Abstract
Aims
To investigate swept-source optical coherence tomography (OCT) angiography in the optic nerve head (ONH) and parafoveal regions in patients with multiple sclerosis (MS).
Methods
Fifty-two MS eyes and 21 healthy control (HC) eyes were included. There were two MS subgroups: 38 MS eyes without an optic neuritis (ON) history (MS −ON), and 14 MS eyes with an ON history (MS +ON). The OCT images were captured by high-speed 1050 nm swept-source OCT. The ONH flow index (FI) and parafoveal FI were quantified from OCT angiograms.
Results
The mean ONH FI was 0.160±0.010 for the HC group, 0.156±0.017 for the MS−ON group, and 0.140±0.020 for the MS+ON group. The ONH FI of the MS+ON group was reduced by 12.5% compared to HC eyes (p=0.004). A higher percentage of MS+ON eyes had abnormal ONH FI compared to HC patients (43% vs 5%, p=0.01). Mean parafoveal FIs were 0.126±0.007, 0.127±0.010, and 0.129±0.005 for the HC, MS−ON, and MS +ON groups, respectively, and did not differ significantly among them. The coefficient of variation (CV) of intravisit repeatability and intervisit reproducibility were 1.03% and 4.53% for ONH FI, and 1.65% and 3.55% for parafoveal FI.
Conclusions
Based on OCT angiography, the FI measurement is feasible, highly repeatable and reproducible, and it is suitable for clinical measurement of ONH and parafoveal perfusion. The ONH FI may be useful in detecting damage from ON and quantifying its severity.
INTRODUCTION
Multiple sclerosis (MS), characterised by demyelination, axonal injury and gliosis, inflammation, and diffuse axonal degeneration throughout the central nervous system, is generally considered an inflammatory autoimmune disease.1 Optic neuritis (ON), a common feature of MS, may affect blood perfusion of the larger ocular vessels and possibly damage visual acuity.2 Vascular abnormalities, which may be caused by the abnormal function of cerebral endothelial cells, may play an important role in the formation of MS lesions and disease progression.3 Several studies have reported that vascular abnormalities, such as ischaemic stroke and global cerebral hypoperfusion, exist in patients with MS, and patients with MS with vascular risk factors have a more rapid disability progression than those who do not.4,5 However, characterisation of cerebral blood flow can be challenging. Studying the circulation of the optic nerve head (ONH) and parafoveal areas of the eye in MS patients may provide insight into the role of the more global vascular changes in the pathogenesis of MS.
Different methods have been used to detect the ocular blood perfusion in clinical practice and experimental research. Fluorescein angiography (FA) and indocyanine green angiography provide qualitative evaluation of retinal and choroidal circulation, but do not provide objective quantitative measurements.6,7 Although laser Doppler flowmetry (LDF) and laser speckle flowgraphy (LSFG) can measure retinal blood flow, the results are too variable to be used in clinical diagnosis.8,9 MRI ocular blood flow measurement has low spatial resolution and long acquisition time, is susceptible to movement artefacts, is limited by assumptions in the blood flow calculation, and has relatively high costs.10 Ultrasound colour Doppler imaging (CDI) can provide independent haemodynamic measurements of the retinal and uveal vascular beds, but due to limited resolution, it cannot provide precise measurements of retinal microcirculation.11
As a non-invasive imaging technique, optical coherence tomography (OCT) has been commonly used worldwide for clinical diagnosis of some ocular diseases. Moreover, OCT has also been explored in a research capacity in MS. Using commercial OCT, it has been demonstrated that retinal structure defects occur in MS patients, such as thinning of the retinal nerve fibre layer (RNFL) and the combined retinal ganglion cell and inner plexiform layers.12 These changes are due to retrograde axonal degeneration of the optic nerve axons after clinically apparent and subclinical ON, and possibly due to primary neurodegenerative pathology. OCT technology can provide retinal structural information and also blood perfusion information in some retinal diseases. Doppler OCT has been able to obtain precise measurement of total retinal blood flow calculated from the Doppler frequency shift of backscattered light. While appropriate for large vessels around ONH, Doppler OCT is not sensitive enough to accurately measure the low velocities of small vessels.13
Recently, we developed a method of measuring local circulation using high-speed OCT to perform quantitative angiography. Using the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm, ONH perfusion can be quantified.14 The purpose of this study was to measure the microcirculation in the ONH and parafoveal retinal regions of MS eyes with and without a history of ON compared with healthy control (HC) eyes, and to investigate the retinal haemodynamic changes in MS. To the best of our knowledge, this is the first study using OCT angiography in MS.
METHODS
Study population
This study was performed at the Casey Eye Institute at the Oregon Health & Science University (OHSU, Portland, Oregon, USA). The research protocols were approved by the institutional review boards and carried out in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject after explanation of the nature of this study.
The HC subjects were part of the Advanced Imaging for Glaucoma Study (AIGS). Their inclusion criteria were a normal Humphrey Swedish Interactive Threshold Algorithm (SITA) 24-2 standard visual field within 95% limits of the normal reference, intraocular pressures (IOP) of less than 21 mm Hg in both eyes, glaucoma hemifield test within 97% limits, normal appearing ONH and RNFL, central corneal thickness of more than 500 μm, an open anterior chamber angle as observed by gonioscopy, and no history of ocular or systemic corticosteroid use (http://www.AIGStudy.net).
All patients with MS were referred by the MS Center of Oregon at OHSU. All patients with MS fulfilled the 2005 panel MS criteria. Disability was assessed by the self-reported Expanded Disability Status Scale score (EDSS) and the physician-rated European Database for Multiple Sclerosis (EDMUS) Grading Gcale. MS inclusion criteria were physician-confirmed diagnosis of MS (any subtype acceptable, eg, relapsing-remitting, secondary progressive, primary progressive), age 18–70 years old, able to comply with study procedures (transfer to a chair), and corrected visual acuity of at least 20/200 in either eye. Exclusion criteria were intravenous or oral steroids in the prior 30 days, MS exacerbation in the prior 60 days, evidence on ophthalmological exam within the last year of other ocular diseases or pathology that would confound the assessment (eg, glaucoma, diabetic or hypertensive retinal disease, amblyopia, etc.), previous intraocular surgery except for uncomplicated cataract extraction with posterior chamber intraocular lens implantation, inability to cooperate with OCT scanning, or refractive error greater than +3 or −7 dioptres. A medical history of ON prior to enrolment was determined by self-report and physician report, and confirmed by record review. Patients with an ongoing attack of ON in either eye were excluded from this study.
MS eyes were categorised into two groups. Patients without ON were placed in the MS−ON group, and those with ON were placed in the MS+ON group.
OCT data acquisition and processing
The prototype high-speed swept-source OCT system operated at an axial scan speed of 100 KHz using 1050 nm wavelength laser (Axsun Technologies, Billerica, Massachusetts, USA) with a tuning range of 100 nm. The image resolution was 5.3 μm axially and 18 μm laterally.
A 3×3×3 mm three-dimensional (3D) volumetric scan centred on the ONH/fovea was captured for blood flow measurements. In horizontal priority (x-fast) scans, the beam is moved horizontally (x dimension) to form a 3 mm-long line scan for cross-sectional imaging (B-scan). Each B-scan contains 200 spots (axial scans). At each vertical (y) position, eight consecutive B-scans were captured in order to detect motion. The B-scan is then shifted slightly to a new position along the slow (vertical) axis. A total of 200 slow-axis locations were sampled to form a 3D data cube. A total of 1600 B-scans were acquired in approximately 3.5 s. In vertical priority (y-fast) scans, the role of the x and y axes were reversed. Four 3D scans, comprising two horizontal priority (x-fast) scans and two vertical priority (y-fast) scans were obtained in one session.
The SSADA algorithm was used to distinguish flowing blood from static tissue.14 As seen in real-time OCT reflectance images, the amplitude of signal backscattered from non-static tissue varies rapidly over time.14 By calculating the decorrelation of signal amplitude from consecutive B-scans, a contrast between static and moving tissue is created. Blood vessels are characterised by motion-induced decorrelation in the lumen. The faster blood particles move across the laser beam, the higher is the decorrelation of the detected signals within a velocity range set by the scan parameters. Thus, decorrelation is approximately linear to flow velocity, that is, the distance travelled by red blood cells flowing across the light beam per unit time.15 However, beyond a saturation velocity that is defined by the time interval between consecutive OCT B-scans, the decorrelation increases more slowly with velocity and eventually reaches an upper boundary.15
Eye motion causes two types of artefacts in SSADA. First, motion between consecutive B-scans at the same nominal position causes decorrelation that can appear as flow. Second, motion between B-scan positions distorts the transverse position of scans along the slow scan axis. To correct the first type of motion error, B-scans with very large (saccadic) bulk motion artefacts were removed. Furthermore, decorrelation due to bulk tissue motion was calculated by histogram analysis and subtracted from each cross-sectional SSADA frame.14 To correct the second type of motion artefact, we used an image registration algorithm that registered four orthogonal raster-scanned volumes.16 Motion correction was first performed on the structural OCT data. The motion correction algorithm generated 3D displacement fields that map A-scans from the input volumes into a common motion-corrected space. The same displacement fields were applied to the decorrelation (flow) data to produce motion-corrected flow data volumes. Flow data from four input volumes were weighted and merged, improving the signal-to-noise ratio in the flow signal, and reducing the flow measurement variation due to local flow changes caused by the cardiac cycle.
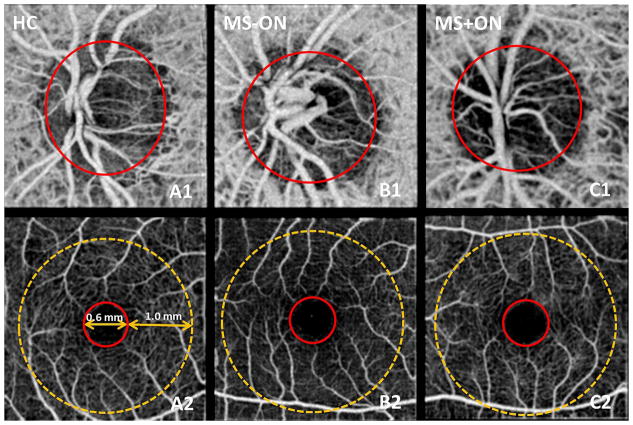
To measure ONH perfusion, cross-sectional registered intensity images and flow images were summarised and viewed as an en face maximum projection. The elliptical boundary was manually delineated on the en face intensity images and transferred to the OCT angiogram for disc region segmentation. The ONH flow index (FI) was defined as the average flow signal (decorrelation value) within the whole ONH. To measure parafoveal retinal perfusion, retinal circulation was segmented along the boundary set at retina pigment epithelium in intensity images and separately projected into en face view. The fovea was manually centred at the avascular zone on the OCT angiogram. The parafoveal retinal FI was defined as the average flow signal within the annular zone of 0.6 to 2.6 mm diameter around the foveal centre (figure 1).14 The central 0.6 mm diameter area (foveal avascular zone) of normal controls was used to determine the noise floor. The decorrelation values above noise floor are flow signals from either large vessels (bright signals in figure 1) or capillaries (weak signals in figure 1), and used for the calculation of FI. FI is a dimensionless parameter between 0 and 1. Due to the non-linear relationship between decorrelation and flow velocity, the FI mainly measured the area (or calibre) of large vessels and the area (or vessel density) and velocity of capillaries.
Figure 1.
En face optical coherence tomography angiograms of the optic nerve head (ONH) and macula in representative healthy control group (HC; A1, A2), multiple sclerosis without optic neuritis group (MS−ON; B1, B2), and multiple sclerosis with optic neuritis group (MS+ON; C1, C2). In the ONH angiograms (A1–C1), the flow index (FI) was averaged over the whole ONH (solid red circles). In comparison with the HC and MSON examples, the whole ONH microvascular network in MS+ON eye showed attenuation. In the macula retinal angiograms (A2–C2), the parafoveal FI was averaged over an annulus between diameters 0.6 mm (red circle) and 2.6 mm (yellow dotted circle). The parafoveal microvascular network in these examples showed no clear differences.
Repeatability and reproducibility
Intravisit repeatability of the ONH and parafoveal FIs were calculated from three patients with HC with three sets of scans within a single visit by a single operator. Intervisit reproducibility of the ONH and parafoveal FIs was calculated from three patients with HC with three sets of scans obtained on three separate visits by the same operator.
Statistical analysis
Statistical analyses were performed with commercial software (SPSS V.13.0; SPSS, Chicago, Illinois, USA). The coefficient of variation (CV) was calculated to assess repeatability and reproducibility of the ONH and parafoveal FIs. Best corrected visual acuity (BCVA) was converted to a log of the minimum angle of resolution (logMAR) for statistical analysis, and two-tailed independent sample t tests were used to compare the mean logMAR BCVA values of each group. The Mann–Whitney U test was used to analyse the FI differences between each group. The χ2 test was performed to compare the percentile differences between groups. All the tests had a significance level of 5%.
RESULTS
A total of 36 patients with MS (70 eyes) and 27 patients with HC (one eye for each patient, 27 eyes) were studied. For all patients, 24 eyes were excluded due to low signal strength (8 eyes), failed 3D registration (6 eyes), poor scan centration (10 eyes) for either ONH or macula scans. Therefore, 35 patients with MS (52 eyes) and 21 patients with HC (21 eyes) were included in the final analysis (table 1). Patients with HC tended to be older than the patients with MS, but were well matched for IOP and visual acuity. Patients with MS −ON were slightly older with shorter disease duration than the patients with MS +ON. The two MS subgroups were well matched for disability (EDSS, EDMUS), visual acuity and IOP.
Table 1.
Characteristics of the study groups
| Group (patients/eyes)
|
|||
|---|---|---|---|
| HC (21/21) | MS −ON (25/38) | MS +ON (10/14) | |
| Age (yrs) | 50±9.6 | 47±12.7 | 40±8.9 |
| High-contrast LogMAR BCVA | 0.01±0.1 | 0.04±0.1 | 0.01±0.1 |
| Low-contrast LogMAR BCVA | none | 0.65±0.2 | 0.72±0.2 |
| EDSS | n/a | 3.8±1.7 | 3.2±1.9 |
| EDMUS | n/a | 2.5±2.0 | 3.0±2.0 |
| IOP (mm Hg) | 15±2.3 | 15±3.1 | 14±2.2 |
| MS duration (yrs) | n/a | 12±8.4 | 17±10.4 |
BCVA, best corrected visual acuity; EDMUS, European Database for Multiple Sclerosis Grading Scale; EDSS, Expanded Disability Status Scale; HC, healthy control; IOP, intraocular pressure; MAR, minimum angle of resolution; MS, multiple sclerosis; MS+ON, MS with history of ON; MS−ON, MS without history of ON; n/a, not applicable; ON, optic neuritis.
Typical examples of ONH angiograms and macular retinal angiograms for HC and MS groups showed notable attenuation of ONH circulation in the MS+ON group (figure 1). The mean ONH FI in MS+ON group was significantly lower than the HC and MS−ON groups (p=0.004 for both), while there was no significant difference between the HC and MS−ON groups (p=0.924; figure 2). There were no significant differences in the mean parafoveal FIs among the three groups.
Figure 2.
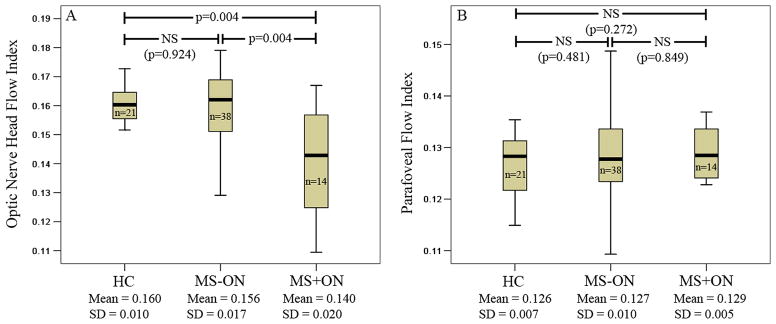
Box plots showing the optic nerve head (ONH) flow index (FI) (A) and the parafoveal FI (B) in healthy control (HC), MS −ON, and MS +ON groups. The median (dark bold line), IQR (box) and the whole range of values (whiskers) are shown. Mann–Whitney U tests showed significant reduction in the ONH FI of the MS +ON group compared to HC and MS −ON groups (p values shown in panel A). MS, multiple sclerosis, NS, non-significant; n, number of eyes.
Using the normative distribution of FI in the HC group, FI values were classified as within normal limits, borderline, or abnormal (table 2). Abnormal values were defined as those more than −2.33 SDs below the mean (1st percentile cutoff for normal distribution). Borderline values were defined as those between 1.65 and 2.33 SD below the mean (between 1st and 5th percentile cutoffs). A significantly higher percentage of MS+ON eyes had an abnormally low ONH FI compared to HC eyes (43% vs 5%, p=0.01). Twenty-one per cent of MS−ON eyes also had an abnormally reduced ONH FI (p=0.198). In the MS−ON group, the parafoveal FI was within the normal range for all eyes except one, thus, there were no significant differences among the three groups (table 2).
Table 2.
Incidence of flow index reduction in the healthy control (HC) and multiple sclerosis subgroups
| Groups (n eyes) | ONH flow index (%)
|
Parafoveal flow index (%)
|
||||
|---|---|---|---|---|---|---|
| Within normal (>5%) | Borderline (1–5%) | Abnormal (<1%) | Within normal (>5%) | Borderline (1–5%) | Abnormal (<1%) | |
| HC (n=21) | 20 (95) | 0 | 1 (5) | 21 (100) | 0 | 0 |
| MS total (n=52) | 36 (69) | 2 (4) | 14 (27) | 48 (92) | 2 (4) | 2 (4) |
| MS −ON (n=38) | 29 (76) | 1 (3) | 8 (21) | 34 (90) | 2 (5) | 2 (5) |
| MS + ON (n=14) | 7 (50)* | 1 (7) | 6 (43)* | 14 (100) | 0 | 0 |
The cutoff points of 1st and 5th percentiles were set at 2.33 and 1.65 SDs below the mean, respectively, according to normal distribution.
Significantly different from the HC group at the p<0.05 level using Fisher’s exact test.
MS, multiple sclerosis; ONH, optic nerve head.
Three HC eyes were scanned to assess intravisit repeatability and intervisit reproducibility of repeated measurements by pooled CV. The repeatability and reproducibility were 1.03% and 4.53% for ONH FI, and 1.65% and 3.55% for parafoveal FI, respectively. The intersubject variability of ONH and parafoveal FIs, which was evaluated by the CV in 21 HC eyes, was 6.3% and 7.7%, respectively.
DISCUSSION
As interest in the contribution of vascular disease to MS and ON pathophysiology is growing, we used OCT angiography to examine the ONH and retinal microcirculation changes in MS eyes with or without ON history. Our results showed that the ONH FI of MS +ON eyes was significantly lower than the values of the HC group and the MS −ON group. We also found that the MS +ON eyes had a significantly higher percentage of abnormally low ONH FI than the HC eyes. Moreover, ~21% of MS −ON eyes, despite a normal visual acuity, showed abnormal low ONH FI.
There are several possible reasons for the decreased blood perfusion in ONH of MS patients. First, MS damages and reduces the number of nerve fibres in the optic nerve, ONH and RNFL which, in turn, reduces metabolic activities. The reduced metabolic load lowers blood flow via autoregulatory mechanisms. MS is associated with a loss of peripapillary RNFL, a structural marker of axonal degeneration, using structural OCT system.17 Fisher et al12 found that MS +ON eyes demonstrated the greatest reduction in RNFL thickness, while MS −ON eyes had a lesser degree of RNFL reduction, when compared to disease-free control eyes. Our finding of the greatest loss of ONH FI in MS +ON eyes is consistent with this work. Second, there could be primary vascular dysfunctions, such as the endothelial abnormalities that have been noted in the brains of patients with MS.3–5
No significant differences in parafoveal FI were found among the three groups in this study. MS and ON are associated with macular ganglion cell loss, therefore, this result was unexpected, especially in contrast with the reduction in ONH FI. A possible explanation is that MS and ON only affect the innermost retinal layers, therefore, the middle retinal layers still had sufficient functionality to maintain autoregulation of macular blood flow in the normal range. Another explanation is that MS and ON often cause only minimal damage to foveal function, as evidenced by only 12% (6 eyes) of MS eyes having reduction in BCVA of two or more lines. Our results suggest that parafoveal FI is a relatively insensitive way to detect the damage caused by MS. Yet another possible explanation is that MS produces primary vascular dysfunctions in the optic nerve, which does affect the retina.
There are several other techniques that can detect decreased ONH perfusion in MS and ON patients. Using FA, Duker et al18 showed that ON is associated with delayed venous filling accompanied by venous dilation and tortuosity. Using colour Doppler ultrasonography, Modrzejewska et al19 found that the reduction of blood flow parameters in the central retinal artery and short posterior ciliary artery occurred in the ON-affected eyes and the fellow eyes. Using CDI, Akarsu et al20 also demonstrated that MS +ON was associated with impaired retrobulbar haemodynamics. Conversely, Hradilek et al21, also using CDI, failed to find an orbital haemodynamic difference between eyes with chronic ON and the control group.
Compared with the techniques mentioned above, OCT angiography is more repeatable, more reproducible, and less variable. It has been reported that 24 h reproducibility of LDF and CDI were between 1.6% and 18.5% CV.22 For LSFG, the inter-session reproducibilities of retinal veins and arteries were 8.4 ±5.6% and 10.9±9.9% CV.23 Using modified laser speckle technology, the ONH blood velocity reproducibility with 1 min and 24 h intervals were 11.7% and 13%, respectively.24 Likewise, intersubject variability of HC patients were much higher in MRI (~33%), LDF (17~21%), and LSFG (~38.4%) compared to OCT angiography (~7%).10,24,25 The higher repeatability and reproducibility suggests that OCT angiography may be more suitable than other imaging techniques for detecting and monitoring of abnormal ONH and parafoveal perfusion changes.
OCT angiography requires high scan speed. Therefore, a prototype OCT system with higher speed (100 kHz) than commercially available OCT instruments was used in this study. The next generation of commercial OCT systems with 70 kHz speed are fast enough for OCT angiography. Therefore, this new method of assessing ONH circulation could be easily accessible in the near future. Although MRI is still needed to detect the characteristic white matter lesions in the brain and establish MS diagnosis, OCT evaluation of optic nerve structure and perfusion could be a relatively low-cost method for quantifying the damage caused by MS.
The current study had some limitations. The study sample size was relatively small. Some (~15%) eyes were excluded due to weak signal (media opacity, dry eye, poor beam focusing or position), failed 3D registration (excessive eye motion or blink), or poor scan centration. Therefore, the technique requires careful operator technique and some patient selection. Further studies with histopathological or other correlations are necessary to help understand the mechanisms of the changes of ONH microcirculation. However, our results are promising.
In conclusion, OCT angiography with the SSADA algorithm is highly repeatable and reproducible for FI measurement of the ONH and parafoveal regions. It was able to detect reduction of ONH perfusion in a significant portion of MS patients with or without a history of ON. Additional studies will explore further the structure-function-perfusion relationships in MS to help determine if perfusion can provide early insights into MS pathology and progression. This new technology may be useful in the detection and monitoring of optic nerve damage caused by MS and ON.
Acknowledgments
Funding This work was supported by NIH grants: Clinical and Translational Science Award Grant (UL1TR000128), R01 EY023285, R01 EY013516, R01-EY11289, and P30EY010572. Additional supports were AFOSR FA9550-10-1-0551, FA9550-12-1-0499, German Research Foundation DFG-HO-1791/11-1, and a grant from Research to Prevent Blindness.
Footnotes
Competing interests DH has a significant financial interest in Carl Zeiss Meditec. Oregon Health & Science University (OHSU), Yali Jia, and David Huang have a significant financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. JGF, BP and JH receive royalties from intellectual property owned by MIT and licensed to Optovue, Inc. Other authors do not have financial interest in the subject of this article.
Patient consent Obtained.
Ethics approval Oregon Health & Science University Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors XW: data analysis and interpretation, drafting the article; YJ: data analysis and interpretation, critically revising for intellectual content; RS: data acquisition, critically revising for intellectual content; BP, JJL, BB, JH, JGF: technical support; QW: critically revising for intellectual content; DH: providing conception and design; data analysis and interpretation, critically revising for intellectual content; obtaining funding and supervision.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Percy AK, Nobrega FT, Kurland LT. Optic neuritis and multiple sclerosis. An epidemiologic study. Arch Ophthalmol. 1972;87:135–9. doi: 10.1001/archopht.1972.01000020137004. [DOI] [PubMed] [Google Scholar]
- 3.Plumb J, McQuaid S, Mirakhur M, et al. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12:154–69. doi: 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen NB, Lichtman JH, Cohen HW, et al. Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology. 2008;30:234–8. doi: 10.1159/000128103. [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–7. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal RV, Biswas J, Gunasekaran D. Indocyanine green angiography in posterior uveitis. Indian J Ophthalmol. 2013;61:148–59. doi: 10.4103/0301-4738.112159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–8. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avila CP, Jr, Bartsch DU, Bitner DG, et al. Retinal blood flow measurements in branch retinal vein occlusion using scanning laser Doppler flowmetry. Am J Ophthalmol. 1998;126:683–90. doi: 10.1016/s0002-9394(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama T, Araie M, Riva CE, et al. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88:723–9. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 10.Peng Q, Zhang Y, Nateras OS, et al. MRI of blood flow of the human retina. Magn Reson Med. 2011;65:1768–75. doi: 10.1002/mrm.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell DG. Color Doppler imaging: principles, limitations, and artifacts. Radiology. 1990;177:1–10. doi: 10.1148/radiology.177.1.2204956. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–5. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokayer J, Jia Y, Dhalla A, et al. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed Opt Express. 2013;4:1909–24. doi: 10.1364/BOE.4.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus MF, Potsaid B, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012;3:1182–99. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson AP, Trip SA, Schlottmann PG, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 18.Duker JS, Sergott RC, Savino PJ, et al. Optic neuritis with secondary retinal venous stasis. Ophthalmology. 1989;96:475–80. doi: 10.1016/s0161-6420(89)32871-5. [DOI] [PubMed] [Google Scholar]
- 19.Modrzejewska M, Karczewicz D, Wilk G. Assessment of blood flow velocity in eyeball arteries in multiple sclerosis patients with past retrobulbar optic neuritis in color Doppler ultrasonography. Klin Oczna. 2007;109:183–6. [PubMed] [Google Scholar]
- 20.Akarsu C, Tan FU, Kendi T. Color Doppler imaging in optic neuritis with multiple sclerosis. Graefes Arch Clin Exp Ophthalmol. 2004;242:990–4. doi: 10.1007/s00417-004-0948-1. [DOI] [PubMed] [Google Scholar]
- 21.Hradilek P, Stourac P, Bar M, et al. Colour Doppler imaging evaluation of blood flow parameters in the ophthalmic artery in acute and chronic phases of optic neuritis in multiple sclerosis. Acta Ophthalmol. 2009;87:65–70. doi: 10.1111/j.1755-3768.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 22.Luksch A, Lasta M, Polak K, et al. Twelve-hour reproducibility of retinal and optic nerve blood flow parameters in healthy individuals. Acta Ophthalmol. 2009;87:875–80. doi: 10.1111/j.1755-3768.2008.01388.x. [DOI] [PubMed] [Google Scholar]
- 23.Aizawa N, Yokoyama Y, Chiba N, et al. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol. 2011;5:1171–6. doi: 10.2147/OPTH.S22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamaki Y, Araie M, Tomita K, et al. Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn J Ophthalmol. 1997;41:49–54. doi: 10.1016/s0021-5155(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 25.Kimura I, Shinoda K, Tanino T, et al. Scanning laser Doppler flowmeter study of retinal blood flow in macular area of healthy volunteers. Br J Ophthalmol. 2003;87:1469–73. doi: 10.1136/bjo.87.12.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]