Abstract
Objective
To summarize the epidemiology of sarcomas occurring in the head and neck and identify prognostic factors for patient survival.
Study Design and Setting
Cross-sectional analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program.
Methods
The SEER 18 registries, comprising sarcoma diagnoses made from 1973 to 2010, were queried for sarcomas arising in the head and neck. Pediatric and adult patients were analyzed separately, and multivariate and propensity-matched analyses were performed to identify predictors of disease-specific survival.
Results
In all, 11,481 adult cases and 1244 pediatric cases were identified. In adults, the most common histologic subtypes were malignant fibrous histiocytoma (MFH), Kaposi sarcoma, and hemangiosarcoma, while in the pediatric cohort, the most common histologic subtypes were rhabdomyosar-coma, MFH, and osteosarcoma. Cause-specific 2-, 5-, and 10-year survival rates were 76%, 66%, and 61% for adults and 84%, 73%, and 71% for pediatric patients. Multivariate analysis performed for adults revealed that male gender, absence of radiation therapy, and stage I disease were associated with improved cause-specific survival reaching statistical significance. However, a propensity-matched model demonstrated no significant difference in cause-specific survival between patients who received radiation and those who did not.
Conclusion
Sarcomas, a heterogeneous group of malignant mesenchymal tumors, are uncommonly found in the head and neck. This study represents the largest analysis of patients with head and neck sarcomas in the literature and demonstrates the impact of age, gender, primary site, histology, and radiation status on overall prognosis.
Keywords: head and neck sarcomas, SEER
Introduction
Sarcomas are a heterogeneous group of mesenchymal malignant neoplasms. They account for 1% to 2% of all head and neck malignancies, while between 4% and 10% of all adult sarcomas occur in the head and neck.1,2 Metastases are relatively uncommon and are only seen in approximately 10% of patients at presentation.3
The 5-year survival of head and neck sarcomas has been reported at approximately 60%.4–7 While sarcomas not arising in the head and neck largely confer mortality via metastatic disease, patients with sarcomas in the head and neck primarily succumb to local recurrence.6,8,9 This has generally been attributed to the proximity of vital structures in the head and neck and may be related to the inherent difficulty in obtaining wide margins during surgical resection while limiting concomitant morbidity.10,11 Surgical resection has been established as the mainstay of therapy.10,12,13 While chemotherapy has emerged as a highly effective treatment for certain sarcomas, including rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma,14–17 the efficacy of chemotherapy in neoadjuvant, and adjuvant fashion for other histologies in improving overall survival remains in debate.18–21 On the other hand, radiation therapy is an important component of multimodality therapy, particularly in treating patients with high-grade tumors or positive margins following surgical resection.11,13,22
Given the relative infrequency of head and neck sarcomas, previous series have been limited by modest sample size. In addition, previous studies, with few exceptions,3,23 have demonstrated a bias toward pathologies treated exclusively by head and neck surgeons, downplaying the prevalence of a large subset of sarcomas in the head and neck—those involving the skin and soft tissues.
The objectives of this study were to characterize the epidemiology of head and neck sarcomas and identify prognostic factors affecting cause-specific survival. To investigate this, a retrospective analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 18 registries was conducted. The SEER 18 data represent 27.8% of the US population and include records obtained from hospital registries, pathology laboratories, and physician offices. The robust source data has ensured that this is the largest series of head and neck sarcomas to date.24
Methods
As no identifiable private information was available, this study did not involve human subjects as defined by the Department of Health and Human Services and was hence exempt from Institutional Review Board approval at the University of California, Los Angeles. The SEER 18 registries, comprising data from 1973 to 2010, were used for analysis. Inclusion criteria consisted of the following: records with a diagnosis of a specific subtype of sarcoma or sarcoma NOS; records reporting a primary site in the head and neck, excluding the central nervous system and orbit; records corresponding to 1 primary tumor or the first primary tumor in patients with 2 or more tumors. Of note, American Joint Committee on Cancer (AJCC) stage was consistently reported in the SEER 18 registries beginning only in 2004.
Patients were stratified into 2 main age groups, which were treated separately for all statistical analyses described in the following: ages 0 through 19 (‘‘pediatric cohort’’) and ages 20 and above (‘‘adult cohort’’). Statistical analyses were performed using SPSS (version 20.0, IBM Corporation, Armonk, New York). Descriptive data were extracted, including gender; age at diagnosis; primary site; histologic subtype; tumor, node, metastasis (TNM) stage and stage group; and use of surgery and/or radiation. Chemotherapy status was not reported in the SEER 18 registries and was therefore intentionally excluded from analysis.
Kaplan-Meier analysis was performed and plots were generated using SPSS as stratified by histologic subtype, AJCC stage (for cases diagnosed in 2004 and subsequent), and radiation status. Predictors of overall and cause-specific survival were evaluated using multivariate Cox proportional hazard models, including the variables of gender, stage, decade of diagnosis, and radiation status.
A propensity score matched model was used to investigate the effects of radiation on survival. In this analysis, a propensity score representing the probability of receiving radiation treatment was first calculated for each patient by using a logistic regression model including the variables of gender, age, decade of diagnosis, primary site, histologic subtype, stage at initial diagnosis, and presence of metastases. After 1:1 matching, there were 2 groups of size 462 in the adult cohort and 2 groups of size 94 in the pediatric cohort, corresponding to subjects who received radiation versus those who did not. Following this matching, the effect of radiation on survival was compared.
Results
A total of 12,725 cases of head and neck sarcoma were identified in the SEER 18 registries, affecting 11,481 adults and 1244 pediatric patients. In the entire study population, there was a male preponderance (n = 8989, 71%, vs female, n = 3736, 29%). The median age group affected was 50 to 54 years. More cases were diagnosed in the 2000s (n = 7075, 56%) than over any other decade (1970s, 1980s, and 1990s).
Adult Cohort
The male gender predominated, with 8330 cases (73%) reported; females accounted for only 3151 cases (27%). The median age group affected was 55 to 59 years. The most common primary site was the skin and soft tissues (n = 7244, 63%), followed by the bones of the skull and face (n = 1277, 11%) and the oral cavity (n = 1108, 10%; Figure 1A). The most common histologic subtypes were malignant fibrous histio-cytoma (MFH, n = 3563, 31%), Kaposi sarcoma (KS, n = 2311, 20%), and hemangiosarcoma (n = 895, 8%; Figure 1B).
Figure 1.
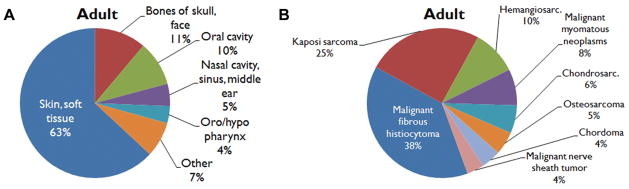
Adult cohort: distribution of (A) primary site and (B) histology.
Complete TNM stage information, including stage grouping, was reported for 1022 patients, representing 9% of the adult cohort. In these patients, stage I accounted for 387 patients (38%), stage II accounted for 322 patients (32%), stage III accounted for 111 patients (11%), and stage IV accounted for 202 patients (20%).
In all, 5835 adult patients (51%) underwent surgery without radiation therapy, 993 patients (9%) underwent radiation therapy without surgery, and 2063 patients (18%) underwent both radiation and surgery. In addition, 2244 patients (20%) received neither radiation therapy nor surgery, and treatment details were not fully reported in 346 patients (3%).
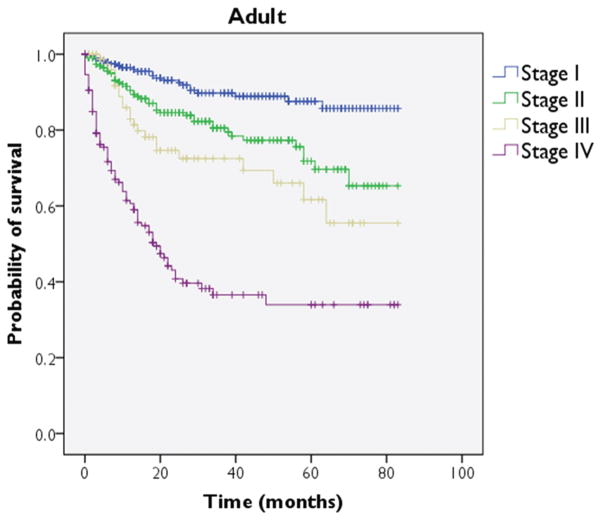
Overall 2-, 5-, and 10-year survival rates for all adult sarcoma patients were 76%, 66%, and 61%, respectively. MFH and liposarcoma demonstrated 5-year survival rates of 90% or higher, while particularly poor prognoses were noted with KS, rhabdomyosarcoma, and hemangiosarcoma, each with 5-year survival under 50% (Table 1). A Kaplan-Meier plot was generated to depict survival as stratified by stage (Figure 2); this comparison reached significance on log-rank testing (P <.001).
Table 1.
Two-, 5-, and 10-Year Cause-Specific Survival Rates for Adult Sarcomas, Stratified by Histologic Type (%).
| 2-Year | 5-Year | 10-Year | |
|---|---|---|---|
| All adult sarcomas | 76 | 66 | 61 |
| Chondrosarcoma | 93 | 87 | 77 |
| Chordoma | 90 | 80 | 65 |
| Hemangiosarcoma | 59 | 44 | 40 |
| Kaposi sarcoma | 49 | 32 | 29 |
| Liposarcoma | 97 | 96 | 90 |
| Malignant fibrous histiocytoma | 94 | 90 | 88 |
| Malignant myomatous neoplasm | 87 | 81 | 73 |
| Malignant nerve sheath tumor | 80 | 69 | 62 |
| Osteosarcoma | 71 | 59 | 52 |
| Rhabdomyosarcoma | 55 | 36 | 32 |
Figure 2.
Adult cohort: Kaplan-Meier survival plot for cause-specific survival as stratified by stage.
Multivariate analysis was performed to identify factors predicting cause-specific mortality in the adult cohort (Table 2). Male gender (hazard ratio [HR] = 1.39, 95% confidence interval [CI], 1.27–1.51, P <.001) and administration of radiation (HR = 1.83, 95% CI, 1.70–1.97, P < .001) were associated with increases in risk of cause-specific death reaching significance (P < .05). When compared to stage I disease, higher staged disease (II, III, IV) conferred statistically significant increases in the risk of cause-specific mortality (P < .05 for all). When compared to a diagnosis of sarcoma made in the 2000s, a diagnosis made in the 1970s, 1980s, and 1990s conferred statistically significant increases in the risk of cause-specific mortality (HR = 1.51, 1.90, and 2.00, respectively; P <.001 for all).
Table 2.
Hazard Ratios for Cause-Specific Mortality Obtained by Multivariate Regression, Adult Cohort.
| Covariate (reference group) | Hazard Ratio (95% confidence interval) | P Value |
|---|---|---|
| Gender (female) | ||
| Male | 1.39 (1.27, 1.51) | <.001 |
| Decade of diagnosis (2000s) | ||
| 1990s | 2.00 (1.83, 2.18) | <.001 |
| 1980s | 1.90 (1.71, 2.12) | <.001 |
| 1970s | 1.51 (1.28, 1.80) | <.001 |
| Radiation status (not given) | ||
| Given | 1.83 (1.70, 1.97) | <.001 |
| Stage (I) | ||
| II | 1.94 (1.17, 3.22) | .01 |
| III | 2.76 (1.54, 4.96) | .001 |
| IV | 8.16 (5.12, 12.99) | <.001 |
The association of radiation with poorer prognosis was investigated further using a propensity score matched model. A propensity score representing the probability of receiving radiation treatment was calculated using a logistic regression model that identified variables associated with radiation use. The variables identified were gender, age, decade of diagnosis, site, histology, stage, and presence of metastases. After 1:1 matching, 2 groups of size 462 each were identified. The hazard ratio of cause-specific mortality when comparing the use of radiation to no radiation was 1.08 (95% CI, 0.79–1.47, P = .650), suggesting that radiation had no statistically significant impact on cause-specific mortality when controlling for the additional variables listed.
Pediatric Cohort
Males and females were affected nearly equally, with 659 cases (53%) reported in males and 585 cases (47%) reported in females. The median age group affected was 5 to 9 years. The most common primary site was the skin and soft tissues (n = 576, 46%), followed by the bones of the skull and face (n = 260, 21%) and the nasal cavities, paranasal sinuses, and middle ear (n = 157, 12.6%; Figure 3A). The most common histologic subtypes were rhabdomyosarcoma (n = 592, 48%), MFH (n = 140, 11%), osteosarcoma (n = 97, 8%), and Ewing sarcoma (n = 76, 6%; Figure 3B).
Figure 3.
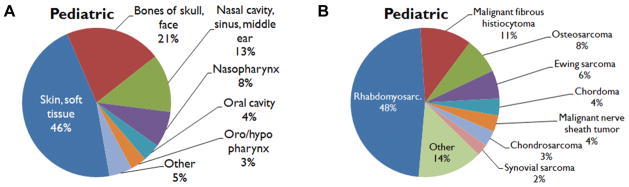
Pediatric cohort: distribution of (A) primary site and (B) histology.
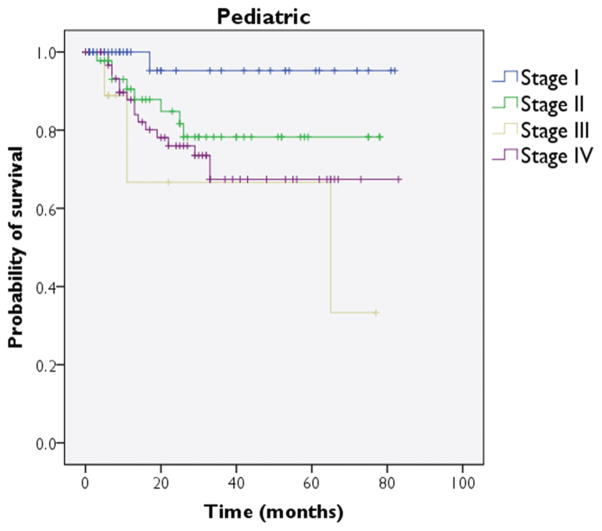
Complete TNM stage information, including stage grouping, was reported for 177 patients, representing 14% of the pediatric cohort. In these patients, stage I accounted for 52 patients (29%), stage II accounted for 52 patients (29%), stage III accounted for 10 patients (6%), and stage IV accounted for 63 patients (36%) (Figure 4).
Figure 4.
Pediatric cohort: Kaplan-Meier survival plot for cause-specific survival as stratified by stage.
Four hundred and thirty pediatric patients (35%) underwent surgery without radiation therapy, 316 patients (25%) underwent radiation therapy without surgery, and 374 patients (30%) underwent both radiation and surgery. Eighty-eight patients (7%) received neither radiation therapy nor surgery, and treatment details were not fully reported in 36 patients (3%).
Overall 2-, 5-, and 10-year survival rates for all pediatric sarcoma patients were 84%, 73%, and 71%, respectively. Chondrosarcoma and MFH demonstrated 5-year survival rates of 90% or higher, while rhabdomyosarcoma, Ewing sarcoma, and chordoma were associated 5-year survival rates below 70% (Table 3). A Kaplan-Meier plot was generated to depict survival as stratified by stage (Figure 2); this comparison reached significance on log-rank testing (P = .027).
Table 3.
Two-, 5-, and 10-Year Survival Rates for Pediatric Sarcomas, Stratified by Histologic Type (%).
| Histology | 2-Year | 5-Year | 10-Year |
|---|---|---|---|
| All pediatric sarcomas | 84 | 73 | 71 |
| Chondrosarcoma | 97 | 90 | 86 |
| Chordoma | 82 | 68 | 55 |
| Ewing sarcoma | 77 | 68 | 65 |
| Malignant fibrous histiocytoma | 96 | 93 | 93 |
| Osteosarcoma | 86 | 77 | 75 |
| Rhabdomyosarcoma | 81 | 68 | 65 |
Multivariate analysis was performed to identify factors predicting cause-specific mortality in the pediatric cohort (Table 4). Administration of radiation (HR = 2.01, 95% CI, 1.55–2.60, P < .001) was significantly associated with an increase in the risk of cause-specific mortality. When compared to stage I disease, stage III disease at presentation was significantly associated with increased cause-specific mortality (HR = 12.12, 95% CI, 1.26–116.9, P = .03), but stage II and stage IV disease did not reach similar significance. When compared to a diagnosis of sarcoma made in the 2000s, a diagnosis made in the 1970s conferred a significantly higher risk of cause-specific mortality (HR = 1.75, 95% CI, 1.23–2.50, P = .002). A similar trend was noted for sarcomas diagnosed in the 1980s, but this did not reach significance (HR = 1.34, 95% CI, 0.96–1.85, P = .08). No significant trends were noted when considering gender or age at diagnosis.
Table 4.
Hazard Ratios for Cause-Specific Mortality Obtained by Multivariate Regression, Pediatric Cohort.
| Covariate (reference group) | Hazard Ratio (95% confidence interval) | P Value |
|---|---|---|
| Gender (female) | ||
| Male | 1.14 (0.91, 1.44) | .249 |
| Decade of diagnosis (2000s) | ||
| 1990s | 0.89 (0.65, 1.21) | .455 |
| 1980s | 1.34 (0.96, 1.85) | .084 |
| 1970s | 1.75 (1.23, 2.50) | .002 |
| Radiation status (not given) | ||
| Given | 2.01 (1.55, 2.60) | <.001 |
| Stage (I) | ||
| II | 4.70 (0.59, 37.66) | .145 |
| III | 12.12 (1.26, 116.9) | .031 |
| IV | 6.33 (0.84, 47.91) | .074 |
The association of radiation with poorer prognosis was investigated further using a propensity score matched model. As before, logistic regression revealed the following variables to be associated with radiation use: gender, age, decade of diagnosis, site, histology, stage, and presence of metastases. After 1:1 matching, 2 groups of size 94 each were identified. The hazard ratio of cause-specific mortality when comparing the use of radiation to no radiation was 1.43 (95% CI, 0.69–2.95, P = .339), suggesting that radiation had no statistically significant impact on cause-specific mortality when controlling for the additional variables listed.
Discussion
Head and neck sarcomas represent a heterogeneous group of malignancies. While the most common entities encountered in the clinical practice of head and neck surgeons include osteosarcoma, chondrosarcoma, and rhabdomyosarcoma, among others,11 the epidemiologic data presented here demonstrate that MFH and KS together represent over a majority of head and neck sarcomas in adults. This discrepancy likely reflects the number of sarcomas diagnosed and managed either medically or surgically by providers outside of the specialty of head and neck surgery.
Adult Cohort
Among adult patients, the most common histologic subtypes were malignant fibrous histiocytoma, Kaposi sarcoma, and hemangiosarcoma. MFH predominantly affects middle- and late-aged adults and is more commonly found in the proximal extremities than in the head and neck.25 It usually involves the fascia or skeletal muscle and is only rarely confined to the epidermis and dermis.26 It is generally considered to be the most common histologic subtype among radiation-induced sarcomas of the head and neck.27 MFH is classified into common types (including storiform and pleomorphic subtypes) as well as the less common myxoid, xanthogranulomatous, and giant cell types.25 In the head and neck, the sinonasal tract and the major salivary glands have also been reported as primary sites.28,29 Wide excision has classically been considered as the standard of care, with postoperative radiation considered in aggressive disease or positive margins.30 While some report worse survival in MFH arising in the head and neck as compared to disease involving the extremities,31 the data here demonstrate 5-and 10-year cause-specific survival rates of 90% and 88%, respectively, approximating survival reported for MFH involving all body sites.32
The present study reiterates previously reported findings that advanced TNM stage group (III and IV) are associated with worse prognosis.3 Other factors that have been consistently reported to influence survival include margin status, size of tumor exceeding 5 cm, and high-grade histology, which were not consistently reported in the SEER registries and not included in the present analysis.2,23 In the current work, male gender was significantly associated with a poorer prognosis. This finding has not previously been reported among adult head and neck sarcomas, although the male gender has been associated with a poorer prognosis among soft tissue sarcomas of all body sites.33
In this study, sarcomas of the oral cavity portended a particularly grave prognosis, and primarily represented Kaposi sarcoma, accounting for 78% of all oral cavity sarcomas. KS, previously a rare entity, sprang to the forefront of medical attention after a surge in diagnoses following the advent of HIV and AIDS.34,35 The etiologic agent of KS and its association with AIDS remained unclear for nearly 15 years following the resurgence of KS,36 but human herpesvirus-8 was eventually implicated.37–39 Aggressive treatment of KS with antiretroviral therapy directed at optimizing immune function in the setting of HIV infection has become accepted as the standard of care,40 with local and systemic chemotherapy as well as radiation reserved for aggressive or locally symptomatic cases.41 Before these paradigms in the early- to mid-1990s, though, KS was associated with early death,35 likely accounting for the poor prognosis of oral cavity sarcomas reported here.
The findings in this study echo observations noted in sarcomas involving sites beyond the head and neck. While direct comparisons must not be undertaken lightly, partially due to varying distributions of histologic subtypes in head and neck compared to non–head and neck sarcomas, several trends emerge. In a SEER analysis of sarcomas of all body sites, stage and age at diagnosis were both confirmed as independent poor predictors of prognosis in musculoskeletal sarcomas.42 A similar analysis of soft tissue sarcomas only revealed that increasing age and male gender were also associated with poorer outcomes,33 similar to our findings.
On initial multivariate analysis, radiation appeared to be associated with an increase in cause-specific mortality. In our propensity-matched model, however, factors predicting radiation were used to perform 1:1 matching, including TNM stage group, between 2 groups of 462 each. Following this matching, regression analysis led to no significant difference comparing the use of radiation to no radiation (HR = 1.08, P = .650). Ultimately, radiation continues to be clinically indicated for large tumors, aggressive histology, and negative margins and remains an integral part of sarcoma treatment in such situations.43–45
Pediatric Cohort
Unlike the adult cohort, the gender distribution was roughly equal, with only a slight male predominance (53% of cases). There was no substantial preference for 1 treatment modality over another (surgery alone, 35% of patients; radiation alone, 25%; both surgery and radiation, 30%). Among pediatric patients, rhabdomyosarcoma accounted for nearly a majority of cases (48%), followed by malignant fibrous histiocytoma, osteosarcoma, and Ewing sarcoma, in that order.
Rhabdomyosarcoma predominantly affects the pediatric population, with 65% of rhabdomyosarcoma cases in the current series being diagnosed in patients aged 19 and under. It represents approximately 5% of all pediatric tumors. As confirmed in the present series, rhabdomyosarcoma accounts for approximately half of all pediatric sarcomas.46 The most common primary site for rhabdomyosarcoma is the head and neck, representing about 40% of cases.47 Rhabdomyosarcoma is classically segregated into 2 main subtypes, embryonaland alveolar, with variable emphasis placed on the botryoid and pleomorphic variants.48
The Intergroup Rhabdomyosarcoma Study Group (IRSG) has proposed a surgicopathologic grading system as well as a pretreatment TNM staging system.49 In this schema, group I represents localized disease that is completely resected, group II represents microscopic disease remaining, group III represents incomplete resection or biopsy with gross residual disease, and group IV accounts for distant metastases present at time of diagnosis. Treatment of non-metastatic disease primarily involves surgery followed by adjuvant chemotherapy, usually a combination of vincris-tine, dactinomycin, and cyclophosphamide, although other regimens are often utilized. Radiation therapy is more commonly used in adjunctive fashion for group III disease.50 The overall 5-year progression-free survival of all pediatric patients with rhabdomyosarcoma has been reported as 62% to 83%,47 in agreement with the current work suggesting a cause-specific survival rate of 68% at 5 years.
Unlike the adult cohort, gender did not reach statistical significance as a predictor of cause-specific mortality. The administration of radiation was significantly associated with a higher risk of cause-specific mortality on initial multivariate analysis. However, similar to the adult findings, this association of radiation with worse outcomes was not confirmed by the propensity matched model (HR of radiation versus no radiation = 1.43, P = .339).
Conclusion
To our knowledge, this study represents the largest series on head and neck sarcomas. Otolaryngologists must remain cognizant of the prevalence of sarcomas routinely diagnosed and treated by providers outside of our specialty, including surgical oncologists and dermatologists. The epidemiologic and survival data presented here provide insights for patient counseling and clinical decision making. Surgery as primary therapy is the mainstay of treatment of head and neck sarcomas; radiation therapy continues to play an important role when surgery is unfeasible or in an adjunctive setting, either preceding or following surgery, in treating aggressive tumors or positive margins. Further investigation is needed to clarify the role of chemotherapy in adult sarcomas of the head and neck.
Acknowledgments
Funding source: This research was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124, specifically providing salary support for Mr Grogan.
Footnotes
Author Contributions
Kevin A. Peng, study design, data collection, statistical analysis, drafting manuscript, editing manuscript; Tristan Grogan, data collection, statistical analysis, drafting manuscript, editing manuscript; Marilene B. Wang, study design, editing manuscript.
Disclosures
Competing interests: None.
Sponsorships: None.
This article was presented as a poster at the 2013 AAO-HNSF Annual Meeting & OTO EXPO; September 29–October 3, 2013; Vancouver, British Columbia, Canada.
References
- 1.Kraus DH. Sarcomas of the head and neck. Curr Oncol Rep. 2002;4:68–75. doi: 10.1007/s11912-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 2.Kraus DH, Dubner S, Harrison LB, et al. Prognostic factors for recurrence and survival in head and neck soft tissue sarcomas. Cancer. 1994;74:697–702. doi: 10.1002/1097-0142(19940715)74:2<697::aid-cncr2820740224>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Farhood AI, Hajdu SI, Shiu MH, et al. Soft tissue sarcomas of the head and neck in adults. Am J Surg. 1990;160:365–369. doi: 10.1016/s0002-9610(05)80544-6. [DOI] [PubMed] [Google Scholar]
- 4.Greager JA, Patel MK, Briele HA, et al. Soft tissue sarcomas of the adult head and neck. Cancer. 1985;56:820–824. doi: 10.1002/1097-0142(19850815)56:4<820::aid-cncr2820560420>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo HJ, Koness RJ, MacFarlane JK, et al. Head and neck sarcoma: report of the Head and Neck Sarcoma Registry. Society of Head and Neck Surgeons Committee on Research. Head Neck. 1992;14:1–7. doi: 10.1002/hed.2880140102. [DOI] [PubMed] [Google Scholar]
- 6.Dudhat SB, Mistry RC, Varughese T, et al. Prognostic factors in head and neck soft tissue sarcomas. Cancer. 2000;89:868–872. [PubMed] [Google Scholar]
- 7.Colville RJ, Charlton F, Kelly CG, et al. Multidisciplinary management of head and neck sarcomas. Head Neck. 2005;27:814–824. doi: 10.1002/hed.20232. [DOI] [PubMed] [Google Scholar]
- 8.Eeles RA, Fisher C, A’Hern RP, et al. Head and neck sarcomas: prognostic factors and implications for treatment. Br J Cancer. 1993;68:201–207. doi: 10.1038/bjc.1993.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bree R, van der Valk P, Kuik DJ, et al. Prognostic factors in adult soft tissue sarcomas of the head and neck: a single-centre experience. Oral Oncol. 2006;42:703–709. doi: 10.1016/j.oraloncology.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Tran LM, Mark R, Meier R, et al. Sarcomas of the head and neck. Prognostic factors and treatment strategies. Cancer. 1992;70:169–177. doi: 10.1002/1097-0142(19920701)70:1<169::aid-cncr2820700127>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Sturgis EM, Potter BO. Sarcomas of the head and neck region. Curr Opin Oncol. 2003;15:239–252. doi: 10.1097/00001622-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Penel N, Van Haverbeke C, Lartigau E, et al. Head and neck soft tissue sarcomas of adult: prognostic value of surgery in multimodal therapeutic approach. Oral Oncol. 2004;40:890–897. doi: 10.1016/j.oraloncology.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Weber RS, Benjamin RS, Peters LJ, et al. Soft tissue sarcomas of the head and neck in adolescents and adults. Am J Surg. 1986;152:386–392. doi: 10.1016/0002-9610(86)90309-0. [DOI] [PubMed] [Google Scholar]
- 14.Rosen G, Caparros B, Nirenberg A, et al. Ewing’s sarcoma: ten-year experience with adjuvant chemotherapy. Cancer. 1981;47:2204–2213. doi: 10.1002/1097-0142(19810501)47:9<2204::aid-cncr2820470916>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Wolden SL, Anderson JR, Crist WM, et al. Indications for radio-therapy and chemotherapy after complete resection in rhabdo-myosarcoma: a report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 17.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 18.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 19.Glenn J, Kinsella T, Glatstein E, et al. A randomized, prospective trial of adjuvant chemotherapy in adults with soft tissue sarcomas of the head and neck, breast, and trunk. Cancer. 1985;55:1206–1214. doi: 10.1002/1097-0142(19850315)55:6<1206::aid-cncr2820550612>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for "high-risk" adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 21.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 22.Chen SA, Morris CG, Amdur RJ, et al. Adult head and neck soft tissue sarcomas. Am J Clin Oncol. 2005;28:259–263. doi: 10.1097/01.coc.0000158440.27229.d6. [DOI] [PubMed] [Google Scholar]
- 23.Bentz BG, Singh B, Woodruff J, et al. Head and neck soft tissue sarcomas: a multivariate analysis of outcomes. Ann Surg Oncol. 2004;11:619–628. doi: 10.1245/ASO.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence -SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (1973–2010 varying) - Linked To County Attributes - Total U.S., 1969–2011 Counties. April 2013 ed: National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; 2013.
- 25.Enjoji M, Hashimoto H, Tsuneyoshi M, et al. Malignant fibrous histiocytoma. A clinicopathologic study of 130 cases. Acta Pathol Jpn. 1980;30:727–741. [PubMed] [Google Scholar]
- 26.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Patel SG, See AC, Williamson PA, et al. Radiation induced sarcoma of the head and neck. Head Neck. 1999;21:346–354. doi: 10.1002/(sici)1097-0347(199907)21:4<346::aid-hed9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Barnes L, Kanbour A. Malignant fibrous histiocytoma of the head and neck. A report of 12 cases. Arch Otolaryngol Head Neck Surg. 1988;114:1149–1156. doi: 10.1001/archotol.1988.01860220083030. [DOI] [PubMed] [Google Scholar]
- 29.Blitzer A, Lawson W, Biller HF. Malignant fibrous histiocytoma of the head and neck. Laryngoscope. 1977;87:1479–1499. doi: 10.1288/00005537-197709000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Ogura JH, Toomey JM, Setzen M, et al. Malignant fibrous histio-cytoma of the head and neck. Laryngoscope. 1980;90:1429–1440. [PubMed] [Google Scholar]
- 31.Sabesan T, Xuexi W, Yongfa Q, et al. Malignant fibrous histiocytoma: outcome of tumours in the head and neck compared with those in the trunk and extremities. Br J Oral Maxillofac Surg. 2006;44:209–212. doi: 10.1016/j.bjoms.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Pezzi CM, Rawlings MS, Jr, Esgro JJ, et al. Prognostic factors in 227 patients with malignant fibrous histiocytoma. Cancer. 1992;69:2098–2103. doi: 10.1002/1097-0142(19920415)69:8<2098::aid-cncr2820690815>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez JC, Perez EA, Franceschi D, et al. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–14. doi: 10.1016/j.jss.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease C. Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 35.Friedman-Kien AE, Laubenstein LJ, Rubinstein P, et al. Disseminated Kaposi’s sarcoma in homosexual men. Ann Intern Med. 1982;96:693–700. doi: 10.7326/0003-4819-96-6-693. [DOI] [PubMed] [Google Scholar]
- 36.Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 37.Dupin N, Grandadam M, Calvez V, et al. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi’s sarcoma. Lancet. 1995;345:761–762. doi: 10.1016/s0140-6736(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 38.Kedes DH, Operskalski E, Busch M, et al. The seroepidemiol-ogy of human herpesvirus 8 (Kaposi’s sarcoma-associated her-pesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nature Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 39.Huang YQ, Li JJ, Kaplan MH, et al. Human herpesvirus-like nucleic acid in various forms of Kaposi’s sarcoma. Lancet. 1995;345:759–761. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 40.Lebbe C, Blum L, Pellet C, et al. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi’s sarcoma. Aids. 1998;12:F45–9. doi: 10.1097/00002030-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445–2451. doi: 10.1200/JCO.1998.16.7.2445. [DOI] [PubMed] [Google Scholar]
- 42.Ng VY, Scharschmidt TJ, Mayerson JL, et al. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597–2604. [PubMed] [Google Scholar]
- 43.Le QT, Fu KK, Kroll S, et al. Prognostic factors in adult soft-tissue sarcomas of the head and neck. Int J Radiat Oncol Biol Phys. 1997;37:975–984. doi: 10.1016/s0360-3016(97)00103-x. [DOI] [PubMed] [Google Scholar]
- 44.Tepper JE, Suit HD. Radiation therapy alone for sarcoma of soft tissue. Cancer. 1985;56:475–479. doi: 10.1002/1097-0142(19850801)56:3<475::aid-cncr2820560311>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Suit HD, Mankin HJ, Wood WC, et al. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer. 1985;55:2659–2667. doi: 10.1002/1097-0142(19850601)55:11<2659::aid-cncr2820551122>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 46.Wiener ES. Head and neck rhabdomyosarcoma. Semin Pediatr Surg. 1994;3:203–206. [PubMed] [Google Scholar]
- 47.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 48.Tsokos M. The diagnosis and classification of childhood rhab-domyosarcoma. Semin Diagn Pathol. 1994;11:26–38. [PubMed] [Google Scholar]
- 49.Maurer HM, Moon T, Donaldson M, et al. The intergroup rhabdomyosarcoma study: a preliminary report. Cancer. 1977;40:2015–2026. doi: 10.1002/1097-0142(197711)40:5<2015::aid-cncr2820400505>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 50.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdo-myosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]