chironomus riparius larvae. Chemosphere. 2015; (In press). [DOI] [PubMed] [Google Scholar]
- 28. Planelló R, Servia MJ, Gómez-Sande P, Herrero O, Cobo F, Morcillo G. Transcriptional responses, metabolic activity and mouthpart deformities in natural populations of Chironomus riparius larvae exposed to environmental pollutants. Environ Toxicol. 2013; 10.1002/tox.21893 [DOI] [PubMed] [Google Scholar]
- 29. Govinda S, Kutlow T, Bentivegna CS. Identification of a putative ribosomal protein mRNA in Chironomus riparius and its response to cadmium, heat shock, and actinomycin D. J Biochem Mol Toxicol. 2000;14: 195–203. Available: http://www.ncbi.nlm.nih.gov/pubmed/10789497 [DOI] [PubMed] [Google Scholar]
- 30. Planelló R, Martínez-Guitarte JL, Morcillo G. Ribosomal genes as early targets of cadmium-induced toxicity in Chironomus riparius larvae. Sci Total Environ. 2007;373: 113–21. 10.1016/j.scitotenv.2006.10.038 [DOI] [PubMed] [Google Scholar]
- 31. Nair PMG, Choi J. Characterization of a ribosomal protein L15 cDNA from Chironomus riparius (Diptera; Chironomidae): transcriptional regulation by cadmium and silver nanoparticles. Comp Biochem Physiol B Biochem Mol Biol. 2011;159: 157–62. 10.1016/j.cbpb.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 32. Park K, Kwak IS. Gene expression of ribosomal protein mRNA in Chironomus riparius: effects of endocrine disruptor chemicals and antibiotics. Comp Biochem Physiol Part C, Toxicol Pharmacol. Elsevier Inc.; 2012;156: 113–20. 10.1016/j.cbpc.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 33. Wülker W, Götz P. Die Verwendung der Imaginalscheiben zur Bestimmung des Entwicklungszustandes von Chironomus-larven (Dipt.). Zeitschrift für Morphol der Tiere. 1968;62: 363–388. 10.1007/BF00401562 [DOI] [Google Scholar]
- 34. Park K, Kwak IS. Alcohol dehydrogenase gene expression in Chironomus riparius exposed to di(2-ethylhexyl) phthalate. Comp Biochem Physiol Part C, Toxicol Pharmacol. Elsevier Inc.; 2009;150: 361–7. 10.1016/j.cbpc.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 35. Planelló R, Herrero O, Martínez-Guitarte JL, Morcillo G. Comparative effects of butyl benzyl phthalate (BBP) and di(2-ethylhexyl) phthalate (DEHP) on the aquatic larvae of Chironomus riparius based on gene expression assays related to the endocrine system, the stress response and ribosomes. Aquat Toxicol. 2011;105: 62–70. 10.1016/j.aquatox.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 36. Planelló R, Martínez-Guitarte JL, Morcillo G. Effect of acute exposure to cadmium on the expression of heat-shock and hormone-nuclear receptor genes in the aquatic midge Chironomus riparius . Sci Total Environ. Elsevier B.V.; 2010;408: 1598–603. 10.1016/j.scitotenv.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 37. Morales M, Martínez-Paz P, Ozáez I, Martínez-Guitarte JL, Morcillo G. DNA damage and transcriptional changes induced by tributyltin (TBT) after short in vivo exposures of Chironomus riparius (Diptera) larvae. Comp Biochem Physiol C Toxicol Pharmacol. 2013;158: 57–63. 10.1016/j.cbpc.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 38. Park K, Park J, Kim J, Kwak IS. Biological and molecular responses of Chironomus riparius (Diptera, Chironomidae) to herbicide 2,4-D (2,4-dichlorophenoxyacetic acid). Comp Biochem Physiol Part C, Toxicol Pharmacol. Elsevier Inc.; 2010;151: 439–46. 10.1016/j.cbpc.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 39. Martínez-Guitarte JL, Planelló R, Morcillo G. Characterization and expression during development and under environmental stress of the genes encoding ribosomal proteins L11 and L13 in Chironomus riparius . Comp Biochem Physiol B Biochem Mol Biol. 2007;147: 590–6. 10.1016/j.cbpb.2007.03.015 [DOI] [PubMed] [Google Scholar]
- 40. Lüchmann KH, Dafre AL, Trevisan R, Craft JA, Meng X, Mattos JJ, et al. A light in the darkness: new biotransformation genes, antioxidant parameters and tissue-specific responses in oysters exposed to phenanthrene. Aquat Toxicol. 2014;152: 324–34. 10.1016/j.aquatox.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 41.Siegel S, Castellan NJ Jr. Non-parametric statistics for the behavioural sciences. McGraw Hill Int. McGraw-Hill Humanities/Social Sciences/Languages; 1988. pp. 213–214. Available: http://www.ncbi.nlm.nih.gov/nlmcatalog/8810006
- 42. Richards G. The radioimmunoassay of ecdysteroid titers in Drosophila melanogaster . Mol Cell Endocrinol. 1981;21:181–197. 10.1016/0303-7207(81)90013-7 [DOI] [PubMed] [Google Scholar]
- 43. Riddiford LM. Juvenile hormone: the status of its "status quo" action. Arch Insect Biochem Physiol. 1996;32(3–4):271–86. [DOI] [PubMed] [Google Scholar]
- 44. Warren JT, Gilbert LI. Metabolism in vitro of cholesterol and 25-hydroxycholesterol by the larval prothoracic glands of Manduca sexta . Insect Biochem Mol Biol. 1996;26(8–9):917–29. 10.1016/S0965-1748(96)00058-6 [DOI] [PubMed] [Google Scholar]
- 45. Langelan RE, Fisher JE, Hiruma K, Palli SB, Riddiford LM. Patterns of MHR3 expression in the epidermis during a larval molt of the tobacco hornworm Manduca sexta . Dev Biol. 2000; 227:481–494. 10.1006/dbio.2000.9895 [DOI] [PubMed] [Google Scholar]
- 46. Parvy JP, Blais C, Bernard F, Warren JT, Petryk A, Gilbert LI, O'Connor MB, Dauphin-Villemant C. A role for βFTZ-F1 in regulating ecdysteroid titers during postembryonic development in Drosophila melanogaster . Dev Biol. 2005;282: 84–94. 10.1016/j.ydbio.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 47. Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60: 1–73. Available: http://www.ncbi.nlm.nih.gov/pubmed/11037621 [DOI] [PubMed] [Google Scholar]
- 48. Planelló R, Martínez-Guitarte JL, Morcillo G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius . Chemosphere. 2008;71: 1870–6. 10.1016/j.chemosphere.2008.01.033 [DOI] [PubMed] [Google Scholar]
- 49. Ozáez I, Martínez-Guitarte JL, Morcillo G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius . Sci Total Environ. 2013;456–457: 120–6. 10.1016/j.scitotenv.2013.03.081 [DOI] [PubMed] [Google Scholar]
- 50. Ozáez I, Martínez-Guitarte JL, Morcillo G. The UV filter benzophenone 3 (BP-3) activates hormonal genes mimicking the action of ecdysone and alters embryo development in the insect Chironomus riparius (Diptera). Environ Pollut. 2014;192: 19–26. 10.1016/j.envpol.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 51. Herrero O, Planelló R, Morcillo G. The plasticizer benzyl butyl phthalate (BBP) alters the ecdysone hormone pathway, the cellular response to stress, the energy metabolism, and several detoxication mechanisms in Chironomus riparius larvae. Chemosphere.2015;128; 266–77. 10.1016/j.chemosphere.2015.01.059 [DOI] [PubMed] [Google Scholar]
- 52. Berger EM, Dubrovsky EB. Juvenile hormone molecular actions and interactions during development of Drosophila melanogaster . Vitam Horm. 2005;73: 175–215. 10.1016/S0083-6729(05)73006-5 [DOI] [PubMed] [Google Scholar]
- 53.Westerlund S. Measuring juvenile hormone and ecdysteroid titers in insect haemolymph simultaneously by LC-MS: The basis for determining the effectiveness of plant-derived alkaloids as insect growth regulators. PhD. thesis, University of Bayreuth. 2004
- 54. Henrich VC, Brown NE. Insect nuclear receptors: A developmental and comparative perspective. Insect Biochem Mol Biol. 1995;25: 881–897. 10.1016/0965-1748(95)00030-Y [DOI] [PubMed] [Google Scholar]
- 55. Wang SF, Li C, Zhu J, Miura K, Miksicek RJ, Raikhel AS. Differential expression and regulation by 20-hydroxyecdysone of mosquito ultraspiracle isoforms. Dev Biol. 2000;218: 99–113. 10.1006/dbio.1999.9575 [DOI] [PubMed] [Google Scholar]
- 56. Cho W-L, Kapitskaya MZ, Raikhel AS. Mosquito ecdysteroid receptor: Analysis of the cDNA and expression during vitellogenesis. Insect Biochem Mol Biol. 1995;25: 19–27. 10.1016/0965-1748(94)00045-J [DOI] [PubMed] [Google Scholar]
- 57. Kapitskaya M, Wang S, Cress DE, Dhadialla TS, Raikhel AS. The mosquito ultraspiracle homologue, a partner of ecdysteroid receptor heterodimer: cloning and characterization of isoforms expressed during vitellogenesis. Mol Cell Endocrinol. 1996;121: 119–132. 10.1016/0303-7207(96)03847-6 [DOI] [PubMed] [Google Scholar]
- 58. Parthasarathy R, Palli SR. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti . J Insect Physiol. 2007;53: 216–29. 10.1016/j.jinsphys.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 59. Rauch P, Grebe M, Elke C, Spindler K-D, Spindler-Barth M. Ecdysteroid receptor and ultraspiracle from Chironomus tentans (Insecta) are phosphoproteins and are regulated differently by molting hormone. Insect Biochem Mol Biol. 1998;28: 265–275. 10.1016/S0965-1748(98)00026-5 [DOI] [PubMed] [Google Scholar]
- 60. Karim FD, Thummel CS. Ecdysone coordinates the timing and amounts of E74A and E74B transcription in Drosophila . Genes Dev. 1991;5: 1067–79. Available: http://www.ncbi.nlm.nih.gov/pubmed/2044954 [DOI] [PubMed] [Google Scholar]
- 61. Blariza MJ, Soria NW, Torres AG, Grosso CG, García BA. cDNA isolation and characterization of two vitellogenin genes in the Chagas’ disease vector Triatoma infestans (Hemiptera, Reduviidae). Gene. 2014;543: 118–24. 10.1016/j.gene.2014.03.054 [DOI] [PubMed] [Google Scholar]
- 62. Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol. 2014;5: 103 10.3389/fphys.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swevers L, Iatrou K. The ecdysone regulatory cascade and ovarian development in lepidopteran insects: insights from the silkmoth paradigm. Insect Biochem Mol Biol. 2003;33: 1285–1297. 10.1016/j.ibmb.2003.06.012 [DOI] [PubMed] [Google Scholar]
- 64. Comas D, Piulachs MD, Bellés X. Induction of vitellogenin gene transcription in vitro by juvenile hormone in Blattella germanica . Mol Cell Endocrinol. 2001;183: 93–100. Available: http://www.ncbi.nlm.nih.gov/pubmed/11604229 [DOI] [PubMed] [Google Scholar]
- 65. Parthasarathy R, Sun Z, Bai H, Palli SR. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum . Insect Biochem Mol Biol. 2010;40: 405–14. 10.1016/j.ibmb.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Parthasarathy R, Sheng Z, Sun Z, Palli SR. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum . Insect Biochem Mol Biol. 2010;40: 429–39. 10.1016/j.ibmb.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Servia MJ, Heydorff M, Péry ARR, Garric J, Lagadic L. Sex- and Developmental Stage-Related Changes in Energy Reserves in Fourth-instar Larvae of the Midge Chironomus riparius Meigen (Diptera: Chironomidae): Implications for Ecotoxicity Testing. Environ Entomol. Entomological Society of America; 2006;35: 865–874. 10.1603/0046-225X-35.4.865 [DOI] [Google Scholar]
- 68. Fink AL. Chaperone-Mediated Protein Folding. Physiol Rev. 1999;79: 425–449. Available: http://physrev.physiology.org/content/79/2/425.full-text.pdf+html [DOI] [PubMed] [Google Scholar]
- 69. Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125: 443–51. 10.1016/j.cell.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 70. Helps NR, Adams SM, Brammar WJ, Varley JM. The Drosophila melanogaster homologue of the human BBC1 gene is highly expressed during embryogenesis. Gene. 1995;162: 245–248. 10.1016/0378-1119(95)00356-B [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcriptional levels of genes, from natural population of C. riparius, involved in the ecdysone-related pathway (EcR, usp, E74 and vg), the folding and maturation of steroid hormone receptors (hsp70 and hsc70), and the synthesis of the ribosomal protein L13. Gene expression was measured during 3rd, 4th and pupa stages of development. The mRNA values were calculated relative to actin, GAPDH and 26s as reference genes. Each bar is the mean ± SE obtained from four independent samples, each with three experimental replicates (a total of 20 larvae of each stage or phase, and separate sex were used). Significant differences among groups: *p≤ 0.05; **p≤ 0.005. Different letters indicate significant differences across groups (p≤ 0.05; p≤ 0.005).
(TIF)
Cluster analysis arranges biological samples into groups based on the expression levels. Relationships among samples are represented by a dendrogram whose branch lengths reflect the degree of similarity between them as assessed by a pairwise similarity genes response. Two groups of developmental stages or phases are clearly separated, corresponding to 3rd and early 4th instar larva (groups 1–4), and to late 4th instar larva and pupa, respectively (groups 5–7). The root represents the whole data set. A leaf represents a single object in the data set. An internal node represents the union of all objects in its sub-tree. The weight of an internal node represents the distance between its two child nodes.
(TIF)
Data Availability Statement
All relevant data are within the paper.